Abstract
Insulin-stimulated glucose uptake requires the fusion of GLUT4 transporter-containing vesicles with the plasma membrane, a process that depends on the SNARE (soluble N-ethylmaleimide-sensitive fusion factor attachment receptor) proteins VAMP2 (vesicle-associated membrane protein 2) and syntaxin 4 (Stx4)/SNAP23 (soluble N-ethylmaleimide-sensitive fusion factor attachment protein 23). Efficient SNARE-dependent fusion has been shown in many settings in vivo to require the generation of both phosphatidylinositol-4,5-bisphosphate (PIP2) and phosphatidic acid (PA). Addition of PA to Stx4/SNAP23 vesicles markedly enhanced the fusion rate, whereas its addition to VAMP2 vesicles was inhibitory. In contrast, addition of PIP2 to Stx4/SNAP23 vesicles inhibited the fusion reaction, and its addition to VAMP2 vesicles was stimulatory. The optimal distribution of phospholipids was found to trigger the progression from the hemifused state to full fusion. These findings reveal an unanticipated dependence of SNARE complex-mediated fusion on asymmetrically distributed acidic phospholipids and provide mechanistic insights into the roles of phospholipase D and PIP kinases in the late stages of regulated exocytosis.
Keywords: SNAP23, syntaxin 4, VAMP2
Intracellular trafficking of secretory proteins, integral membrane proteins, and lipids requires the movement and fusion of cargo-containing phospholipid membrane vesicles from donor to acceptor compartments. The fusion of transport vesicles into acceptor membranes is mediated by a complex of proteins known as t-SNAREs or Q-SNARES, which are found on target/acceptor membranes and are composed of the syntaxin (Stx) and SNAP (soluble N-ethylmaleimide-sensitive fusion factor attachment protein) family of proteins, and v-SNAREs or R-SNAREs, which are members of the VAMP (vesicle-associated membrane protein)/synaptobrevin family and are found on vesicle/donor membranes (1–4). Specificity of the membrane fusion process is achieved through both subcellular compartmentalization of these components and selectivity of the binding interactions between the various SNARE (soluble N-ethylmaleimide-sensitive fusion factor attachment receptor) proteins (5–7).
The mechanism underlying membrane bilayer fusion requires the formation of high-affinity, parallel, four-α-helix bundles containing one coiled-coil Stx domain, two coiled-coil SNAP domains, and a coiled-coil VAMP domain (8, 9). Recent studies have suggested that this process proceeds through a hemifused state in which the outer membrane leaflets fuse first, followed by fusion of the inner membrane leaflets (10–13). Formation of the hemifused state is characterized by significant physical constraints, because the outer leaflet needs to bend in a tight negative curve to preserve membrane integrity. Similarly, progress to full fusion then requires the inner leaflet to achieve a positive curvature (14). Recently, we reported that phospholipase D (PLD) 1 activity enhanced insulin-stimulated fusion of GLUT4 vesicles into the plasma membrane, whereas reduction of PLD1 by RNAi gene silencing was inhibitory (15), consistent with earlier studies that demonstrated a functional requirement for PLD1 as a downstream effector in secretagogue-stimulated granule exocytosis in PC12 cells (16) and prospore membrane formation in yeast (17). PLD1 generates phosphatidic acid (PA), a phospholipid that favors the formation of negative curvature, on one or both of the external leaflets undergoing fusion. Because the PLD1-RNAi phenotype could be rescued by exposure of one of the inner leaflets to lysophosphatidylcholine, a positive curvature-favoring phospholipid, it is suggested that membrane curvature might play an important physiological role in GLUT4 vesicle fusion with the plasma membrane. A second acidic phospholipid that is known to be important for the regulated exocytosis fusion event in neuronal cells is phosphatidylinositol-4,5-bisphosphate (PIP2) (18), which may undertake a number of roles, including promoting inner membrane leaflet positive curvature (14). In the current study, we have examined acidic phospholipid requirements for SNARE-dependent membrane fusion, and we demonstrate an unexpected asymmetric and reciprocal requirement for PA and PIP2 in donor and acceptor membranes.
Results and Discussion
The v-SNARE VAMP2 and t-SNARE Complex Stx4/SNAP23 Suffice to Drive Membrane Fusion in Vitro.
Because membrane fusion displays selectivity among different pairs of v- and t-SNARE proteins (6, 7), we initially set out to determine whether the SNARE complexes involved in insulin-stimulated GLUT4 translocation were capable of mediating in vitro membrane fusion. Purified Stx4/SNAP23 complexes were reconstituted into phospholipids composed of 85% phosphatidylcholine (PC) and 15% phosphatidylserine (PS). In parallel, VAMP2 was reconstituted into phospholipids containing 82% PC and 15% PS plus 1.5% NBD-PE [N-(7-nitro-2,1,3-benzoxadiazole-4-yl)-phosphatidylethanolamine] and 1.5% rhodamine-PE as the fluorescent phospholipid reporters. The close proximity of the NBD-PE with rhodamine-PE results in fluorescence quenching such that the NBD-PE fluorescence is reduced by >90%.
As reported in ref. 19, we observed that the efficiency of VAMP2 reconstitution was more efficient than that for the t-SNARE complex. Specifically, 18 nmol (300 μg) of VAMP2 mixed with 300 nmol of phospholipids incorporated with 78% efficiency (14.4 nmol) into vesicles (Fig. 1A, lanes 1 and 2). In contrast, only 31% (1.5 nmol) of the 4.8-nmol (300 μg) input of Stx4/SNAP23 complexes became incorporated (Fig. 1A, lanes 3 and 4). This reconstitution corresponded to a 1:200 protein:lipid ratio for the Stx4/SNAP23 complex (≈75 copies per proteoliposome) compared with ≈1:20 for VAMP2 (≈750 copies per proteoliposome). Thus, because of the difference in mass and reconstitution efficiency, we standardized each reaction mixture by adding at least 10-fold more acceptor Stx4/SNAP23 vesicles than fluorescent donor VAMP2 vesicles, following methodology that had been described previously for other SNARE isoforms (19).
