Abstract
Concentrations and isotopic compositions of ethane and propane in cold, deeply buried sediments from the southeastern Pacific are best explained by microbial production of these gases in situ. Reduction of acetate to ethane provides one feasible mechanism. Propane is enriched in 13C relative to ethane. The amount is consistent with derivation of the third C from inorganic carbon dissolved in sedimentary pore waters. At typical sedimentary conditions, the reactions yield free energy sufficient for growth. Relationships with competing processes are governed mainly by the abundance of H2. Production of C2 and C3 hydrocarbons in this way provides a sink for acetate and hydrogen but upsets the general belief that hydrocarbons larger than methane derive only from thermal degradation of fossil organic material.
Keywords: ethanogenesis, hydrocarbon gases, marine sediments, propanogenesis, stable carbon isotopes
Leg 201 of the Ocean Drilling Program was dedicated to the study of microbial life in deeply buried marine sediments (1, 2). Cores were obtained from open-ocean sites in the Equatorial Pacific, where sediments deposited 40 million years ago are underlain by seafloor basalts through which oxygenated seawater is flowing, and from the Peruvian Margin, where drilling penetrated sediments up to 15 million years old (Fig. 1). Temperatures in sediments ranged from 2°C to 25°C. All sites are isolated from reservoirs of fossil hydrocarbons. At both open-ocean and ocean-margin sites, treatment of sediments with strong base released ethane and propane (Fig. 2). When the treatment was repeated with fresh sediment and isotopically labeled water (δD = +4000‰), no excess deuterium appeared in the ethane or propane. Therefore, we conclude that the hydrocarbons were strongly sorbed, indigenous constituents of the sediment and did not derive from a chemical reaction between the strong base and an organic substrate.
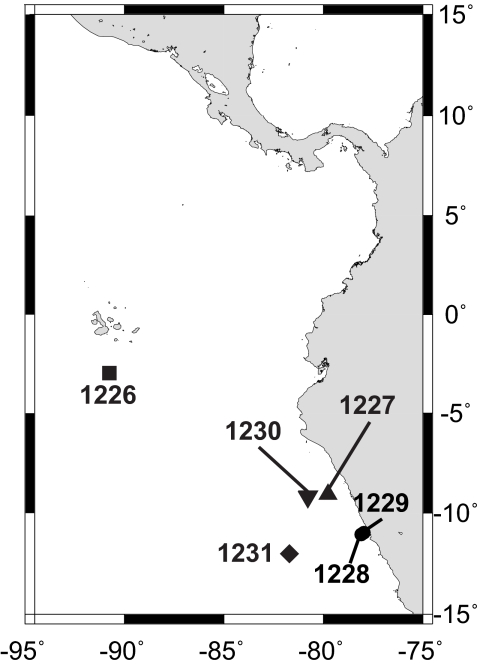
Fig. 1.
Map showing the locations of studied ODP drill sites.
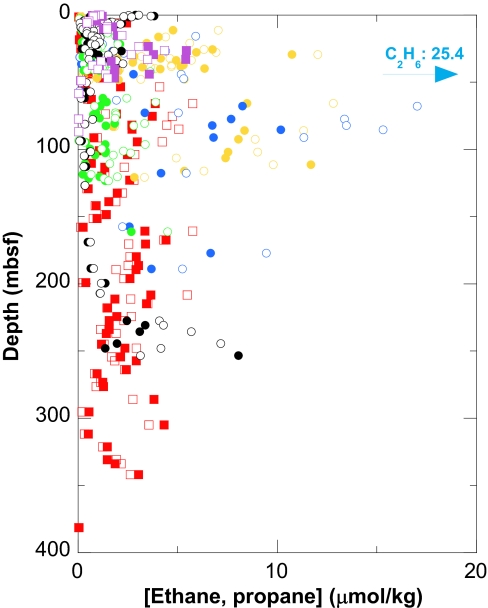
Fig. 2.
Concentrations of ethane (filled symbols) and propane (open symbols) in sediments at ODP Sites 1226–1231, Equatorial Pacific and Peru Margin, expressed in μmol per kg of dry sediment: Site 1226 (red squares), Site 1227 (orange circles), Site 1228 (blue circles), Site 1229 (green circles), Site 1230 (black circles), and Site 1231 (purple squares). Sediments were deposited under a wide range of environmental conditions. In most samples, the bulk of the ethane and propane was sorbed to the sediment, i.e., dissolved ethane and propane were below the detection limit.
Earlier reports describe sediments offshore Peru (3) and Spitsbergen (4), from which similar mixtures of hydrocarbons could be released by treatment with hot solutions of phosphoric acid. In each case, the carbon-isotopic compositions and abundance ratios (C1/C2+) led to reluctant suggestions that the gases must be of thermogenic origin and thus have migrated into the unconsolidated seafloor sediments: “the [postulated] migration of C2+ hydrocarbons… is somehow related to these fluids [brines that might have flowed from one basin to another]” (3); and “… elevated seepages [of thermogenic hydrocarbons] occurred irregularly but are not currently active… it remains speculative whether the detected hydrocarbon anomalies are related to reservoirs and/or active source rocks” (4). No mechanism for sorbing the putatively migrated hydrocarbons more strongly than indigenous microbial products has been offered.
