Abstract
Rhizobia are nitrogen-fixing bacteria that establish endosymbiotic associations with legumes. Nodule formation depends on various bacterial carbohydrates, including lipopolysaccharides, K-antigens, and exopolysaccharides (EPS). An acidic EPS from Rhizobium sp. strain NGR234 consists of glucosyl (Glc), galactosyl (Gal), glucuronosyl (GlcA), and 4,6-pyruvylated galactosyl (PvGal) residues with β-1,3, β-1,4, β-1,6, α-1,3, and α-1,4 glycoside linkages. Here we examined the role of NGR234 genes in the synthesis of EPS. Deletions within the exoF, exoL, exoP, exoQ, and exoY genes suppressed accumulation of EPS in bacterial supernatants, a finding that was confirmed by chemical analyses. The data suggest that the repeating subunits of EPS are assembled by an ExoQ/ExoP/ExoF-dependent mechanism, which is related to the Wzy polymerization system of group 1 capsular polysaccharides in Escherichia coli. Mutation of exoK (NGRΩexoK), which encodes a putative glycanase, resulted in the absence of low-molecular-weight forms of EPS. Analysis of the extracellular carbohydrates revealed that NGRΩexoK is unable to accumulate exo-oligosaccharides (EOSs), which are O-acetylated nonasaccharide subunits of EPS having the formula Gal(Glc)5(GlcA)2PvGal. When used as inoculants, both the exo-deficient mutants and NGRΩexoK were unable to form nitrogen-fixing nodules on some hosts (e.g., Albizia lebbeck and Leucaena leucocephala), but they were able to form nitrogen-fixing nodules on other hosts (e.g., Vigna unguiculata). EOSs of the parent strain were biologically active at very low levels (yield in culture supernatants, ∼50 μg per liter). Thus, NGR234 produces symbiotically active EOSs by enzymatic degradation of EPS, using the extracellular endo-β-1,4-glycanase encoded by exoK (glycoside hydrolase family 16). We propose that the derived EOSs (and not EPS) are bacterial components that play a crucial role in nodule formation in various legumes.
Legumes establish mutualistic associations with nitrogen-fixing bacteria, commonly referred to as rhizobia. Most rhizobia enter roots of host plants via root hairs, where they induce the formation of infection threads. Bacterial invasion and formation of nodules containing nitrogen-fixing bacteroids (the Fix+ phenotype) depend on a molecular dialogue between the symbiotic partners. Host plants secrete flavonoids that induce the synthesis of rhizobial lipo-chito-oligosaccharides called Nod factors (24, 26) and perceive them via a specific signal transduction pathway that culminates in expression of symbiosis-specific plant genes required for nodule development (34). In many symbiotic associations, nodule formation also depends on rhizobial macromolecules that include exopolysaccharides (EPS), lipopolysaccharides, K-antigens, and cyclic β-glucans. These extracellular polysaccharides (or the oligosaccharides derived from them) are crucial in the early stages of nodule formation that begins after rhizobia have entered the host plant (3, 8, 17, 19, 25, 33, 36).
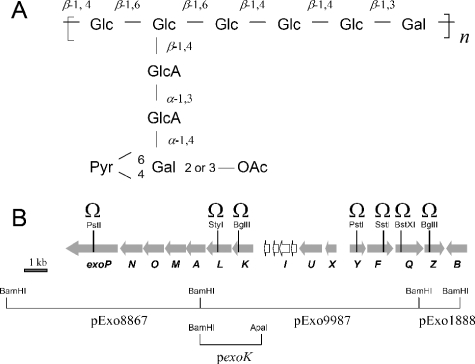
In addition to a protective role, rhizobial EPS have vital but poorly understood roles in infection of legume roots (2). The EPS structures of various rhizobia, including Rhizobium sp. strain NGR234 (also called Sinorhizobium fredii NGR234), which nodulates more than 112 genera of legumes (30), have been described. The repeating subunit of the acidic EPS of NGR234 is a nonasaccharide consisting of glucosyl (Glc), galactosyl (Gal), glucuronosyl (GlcA), and 4,6-pyruvylated galactosyl (PvGal) residues (9) (Fig. 1A). Mutants of NGR234 deficient in EPS synthesis formed nonmucoid (“dry”) colonies on agar plates and lost the capacity to induce the formation of nitrogen-fixing nodules on Leucaena leucocephala (Fix− phenotype). Instead, small empty nodules (pseudonodules) were formed (5). A number of genes of NGR234 involved in synthesis of EPS have been identified in an exo cluster (6, 15, 45), which has been sequenced (38) (Fig. 1B). This cluster is located on the pNGR234b megaplasmid and is similar to the exo cluster of Sinorhizobium meliloti strain 1021 that is required for synthesis of succinoglycan (also called EPS I) (13). Based on known or assumed functions of exo genes in S. meliloti (2, 32), we predict that EPS synthesis in NGR234 depends on the glycosyl transferases (ExoY, ExoA, ExoL, ExoM, ExoO, and ExoU) that form an undecaprenol diphosphate-linked repeating subunit at the cytoplasmic face of the inner membrane. Probably, the lipid-linked subunits are then flipped across the inner membrane and finally assembled by an ExoQ/ExoP/ExoF-dependent polymerization system that is similar to the Wzy polymerization pathway of group 1 capsular polysaccharides in Escherichia coli (41). In this model, ExoQ is a putative polymerase and ExoP is a copolymerase belonging to the MPA1 (cytoplasmic membrane-periplasmic auxiliary protein 1) family. ExoF is probably an outer membrane auxiliary protein required for EPS export. Related genes required for secretion and export of EPS (pssL, pssT, pssP, and pssN) have been identified in Rhizobium leguminosarum bv. trifolii (reference 22 and references therein).
FIG. 1.
(A) Repeating subunit of the acidic EPS from NGR234 (9). Additional acetyl groups are present at unidentified positions. (B) Genetic map of the exo cluster of pNGR234b (38). The exs genes and neighboring exoB gene are not shown. BamHI fragments were cloned into pBluescript II KS(+), yielding pExo8867, pExo9987, and pExo1888. Seven exo genes were mutated by insertion of Ω interposons at the restriction sites indicated. The pexoK plasmid is a pLAFR6 derivative carrying the 2.9-kb BamHI-ApaI fragment indicated. Computer analyses predicted that exoLAMON and exoFQZ form operons. Biochemical characteristics and symbiotic phenotypes of NGRΩexoK, NGRΩexoP, and NGRΩexoZ can be attributed to site-specific mutations.
Low-molecular-weight (LMW) forms of EPS (called exo-oligosaccharides [EOSs]) have been identified in supernatants of rhizobial cultures. Nodulation experiments with purified LMW EPS applied to roots indicated that EOSs could complement the symbiotic defects of exo mutants under certain growth conditions (1, 10, 14, 40). Similar complementation effects were observed when exo mutants were coinoculated with noninvasive strains that synthesize EPS (17, 18). Although not always conclusive, the results of such complementation experiments suggest that EOSs are symbiotically active molecules in certain plants. Synthesis of EOSs seems to be controlled either by enzymes involved in export and polymerization of the lipid-linked repeating EPS subunit or by extracellular glycanases that release EOSs from high-molecular-weight (HMW) forms (14). Glycanases that cleave EPS have been identified in S. meliloti and R. leguminosarum bv. viciae. Corresponding mutants produced lower levels of EOSs without altering the symbiotic phenotype (12, 43). The previously characterized glycanase ExoK secreted by S. meliloti (43, 44) exhibits a high level of sequence similarity [e−129] to the putative ExoK protein of NGR234.
In this study, we mutated exo genes of NGR234 involved in synthesis of the repeating subunit (exoL, exoY, and exoZ), polymerization (exoF, exoP, and exoQ), and production of EOSs (exoK). We provide evidence that EOSs of NGR234 are required for symbiosis with certain plants.
MATERIALS AND METHODS
Construction of mutants.
Standard molecular techniques, as well as cultivation of E. coli, were performed as described by Sambrook et al. (35). Bacterial strains and plasmids used in this study are listed in Table 1. BamHI fragments from a cosmid carrying the sequenced exo cluster of Rhizobium sp. strain NGR234 (accession number AY316746) were cloned into pBluescript II KS(+). pExo1888, pExo8867, and pExo9987 (Fig. 1B) were used to construct the mutants. A spectinomycin resistance Ω interposon was excised from pHP45Ω (28) with SmaI and ligated into the sites indicated in Table 1. When necessary, the Klenow fragment was used to generate blunt ends. E. coli DH5α carrying the mutated genes cloned into a suicide vector (Table 1) was crossed with NGR234 by a triparental mating procedure using the pRK2013 “helper” plasmid. Replacement of the mutated gene was forced by selecting for the resistance of the interposon marker (Spr) and resistance to sucrose (5%, wt/vol). Double-crossover events at homologous sites were confirmed by Southern blot analyses using rhizobial DNA (7).