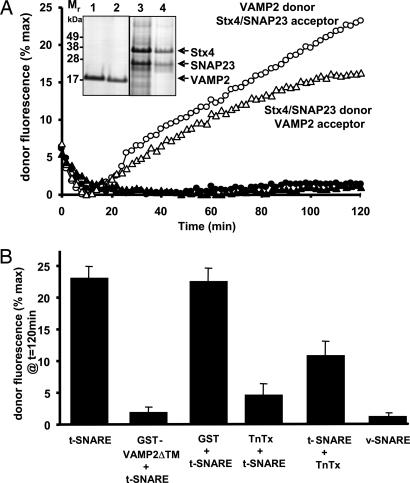
Fig. 1.
The v-SNARE VAMP2 and the t-SNAREs Stx4 and SNAP23 suffice to drive membrane fusion. (A) Three hundred micrograms of purified human VAMP2 (18 nmol) was mixed with 300 nmol of phospholipids (82% PC/15% PS/1.5% NBD-PE/1.5% rhodamine-PE) in octyl-β-d-glucopyranoside. The mixture was then rapidly diluted and subjected to Histodenz gradient flotation and dialysis. Aliquots (1 nmol of phospholipids, equivalent to 1/300 of the reconstituted vesicles) of the initial input (lane 1) and final recovered vesicles (lane 2) were subjected to SDS/PAGE. Similarly, 300 μg of the purified mouse Stx4/SNAP23 complex (4.8 nmol) was mixed with 300 nmol of phospholipids (85% PC/15% PS) in octyl-β-d-glucopyranoside. The mixture was then rapidly diluted and subjected to Histodenz gradient flotation and dialysis. Aliquots (2 nmol of phospholipids equivalent to 1/150 of the reconstituted vesicles) of the initial input (lane 3) and final recovered vesicles (lane 4) were subjected to SDS/PAGE. The SNARE proteins were then visualized by Coomassie blue staining. VAMP2 (750 copies per vesicles) fluorescent reporter liposomes were mixed with Stx4/SNAP23 (75 copies per vesicle) liposomes (○), or Stx4/SNAP23 fluorescent reporter vesicles were mixed with VAMP2 vesicles (▵). As a control, symmetric donor and acceptor membranes were mixed: VAMP2 donor and acceptor vesicles (●) or Stx4/SNAP23 donor and acceptor vesicles (▴). The increases in NBD-PE fluorescence were monitored and normalized as a percentage of the maximum fluorescence after Triton X-100 solubilization. (B) Acceptor t-SNARE fluorescent reporter vesicles were preincubated with soluble GST-Syb2ΔTM (10 μg) before mixing with v-SNARE membranes. v-SNARE fluorescent donor vesicles were treated with tetanus toxin (TnTx) for 2 h at 37°C before mixing and incubation with t-SNARE acceptor membranes. Donor v-SNARE and acceptor t-SNARE membranes were preincubated before addition of tetanus toxin. The fusion reaction was initiated by warming the samples to 37°C, and the change in fluorescence was determined 2 h later. The percentage of NBD-PE fluorescence at 120 min compared with the maximum amount of fluorescence dequenching (Triton X-100 solubilization) was determined. Data are averages ± SD from three independent experiments.
Incubation of the fluorescent donor VAMP2 vesicles with Stx4/SNAP23 acceptor membranes at 37°C resulted in a time-dependent increase in membrane fluorescence (Fig. 1A, open circles). As previously documented for other v- and t-SNARE pairs, this time frame and extent of membrane increase in fluorescence is characteristic of membrane fusion (19–22). Switching the fluorescent probes to the Stx4/SNAP23 vesicles similarly resulted in a time-dependent membrane fusion with VAMP2 vesicles (Fig. 1A, open triangles). As expected, the extent of fusion under these conditions was reduced, reflecting the reduced amount of Stx4/SNAP23 protein per individual vesicle, which readily becomes saturated after a limited number of fusion events.
To demonstrate specificity of the v- and t-SNARE interaction, we incubated Stx4/SNAP23 fluorescent vesicles with Stx4/SNAP23 acceptor vesicles or VAMP2 fluorescent vesicles with VAMP2 acceptor vesicles. These homotypic combinations did not exhibit membrane fusion (Fig. 1A, filled circles and triangles, respectively). Moreover, the Stx4/SNAP23 vesicle fusion reaction with VAMP2 vesicles was specific; incubation in the additional presence of a soluble form of VAMP2 lacking the transmembrane domain (GST-VAMP2ΔTM) or pretreatment of the donor liposomes with a VAMP2-specific protease (tetanus toxin) inhibited the fusion reaction (Fig. 1B). However, if tetanus toxin was added after the mixing of donor and acceptor vesicles, a partial protection of membrane fusion was observed, confirming that, during the preincubation step, the SNARE proteins form a stable ternary complex that is toxin-resistant (Fig. 1B). These data confirm that Stx4/SNAP23 acceptor and proteoliposome donor membranes undergo SNARE-dependent membrane fusion in a manner analogous to other SNARE isoforms.
Addition of PA to the Stx4/SNAP23 Acceptor Membranes Enhances Membrane Fusion.