Ethane and propane with similar isotopic characteristics and abundance ratios have recently been reported in Cretaceous marine shales in the Western Canadian sedimentary basin (5). Previous work has also pointed to the existence of microbially mediated pathways that yield hydrocarbons with two or more carbon atoms (6–12). In the present case, the occurrences of ethane and propane in each core are well correlated with pertinent biogeochemical factors and chances that migration could supply the ethane and propane are profoundly more remote. Accordingly, we explore the possibility that the gases are previously unrecognized products of the subsurface microbial community.
Results and Discussion
Distribution of Ethane and Propane in Sediments.
The gases were detected in all depth zones studied, to 380 m below sea floor (mbsf; Fig. 2). Within each borehole, concentration profiles are more consistent with production in situ than with transport from greater depths. Intact prokaryotic cells are present in all sediments studied and chemical compositions of pore fluids indicate microbial activity at all depths (1, 2, 13). DNA- and RNA-based culture-independent studies of the compositions of microbial communities in these sediments indicate the presence of diverse Archaea and Bacteria with largely unknown properties. Many of these phylotypes are apparently widespread in subsurface environments (14–17). The geochemical environments probed are highly diverse. Concentrations of organic carbon range from ≈0.1% at the open-ocean Sites 1226 and 1231 to 12% at near-shore Sites 1227–1230, underlying highly productive surface waters off Peru (18). Concentrations of dissolved methane vary between sites by at least six orders of magnitude (1). Sulfate is consumed by respiration at relatively shallow depths at sites off Peru but is supplied to deeper sediments at Sites 1228 and 1229 by subsurface brines. It is never fully consumed at open-ocean Sites 1226 and 1231. Despite this high geochemical diversity, concentrations of ethane and propane are rather uniform and range from ≈1 to 25 μmol per kg of dry sediment (Fig. 2). Average concentrations of ethane and propane are highest at coastal Sites 1227, 1228, and 1229 (Table 1, which is published as supporting information on the PNAS web site), intermediate at the open-ocean Sites 1226 and 1231, and lowest at the organic-carbon-rich, methane-hydrate-bearing Site 1230. At Sites 1226 and 1228, ethane and propane together account for roughly as much carbon as methane (Table 1). They account for 30-fold less carbon than methane at Site 1231 and still less at Sites 1227, 1229, and 1230. At all sites except 1230, the combined concentration of sorbed ethane and propane is comparable to or higher than the sum of dissolved, volatile fatty acids (1).
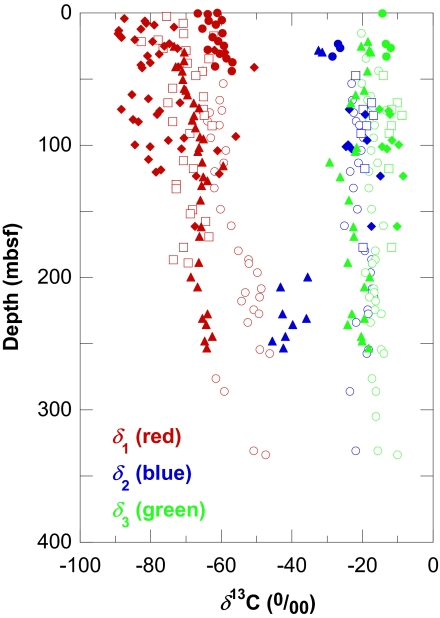
Sites 1226 and 1231 are remote not only from continental sources of hydrocarbons but also from the active margin and thick piles of sediments (Fig. 1). At these sites, relatively thin packages of cold, organic-lean sediment overly a basement through which oxygenated seawater circulates. The ethane and propane must have been produced in situ, and microbial catalysis is, by far, the most likely source. At Site 1231, where ethane and propane were detected in the top 47 mbsf (Fig. 2), the concurrent presence of dissolved manganese, dissolved iron (1), and sorbed methane with δ13C of −57 to −65‰ (Fig. 3), testifies to microbial activity in this sediment interval.
Fig. 3.
Carbon-isotopic compositions of methane (δ1, red symbols), ethane (δ2, blue), and propane (δ3, green) at ODP Sites 1226 (open circles), 1228 (open squares), 1229 (diamonds), 1230 (triangles), and 1231 (filled circles) [δn ≡ (13Rn/13Rvpdb) − 1, where 13R ≡ 13C/12C and vpdb designates the Vienna PeeDee Belemnite isotopic standard. Reported values of δ are customarily multiplied by 1,000 and expressed in permil units (‰).] Note that δ1 reflects the isotopic compositions of mixtures of sorbed and dissolved methane with variable relative proportions (e.g., Site 1226, CH4,aq. < CH4,sol.; Site 1228: CH4,aq. < CH4,sol.; Site 1229: CH4,aq. ≈ CH4,sol.; Site 1230: CH4,aq. > CH4,sol.; Site 1231: CH4,aq. ≪ CH4,sol.), whereas δ2 and δ3 largely pertain to the sorbed fraction of the respective gases.