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid(s) | Characteristicsa | Reference(s) or source |
|---|---|---|
| Rhizobium sp. strains | ||
| NGR234 | Rifr derivative of wild-type Rhizobium sp. strain NGR234 isolated from Lablab purpureus (Rifr) | 39 |
| NGRΩexoF | NGR234 carrying an Ω insertion in the SstI site of exoF (Rifr Spr) | This study |
| NGRΩexoK | NGR234 carrying an Ω insertion in the BglI site of exoK (Rifr Spr) | This study |
| NGRΩexoL | NGR234 carrying an Ω insertion in the StyI site of exoL (Rifr Spr) | This study |
| NGRΩexoP | NGR234 carrying an Ω insertion in the C-terminal PstI site of exoP (Rifr Spr) | This study |
| NGRΩexoQ | NGR234 carrying an Ω insertion in the BstXI site of exoQ (Rifr Spr) | This study |
| NGRΩexoY | NGR234 carrying an Ω insertion between the two PstI sites of exoY (Rifr Spr) | This study |
| NGRΩexoZ | NGR234 carrying an Ω insertion in the BglII site of exoZ (Rifr Spr) | This study |
| E. coli DH5α | supE44 ΔlacU169 (φ80lacZΔM15) hsdR17 recA1 endA1 gyrA96 thi-1 relA1 | BRL, Bethesda, MD |
| Plasmids | ||
| pBluescript II KS(+) | High-copy-number ColE1-based phagemid (Apr) | Stratagene, La Jolla, CA |
| pExo1888 | pBluescript II KS(+) derivative carrying a 1.8-kb BamHI fragment of NGR234 containing exoZ (Apr) | This study |
| pExo8867 | pBluescript II KS(+) derivative carrying an 8.8-kb BamHI fragment of NGR234 containing exoP (Apr) | This study |
| pExo9987 | pBluescript II KS(+) derivative carrying a 9.9-kb BamHI fragment of NGR234 containing exoQ, exoF, exoY, exoK, and exoL (Apr) | This study |
| pHP45Ω | Vector containing an omega (Ω) interposon (Apr Spr) | 28 |
| pJQ200SK, pJQ200mp18, pJQ200uc1 | Suicide vectors used in directed mutagenesis (Ger) | 31 |
| pRK2013 | Tra+ helper plasmid for mobilization (Kmr) | 11 |
| pLAFR6 | Broad-host-range vector containing transcriptional terminators flanking cloning sites (Tcr) | D. Dahlbeck and B. Staskawicz, unpublished data |
| pFAJ1815 | Mini-Tn5 transposon derivative containing mTn5gfp-pgusA (constitutively expressed gusA) | 42 |
| pexoK | pLAFR6 derivative carrying a 2.9-kb BamHI-ApaI fragment of NGR234 containing exoK (Tcr) | This study |
| pEX41 | pSUP104 derivative carrying a 3.6-kb fragment of S. meliloti 1021 containing exoK (Tcr) | 21 |
| pEX80 | pSUP104 derivative carrying an 8-kb fragment of S. meliloti 1021 containing various exo genes but lacking exoK (Tcr) | 21 |
| pEX312 | pLAFR1 derivative carrying the exo gene cluster of S. meliloti 1021 (from exoP to exoB) (Tcr) | 21 |
| pD2 | pLAFR1 derivative carrying a fragment of S. meliloti 1021 containing exs genes (including exsH) (Tcr) | 19, 21 |
Apr, Ger, Kmr, Rifr, Spr, and Tcr, resistance to ampicillin, gentamicin, kanamycin, rifampin, spectinomycin, and tetracycline, respectively.
Strains carrying pexoK and plasmids with exo genes of S. meliloti.
pExo9987 was digested with ApaI, and the 2.9-kb fragment containing exoK was cloned into pBluescript II KS(+). The construct was transferred into the HindIII and KpnI sites of pLAFR6, yielding plasmid pexoK (Fig. 1B). Then this plasmid was conjugated into NGRΩexoK (and its parent strain, NGR234) using a triparental mating procedure and the helper plasmid pRK2013. NGRΩexoK derivatives carrying exo genes of S. meliloti on plasmids pEX41, pEX80, pEX312, and pD2 (21) were obtained in a similar manner.
Visualization of strains constitutively expressing gusA.
The mini-Tn5 transposon derivative pFAJ1815 containing Tn5gfp-pgusA was introduced into NGR234, NGRΩexoK, and NGRΩexoZ as described by Xi et al. (42). Tagged colonies that constitutively expressed gusA (which encodes β-glucuronidase [GUS]) were selected on agar plates containing 5-bromo-4-chloro-3-indolyl-β-d-glucuronic acid. Nodulation tests with various host plants indicated that the Tn5gfp-pgusA insertion did not alter the symbiotic phenotype. Roots were harvested, immersed in ice-cold 90% (vol/vol) acetone for 30 min, washed several times with sodium phosphate buffer (200 mM, pH 7.2), and incubated with the substrate 5-bromo-4-chloro-3-indolyl-β-d-glucuronic acid (0.5 mg ml−1 in sodium phosphate buffer) under reduced pressure for 2 h at room temperature. The samples were then incubated at 37°C and washed with the sodium phosphate buffer at room temperature. After fixation with glutaraldehyde (2.5%, vol/vol) and stepwise dehydration with increasing volumes of ethanol (10%, 30%, 50%, and 70% [vol/vol] over a 24-h period), the stained nodules and pseudonodules were photographed using a binocular microscope.
Purification of EPS and EOSs.
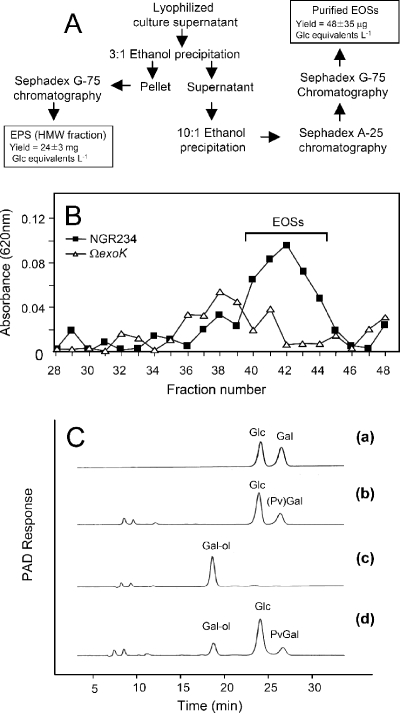
NGR234 and its derivatives were cultivated in modified GMS medium (5 g liter−1 mannitol, 1 g liter−1 glutamic acid, 6 mM K2HPO4; adjusted with KOH to pH 7). After autoclaving, the medium was supplemented with 1 mM MgSO4, 250 μM CaCl2, 100 μg liter−1 MnCl2 · 4H2O, 10 μg liter−1 H3BO3, 10 μg liter−1 ZnSO4 · 7H2O, 10 μg liter−1 CoCl2 · 6H2O, 10 μg liter−1 CuSO4 · 5H2O, 2.5 mg liter−1 FeCl3 · 6H2O, and 1 ml liter−1 Gamborg's B5 vitamin solution (Sigma-Aldrich, St. Louis, MO). To purify EPS (see Fig. 4A), 1 ml of a bacterial preculture (optical density at 600 nm, 1) was added to 500 ml modified GMS medium (in a 2.5-liter Erlenmeyer flask). The bacteria were then grown on a rotary shaker (220 rpm) at 24°C for 5 days. At the time of harvest (optical density at 600 nm, ∼2.6), the culture was centrifuged twice, and the clear supernatant was freeze-dried. The lyophilized powder was then suspended in water to obtain a fivefold-concentrated supernatant fraction, to which ethanol was added in a stepwise fashion. Addition of 3 volumes of ethanol resulted in precipitation of HMW forms of EPS, whereas LMW molecules precipitated only with seven additional volumes of ethanol. The precipitated HMW fraction was either dialyzed against water or purified further on a Sephadex G-75 size exclusion chromatography (SEC) column (960 by 12 mm) with a volatile running buffer (100 mM pyridine/acetate, pH 5). Fractions (1.5 ml) were collected, aliquots were assayed with anthrone-sulfuric acid (20), and the HMW forms of EPS (corresponding to fractions 14 to 26) were lyophilized. The ethanol-precipitated LMW fraction was air dried, resuspended in 25 mM Tris-HCl (pH 7.6), and loaded on a DEAE-Sephadex A-25 column (10). After the column was washed with the same buffer, EOSs were eluted with 25 mM Tris-HCl (pH 7.6) containing 100 mM NaCl. This material was lyophilized and loaded on a Sephadex G-75 SEC column as described above. Fractions were analyzed colorimetrically for carbohydrates using the anthrone method (20). The EOSs in fractions 40 to 44 (see Fig. 4B) were pooled and lyophilized.
FIG. 4.
Characterization of EOSs. (A) Purification steps and yield of HMW forms of EPS and EOSs of NGR234. Bacterial supernatants were precipitated with ethanol in a stepwise fashion. EOSs were applied to a DEAE-Sephadex A-25 column in 25 mM Tris-HCl (pH 7.6) and eluted with 100 mM NaCl added to the same buffer. The final purification step was SEC on a Sephadex G-75 column. (B) SEC (Sephadex G-75) chromatography of EOSs. Fractions (1.5 ml) were collected, and the carbohydrates were analyzed using the anthrone method (absorbance at 620 nm). EOSs produced by NGR234 eluted in fractions 40 to 44. (C) Effect of NaBH4 reduction. HMW forms of EPS and EOSs were subjected to NaBH4 reduction and complete acid hydrolysis. Glc, Gal, and the resulting galactitol were analyzed by Dionex chromatography. Uronic acids were not analyzed. Chromatogram a shows the results for Glc and Gal standards. As shown in chromatogram b, purified HMW forms of EPS from NGR234 resulted in formation of Glc, PvGal, and Gal. PvGal and Gal coeluted [peak (Pv)Gal]. The ratio of Glc to (Pv)Gal was 5:2, as determined by integration of peak areas. As shown in chromatogram c, the Gal standard treated with NaBH4 was completely converted to galactitol. Chromatogram d shows that NaBH4 reduction of EOSs from NGR234 resulted in the formation of Glc, PvGal, and galactitol (PvGal/galactitol ratio, 1:1).