In vitro fusion reactions using reconstituted neuronal SNARE proteins are typically performed by using liposomes composed of PC plus an additional 15–25% PS (19, 20, 22, 23). The addition of PA resulted in a concentration-dependent increase in VAMP2 incorporation, whereas PA had no significant effect on the efficiency of Stx4 incorporation (Fig. 2A). Under these conditions, reconstitution with pure PC resulted in vesicles containing ≈125 copies of VAMP2, whereas the addition of 3% PA (97% PC/3% PA) or 10% PA (90% PC/10% PA) resulted in the incorporation of ≈375 and ≈750 copies per vesicle, respectively. The differences in VAMP2 reconstitution efficiency occurred independently of vesicle size; dynamic light scattering demonstrated identical vesicle diameters (60 ± 14 nm). Because the efficiency of VAMP2 reconstitution depends on the specific type and concentration of phospholipids, we adjusted the VAMP2 protein input during the various reconstitution reactions to obtain vesicles containing an average of ≈125 VAMP2 copies. Immunoblotting demonstrated the presence of equivalent amounts of VAMP2 protein for each phospholipid reconstitution and assay condition (Fig. 2C).
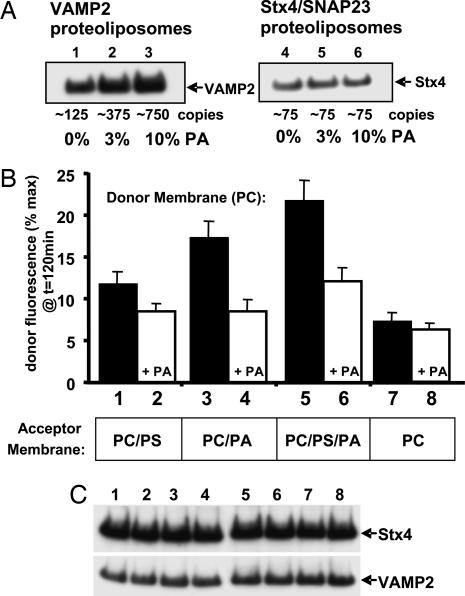
Fig. 2.
Incorporation of PA in donor and acceptor membranes modifies the efficiency of SNARE-mediated fusion in an asymmetric manner. (A) Purified VAMP2 (lanes 1–3) and Stx4/SNAP23 (lanes 4–6) were reconstituted into PC vesicles containing 0% (lanes 1 and 4), 3% (lanes 2 and 5), or 10% (lanes 3 and 6) PA and immunoblotted with the 6× His-tag antibody. The estimated copy numbers of v- and t-SNAREs incorporated per vesicle are indicated. (B) Purified VAMP2 (≈125 copies per vesicle) was reconstituted into 97% PC (filled bars) or 87% PC/10% PA (+PA, open bars) containing 1.5% NBD-PE and 1.5% rhodamine-PE. The VAMP2 v-SNARE donor membranes were preincubated overnight at 4°C with reconstituted Stx4/SNAP23 t-SNARE acceptor membranes (≈75 copies per vesicle) containing 85% PC/15% PS (lanes 1 and 2), 90 PC/10% PA (lanes 3 and 4), 75% PC/10% PA/15% PS (lanes 5 and 6), or 100% PC (lanes 7 and 8). Data are averages ± SD from three independent experiments. (C) After the above fusion reactions, each sample was subjected to immunoblotting using the 6× His-tag antibody to verify equal amounts of the VAMP2 and Stx4 proteins.
We next determined the effect of altering phospholipid composition on the extent of membrane fusion (Fig. 2B). Even at relatively low v-SNARE protein density (≈125 copies), the PC donor membranes underwent ≈12% of the maximum extent of fusion after 120 min of incubation (Fig. 2B, bar 1). The addition of 10% PA to the donor membranes inhibited this fusion by ≈30% (Fig. 2B, bar 2). Unexpectedly, the replacement of the 15% PS with 10% PA in the acceptor membranes increased membrane fusion by ≈50% (Fig. 2B, bar 3). The enhanced fusion driven by PA in the acceptor membrane was again inhibited by the presence of PA in the donor membrane (Fig. 2B, bar 4). The more physiological combination of PC/PS/PA in the acceptor membrane promoted the highest level of fusion (Fig. 2B, bar 5), and, again, the presence of PA in the donor membrane almost completely eliminated the gain in the rate of fusion (Fig. 2B, bar 6). In contrast, pure PC acceptor vesicles supported only a relatively low rate of fusion, which was not further suppressed by the presence of PA in the donor membrane (Fig. 2B, bars 7 and 8). As a control, there was no detectable fusion in the SNARE protein-free liposomes containing PA or PS under these conditions.
We previously demonstrated that PLD1 activity in 3T3L1 adipocytes increases the sensitivity of insulin-stimulated GLUT4 translocation and glucose uptake by promoting the fusion of GLUT4 vesicles with the plasma membrane (15). However, because PLD1 is an intracellular protein, in vivo PLD1 can only generate PA in the outer leaflet of vesicles and/or the inner plasma membrane leaflet. Because vesicle reconstitution using PA results in PA incorporation into both leaflets, we next generated vesicles containing PA in only the outer leaflet by reconstituting Stx4/SNAP23 and VAMP2 into 100% PC vesicles and incubating them with bacterial PLD to convert a fraction of the externalized PC into PA. Incubation of the Stx4/SNAP23 liposomes with 1 or 5 units of PLD increased the extent (Fig. 3A) and rate of membrane fusion (Fig. 6A, which is published as supporting information on the PNAS web site). Under these conditions, PLD incubation generated an approximate formation of 1% and 5% PA, respectively (data not shown). The effect of PLD was specific; treatment of the vesicles with EGTA prevented the enhancement of membrane fusion. As a control, there was no detectable fusion when protein-free liposomes were pretreated with PLD (data not shown).