Similarly, at Site 1230, discrete maxima of both hydrocarbons were observed at specific sediment horizons and appear to be linked to microbial processes. Here, concentrations of ethane and propane are bimodally distributed, with peaks of ≈4 μmol/kg at the sediment surface and ≈8 μmol/kg at ≈250 mbsf. In the deep sediment interval, a relatively high proportion of the gases was dissolved in porewater rather than sorbed to the sediment because it was already detected by applying extraction protocols that are largely limited to dissolved gases (1). The surface maximum is best explained by a coupling of hydrocarbon production with overall rates of microbial remineralization of organic carbon, which are typically highest close to the sediment–water interface. The lower maximum coincides with an almost 3-fold decline of acetate concentration from 147 to 65 μM between ≈207 and 253 mbsf (1) and probably represents another zone of increased hydrocarbon production. The up- and/or downward decrease from these two zones of increased hydrocarbon production might then represent loss by diffusion or loss of sorbed hydrocarbons by slow desorption followed by consumption.
Proposed Reactions and Thermodynamics.
Reactions 1 and 2 show how ethanogenesis and propanogenesis can provide sinks for two major products of fermentation, namely acetate and hydrogen. In this way, where energetically favorable, these processes can help to extend anaerobic microbial processes. The potential of acetate as a precursor of ethane, analogous to the role of CO2 as the precursor of methane, was foreseen by Claypool in 1999.‡‡
These processes are exergonic under conditions prevailing in a wide range of marine subsurface settings. Specifically, both processes are feasible under conditions that support methanogenesis and homoacetogenesis (Fig. 4). Carbon-chain-elongation processes analogous to propanogenesis have been observed in fermentative communities, e.g., production of propionate from bicarbonate, acetate, and hydrogen (19). The latter process is a six-electron reduction. Under conditions pertinent to these sediments, namely T = 10°C, P = 200 bar, [DIC] = 10 mM, pH = 8, and activities of 10 μM for acetate and 100 nM each for propane, propanoate, and H2, its energy yield per electron transferred is identical to that in reaction 2, which requires a 12-electron reduction. This finding suggests that, under these conditions, formation of propane and of propionate are equally feasible.
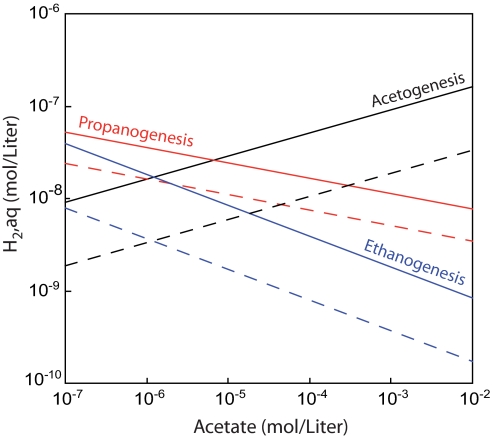
Fig. 4.
Conditions yielding ΔG = −15 kJ (thick, solid lines) and ΔG = 0 kJ (dotted lines) for ethanogenesis (reaction 1, blue), propanogenesis (reaction 2, red), and homoacetogenesis (black) as a function of concentrations of acetate and hydrogen, assuming conditions considered typical for marine sediments (e.g., ref. 1): T = 10°C, [DIC] = (10 mM), pH = 8, [ethane]aq = 20 nM, [propane]aq = 20 nM, P = 200 bar. The horizontal axis depicts the range of acetate concentrations observed in marine sediments.
Potential Habitats.
Hypothetical sedimentary niches can be identified on the basis of thermodynamic constraints. Values of ΔG for reactions 1 and 2 decrease by ≈0.5 and 1 kJ, respectively, for each 1°C decline in temperature; this favors colder settings like those encountered in the majority of Leg 201 sediments (2–20°C). In the presence of sulfate, ΔG for hydrogenotrophic sulfate reduction is generally more negative than that of reactions 1 and 2. Thus, the presence of ethane and propane, in addition to methane, in sulfate-bearing sediments such as the upper 10 m of Site 1230 and the sulfate-bearing Sites 1226, 1228, and 1231, suggests the existence of microenvironments in which competitive pressure for substrates is reduced and/or sulfate reduction is inhibited. On the other hand, in sulfate-free sediments, reactions 1 and 2 may be energetically favored over other hydrogenotrophic or acetotrophic processes.