Visualization of EPS production on agar plates.
NGR234 and its derivatives were cultivated on modified GMS agar plates supplemented with 5% (wt/vol) sucrose and 100 mg liter−1 Congo red. Cultures were incubated at room temperature for 2 weeks and then photographed. EPS-producing strains had a “mucoid” phenotype that could easily be distinguished from mutants deficient in EPS synthesis (“nonmucoid” phenotype).
Chemical analyses of HMW forms of EPS.
Glycosyl compositions were determined for per-O-(trimethylsilyl) (TMS) methyl glycoside derivatives by gas chromatography (GC)-mass spectrometry (MS) analysis. Samples were dissolved in 2 M methanolic HCl using sonication for at least 5 h. Then the samples were hydrolyzed for 16 h at 80°C and subjected to N acetylation and trimethylsilylation (Tri-Sil reagent; Pierce Chemical Co., Woburn, MA). The resulting TMS-methyl glycosides were analyzed by GC-MS using a 30-m DB-5 capillary column equipped with a mass selective detector.
Glycosyl linkage analyses were performed using the Hakomori procedure, combined with carboxyl reduction to detect the uronic acid linkages. Sonication was used to help dissolve the samples in dimethyl sulfoxide. About 1 h of sonication was required to obtain clear solutions. The samples were then treated with 2 M potassium methylsulfonyl carbanion for 8 h at room temperature, and this was followed by addition of a 500- to 100-fold molar excess of methyl iodide and incubation for 8 h at room temperature. The partially methylated material was isolated by extraction into methylene chloride and then treated with triethylborodeuteride (Superdeuteride; Sigma-Aldrich) for 3 h at room temperature in order to reduce the carboxyl groups of uronic acid. The residue was isolated, desodiated by treatment with a cation-exchange resin that was eluted with ethanol-water, and subjected to sequential hydrolysis (2 M trifluoroacetic acid, 2 h, 121°C), reduction (NaBD4), and acetylation (trifluoroacetic acid-acetic anhydride [1:1, vol/vol] for 10 min at 50°C). The resulting partially methylated alditol acetates were analyzed by GC-MS using a 30-m SP-2330 capillary column equipped with a mass selective detector.
Nuclear magnetic resonance (NMR) analyses were performed with a 600-MHz Varian spectrometer (Varian Inc., Palo Alto, CA). Samples were analyzed in D2O using sonication to increase solubilization. Purified EPS from NGR234 and NGRΩexoZ were subjected to 1D-proton, 1H-1H correlation spectroscopy (COSY), and 1H-13C heteronuclear single quantum coherence (HSQC) analyses. COSY data were collected at 40 transients per increment with a 256 increment × 2,400 data point matrix size. HSQC was conducted with 180 to 200 transients and 128 increments using an acquisition time of 0.20 s.
Chemical analysis of EOSs.
Purified EOS fractions from NGR234 and NGRΩexoK were examined by matrix-assisted laser desorption ionization—time of flight (MALDI-TOF) analysis (positive ionization mode) using the Voyager 1247 system (Applied Biosystems, Foster City, CA). The predicted molecular masses of the oligosaccharides were calculated using the following average incremental masses: hexose, 162.14 Da; hexuronic acid, 176.12 Da; pyruvate, 70.05 Da; acetate, 42.04 Da; and free reducing ends, 18.02 Da. The degree of polymerization (d.p.) of purified EOSs was determined as described by Gonzalez et al. (14). Briefly, the reducing ends of the oligosaccharides were reduced with 0.1 M NaBH4 in 5 mM NaOH at 37°C for 7 h, neutralized with acetic acid, and desalted on a Dowex 50W-X4 (H+ form) column. The sample was then subjected to hydrolysis with 4 M trifluoroacetic acid at 100°C for 3.5 h, and the dried monosaccharides (Glc and Gal and their alditols) were analyzed by anion-exchange high-performance liquid chromatography coupled with a pulsed amperometric detection system (Dionex Corp., Sunnyvale, CA). Monosaccharides and their alditols were separated on a CarboPac MA-1 column (4 by 250 mm; Dionex) with an isocratic NaOH concentration of 0.5 M and a flow rate of 0.4 ml/min (14).
Nodulation tests.
Albizia lebbeck (L.) Benth., Albizia procera (Roxb.) Benth., Kennedia rubicunda (Schneev.) Vent., L. leucocephala (Lam.) De Wit cv. Peru and cv. Cunninghami, and Sophora tomentosa L. were purchased from AustraHort (Bomaderry, N.S.W., Australia). Chamaecrista fasciculata (Michx.) Greene was obtained from Prairie Moon Nursery (Winona, MN), and Vigna unguiculata (L.) Walp. cv. Red Caloona was obtained from Rawlings Seeds (Orpington, Kent, United Kingdom). All seeds were surface sterilized by treatment with concentrated sulfuric acid followed by 5% (vol/vol) hydrogen peroxide and then left to germinate on water agar plates. Plantlets (root length, 1 to 3 cm) were transferred to sterilized Magenta jars (one plant per jar). The upper assembly was filled with vermiculite, and the lower assembly was filled with B&D nutrient solution (4). Rhizobial strains were cultivated in modified GMS medium (see above) on a rotary shaker (150 rpm) at 27°C prior to inoculation. Plants were inoculated with ∼109 bacteria and grown at a day temperature of 26°C and a night temperature of 20°C with a 16-h light phase (including a 1-h stepped “sunrise” and a 1-h stepped “sunset”; maximum intensity of illumination, 350 μmol m−2 s−1 photosynthetically active radiation). Plants were harvested at different times postinoculation. Dry weights of nodules, roots, and leaves were determined after lyophilization. When indicated below, the dried material was pulverized in a Retch mill and used for determination of the nitrogen content with a LECO-1000 CHN analyzer (LECO, St. Joseph, MI). Data from nodulation experiments were statistically analyzed using the nonparametric Kruskal-Wallis rank sum test, which is suitable for unequal replications. A P value of ≤0.05 was considered significant in this study.
RESULTS
Characterization of exo mutants.
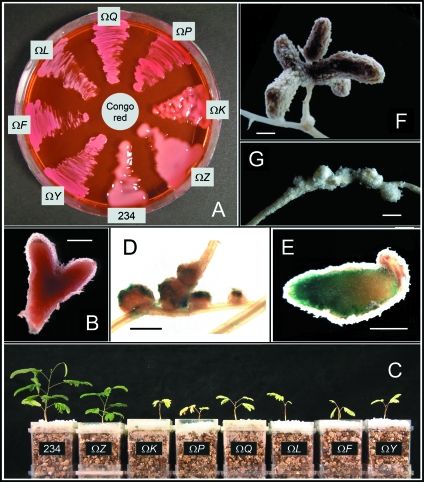
Knockout mutants constructed by inserting Ω interposons into various open reading frames of the exo cluster (Fig. 1B and Table 1) formed nonmucoid colonies when they were grown on GMS agar plates, a phenotype that is typical of exo mutants that are deficient in EPS synthesis (5). In contrast, a mucoid layer surrounded the colonies of the parent strain. The differences between NGR234 and mutants that did not produce EPS were most pronounced, however, when GMS agar plates were supplemented with the dye Congo red and high levels of sucrose. Under these conditions, the NGRΩexoF, NGRΩexoL, NGRΩexoP, NGRΩexoQ, and NGRΩexoY mutants were red and nonmucoid, whereas NGRΩexoK and NGRΩexoZ were mucoid, suggesting that they produced EPS (Fig. 2A).
FIG. 2.
Phenotypes of Exo− mutants. (A) Parent strain NGR234 and Exo− mutants were grown on GMS agar plates containing 5% (wt/vol) sucrose and Congo red. NGRΩexoF (ΩF), NGRΩexoL (ΩL), NGRΩexoP (ΩP), NGRΩexoQ (ΩQ), and NGRΩexoY (ΩY) formed nonmucoid (“dry”) streaks and absorbed Congo red. NGR234 (234), NGRΩexoK (ΩK), and NGRΩexoZ (ΩZ) formed mucoid colonies. (B) Typical nitrogen-fixing nodule of L. leucocephala cv. Peru induced by NGR234 and harvested 92 days postinoculation. Nodules of L. leucocephala are often branched at this stage. (C) L. leucocephala cultivated in Magenta jars and inoculated with NGR234, as well as NGRΩexoF, NGRΩexoK, NGRΩexoL, NGRΩexoP, NGRΩexoQ, NGRΩexoY, and NGRΩexoZ. Plants were photographed 61 days postinoculation. Only nodules containing NGR234 and NGRΩexoZ fixed nitrogen. (D) Pseudonodules of L. leucocephala induced by NGRΩexoK expressing the gusA gene (42 days postinoculation; Fix− phenotype). Bacteria colonized the surface of pseudonodules, whereas GUS staining was not detected within pseudonodules. (E) Nitrogen-fixing nodule induced by a NGR234 derivative expressing the gusA gene (59 days postinoculation; Fix+ phenotype). Nodulated roots were stained for GUS activity. (F) Typical nodule of A. lebbek containing nitrogen-fixing bacteroids of NGR234 (87 days postinoculation; Fix+ phenotype). (G) Pseudonodules of A. lebbek induced by NGRΩexoK (87 days postinoculation; Fix− phenotype). Bars = 1 mm.