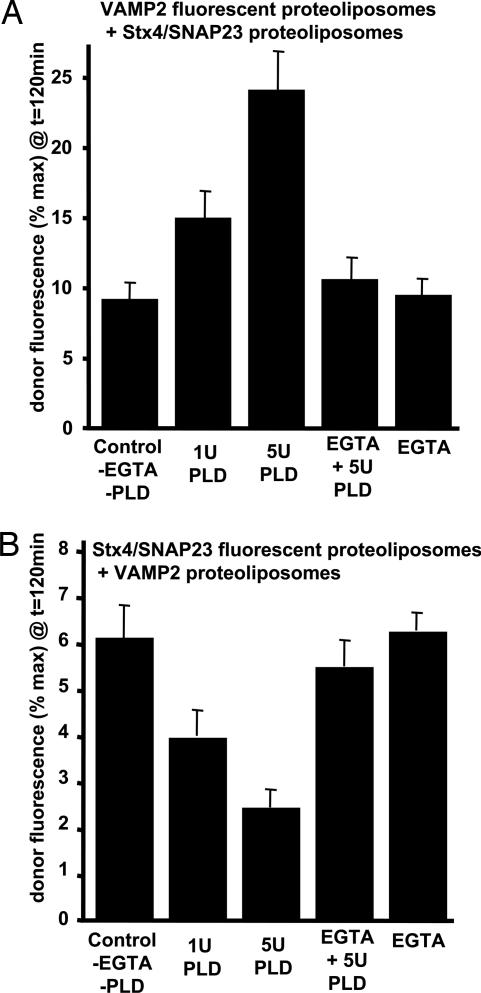
Fig. 3.
PLD activity modifies the efficiency of SNARE-mediated fusion in an asymmetric manner. (A) Reconstituted Stx4/SNAP23 donor membrane (≈75 copies per proteoliposome) in 100% PC were incubated with bacterial PLD and/or 1 mM EGTA as indicated for 10 min at 37°C. After neutralization of the PLD activity by addition of 1 mM EGTA, the vesicles were incubated overnight at 4°C with VAMP2 fluorescent vesicles (≈125 copies per vesicle) in 97% PC. The increases in NBD-PE fluorescence were monitored and normalized as a percentage of the maximum fluorescence after Triton X-100 solubilization. Data are plotted as the percentage of fluorescent dequenching at 120 min for the PLD-treated Stx4/SNAP23 vesicles. (B) Reconstituted VAMP2 donor membranes (≈125 copies per vesicle) in 100% PC were incubated with bacterial PLD and/or 1 mM EGTA as indicated for 10 min at 37°C. After neutralization of the PLD activity by addition of 1 mM EGTA, the vesicles were incubated overnight at 4°C with Stx/SNAP23 fluorescent vesicles (≈75 copies per vesicle) in 97% PC. The increases in NBD-PE fluorescence were monitored and normalized as a percentage of the maximum fluorescence after Triton X-100 solubilization. Data are plotted as the percentage of fluorescent dequenching at 120 min for the PLD-treated VAMP2 vesicles. All data are averages ± SD from three independent experiments.
Because bacterial PLD is relatively nonselective for phospholipid head groups, it also hydrolyzes the outer leaflet rhodamine-PE and NBD-PE. Accordingly, for the VAMP2-reconstituted PC vesicles treated with PLD, the fluorescent reporters were reconstituted instead into the Stx4/SNAP23 vesicles. Under these conditions, there was a dose-dependent inhibition of the extent (Fig. 3B) and rate of fusion (Fig. 6B). Coomassie blue staining demonstrated that the PLD did not degrade the VAMP2 or Stx4/SNAP23 proteins (Fig. 6C). Taken together, these data indicate that the ability of PA to enhance or inhibit liposome fusion occurs asymmetrically, with enhancement occurring when it is present in the acceptor membrane (Stx4/SNAP23) and inhibition occurring when it is present in the donor membrane (VAMP2).
PIP2 in the VAMP2 Donor Membrane Enhances Membrane Fusion.
PIP2 is also a negatively charged phospholipid, but it possesses biophysical properties that differ from those of PA. PIP2 has a bulky head group that promotes the formation of positive membrane curvature, whereas PA promotes the formation of negative membrane curvature. Similar to the reconstitution of VAMP2 into PA-containing liposomes, the presence of PIP2 also increased the efficiency of VAMP2 incorporation in a dose-dependent manner without any significant effect on the incorporation of Stx4/SNAP23 (Fig. 4A).
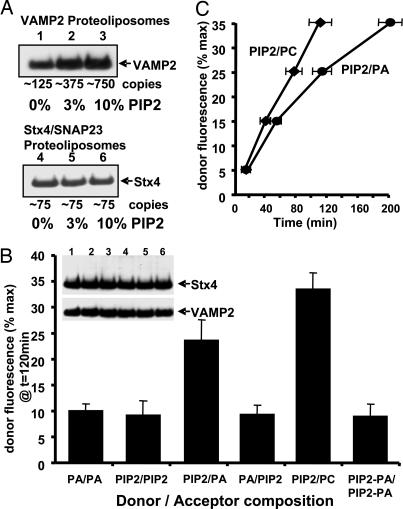
Fig. 4.