Hydrogenotrophic methanogenesis will be more exergonic than reactions 1 and 2 in most sedimentary environments unless the porewater is saturated with methane as is the case in hydrate-bearing sediments. If the concentration of methane in porewater is 10 μM (typical of sulfate-bearing, near-shore sediments off Peru; ref. 1), 2.1 nM H2 will yield ΔG = −15 kJ for the reduction of CO2 to methane, thus providing the minimum required for a biologically useful energy source (20). At these conditions and with the acetate levels commonly observed in marine sediments, methanogenesis will be more exergonic than reactions 1 and 2 (Fig. 4). Thus, the coexistence of ethane and propane with methane and sulfate at low-methane Sites 1226 and 1231 suggests that the C2 and C3 hydrocarbons are cometabolic products. Alternatively, kinetic factors or levels of cofactors determine the importance of these reactions. In contrast, if the concentration of methane is 100 mM, a H2 concentration of 21 nM is required for ΔG = −15 kJ for methanogenesis. At these or higher levels of H2, reactions 1 and 2 will be the energetically favored hydrogenotrophic processes over a wide range of acetate concentrations (Fig. 4).
Ethanogenic and propanogenic organisms must compete with aceticlastic methanogens for acetate. The concentration of methane will be a determining factor. Over the range of acetate concentrations encountered at Leg 201 Sites, 1–200 μM (1), concentrations of methane corresponding to ΔG = −15 kJ for aceticlastic methanogenesis will range from 85 μM to 16 mM. In other words, at acetate concentrations of ≈1 μM, as typically found at the near-shore Peru Margins Sites 1227–1229, the concentration of methane must be <85 μM for aceticlastic methanogenesis to be a viable sink for acetate. However, the sulfate-free sediments at these sites contain generally high methane concentrations so that aceticlastic methanogenesis is unattractive from an energetic point of view. This finding is consistent with the δ-values of methane, which suggest CO2 reduction as predominant source (ref. 21 and Fig. 3). On the other hand, in low-acetate, low-methane sediments at Sites 1226 and 1231, aceticlastic methanogenesis is energetically feasible. A potential contribution of this process to the pool of methane is consistent with its generally higher δ-values (Fig. 3). At the hydrate-bearing, high-acetate Site 1230, dissolved methane concentrations are probably too high for aceticlastic methanogenesis to be important. As shown in Fig. 4, homoacetogenic reduction of CO2 will only be competitive at low acetate concentrations.
As indicated by the stoichiometry, when concentrations of hydrogen are high, the value of ΔG for propanogenesis can be more favorable than that for ethanogenesis. For example, for conditions used for the construction of Fig. 4, 70 nM H2 would result in equal values of ΔG for reactions 1 and 2, i.e., at acetate concentrations of 1 and 10 μM we obtain values of −30 and −24.5 kJ, respectively. At concentrations of hydrogen >70 nM, reaction 2 will be more exergonic than reaction 1. The frequently observed higher concentrations of propane relative to those of ethane (Fig. 2) could reflect such high hydrogen concentrations.
Our knowledge of in situ concentrations of H2 is limited. On the drill ship, incubation-based techniques that have been used successfully to measure concentrations of H2 in microbially active surface sediments and soils (22–24) were applied to core samples at all Leg 201 sites (1). Indicated concentrations of H2 were generally very low, typically 1 nM and lower, and largely invariant despite enormous variations in microbial activities and geochemical conditions. For example, in deep sediments from the methane-hydrate-bearing Site 1230, in which geochemical evidence for in situ production of methane by reduction of CO2 is strong, the indicated concentrations of H2 are not high enough to allow this process to be exergonic (data not shown). This obviously incorrect result indicates that the onboard, incubation-based analyses of the samples recovered from the cores do not accurately represent the concentrations of H2 available in situ. Consistent with this observation, analyses of H2 in gas voids formed in core liners, and in gases released from the pressure core sampler at the hydrate-bearing Site 1230, suggest that in situ levels of H2 were up to two orders of magnitude higher than indicated by the incubation technique (Leg 201 Shipboard Party, unpublished observations).
Isotopic Constraints.
Isotopic compositions of methane in all samples point to microbial sources (Fig. 3). Relative to methane, ethane and propane are enriched in 13C. The magnitude and variability of this enrichment are not characteristic of thermogenic natural gas (e.g., ref. 25). Moreover, ethane and especially propane (Figs. 3 and 5B) are enriched in 13C relative to ethane and propane in most natural gas (25). Finally, the average molar ratio of ethane to propane is 1 (n = 254, average ratios at Sites 1226–1231 range from 0.6 to 1.5, 17 ≤ n ≤ 66; see Fig. 2 and Table 1). This finding contrasts with the abundance ratios in thermogenic gas, where ethane is typically far more abundant than propane (27). The high relative abundance of propane argues against a mechanism involving its selective degradation as explanation for the strong 13C-enrichment relative to ethane (compare ref. 28).
Fig. 5.