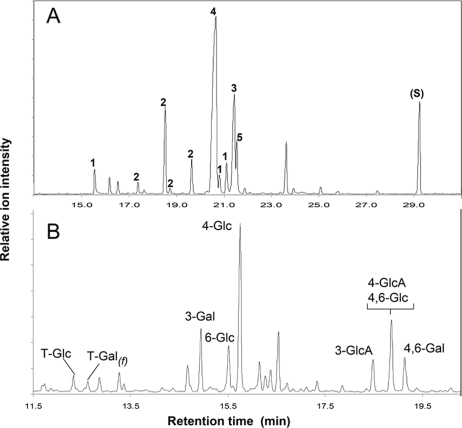
As determined by the anthrone-sulfuric acid carbohydrate assay, the carbohydrate levels in supernatants from cultures of mutant bacteria that exhibited a nonmucoid phenotype were about 10-fold lower than the carbohydrate levels in supernatants from the strains that produced mucoid colonies (data not shown). To confirm that the nonmucoid colony phenotype correlated with a deficiency of EPS synthesis, we analyzed the extracellular glycosyl compositions of bacterial suspensions. As shown in Table 2, all samples contained Glc and Gal residues. Striking differences were found for GlcA and PvGal, the residues which form the side chains of the repeating EPS subunit (Fig. 1A). Both of these residues were present in supernatants of parent strain NGR234, as well as in the NGRΩexoK and NGRΩexoZ mutants. Detectable amounts of GlcA and PvGal did not accumulate in the culture supernatants of all other exo mutants, however. Hence, GlcA and PvGal are specific to EPS, whereas Glc and Gal residues are also present in other secreted molecules. Further purification of ethanol-precipitated EPS by SEC on Sephadex G-75 showed that the glycosyl composition of HMW fractions (Table 2) agreed with the glycosyl composition described previously for EPS (9). As expected, the Glc/GlcA/Gal/PvGal ratio was 5:2:1:1 (Fig. 1A). A typical GC-MS chromatogram of purified EPS is shown in Fig. 3A, and similar results were obtained for HMW forms of EPS purified from the NGRΩexoK and NGRΩexoZ mutants (Table 2).
TABLE 2.
Glycosyl compositions of ethanol-precipitated bacterial culture supernatants and of EPS (HMW fraction) purified by gel filtration
| Strain | Glycosyl composition (mol%)a
|
|||
|---|---|---|---|---|
| Gal | Glc | GlcA | PvGal | |
| Culture supernatant precipitated with ethanolb | ||||
| NGR234 | 20.2 | 52.3 | 11.3 | 8.9 |
| NGRΩexoF | 46.6 | 53.4 | 0c | 0c |
| NGRΩexoK | 21.2 | 46.4 | 6.0 | 12.3 |
| NGRΩexoL | 54.8 | 45.2 | 0 | 0 |
| NGRΩexoP | 38.8 | 61.2 | 0 | 0 |
| NGRΩexoQ | 38.1 | 61.9 | 0 | 0 |
| NGRΩexoY | 63.6 | 36.4 | 0 | 0 |
| NGRΩexoZ | 19.9 | 49.9 | 9.7 | 5.4 |
| Purified EPS (HMW fraction) after Sephadex G-75 chromatography | ||||
| NGR234 | 16.4 | 51.4 | 13.5 | 12.2 |
| NGRΩexoK | 21.9 | 53.4 | 9.4 | 9.9 |
| NGRΩexoZ | 14.9 | 51.2 | 13.6 | 8.8 |
Gal, galactose; Glc, glucose; GlcA, glucuronic acid; PvGal, 4,6-pyruvylated galactose.
Ethanol-precipitated culture supernatants contained variable amounts of xylose and mannose. All samples also contained trace amounts of 3-deoxy-d-manno-2-octulosonic acid and galacturonic acid.
≤0.01 mol% based on a detection limit of ∼1 ng by GC-MS.
FIG. 3.
Representative GC-MS analysis of purified EPS (HMW forms). (A) Glycosyl composition analysis: total-ion chromatogram of the TMS-α/β-methyl glycosides derived from EPS (NGR234). Peak 1, glucuronic acid; peak 2, galactose; peak 3, glucose; peak 4, glucose and 4,6-pyruvate acetal of galactose coelute; peak 5, 4,6-pyruvate acetal of galactose; peak S, internal standard. Unlabeled peaks are components arising from the culture medium. (B) Glycosyl linkage analysis: total-ion chromatogram of the partially methylated alditol acetate derivatives prepared from NGR234. For an explanation of abbreviations see Table 4.
To characterize the symbiotic phenotypes of the different exo mutants, nodulation tests were performed with the NGR234 hosts L. leucocephala and V. unguiculata. NGR234 and all the exo mutants induced the formation of nitrogen-fixing nodules on V. unguiculata (Fix+ phenotype), suggesting that exo genes are not required for nodulation of V. unguiculata. When inoculated onto L. leucocephala, however, the five nonmucoid colony mutants (the mutants with mutations in exoF, exoL, exoP, exoQ, and exoY) were unable to establish nitrogen-fixing symbioses (Fix− phenotype), whereas the parent strain and NGRΩexoZ formed fully Fix+ nodules. Interestingly, L. leucocephala plants inoculated with NGRΩexoK displayed a Fix− phenotype (Fig. 2C). Nitrogen-fixing nodules containing NGR234 or NGRΩexoZ were pink. With increasing age, the meristematic zone of the “indeterminate” nodules often branched, resulting in two or more nodule compartments (Fig. 2B). The amounts of nitrogen in plants inoculated with NGR234 or NGRΩexoZ were about 10-fold higher than the amounts of nitrogen in plants inoculated with the other mutants. All interactions with L. leucocephala that showed a Fix− phenotype resulted in the production of small spherical nodule structures (called “pseudonodules”). To visualize the bacteria, mutant derivatives expressing gusA were inoculated onto L. leucocephala. Bacteria expressing GUS were found in nitrogen-fixing nodules whose formation was induced by NGR234 and NGRΩexoZ, but not in pseudonodules whose formation was induced by NGRΩexoK expressing gusA (Fig. 2D shows a pseudonodule whose formation was induced by NGRΩexoK expressing gusA). “Free-living” bacteria occasionally colonized the outer surface of pseudonodules. In contrast, nodules whose formation was induced by the gusA-expressing derivative of NGR234 were highly stained (Fig. 2E). These findings indicated that the pseudonodules that we observed were small, uninfected nodules and that the mutant strains that induced the formation of these structures could not invade these “empty nodules.”
NGRΩexoK produces HMW forms of EPS but not EOSs.
ExoK of S. meliloti is an extracellular glycanase that belongs to glycosyl hydrolase family 16 (27). This enzyme cleaves succinoglycan and thus contributes to the formation of LMW succinoglycan (43, 44). Therefore, we tested whether production of EOSs by NGR234 and production of EOSs by NGRΩexoK differ. An overview of the various purification steps is shown in Fig. 4A. An LMW EPS fraction was separated from HMW forms by stepwise precipitation with ethanol. EOSs from NGR234 (containing GlcA residues) were negatively charged under basic conditions and were purified further by anion-exchange chromatography on a DEAE-Sephadex A-25 column. This purification step removed about 50% of other oligosaccharides, as determined by the anthrone test. In a final purification step, the EOSs were fractioned by SEC on a Sephadex G-75 column. Differences between NGR234 and NGRΩexoK occurred in fractions 40 to 44, which were combined. The carbohydrate concentrations in this combined EOS fraction of NGR234 (determined using the anthrone test and expressed in Glc equivalents) were approximately 50 μg per liter of culture supernatant. This yield is about 500-fold lower than the yield obtained for polymeric EPS (HMW forms) (∼24 mg per liter of culture) (Fig. 4A).
LMW succinoglycans of S. meliloti consist of fractions with different degrees of polymerization, including the monomer (d.p., 1), the dimer (d.p., 2), and the trimer (d.p., 3). We used these fractions as molecular weight standards to calibrate Sephadex G-75 columns. The material in the purified EOS fraction (Fig. 4B, fractions 40 to 44) corresponded to an estimated molecular mass of 1 to 2 kDa. This suggests that the EOSs of NGR234 are monomers (d.p., 1). To confirm this, EOSs from NGR234 were subjected to NaBH4 reduction (14), which converted Gal to galactitol (Gal-ol) at the reducing end of the oligosaccharide. After complete acidic hydrolysis, the monomeric sugars (except GlcA) were analyzed by Dionex chromatography (Fig. 4C). Complete conversion of a Gal standard to Gal-ol was observed under the test conditions. When applied to EOSs of NGR234, this procedure resulted in formation of Glc, PvGal, and Gal-ol, whereas Glc-ol was not formed. Quantitative analysis of peaks indicated that the amounts of PvGal and Gal-ol were nearly equal (ratio of Glc to Gal, approximately 5:2). These results are consistent with the hypothesis that a repeating nonasaccharide subunit of EPS contains PvGal and Gal residues at a 1:1 ratio (Fig. 1A). NaBH4 reduction of polymeric EPS was also performed. Glc and (pyruvylated) Gal were found at the expected 5:2 ratio, whereas the amount of Gal-ol formed was below the detection threshold (Fig. 4C).