Incorporation of PIP2 in donor and acceptor membranes modifies the efficiency of SNARE-mediated fusion in an asymmetric manner. (A) Purified VAMP2 (lanes 1–3) and Stx4/SNAP23 (lanes 4–6) were reconstituted into PC containing 0% (lanes 1 and 4), 3% (lanes 2 and 5), or 10% (lanes 3 and 6) PIP2 and immunoblotted with the 6× His-tag antibody. The estimated copy numbers of v- and t-SNAREs incorporated per vesicle are indicated. (B) VAMP2 donor membranes (≈750 copies per vesicle) were incubated with Stx4/SNAP23 acceptor membranes (≈75 copies per vesicle) for 2 h at 37°C. The vesicles contained 90% PC plus 10% PA, 10% PIP2, or 5% PA/5% PIP2 as indicated. Results are expressed as the percentage of fluorescent dequenching at 120 min. At the end of the reaction, for each assay, the use of equal amounts of VAMP2 and Stx4 was verified by 6× His-tag immunoblotting. Data are averages ± SD from three independent experiments. (C) Time dependence of vesicle fusion when 87% PC/10% PIP2 VAMP2 donor membranes were mixed with 100% PC or 90% PC/10% PA-containing Stx4/SNAP23 acceptor membranes. Data are averages ± SD from three independent experiments.
Because the incorporation of VAMP2 was equivalent in both 10% PA- and 10% PIP2-containing vesicles, we used a higher amount of VAMP2 protein (≈750 copies per vesicle). Reconstitution of VAMP2 at 750 copies per vesicle with the hydrophilic dye 5(6)-carboxyfluorescein demonstrated that, at this protein density, these vesicles were fully sealed and had no appreciable leakiness (Fig. 7, which is published as supporting information on the PNAS web site). Similarly, Stx4/SNAP23 vesicles reconstituted at 75 copies were also impermeant to 5(6)-carboxyfluorescein.
A comparison of different combinations of PA and PIP2 in the donor and acceptor liposomes demonstrated that, when the acceptor and donor liposomes had the same lipid composition (PA/PA or PIP2/PIP2), the rate of fusion was relatively slow (Fig. 4B, bars 1 and 2). However, if the VAMP2 vesicles containing PIP2 were mixed with Stx4/SNAP23 vesicles containing PA or just PC, the rate of fusion was enhanced (Fig. 4B, bars 3 and 5). In contrast, if the VAMP2 donor membranes contained PA and the Stx4/SNAP23 membranes contained PIP2, the fusion reaction was reduced (Fig. 4B, bar 4). Similarly, if both the donor and acceptor membranes contained equal amounts of PIP2/PA, then the fusion reaction was also markedly reduced (Fig. 4B, bar 6).
Although the fusion reaction was enhanced when the donor/acceptor membranes contained PIP2/PA, respectively, the extent of fusion was even greater when the donor VAMP2 membrane contained only PIP2 and the acceptor Stx4/SNAP23 membrane contained only PC (Fig. 4B, bar 5). This less efficient fusion reaction with the combination of PIP2/PA could have resulted from a mixing of the phospholipids after successive rounds of membrane fusion (e.g., transfer of PA into the VAMP2 vesicles) that progressively inhibited the fusion reaction. To address this possibility, we compared the time required for various extents of fusion when the donor/acceptor vesicles were PIP2/PC and PIP2/PA, respectively (Fig. 4C). Fusion reactions with the PIP2/PC donor/acceptor membranes displayed a linear time dependence over a range of 5–35% of the maximum extent of fusion, indicating that the rate of fusion remained relatively constant. However, in the PIP2/PA donor/acceptor membrane pair, the time required for each subsequent round of fusion increased, demonstrating that the rate of fusion was not constant but continually slowed as the amount of fusion proceeded. These data are consistent with the transfer of PA from the acceptor Stx4/SNAP23 membrane to the donor VAMP2 membrane, resulting in a continual slowing of the reaction rate.
The Hemifusion Step Is the Rate-Limiting Factor for in Vitro Membrane Fusion.
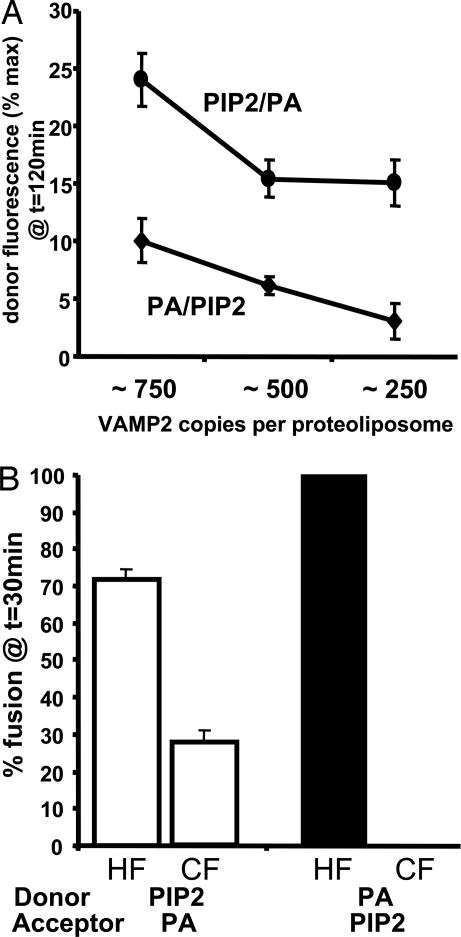
The findings described above demonstrate that the presence of acidic phospholipids strongly improves the incorporation of VAMP2 into vesicles. However, as previously reported, elevating SNARE protein density facilitates the fusion reaction (24). To evaluate the effect of the SNARE density in the fusion rate of PIP2/PA or PA/PIP2 donor/acceptor membrane combinations, we next adjusted the VAMP2 density in donor membranes from ≈750 to ≈250 copies per vesicle (Fig. 5A). As expected, the highest concentrations of VAMP2 in the donor membranes (≈750 copies) promoted the highest extent of fusion. However, the PIP2/PA-reconstituted vesicles were relatively insensitive to SNARE protein density, and there was not a significant difference between ≈500 and 250 VAMP2 copies per vesicle. In contrast, the PA/PIP2 lipid combination was strongly SNARE protein density-sensitive; the fusion reaction was markedly attenuated by decreasing the density to ≈250 VAMP2 copies per vesicle.