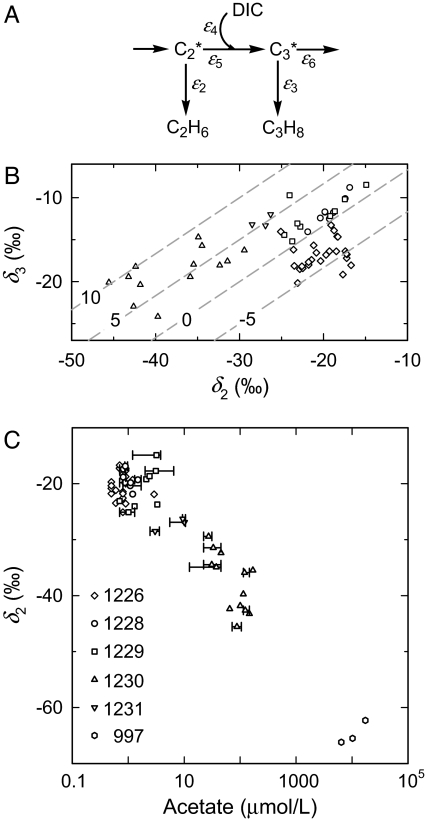
Suggested reaction network and supporting geochemical evidence. (A) A schematic reaction network showing precursors and isotope effects related to ethane and propane. (B) Values of δ2 versus δ3 from samples that yielded both results, symbols are defined in C. Lines are δ3 = 2δ2/3 + x, with values of x specified. (C) Apparent linear relationship between δ2 and log[acetate] for ODP Sites 1226, 1228–1231, and 997, Blake Ridge, suggesting that acetate is a precursor of ethane. When necessary, acetate concentrations (1) were linearly interpolated between two data points to match the sampling interval of the closest hydrocarbon gas sample; error bars designate the acetate concentrations used for interpolation. No isotopic data for ethane was obtained for Site 1227. Data for ODP Site 997 are derived from refs. 10 (δC2) and 26 ([acetate]).
Abiotic production of hydrocarbons probably requires temperatures higher than and sedimentary compositions different from those encountered in sediments of the Equatorial Pacific and Peru Margin (compare refs. 29 and 30). McCollom and Seewald (29) produced hydrocarbons abiotically at 250°C. The isotopic compositions of ethane and propane were equal. In contrast, propane from the sorbed gases is significantly enriched in 13C relative to coexisting ethane (Fig. 5B). Given the variability of sediment sources at the Leg-201 sites, a scenario in which the ethane and propane were produced by high-temperature, abiological processes elsewhere, sorbed by mineral particles, and subsequently transported and deposited appears inconceivable.
The specific relationship between stable carbon isotopic compositions of ethane and propane can be accounted for by biochemical processes. A minimal reaction network is shown in Fig. 5A. The postulated addition of dissolved inorganic carbon (DIC) to C2* (acetate or a related intermediate) is supported by the observed enrichment of 13C in propane relative to ethane. For this system, the expected relationship between isotopic compositions of propane and ethane is δ3 = 2δ2/3 + x, where x is a linear function of the isotope effects (ε2, …, ε6), δDIC, and f3, the fraction of C3* flowing to propane. The system is open. Provided that times are long compared with the turnover times of C2* and C3*, it will evolve through a series of steady states. For any of these, independent of the isotopic composition of the input, δ′3 = 2δ′2/3 + x, where the primed variables refer to isotopic compositions of increments of carbon flowing to ethane and propane, respectively, and x = [δDIC − ε4 + 2(ε2 − ε5) − 3(1 − f3)(ε3 − ε6)]/3. Notably, x is independent of the branching ratio at C2*. If the reactions do not change, all of the isotope effects are constants and x depends only on δDIC and f3. The presently observable values of δ2 and δ3 represent the integrated products of the evolving system whereas values of δDIC represent the present steady state. The observed scatter of results is therefore not surprising, but the trends qualitatively support the postulated reaction pathways. For an extended treatment of the isotopic constraints of the reaction network, see Supporting Text and Table 2, which are published as supporting information on the PNAS web site.
Assuming that the isotope effects associated with reduction, ε2 and ε3, are larger than ε5 and ε6, respectively, increasing values of x are associated with increasing values of δDIC and/or f3. In fact, higher values of x (see reference lines, Fig. 5B) are associated with samples that have higher values of δDIC (Table 2). Conversely, lower values of x tend to be associated with samples containing higher concentrations of sulfate (Table 2). The latter should favor oxidation rather than reduction of C3* and thus minimization of f3. Notably, these relationships prevail over a wide range of concentrations (3–160 mM) and isotopic compositions (−20 to +20‰) of dic (D. P. Schrag, unpublished data, compare Table 2).
Values of δ2 decrease linearly with log[acetate] (Fig. 5C). Strikingly, the relationship pertains to concentrations of acetate varying by a factor of 10,000. It would be relatively easy to explain if the system were closed and acetate could be viewed as a reactant that was being consumed by some process with a normal isotope effect. In such cases, the δ value of the unconsumed acetate would increase linearly with declining values of log[acetate]. In this scenario: (i) Acetate, or some precursor molecule from which acetate is derived, is consumed by a process with a normal isotope effect. Its isotopic composition therefore follows the relationship shown in Fig. 5C. (ii) When that process stops, a portion of the residual acetate is converted to ethane. The isotopic composition of the ethane therefore monitors that of the residual acetate. This hypothetical sequence deserves attention because it is relatively simple and fits the observations so perfectly. Only further study can determine whether it should be discarded, modified, or accepted.