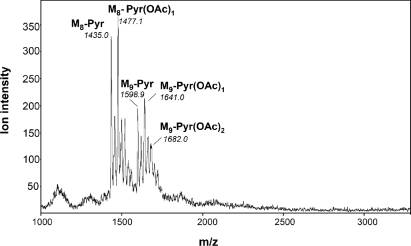
Purified EOSs of NGR234 (Fig. 4B, SEC fractions 40 to 44) were analyzed further by positive-ion MALDI-TOF MS. Interestingly, two ion families were observed, indicating that octasaccharides and nonasaccharides were present (the calculated mass difference for hexose is 162.14 mass units). The proposed sodiated ions, their predicted m/z ratios, and the measured signals (Fig. 5) are shown in Table 3. Consistent with the observed masses, both the octasaccharides and the nonasaccharides contained various O-acetyl and pyruvate-acetal groups. These substituents were also detected during a 1H NMR analysis of the HMW EPS (see below). Analysis of fractions 40 to 44 from NGRΩexoK (Fig. 4B) resulted in MALDI-TOF spectra containing only faint background signals, which were devoid of oligosaccharides. MALDI-TOF analyses of material from neighboring fractions of the parent strain (Fig. 4B, SEC fractions 35 to 39) did not provide conclusive evidence that there were repeating EPS subunits with d.p. of >1, confirming the data obtained by the NaBH4 reduction method (spectra not shown).
FIG. 5.
Positive-ion MALDI-TOF mass spectrum of the purified EOS fraction derived from NGR234. Two major ion families were observed, corresponding to the nonasaccharide and octasaccharide subunits. All ions were sodiated and are listed in Table 3 along with their proposed formulas and the predicted ions. For an explanation of abbreviations see Table 3.
TABLE 3.
Positive-ion MALDI-TOF analysis of components of the EOS fraction purified from NGR234
| Proposed formulaa | [M+Na]+1
|
[(MNa)+Na]+1
|
||
|---|---|---|---|---|
| Predictedb | Observed | Predictedb | Observed | |
| M8 | 1366.1 | NOc | 1388.1 | NO |
| M8-Pyr | 1436.1 | 1435.0 | 1458.1 | 1456.5 |
| M8-Pyr(OAc)1 | 1478.2 | 1477.1 | 1500.2 | 1499.3 |
| M8-Pyr(OAc)2 | 1520.2 | 1519.2 | 1542.2 | 1541.2 |
| M8-Pyr(OAc)3 | 1562.3 | 1561.3 | 1562.3 | 1561.9 |
| M9 | 1528.3 | NO | 1550.2 | NO |
| M9-Pyr | 1598.3 | 1598.9 | 1620.3 | 1619.8 |
| M9-Pyr(OAc)1 | 1640.3 | 1641.0 | 1662.3 | 1663.0 |
| M9-Pyr(OAc)2 | 1682.4 | 1682.0 | 1704.4 | 1702.9 |
| M9-Pyr(OAc)3 | 1724.4 | 1723.8 | ||
M8, octasaccharide subunit consisting of six hexosyl residues and two GlcA residues; M9, nonasaccharide subunit consisting of seven hexosyl residues and two GlcA residues; Pyr, pyruvate attached in acetal linkage; OAc, O-acetyl esters.
Values were calculated from the average incremental masses of the component glycosyl and noncarbohydrate residues as described in Materials and Methods. Sodium adducts of sodiated molecular ions, [MNa]Na+1, arose by charge neutralization of some carboxyl groups with sodium. Other less abundant ions, containing K+, were also observed.
NO, not observed.
Although deficient in synthesis of EOSs, NGRΩexoK produced HMW forms of EPS. As indicated above, NGRΩexoK and parent strain NGR234 secreted large amounts of a polysaccharide that contained GlcA and PvGal (Table 2). To obtain additional evidence that HMW forms of EPS are present in NGRΩexoK, glycosyl linkage analyses were performed using the Hakomori procedure. The results of a gas-liquid chromatography-MS analysis of partially methylated alditol acetates are shown in Table 4, and a representative total ion chromatogram is shown in Fig. 3B. HMW forms of EPS purified from NGR234 and NGRΩexoK produced similar chromatographic patterns comprising essentially identical glycosyl linkages and ratios that are consistent with the hypothesis that a nonasaccharide structure is the repeating subunit of EPS (Fig. 1A). Moreover, NMR analyses (1D-proton, 1H-1H COSY, and 1H-13C HSQC) of purified polymeric EPS from both strains resulted in identical spectra that are consistent with the EPS structure of NGR234 (spectra not shown). Relevant signals from noncarbohydrate substituents and their chemical shifts (δH) arose from lactate H2 (3.19 ppm) and H3 (1.27 ppm), pyruvate-CH3 (1.47 ppm), and O-acetyl-CH3 (1.96 to 2.19 ppm). Together, these findings demonstrate that production of EOSs by NGRΩexoK is impaired, but the synthesis of HMW forms is not affected.
TABLE 4.
Glycosyl linkage analysis of EPS (HMW fraction) from NGR234 and mutant NGRΩexoK
| Glycosyl linkagea | NGR234
|
NGRΩexoK
|
Ratio in EPS structureb | ||
|---|---|---|---|---|---|
| Total area (%) | Area ratio | Total area (%) | Area ratio | ||
| T-Glucose | 0.8 | 0.1 | 0.6 | 0.1 | |
| T-Galactose (f) | 0.4 | 0.0 | 4.0 | 0.3 | |
| 3-Galactose | 13.1 | 1.2 | 14.2 | 1.2 | 1 |
| 6-Glucose | 11.2 | 1.0 | 12.1 | 1.0 | 1 |
| 4-Glucose | 38.5 | 3.4 | 38.2 | 3.2 | 3 |
| 3-Glucuronic acid | 7.9 | 0.7 | 7.0 | 0.6 | 1 |
| 4-Glucuronic acid | 9.4 | 0.8 | 8.1 | 0.7 | 1 |
| 4,6-Glucose | 9.5 | 0.8 | 8.1 | 0.7 | 1 |
| 4,6-Galactose | 9.2 | 0.8 | 7.6 | 0.6 | 1 |
Glycosyl linkages were determined by GC-MS analysis of partially methylated alditol acetate derivatives. T, terminal residue; f, furanose form.
The two strains have similar glycosyl linkage ratios that are consistent with the structure of the nonasaccharide repeating subunit (Fig 1A).
Symbiotic defects of NGRΩexoK can be complemented.
A pLAFR6 derivative containing exoK of NGR234 (including its putative promoter region) was constructed and designated pexoK (Table 1 and Fig. 1B). Then the pLAFR6 vector, as well as pexoK, was mobilized into NGRΩexoK and into NGR234, and the resulting transconjugants were tested to determine their capacities to induce the formation of nodules on L. leucocephala. Complementation was obtained with NGRΩexoK(pexoK), and the complemented mutant regained the ability to induce the formation of pink Fix+ nodules. A few pseudonodules were also seen on the plants. Under nitrogen-limiting conditions, inoculation with NGRΩexoK(pexoK) strongly stimulated plant growth (Fix+ phenotype). Compared to the parent strain, however, NGRΩexoK(pexoK) induced the formation of fewer nodules, suggesting that pexoK is not stably maintained in the absence of selection for tetracycline. Plants inoculated with the control strain, NGRΩexoK(pLAFR6), developed only pseudonodules (Table 5).
TABLE 5.
Nodule formation on L. leucocephala cv. Peru inoculated with NGRΩexoK carrying pexoK and exo plasmids of S. meliloti 1021
| Strain | No. of pink Fix+ nodulesa | Phenotype |
|---|---|---|
| NGR234 (parent) | 12.2 ± 2.0 | Fix+ |
| NGRΩexoK | 0 | Fix− |
| NGRΩexoK(pexoK) | 3.1 ± 0.8 | Fix+ |
| NGRΩexoK(pLAFR6) | 0.2 ± 0.2 | Fix− |
| NGR234(pLAFR6) | 10.3 ± 1.5 | Fix+ |
| NGR234(pexoK) | 9.6 ± 2.7 | Fix+ |
| NGRΩexoK(pEX41) | 0.8 ± 0.3 | Fix+/− |
| NGRΩexoK(pD2) | 0 | Fix− |
| NGRΩexoK(pEX312) | 7.5 ± 0.9 | Fix+ |
| NGRΩexoK(pEX80) | 0 | Fix− |
| No bacteria (control) | 0 | Fix− |
The strains were tested to determine their capacities to induce nitrogen-fixing nodules (time of harvest, 67 days postinoculation). Data for pseudonodules are not shown. The values are means ± standard errors (n = 6).