Fig. 5.
Incorporation of 10% PIP2 into donor vesicles and 10% PA into acceptor vesicles promotes the progression of SNARE-mediated fusion from the hemifused intermediate state to the fully fused state. (A) VAMP2 was reconstituted into vesicles containing 87% PC/10% PA (●) or 87% PC/10% PIP2 (♦) at ≈750, 500, and 250 VAMP2 copies per vesicle. These v-SNARE membranes were incubated with Stx4/SNAP23 (≈75 copies per vesicle) containing 90% PC/10% PA (♦) or 90% PC/10% PIP2 (○). Data are averages ± SD from three independent experiments. (B) Percentage of hemifused (HF) or completely fused (CF) vesicles after incubation of 87% PC/10% PIP2 or 87% PC/10% PA v-SNARE donor membranes with 90% PC/10% PA or 90% PC/10% PIP2 t-SNARE acceptor membranes for 30 min at 37°C. Data are averages ± SD from four independent experiments.
It has been recently reported that SNARE-mediated membrane fusion occurs through a hemifusion intermediate state (10–13). Therefore, we next determined the effect of different phospholipid compositions on the proportion of hemifused versus fused membrane states. This quantification was accomplished by reduction of the outer membrane half-bilayer NBD fluorescence with sodium dithionite as described in refs. 13 and 22. As a consequence, the NBD fluorescence remains on only the inner leaflet that is protected from the sodium dithionite reduction (Fig. 8, which is published as supporting information on the PNAS web site), and, therefore, any increase in fluorescence that is observed corresponds to inner leaflet fusion (Fig. 9A, which is published as supporting information on the PNAS web site). In parallel, an identical fusion reaction was performed without prior sodium dithionite reduction to generate a signal representing the combined fluorescence gain resulting from inner and outer leaflet fusion. The difference between the two signals at any given point in time indicates the extent of hemifusion (outer but not inner leaflet fusion).
By 30 min, ≈30% of the vesicles had undergone complete fusion (Fig. 5B, CF, open bar), with ≈70% remaining in the hemifused state (Fig. 5B, HF, open bar). In parallel, we also determined the time dependence of PA/PIP2 donor/acceptor membrane fusion in the absence and presence of dithionite. In contrast to the PIP2/PA donor/acceptor pair, essentially 100% of the vesicles were in the hemifused state when the donor/acceptor membranes were in the less efficient PA/PIP2 distribution (Fig. 5B, filled bar). The time dependence of membrane fusion in the absence and presence of dithionite for PIP2/PA and PA/PIP2 donor/acceptor membrane pairs is shown in Fig. 9 B and C. Thus, not only are these data consistent with hemifusion being the rate-limiting step for full fusion, but they also suggest that the presence of PA and PIP2 in the t- and v-SNARE membranes, respectively, facilitates more efficient transition from the hemifused state to the fully fused state.
In summary, we have unexpectedly observed that the fusion reaction between Stx4/SNAP23 acceptor and VAMP2 donor membranes displays asymmetric acidic phospholipid dependence. That is, PA in the Stx4/SNAP23 vesicles increased the rate of fusion, whereas the addition of PIP2 was inhibitory. Conversely, the addition of PA to the VAMP2 vesicles was inhibitory, and PIP2 in the Stx4/SNAP23 vesicles stimulated the rate of fusion. The ability of PA to enhance membrane fusion is consistent with the recent observation that insulin induces the plasma membrane translocation of PLD1 along with GLUT4 and that PLD1 activity potentiates insulin-stimulated GLUT4 translocation in adipocytes (15).
Moreover, we have also observed that the efficiency of VAMP2, but not Stx4, protein reconstitution into vesicles is increased in the presence of PA and PIP2, demonstrating a preference for general acidic phospholipids similar to that observed for annexin V (25–29). Thus, the ability of PA in the acceptor membrane to enhance fusion but in the donor membrane to act as an inhibitor could reflect the affinity of VAMP2 to bind either to the trans membrane (stimulatory) or to the cis membrane (inhibitory). However, because VAMP2 apparently has similar affinities for PA and PIP2, we would expect that if this interaction were based simply on charge, then PA and PIP2 would manifest a similar potentiation of fusion when present in the trans membrane bilayer and a similar degree of inhibition when present in the cis membrane bilayer. In fact, we observed the converse: PA and PIP2 are stimulatory on opposite membrane surfaces. Therefore, either the nature of the SNARE protein interaction and the fusogenic properties were specific to the type of lipid head group or another mechanism underlies their reciprocal effects on fusion efficiency.
PA and PIP2 have different physical properties, including charge density, but they also differ in their ability to generate membrane curvature. Because of the relative size of the phospholipid head group, PA favors the formation of negative membrane curvature (concave), whereas PIP2 favors the generation of positive membrane curvature (convex). During the fusion reaction, PA is thought to stabilize and expand the outer membrane leaflets in the first transition from separated donor and acceptor bilayers to the hemifused state (fusion of the outer membrane bilayers only) (30, 31). Similarly, the next transition between the hemifused state and full bilayer fusion requires that the inner bilayer half-membranes adopt a positive curvature; thus, this reaction intermediate favors the presence of phospholipids such as PIP2 (14).
Further studies are now necessary to determine whether this asymmetric phospholipid requirement for Stx4/SNAP23- and VAMP2-dependent membrane fusion is a general phenomenon for all SNARE pairs and to examine the potential contribution of phospholipid asymmetry as a regulatory mechanism for fusion events in vivo.
Materials and Methods
Plasmid Construction.