In an open system, the relationship summarized in Fig. 5C might indicate mixing of ethane from two sources, with the mixing ratio somehow related to log[acetate]. There are, for example, two processes, fermentation and homoacetogenesis, which produce isotopically distinct acetate (31, 32). The resulting variations in δacetate would, after reduction of the acetate to produce ethane, appear as variations in δ2. The processes might be complementary because fermentation produces H2 and acetogenesis consumes it. A logarithmic dependence on [H2] would be expected because relative rates of metabolic processes in marine sediments often depend on their respective free energy yields (33). To obtain the relationship in Fig. 5C it is then required that the concentration of acetate monitors that of H2. Again, further study is required.
Sorbtion of Ethane and Propane.
The combination of low concentrations of ethane and propane in interstitial water and the requirement to use strong base to release them suggests that these hydrocarbons, including methane and sometimes also their larger homologues, were adsorbed to hydrophobic siloxane patches within the interlayer region of clay minerals (34). Expandable clay minerals such as smectite are common constituents of marine sediments and are considered important host phases of organic carbon (35) and potential nucleation sites for gas hydrates (36).
Conclusion
Previous studies have provided independent lines of evidence in support of biologically mediated pathways leading to gaseous hydrocarbons other than methane (6–12). Previous work had focused mostly on ethane and demonstrated that (i) certain methanogenic archaea (7) may be capable of ethane production, (ii) ethylated Coenzyme M (ethyl-S-CoM) can play a mechanistic role (7), and (iii) ethanethiol can serve as a substrate (9). However, propanethiol could not be confirmed as substrate for propane production (9), and mechanisms for its biological production are not known. In connection with the evidence presented here, this finding implies that multiple substrates and mechanisms are associated with the formation of hydrocarbons.
Although concentrations of biogenic ethane and propane are low, the likely significance of these compounds is great. Specifically, they signal the presence of an additional process, probably significant in many environments, for extending the terminal degradation of organic material.
Materials and Methods
All sediments studied were recovered during Leg 201 of the Ocean Drilling Program (1). Details of the sampling and analysis of hydrocarbon gases are reported elsewhere (37) and in Supporting Text.
Concentrations of Hydrocarbon Gases.
In brief, hydrocarbon gases were analyzed by using a headspace technique. Three-ml subcores of fresh sediment were taken immediately after core recovery on the catwalk and placed in headspace vials containing 5 ml of 1 M NaOH. Mixtures were shaken for 1 h; afterward the sediment and the NaOH solution had typically formed a slurry. Sampling of the headspace with a gas-tight syringe was performed at least twice within 4 months after core retrieval. Analysis of the hydrocarbons was performed with a Hewlett Packard 6890 gas chromatograph equipped with a stainless steel column packed with HayeSep S (100–120 mesh) and equipped with a flame ionization detector (FID). Gas chromatography onshore used a Hewlett Packard 5890 gas chromatograph equipped with Poropak-Q column. A calibration resulting from injection of known quantities of hydrocarbon gases was applied to calculate the molar fraction of ethane and propane in the gas mixture. Further details can be found in Supporting Text.
Isotopic Compositions.
Headspace gas samples were sampled by using a 1,000-μl gastight syringe and injected into a six-port 2-position valve with a 500-μl external loop upstream of a Gerstel CIS-4 injector on an HP6890 gas chromatograph that was operated in split mode. The split ratio was adjusted to optimize the signal intensity, typically between 0.1 and 1.0. Gaseous hydrocarbons were separated on a 30 m × 0.3 mm ID Alltech AT-Q column with helium carrier gas flow of 3.0 ml/min. The column temperature was programmed from 50°C with a 2-min hold at 50°C per min to 240°C. An integral fused silica combustion system (38) at 950°C converted all organic components to CO2. Isotopic data were acquired and processed on a Finnigan MAT Delta Plus isotope-ratio-monitoring mass spectrometer using the Isodat NT data package. Overall system accuracy was confirmed to be better than 1‰ based on a standard with ethane and propane in helium at 1,000 ppm (nominal). The standards had been independently analyzed by Isotech (Champaign, IL).
Thermodynamic Calculations.
The standard free energies (ΔG°) of acetotrophic ethanogenesis and propanogenesis were calculated by using SUPCRT92 (39) and thermodynamic data from Shock and Helgeson (40) for a pressure of 20 MPa and temperatures as measured in the boreholes (1). The free energy of reactions at nonstandard conditions (ΔG) was calculated according to ΔG = ΔG° + RT ln Q, where Q is the activity quotient of the reactants and reaction products. For reaction 2, for example, Q = [apropane(aq) × a5H2O]/[aacetate(aq) × aHCO3- × a6H2(aq) × a2H+]. R is the gas constant, and T is the temperature in Kelvin.