Further complementation tests were performed with plasmids carrying exo genes of S. meliloti (Table 5). The NGRΩexoK(pEX41) transconjugant (pEX41 carries a 3.6-kb fragment containing the glycanase encoded by exoK) induced the production of nitrogen-fixing nodules at a low frequency (a single Fix+ nodule per plant; Fix+/− phenotype). A complete Fix− phenotype was observed when pD2 (containing exsH, which encodes another glycanase of S. meliloti) was mobilized into NGRΩexoK. In contrast, NGRΩexoK(pEX312) formed Fix+ nodules. pEX312 includes the genes from exoP to exoB of S. meliloti. NGR234 derivatives carrying exo genes of S. meliloti produce oligosaccharides with the characteristics of LMW succinoglycan of S. meliloti (16). Purification of LMW fractions from NGRΩexoK(pEX312) yielded significant amounts of oligosaccharides which did not bind to DEAE-Sephadex A-25 at pH 7.6 (data not shown). In contrast, NGRΩexoK(pEX80) (pEX80 lacks exoK) did not induce the formation of Fix+ nodules on L. leucocephala. Thus, it seems that endoglycanases ExoK and ExsH of S. meliloti cannot functionally replace ExoK of NGR234, whereas oligosaccharides synthesized by the exo cluster of S. meliloti have a symbiosis-promoting effect similar to that of the EOSs of NGR234.
Symbiotic phenotype of NGRΩexoK with various hosts.
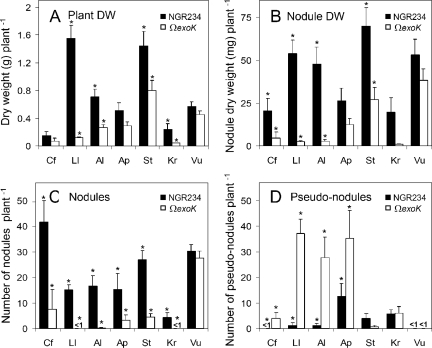
One of only two hosts of NGR234 in the Caesalpinioideae is C. fasciculata (30). Inoculation of C. fasciculata with either NGR234 or NGRΩexoK showed that the mutant has a lower symbiotic potential than the wild type. Fewer and smaller nodules were formed, whereas the number of pseudonodules increased (Fig. 6A to D). The level of nitrogen fixation in nodules of C. fasciculata was low compared with the levels in other plants (nitrogen content with NGR234, 21 mg N g [dry weight]−1; nitrogen content with NGRΩexoK, 13 mg N g [dry weight]−1).
FIG. 6.
Plant growth and nodulation of various legumes inoculated with NGR234 or NGRΩexoK (ΩexoK). At the time of harvest, the following data were acquired for each plant: total plant biomass (dry weight) (A), nodule dry weight (biomass of nodules and pseudonodules) (B), number of fully developed nodules (C), and number of pseudonodules (D). The data are means ± standard errors (n = 5 for V. unguiculata; n = 6 for A. procera and K. rubicunda; n = 7 for A. lebbeck, C. fasciculata, L. leucocephala, and S. tomentosa). The asterisks indicate statistically significant differences (P < 0.05, as determined by the Kruskal-Wallis test) between plants inoculated with NGR234 and NGRΩexoK. Abbreviations: Al, A. lebbeck 69 days postinoculation (Fig. 2F and 2G); Ap, A. procera 77 days postinoculation; Cf, C. fasciculata 69 days postinoculation; Kr, K. rubicunda 77 days postinoculation; Ll, L. leucocephala cv. Cunninghami 80 days postinoculation; St, S. tomentosa 101 days postinoculation; Vu, V. unguiculata 34 days postinoculation; DW, dry weight.
In contrast to the Caesalpinioideae, NGR234 nodulates 90% of the mimosoid species that have been tested and fixes nitrogen in more than one-half of them (30). As shown above, NGRΩexoK induced the formation of only pseudonodules on L. leucocephala cv. Peru. Nodulation assays with L. leucocephala cv. Cunninghami yielded similar results (Fig. 6). When A. lebbeck (Mimoseae; tribe Ingeae) was challenged with NGRΩexoK, either no nodules or one nodule per plant was formed. In contrast to the parent strain, which induced the formation of fully developed nodules, the ExoK− mutant induced the formation of numerous pseudonodules (Fig. 2F and G and 6). NGR234 fixed nitrogen in nodules of A. lebbeck, as assessed by measuring the plant's biomass (Fig. 6A) and its nitrogen content (NGR234, 17 mg N g [dry weight]−1; NGRΩexoK, 14 mg N g [dry weight]−1). A closely related species, A. procera, displayed similar phenotypes, with fewer nodules formed following inoculation with NGRΩexoK (Fig. 6C) (nitrogen content with NGR234, 23 mg N g [dry weight]−1; nitrogen content with NGRΩexoK, 14 mg N g [dry weight]−1).
Most papilionoid species form nitrogen-fixing associations with NGR234. Inoculation of S. tomentosa (tribe Sophoreae) with NGRΩexoK resulted in reduced nodulation efficiency compared with that of the parent strain (Fig. 6B and C). S. tomentosa did not, however, form pseudonodules with NGRΩexoK (Fig. 6D). Differences between plants inoculated with NGR234 and plants inoculated with NGRΩexoK were also observed with K. rubicunda (tribe Phaseoleae). NGRΩexoK occasionally induced the formation of a single nodule on K. rubicunda, whereas NGR234 formed four nodules on average and the nodule biomass per plant was 23-fold higher (Fig. 6B and C). Nodules containing NGR234 fixed nitrogen (NGR234, 14 mg N g [dry weight]−1; NGRΩexoK, 8 mg N g [dry weight]−1). Mutation in the exoK gene did not affect nodulation of V. unguiculata (tribe Phaseoleae).
DISCUSSION
The known function of exo genes in S. meliloti (2, 32) and their sequence similarities to exo genes of NGR234 (38) suggest that ExoY of NGR234 is a galactosyl transferase involved in transfer of Gal to an undecaprenol carrier at the cytoplasmic face of the inner membrane. The putative glucosyl transferase ExoL of NGR234 is probably involved in synthesis of the repeating subunit. ExoZ of S. meliloti is an EPS-modifying acetyltransferase (32), and we propose that ExoZ of NGR234 has the same function. Our preliminary NMR data (1D-proton, 1H-1H COSY, and 1H-13C HSQC) suggest that the O-acetylation pattern of the NGRΩexoZ EPS is indeed different from that of the parent strain. The data presented here also suggest that NGR234 exports and assembles the repeating subunit of EPS by an ExoQ/ExoP/ExoF-dependent mechanism that is similar to the Wzy-dependent polymerization of group 1 capsular polysaccharides in E. coli (41). The export and polymerization system of NGR234 seems to include the putative polymerase ExoQ, the tyrosine autokinase ExoP, and the putative outer membrane auxiliary protein ExoF. Additional components involved in export and polymerization of EPS remain to be identified.
In NGR234, synthesis of EOSs is controlled by a hydrolytic enzyme, the glycanase ExoK. Previous work with S. meliloti provided evidence that symbiotically active LMW succinoglycan is either directly synthesized by the polymerization system or produced by hydrolysis of HMW succinoglycan. Two extracellular glycanases, ExoK and ExsH, have been identified in S. meliloti, and both are able to process succinoglycan into LMW forms (43). In NGR234, no gene related to exsH was found on the part of pNGR234b sequenced (38). This study showed that NGRΩexoK is deficient for synthesis of EOSs, indicating that NGR234 produces all EOSs by degradation. In other words, HMW forms of EPS are precursors of symbiotically active EOSs, an observation that was corroborated by nodulation tests with L. leucocephala. All mutants in which synthesis of EPS was compromised were unable to induce the formation of Fix+ nodules on this host.
NGR234 has a nonredundant EOS synthesis system. Other Rhizobium species with mutated glycanase genes are not completely deficient in production of EOSs. PlyA and PlyB, two extracellular glycanases of R. leguminosarum bv. viciae, are involved in degradation of HMW forms of EPS, but pea plants infected with a corresponding double mutant (in which plyA and plyB were mutated) formed Fix+ nodules (12). Similarly, a derivative of S. meliloti with mutated exoK and exsH glycanase genes induced the formation of Fix+ nodules on Medicago sativa. LMW succinoglycan was not completely eliminated in this mutant, presumably due to direct synthesis by the EPS polymerization system (43). In NGR234, however, synthesis of EOSs depends on a single hydrolase, the glycanase ExoK. Consequently, the results of comparative nodulation tests with NGRΩexoK and NGR234 directly reflect the extent to which EOSs are required for symbiosis with a given host.
Our results indicate that the symbiotically active EOSs of NGR234 are octa- and nonasaccharides. It has been suggested, however, that in S. meliloti trimers (with 24 hexosyl residues) may be the active molecules (1, 14, 40). Mass spectrometric observations have suggested that NGR234 does not seem to produce dimers and trimers of the repeating subunit of EPS. One possibility is that the endo-β-1,4-glycanase ExoK of NGR234 directly releases the repeating subunit from the polymer. Alternatively, a class of EOSs consisting of dimers or trimers may be released by ExoK and then further processed either by ExoK itself or by another hydrolase.