The full-length human VAMP2 coding region was amplified by PCR from pCMV-Sport6-VAMP2 cDNA (IMAGE clone, American Type Culture Collection, Manassas, VA) cloned in-frame with the N-terminal 6× His-tag of the bacterial expression vector TOPO-D pET200 (Invitrogen, Carlsbad, CA), verified by sequencing, and expressed in Bl21 Star (DE3) (Invitrogen). The full-length mouse Stx4 and mouse SNAP23 cDNAs were amplified and subcloned into the dual protein expression vector pET-duet (Novagen, San Diego, CA). The Stx4 coding sequence was cloned in-frame with the N-terminal 6× His-tag of the first multicloning site, whereas the SNAP23 coding sequence was cloned in-frame with the second multicloning site (for further details, see Supporting Methods, which is published as supporting information on the PNAS web site).
Protein Expression and Purification.
The recombinant VAMP2 and t-SNARE proteins were expressed and purified as described in Supporting Methods. Protein purity and molar amounts of the t-SNARE complex Stx4/SNAP23 (1:1 ratio) and VAMP2 were assessed by NuPAGE gel (Invitrogen), Coomassie blue staining, and protein assay (Bradford method, BCA Protein Assay Kit, Pierce, Rockford, IL). The cytosolic domain of VAMP2 (VAMP2ΔTM) was cloned into pET-4T-1 (Novagen) and transformed into Rosetta Escherichia coli. GST-VAMP2ΔTM (1–94) was then purified by glutathione affinity chromatography and eluted with 10 mM reduced glutathione.
SNARE Reconstitution into Liposomes.
The reconstitution and isolation of both v-SNARE- and t-SNARE-containing vesicles was performed as described in ref. 19. Briefly, the SNARE proteins were reconstituted by mixing octyl-β-d-glucopyranoside-soluble SNARE proteins with various detergent-solubilized phospholipids, palmitoyloleoylphosphatidylcholine, dioleoylphosphatidylserine, palmitoyloleoylphosphatidic acid, and PIP2, purchased from Avanti Lipids (Alabaster, AL). After rapid dilution, the vesicles were purified by Histodenz gradient flotation and dialysis (19). SNARE protein copy numbers were calculated based on the vesicle volume and number (determined by dynamic light scattering) and the amount of v-/t-SNARE protein (in moles) present in the sample (19). Recovery of phospholipids was determined as the recovery of NBD-PE and rhodamine-PE fluorescence (Avanti Lipids). To normalize for the different levels of t- and v-SNARE proteins per vesicle, the amount of VAMP2 vesicles added to each fusion reaction was adjusted to give an equivalent molar ratio (1:1) between VAMP2 and Stx4/SNAP23 protein.
Fusion Reactions.
The vesicle fusion assays were performed by using the standard lipid fluorescent mixing assay. In a given reconstitution assay, 1.5% of the total phospholipids contained NBD-PE, and 1.5% contained rhodamine-PE (3% total). Membrane fusion was monitored in black 96-well plates in a Fluoroskan Ascent fluorometer (Thermo Electron, Waltham, MA) preincubated at 37°C. Typically, 5 μl of normalized fluorescent donor vesicles was mixed with 50 μl of acceptor vesicles (nonfluorescent), and the volume was brought to 100 μl with reconstitution buffer (10% glycerol/25 mM Hepes-KOH, pH 7.4/100 mM KCl/2 mM 2-mercaptoethanol). For overnight incubations, wells were sealed with tape and stored in the dark at 4°C under gentle agitation. Fluorescence was monitored every 2 min with the excitation filter set at 460 nm (half-bandwidth 25 nm) and the emission filter set at 538 nm (half-bandwidth 25 nm). Conversion to percentage of maximum fluorescence of NBD-PE by addition of Triton X-100 at the end of reaction was performed as described in ref. 19.
Proteoliposome Treatment.
Pretreatment of VAMP2 vesicles with activated tetanus toxin (Clostridium tetani, Calbiochem, San Diego, CA) was achieved by adding 2 μg of toxin to VAMP2 vesicles for 2 h at 37°C and then cooling to 4°C for 30 min. t-SNARE vesicles were added and incubated overnight at 4°C before the fusion assay. To confirm the assembly of SNARE complexes during overnight preincubation, tetanus toxin was added to the reaction mixture for 2 h at 4°C before the fusion assay. Competition with VAMP2ΔTM was performed by preincubation of the t-SNARE acceptor membranes for 1 h at 37°C with 10 μg of VAMP2ΔTM. v-SNARE donor vesicles plus soluble VAMP2ΔTM were then added to the Stx4/SNAP23 acceptor membranes, and the mixture was incubated overnight at 4°C. Vesicles were incubated with 1 or 5 units of bacterial PLD (from Streptomyces chromofuscus, Sigma, St. Louis, MO) for 10 min at 37°C in reconstitution buffer containing 50 μM CaCl2. The reaction was terminated by addition of 1 mM EGTA, and the vesicles were placed at 4°C. Fluorescent donor membranes were then added to the reaction mixture and incubated overnight at 4°C. Conversion of PC to PA in acceptor membranes was estimated by thin-layer chromatography and phosphate staining.
To assess hemifusion, fluorescent donor vesicles were preincubated in 45 μl of reconstitution buffer containing 1 mM sodium dithionite for 10 min at 37°C and then transferred to 4°C before mixing with acceptor vesicles. The efficiency of NBD reduction to nonfluorescent N-(7-amino-2,1,3-benzoxadiazole-4-yl) was estimated to be between 50% and 60% of the total NBD-PE content. By measuring the total lipid-mixing and inner leaflet-mixing fluorescence signals independently, the percentage of hemifused vesicles could be calculated {defined as 2(PT − PI)/[2(PT − PI) + PI] × 100, where PT is the percentage of maximum for total lipid mixing and PI is the percentage of maximum for inner leaflet mixing} as a function of time (13).