For the construction of Fig. 4, the dependency of aqueous acetate activity on H2,aq activity was calculated for constant ΔGR values of 0 and −15 kJ/mol, assuming pH = 8, aHCO3- = 10 mM, aethane = apropane = 20 nM, and T = 20°C and using
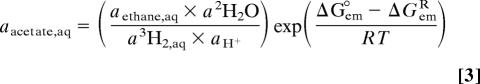 |
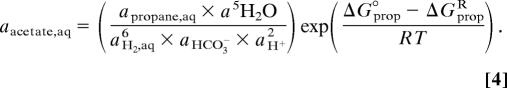 |
Testing for Analytical Artifacts with d-Labeled Water.
To exclude the possibility that ethane and propane were formed by reaction of strong base and an organic substrate, fresh samples of sediments were reanalyzed in the presence of d-labeled water. Under the conditions used (details in supporting information), the value of δd in the propane would have been greater than +500‰ vs. standard mean ocean water if one of the H atoms derived from the reagents. The observed values instead averaged −135‰, indicating that the propane was not formed by a chemical reaction associated with the analyses.
Supplementary Material
Acknowledgments
We thank the Ocean Drilling Program (ODP) Leg 201 shipboard scientific party and ship crew for their extraordinary commitment during the cruise that resulted in many of the supporting data discussed here. We thank Dan Schrag for sharing unpublished carbon isotope data (DIC), Jeff Seewald for providing his GC for postcruise analyses of gases, Glen Gettemy for physical properties data, Yu-Shih Lin for reviewing and correcting the isotopic calculations, Steve D'Hondt and Jerry Dickens for stimulating discussions, and Tori Hoehler and Ken Nealson for their constructive reviews. This research used samples and data provided by the ODP. The ODP is sponsored by the U.S. National Science Foundation (NSF) and participating countries under management of Joint Oceanographic Institutions (JOI), Inc. Funding for this research was provided by the U.S. Science Support Program, NASA Astrobiology Institute, and the Deutsche Forschungsgemeinschaft (Research Center Ocean Margins). This is RCOM publication 418.
Abbreviation
- mbsf
m below sea floor
Footnotes
The authors declare no conflict of interest.
Claypool, G. E., AAPG Hedberg Conference Abstracts, Natural Gas Formation and Occurrence, June 6–11, 1999, Durango, CO, pp. 27–29 (abstr.).
References
- 1.D'Hondt SL, Jørgensen BB, Miller DJ, ODP Leg 201 Shipboard Scientific Party Proceedings of the Ocean Drilling Program; 2003. http://www-odp.tamu.edu/publications/201_IR/201ir.htm. [Google Scholar]
- 2.D'Hondt SL, Jørgensen BB, Miller DJ, Batzke A, Blake R, Cragg BA, Cypionka H, Dickens GR, Ferdelman T, Hinrichs K-U, et al. Science. 2004;306:2216–2221. doi: 10.1126/science.1101155. [DOI] [PubMed] [Google Scholar]
- 3.Whiticar MJ, Suess E. Proc ODP Sci Res. 1990;112:527–538. [Google Scholar]
- 4.Knies J, Damm E, Gutt J, Mann U, Pinturier L. Geochem Geophys Geosyst. 2004;5 doi: 10.1029/2003GC000687. [DOI] [Google Scholar]
- 5.Rowe D, Muehlenbachs A. Nature. 1999;398:61–63. [Google Scholar]
- 6.Davis JB, Squires RM. Science. 1954;119:381–382. doi: 10.1126/science.119.3090.381. [DOI] [PubMed] [Google Scholar]
- 7.Oremland RS. Appl Env Microbiol. 1981;42:122–129. doi: 10.1128/aem.42.1.122-129.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vogel TM, Oremland RS, Kvenvolden KA. Chem Geol. 1982;37:289–298. [Google Scholar]
- 9.Oremland RS, Whiticar MJ, Strohmaier FE, Kiene RP. Geochim Cosmochim Acta. 1988;52:1895–1904. [Google Scholar]
- 10.Paull CK, Lorenson TD, Borowski WS, Ussler W, III, Olsen K, Rodriguez NM. Proc ODP Sci Res. 2000;164:67–78. [Google Scholar]
- 11.Taylor SW, Sherwood Lollar B, Wassenaar LI. Environ Sci Technol. 2000;34:4727–4732. [Google Scholar]
- 12.Mather ID, Wellsbury P, Parkes RJ, Maxwell JR. Proc ODP Sci Res. 2002;180:188–201. [Google Scholar]
- 13.Schippers A, Neretin LN, Kallmeyer J, Ferdelman TG, Cragg BA, Parkes RJ, Jørgensen BB. Nature. 2005;433:861–864. doi: 10.1038/nature03302. [DOI] [PubMed] [Google Scholar]