Low levels of EOSs accumulated in NGR234 culture supernatants (Fig. 4A). The yields of purified EOSs were about 500-fold lower than the yield of EPS (HMW forms). Control experiments, in which a dialysis protocol was used instead of ethanol precipitation, did not result in an increase in the yield of EOSs. Other media and higher growth temperatures resulted in even lower yields (data not shown). Thus, the levels of EOSs in NGR234 supernatants seem to be lower than the levels of Nod factors (29, 37). This is remarkable as other rhizobial strains produce significantly larger amounts of EOSs. S. meliloti, for example, synthesizes equal amounts of LMW succinoglycan and the corresponding polymer. Mutational analyses indicated that ExoK from S. meliloti contributes significantly to the formation of LMW succinoglycan (43). The ExoK enzyme from both strains seems to exhibit weak activity with EPS from NGR234. It has been reported that ExoK of S. meliloti degrades only nascent succinoglycan and that the mature polymer in the culture supernatant is resistant to depolymerization (44). We suggest that ExoK of NGR234 has similar characteristics. Furthermore, it is possible that ExoK has a low affinity for its substrate and that exoK is expressed only at low levels.
MALDI-TOF analysis showed that the purified EOS fraction of NGR234 consists of nonasaccharides and octasaccharides. Both oligosaccharide families are heterogeneous, due to differences in O acetylation. Our data provide evidence that there are up to three acetyl groups per EOS (Table 3). The identification of the nonasaccharide family and the detection of galactitol following borohydride reduction indicate that the endoglycanase ExoK releases the repeating subunit of EPS by hydrolyzing the β-1,4 linkage between Gal and Glc. Hydrolysis at this linkage is in agreement with reported specificities of enzymes belonging to glycoside hydrolase family 16, which hydrolyze either β-1,4 or β-1,3 linkages in Glc- and Gal-containing polymers (27). Octasaccharidic EOS lacks a single hexosyl residue. All EOSs identified by MALDI-TOF analysis had masses consistent with the masses of pyruvylated species, indicating that each octasaccharide or nonasaccharide contains a single nonreducing PvGal residue (Table 3). This was substantiated by the glycosyl composition ratios and by borohydride reduction, which produced equal amounts of PvGal and Gal-ol (Fig. 4C). As Glc-ol was not formed by NaBH4 reduction of EOS, it is likely that Gal residues constitute the reducing termini in all EOSs. Hence, the octasaccharides in the EOS fraction must contain PvGal and Gal residues, and we propose that the octasaccharides lack a single Glc residue compared to the nonasaccharides. Based on the known substrate specificity of enzymes belonging to glycoside hydrolase family 16, it is unlikely that the ExoK endoglycanase directly releases the octasaccharides from polymeric EPS (which is composed of nonasaccharide subunits). Rather, we suggest that the octasaccharides are derived from the nonasaccharides by cleavage of the terminal, nonreducing, β-1,6-linked glucosyl residue of the nonasaccharides by a separate (exo)glycosidase activity.
Our studies indicate that the endo-β-1,4-glycanase ExoK promotes nodulation of various hosts of NGR234. Legumes that form indeterminate nodules (A. lebbeck, A. procera, L. leucocephala, and S. tomentosa), as well as determinate nodules (K. rubicunda), seem to require ExoK for optimal nodulation. The effects of ExoK are most pronounced in plants belonging to the Mimosoideae subfamily. As nodulation of these plants depends on ExoK and EPS, we postulate that EOSs have a role in symbiosis. One possibility is that the secreted ExoK protein acts directly on plants, perhaps by cleaving β-glucans containing β-1,4 and β-1,3 linkages. Cleavage of plant cell wall components might be a crucial event in infection thread growth. In such a model, EOSs would not be essential for symbiosis per se. The nodulation experiments with NGRΩexoK(pEX312) and NGRΩexoK(pEX41) (Table 5) suggested that ExoK of S. meliloti complements symbiotic defects only in combination with other exo genes from this strain. We therefore favor the possibility that the LMW succinoglycan of S. meliloti can functionally replace EOSs from NGR234 (16). Coinoculation tests with Vicia sativa subsp. nigra and various rhizobial strains also supported the hypothesis that EOSs (or EPS) with different structures can promote infection thread growth and release of the bacteria into the nodule tissue (18). It is worth noting that LMW succinoglycan of S. meliloti is symbiotically active when it is applied exogenously to M. sativa roots (1, 14, 40). Djordjevic et al. (10) performed similar complementation experiments with exo mutants of NGR234 and suggested that both EPS and EOSs are symbiotically active on L. leucocephala. Our experiments with NGRΩexoK provide evidence that EOSs are symbiotically active, whereas EPS alone is not sufficient for establishment of symbiosis with various legumes. In contrast to the data reported by Djordjevic et al. (10), L. leucocephala plants treated with filter-sterilized HMW forms of EPS and inoculated with exo mutants were unable to form Fix+ nodules under our test conditions. A weak complementation effect was seen, however, when L. leucocephala was coinoculated with NGRΩexoK and ANU265, a nonnodulating derivative of NGR234 cured of pNGR234a (23).
In summary, our observations show that symbiotically active EOSs derived from EPS play a crucial role in nodule formation in various host plants. In NGR234, synthesis of EOSs depends on genes involved in EPS synthesis, as well as on the endo-β-1,4-glycanase ExoK. The EPS of NGR234 is a precursor of EOSs that are required for establishment of symbiosis with certain plants.
Acknowledgments
We thank J. Michiels (K.U. Leuven, Heverlee, Belgium) for providing pFAJ1815 and L.-X. Wang (University of Maryland, Baltimore) for technical advice. We also thank D. Gerber, Y.-Y. Aung, F. Ares-Orpel, W. J. Deakin, K. Deladoey, and X. Perret (Université de Genève) for their assistance with many aspects of this work.
Financial assistance in Geneva was provided by the Département de l'Instruction Publique du Canton de Genève, by the Université de Genève, and by the Fonds National Suisse de la Recherche Scientifique (projects 31-63893.00 and 3100AO-104097/1). This work was supported in part by National Institutes of Health grant GM39583 (to R.W.C.). The Complex Carbohydrate Research Center was supported in part by Department of Energy grant DE-FG02-93ER20097. Financial support in Guangzhou (to C.S.) was provided by the Department of Science and Technology of Guangdong Province (grant 2005B50201010) and by the Guangdong Natural Foundation (grant 5003358).
REFERENCES
- 1.Battisti, L., J. C. Lara, and J. A. Leigh. 1992. Specific oligosaccharide form of the Rhizobium meliloti exopolysaccharide promotes nodule invasion in alfalfa. Proc. Natl. Acad. Sci. USA 89:5625-5629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Becker, A., and A. Pühler. 1998. Production of exopolysaccharides, p. 97-118. In H. P. Spaink, Á. Kondorosi, and P. J. J. Hooykaas (ed.), The Rhizobiaceae. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 3.Becker, A., N. Fraysse, and L. Sharypova. 2005. Recent advances in studies on structure and symbiosis-related function of rhizobial K-antigens and lipopolysaccharides. Mol. Plant-Microbe Interact. 9:899-905. [DOI] [PubMed] [Google Scholar]
- 4.Broughton, W. J., and C. K. John. 1979. Rhizobia in tropical legumes. III. Experimentation and supply in Malaysia 1927-1976, p. 113-136. In W. J. Broughton, C. K. John, B. Lim, and C. Rajova (ed.), Soil microbiology and plant nutrition. University of Malaysia, Kuala Lumpur, Malaysia.