- 14.Biddle JF, Lipp JS, Lever M, Lloyd K, Sørensen K, Anderson R, Fredricks HF, Elvert M, Kelly TJ, Schrag DP, et al. Proc Natl Acad Sci USA. 2006;103:3846–3851. doi: 10.1073/pnas.0600035103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inagaki F, Nunoura T, Nakagawa S, Teske A, Lever M, Lauer A, Suzuki M, Takai K, Delwiche M, Colwell FS, et al. Proc Natl Acad Sci USA. 2006;103:2815–2820. doi: 10.1073/pnas.0511033103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parkes RJ, Webster G, Cragg BA, Weightman AJ, Newberry CJ, Ferdelman TG, Kallmeyer J, Jørgensen BB, Aiello IW, Fry JC. Nature. 2005;436:390–394. doi: 10.1038/nature03796. [DOI] [PubMed] [Google Scholar]
- 17.Sørensen KB, Lauer A, Teske A. Geobiology. 2004;2:151–161. [Google Scholar]
- 18.Meister P, Prokopenko M, Skilbeck CG, Watson M, McKenzie JA. In: Proceedings of the Ocean Drilling Program: Initial Reports. D'Hondt SL, Jørgensen BB, Miller DJ, Shipboard Scientific Party, editors. Vol 201. College Station, TX: Integrated Ocean Drilling Program–US Implementing Organization/Texas A&M University Publication Services; 2005. http://www-odp.tamu.edu/publications/201_SR/105/105.htm. [Google Scholar]
- 19.Conrad R, Klose M. FEMS Microbiol Ecol. 1999;30:147–155. doi: 10.1111/j.1574-6941.1999.tb00643.x. [DOI] [PubMed] [Google Scholar]
- 20.Schink B, Stams AJM. In: The Prokaryotes: An Evolving Electronic Resource for the Microbiological Community. Dworkin M, Falkow S, Rosenberg E, Schleifer K-H, Stackebrandt E, editors. New York: Springer; 2002. [Google Scholar]
- 21.Whiticar MJ. Chem Geol. 1999;161:291–314. [Google Scholar]
- 22.Hoehler TM, Alperin MJ, Albert DB, Martens CS. Geochim Cosmochim Acta. 1998;62:1745–1756. [Google Scholar]
- 23.Hoehler TM, Albert DB, Alperin MJ, Bebout BM, Martens CS, Des Marais DJ. Antonie Leeuwenhoek. 2002;81:575–585. doi: 10.1023/a:1020517924466. [DOI] [PubMed] [Google Scholar]
- 24.Hoehler TM, Alperin MJ, Albert DB, Martens CS. FEMS Microbiol Ecol. 2001;38:33–41. [Google Scholar]
- 25.Berner U, Faber E. Org Geochem. 1996;24:947–955. [Google Scholar]
- 26.Egeberg PK, Barth T. Chem Geol. 1998;149:25–35. [Google Scholar]
- 27.Tissot BP, Welte DH. Petroleum Formation and Occurrence. Berlin: Springer; 1984. [Google Scholar]
- 28.James AT, Burns BJ. AAPG Bull. 1984;68:957–960. [Google Scholar]
- 29.McCollom TM, Seewald JS. Earth Planet Sci Lett. 2006;243:74–84. [Google Scholar]
- 30.Foustoukos DI, Seyfried WEJ. Science. 2004;304:1002–1005. doi: 10.1126/science.1096033. [DOI] [PubMed] [Google Scholar]
- 31.Gelwicks JT, Risatti JB, Hayes JM. Org Geochem. 1989;14:441–446. doi: 10.1016/0146-6380(89)90009-0. [DOI] [PubMed] [Google Scholar]
- 32.Heuer V, Elvert M, Tille S, Krummen M, Mollar XP, Hmelo LR, Hinrichs K-U. Limn Oceanogr Meth. 2006;4 in press. [Google Scholar]
- 33.Froelich PN, Klinkhammer GP, Bender ML, Luedtke NA, Heath GR, Cullen D, Dauphin P, Hammond D, Hartman B, Maynard V. Geochim Cosmochim Acta. 1979;43:1075–1090. [Google Scholar]
- 34.Sposito G, Skipper NT, Sutton R, Park SH, Soper AK, Greathouse JA. Proc Natl Acad Sci USA. 1999;96:3358–3364. doi: 10.1073/pnas.96.7.3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kennedy MJ, Pevear DR, Hill RJ. Science. 2002;295:657–660. doi: 10.1126/science.1066611. [DOI] [PubMed] [Google Scholar]
- 36.Guggenheim S, van Groos AKF. Geology. 2003;31:653–656. [Google Scholar]
- 37.ODP Leg 201 Shipboard Scientific Party . In: Proceedings of the Ocean Drilling Program: Initial Reports. D'Hondt SL, Jørgensen BB, Miller DJ, Shipboard Scientific Party, editors. Vol 201. College Station, TX: Integrated Ocean Drilling Program–US Implementing Organization/Texas A&M University Publication Services; 2003. http://www-odp.tamu.edu/publications/201_IR/chap_05/chap_05.htm. [Google Scholar]
- 38.Goodman K. Anal Chem. 1998;70:833–837. doi: 10.1021/ac970887q. [DOI] [PubMed] [Google Scholar]
- 39.Johnson JW, Oelkers EH, Helgeson HC. Comput Geosci. 1992;18:899–947. [Google Scholar]
- 40.Shock EL, Helgeson HC. Geochim Cosmochim Acta. 1990;54:915–945. doi: 10.1016/s0016-7037(97)00009-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.