- 5.Chen, H., M. Batley, J. W. Redmond, and B. G. Rolfe. 1985. Alteration of the effective nodulation properties of a fast-growing broad host-range Rhizobium due to changes in exopolysaccharide synthesis. J. Plant Physiol. 120:331-349. [Google Scholar]
- 6.Chen, H., J. X. Gray, M. Nayudu, M. A. Djordjevic, M. Batley, J. W. Redmond, and B. G. Rolfe. 1988. Five genetic-loci involved in the synthesis of acidic exopolysaccharides are closely linked in the genome of Rhizobium sp. strain NGR234. Mol. Gen. Genet. 212:310-316. [Google Scholar]
- 7.Chen, W.-P., and T.-T. Kuo. 1993. A simple and rapid method for the preparation of gram-negative bacterial genomic DNA. Nucleic Acids Res. 21:2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D'Haeze, W., J. Glushka, R. De Rycke, M. Holsters, and R. W. Carlson. 2004. Structural characterization of extracellular polysaccharides of Azorhizobium caulinodans and importance for nodule initiation on Sesbania rostrata. Mol. Microbiol. 52:485-500. [DOI] [PubMed] [Google Scholar]
- 9.Djordjevic, S. P., B. G. Rolfe, M. Batley, and J. W. Redmond. 1986. The structure of the exopolysaccharide from Rhizobium sp. strain ANU280 (NGR234). Carbohydr. Res. 148:87-99. [Google Scholar]
- 10.Djordjevic, S. P., H. Chen, M. Batley, J. W. Redmond, and B. G. Rolfe. 1987. Nitrogen fixation ability of exopolysaccharide synthesis mutants of Rhizobium sp. strain NGR234 and Rhizobium trifolii is restored by the addition of homologous exopolysaccharides. J. Bacteriol. 169:53-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Figurski, D. H., and D. R. Helinski. 1979. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc. Natl. Acad. Sci. USA 76:1648-1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Finnie, C., A. Zorreguieta, M. Nigel, M. Hartley, and J. A. Downie. 1998. Characterization of Rhizobium leguminosarum exopolysaccharide glycanases that are secreted via a type I exporter and have a novel heptapeptide repeat motif. J. Bacteriol. 180:1691-1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glucksmann, M. A., T. L. Reuber, and G. C. Walker. 1993. Family of glycosyl transferases needed for the synthesis of succinoglycan by Rhizobium meliloti. J. Bacteriol. 175:7033-7044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gonzalez, J. E., C. E. Semino, L.-X. Wang, L. E. Castellano-Torres, and G. C. Walker. 1998. Biosynthetic control of molecular weight in the polymerization of the octasaccharide subunits of succinoglycan, a symbiotically important exopolysaccharide of Rhizobium meliloti. Proc. Natl. Acad. Sci. USA 95:13477-13482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gray, J. X., M. A. Djordjevic, and B. G. Rolfe. 1990. Two genes that regulate exopolysaccharide production in Rhizobium sp. strain NGR234: DNA sequences and resultant phenotypes. J. Bacteriol. 172:193-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gray, J. X., H. J. Zhan, S. B. Levery, L. Battisti, B. G. Rolfe, and J. A. Leigh. 1991. Heterologous exopolysaccharide production in Rhizobium sp. strain NGR234 and consequences for nodule development. J. Bacteriol. 173:3066-3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klein, S., A. M. Hirsch, C. A. Smith, and E. R. Signer. 1988. Interaction of nod and exo Rhizobium meliloti in alfalfa nodulation. Mol. Plant-Microbe Interact. 1:94-100. [DOI] [PubMed] [Google Scholar]
- 18.Laus, M. C., A. A. N. van Brussel, and J. W. Kijne. 2005. Exopolysaccharide structure is not a determinant of host-plant specificity in nodulation of Vicia sativa roots. Mol. Plant-Microbe Interact. 18:1123-1129. [DOI] [PubMed] [Google Scholar]
- 19.Leigh, J. A., E. R. Signer, and G. C. Walker. 1985. Exopolysaccharide deficient mutants of Rhizobium meliloti that form ineffective nodules. Proc. Natl. Acad. Sci. USA 82:6231-6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loewus, F. A. 1952. Improvement in anthrone method for determination of carbohydrates. Anal. Chem. 24:219. [Google Scholar]
- 21.Long, S., J. W. Reed, J. Himawan, and G. C. Walker. 1988. Genetic analysis of a cluster of genes required for synthesis of the calcofluor-binding exopolysaccharide of Rhizobium meliloti. J. Bacteriol. 170:4239-4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mazur, A., M. Marczak, J. E. Król, and A. Skorupska. 2005. Topological and transcriptional analysis of pssL gene product: a putative Wzx-like exopolysaccharide translocase in Rhizobium leguminosarum bv. trifolii TA1. Arch. Microbiol. 184:1-10. [DOI] [PubMed] [Google Scholar]
- 23.Morrison, N. A., C. Y. Hau, M. J. Trinick, J. Shine, and B. G. Rolfe. 1983. Heat curing of a Sym plasmid in a fast-growing Rhizobium sp. that is able to nodulate legumes and the nonlegume Parasponia sp. J. Bacteriol. 153:527-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ovtsyna, A. O., and C. Staehelin. 2003. Bacterial signals required for the Rhizobium-legume symbiosis, p. 631-648. In S. G. Pandalai (ed.), Recent research developments in microbiology, vol. 7 (part II). Research Signpost, Trivandrum, India. [Google Scholar]
- 25.Pellock, B. J., H.-P. Cheng, and G. C. Walker. 2000. Alfalfa root nodule invasion efficiency is dependent on Sinorhizobium meliloti polysaccharides. J. Bacteriol. 182:4310-4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perret, X., C. Staehelin, and W. J. Broughton. 2000. Molecular basis of symbiotic promiscuity. Microbiol. Mol. Biol. Rev. 64:180-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Planas, A. 2000. Bacterial 1,3-1,4-β-glucanases: structure, function and protein engineering. Biochim. Biophys. Acta 1543:361-382. [DOI] [PubMed] [Google Scholar]
- 28.Prentki, P., and H. M. Krisch. 1984. In vitro insertional mutagenesis with a selectable DNA fragment. Gene 29:303-313. [DOI] [PubMed] [Google Scholar]
- 29.Price, N. P., B. Relić, F. Talmont, A. Lewin, D. Promé, S. G. Pueppke, F. Maillet, J. Dénarié, J.-C. Promé, and W. J. Broughton. 1992. Broad-host-range Rhizobium species strain NGR234 secretes a family of carbamoylated, and fucosylated, nodulation signals that are O-acetylated or sulphated. Mol. Microbiol. 6:3575-3584. [DOI] [PubMed] [Google Scholar]
- 30.Pueppke, S. G., and W. J. Broughton. 1999. Rhizobium sp. strain NGR234 and R. fredii USDA257 share exceptionally broad, nested host ranges. Mol. Plant-Microbe Interact. 12:293-318. [DOI] [PubMed] [Google Scholar]
- 31.Quandt, J., and M. F. Hynes. 1993. Versatile suicide vectors which allow direct selection for gene replacement in gram negative bacteria. Gene 127:15-21. [DOI] [PubMed] [Google Scholar]
- 32.Reuber, T. L., and G. C. Walker. 1993. Biosynthesis of succinoglycan, a symbiotically important exopolysaccharide of Rhizobium meliloti. Cell 74:269-280. [DOI] [PubMed] [Google Scholar]
- 33.Reuhs, B. L., B. Relić, L. S. Forsberg, C. Marie, T. Ojanen-Reuhs, S. B. Stephens, C. H. Wong, S. Jabbouri, and W. J. Broughton. 2005. Structural characterization of a flavonoid-inducible Pseudomonas aeruginosa A-band-like O antigen of Rhizobium sp. strain NGR234, required for the formation of nitrogen-fixing nodules. J. Bacteriol. 187:6479-6487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Riely, B. K., J.-M. Ané, R. V. Penmetsa, and D. R. Cook. 2004. Genetic and genomic analysis in model legumes bring Nod-factor signaling to center stage. Curr. Opin. Plant Biol. 7:408-413. [DOI] [PubMed] [Google Scholar]
- 35.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 36.Spaink, H. P. 2000. Root nodulation and infection factors produced by rhizobial bacteria. Annu. Rev. Microbiol. 54:257-288. [DOI] [PubMed] [Google Scholar]
- 37.Staehelin, C., J. Granado, J. Müller, A. Wiemken, R. B. Mellor, G. Felix, M. Regenass, W. J. Broughton, and T. Boller. 1994. Perception of Rhizobium nodulation factors by tomato cells and inactivation by root chitinases. Proc. Natl. Acad. Sci. USA 91:2196-2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Streit, W. R., R. A. Schmitz, X. Perret, C. Staehelin, W. J. Deakin, C. Raasch, H. Liesegang, and W. J. Broughton. 2004. An evolutionary hot spot: the pNGR234b replicon of Rhizobium sp. strain NGR234. J. Bacteriol. 186:535-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trinick, M. J. 1980. Relationships amongst the fast-growing rhizobia of Lablab purpureus, Leucaena leucocephala, Mimosa spp., Acacia farnesiana and Sesbania grandiflora and their affinities with other rhizobial groups. J. Appl. Bacteriol. 49:39-53. [Google Scholar]
- 40.Wang, L.-X., Y. Wang, B. Pellock, and G. C. Walker. 1999. Structural characterization of the symbiotically important low-molecular-weight succinoglycan of Sinorhizobium meliloti. J. Bacteriol. 181:6788-6796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Whitfield, C., and A. Paiment. 2003. Biosynthesis and assembly of group 1 capsular polysaccharides in Escherichia coli and related extracellular polysaccharides in other bacteria. Carbohydr. Res. 338:2491-2502. [DOI] [PubMed] [Google Scholar]
- 42.Xi, C., M. Lambrecht, J. Vanderleyden, and J. Michiels. 1999. Bi-functional gfp- and gusA-containing mini-Tn5 transposon derivatives for combined gene expression and bacterial localization studies. J. Microbiol. Methods 35:85-92. [DOI] [PubMed] [Google Scholar]
- 43.York, G. M., and G. C. Walker. 1997. The Rhizobium meliloti exoK gene and prsD/prsE/exsH genes are components of independent degradative pathways which contribute to production of low-molecular-weight succinoglycan. Mol. Microbiol. 25:117-134. [DOI] [PubMed] [Google Scholar]
- 44.York, G. M., and G. C. Walker. 1998. The Rhizobium meliloti ExoK and ExsH glycanases specifically depolymerize nascent succinoglycan chains. Proc. Natl. Acad. Sci. USA 95:4912-4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhan, H. J., J. X. Gray, S. B. Levery, B. G. Rolfe, and J. A. Leigh. 1990. Functional and evolutionary relatedness of genes for exopolysaccharide synthesis in Rhizobium meliloti and Rhizobium sp. strain NGR234. J. Bacteriol. 172:5245-5253. [DOI] [PMC free article] [PubMed] [Google Scholar]