Supplementary Material
Acknowledgments
This work was supported by an American Diabetes Association grant and National Institutes of Health Grants DK33823 (to J.E.P.), DK55811 (to J.E.P.), DK64166 (to M.A.F.), and GM071520 (to M.A.F.).
Abbreviations
- Stx
syntaxin
- PA
phosphatidic acid
- PIP2
phosphatidylinositol-4,5-bisphosphate
- PC
phosphatidylcholine
- PS
phosphatidylserine
- PE
phosphatidylethanolamine
- NBD
N-(7-nitro-2,1,3-benzoxadiazole-4-yl)
- PLD
phospholipase D
Footnotes
The authors declare no conflict of interest.
References
- 1.Hong W. Biochim Biophys Acta. 2005;1744:493–517. [PubMed] [Google Scholar]
- 2.Bonifacino JS, Glick BS. Cell. 2004;116:153–166. doi: 10.1016/s0092-8674(03)01079-1. [DOI] [PubMed] [Google Scholar]
- 3.Fasshauer D, Sutton RB, Brunger AT, Jahn R. Proc Natl Acad Sci USA. 1998;95:15781–15786. doi: 10.1073/pnas.95.26.15781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bock JB, Matern HT, Peden AA, Scheller RH. Nature. 2001;409:839–841. doi: 10.1038/35057024. [DOI] [PubMed] [Google Scholar]
- 5.Scales SJ, Chen YA, Yoo BY, Patel SM, Doung YC, Scheller RH. Neuron. 2000;26:457–464. doi: 10.1016/s0896-6273(00)81177-0. [DOI] [PubMed] [Google Scholar]
- 6.McNew JA, Parlati F, Fukuda R, Johnston RJ, Paz K, Paumet F, Sollner TH, Rothman JE. Nature. 2000;407:153–159. doi: 10.1038/35025000. [DOI] [PubMed] [Google Scholar]
- 7.Whyte JR, Munro S. J Cell Sci. 2002;115:2627–2637. doi: 10.1242/jcs.115.13.2627. [DOI] [PubMed] [Google Scholar]
- 8.Sutton RB, Fasshauer D, Jahn R, Brunger AT. Nature. 1998;395:347–353. doi: 10.1038/26412. [DOI] [PubMed] [Google Scholar]
- 9.Antonin W, Fasshauer D, Becker S, Jahn R, Schneider TR. Nat Struct Biol. 2002;9:107–111. doi: 10.1038/nsb746. [DOI] [PubMed] [Google Scholar]
- 10.Giraudo CG, Hu C, You D, Slovic AM, Mosharov EV, Sulzer D, Melia TJ, Rothman JE. J Cell Biol. 2005;170:249–260. doi: 10.1083/jcb.200501093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu X, Zhang F, McNew JA, Shin YK. J Biol Chem. 2005;280:30538–30541. doi: 10.1074/jbc.M506862200. [DOI] [PubMed] [Google Scholar]
- 12.Reese C, Heise F, Mayer A. Nature. 2005;436:410–414. doi: 10.1038/nature03722. [DOI] [PubMed] [Google Scholar]
- 13.Xu Y, Zhang F, Su Z, McNew JA, Shin YK. Nat Struct Mol Biol. 2005;12:417–422. doi: 10.1038/nsmb921. [DOI] [PubMed] [Google Scholar]
- 14.Lee AG. Biochim Biophys Acta. 2004;1666:62–87. doi: 10.1016/j.bbamem.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 15.Huang P, Altshuller YM, Hou JC, Pessin JE, Frohman MA. Mol Biol Cell. 2005;16:2614–2623. doi: 10.1091/mbc.E04-12-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vitale N, Chasserot-Golaz S, Bailly Y, Morinaga N, Frohman MA, Bader MF. J Cell Biol. 2002;159:79–89. doi: 10.1083/jcb.200203027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nakanishi H, Morishita M, Schwartz CL, Coluccio A, Engebrecht J, Neiman AM. J Cell Sci. 2006;119:1406–1415. doi: 10.1242/jcs.02841. [DOI] [PubMed] [Google Scholar]
- 18.Di Paolo G, Moskowitz HS, Gipson K, Wenk MR, Voronov S, Obayashi M, Flavell R, Fitzsimonds RM, Ryan TA, De Camilli P. Nature. 2004;431:415–422. doi: 10.1038/nature02896. [DOI] [PubMed] [Google Scholar]
- 19.Weber T, Zemelman BV, McNew JA, Westermann B, Gmachl M, Parlati F, Sollner TH, Rothman JE. Cell. 1998;92:759–772. doi: 10.1016/s0092-8674(00)81404-x. [DOI] [PubMed] [Google Scholar]
- 20.Parlati F, Weber T, McNew JA, Westermann B, Sollner TH, Rothman JE. Proc Natl Acad Sci USA. 1999;96:12565–12570. doi: 10.1073/pnas.96.22.12565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sollner TH. Mol Membr Biol. 2003;20:209–220. doi: 10.1080/0968768031000104953. [DOI] [PubMed] [Google Scholar]
- 22.Schuette CG, Hatsuzawa K, Margittai M, Stein A, Riedel D, Kuster P, Konig M, Seidel C, Jahn R. Proc Natl Acad Sci USA. 2004;101:2858–2863. doi: 10.1073/pnas.0400044101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fix M, Melia TJ, Jaiswal JK, Rappoport JZ, You D, Sollner TH, Rothman JE, Simon SM. Proc Natl Acad Sci USA. 2004;101:7311–7316. doi: 10.1073/pnas.0401779101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tucker WC, Weber T, Chapman ER. Science. 2004;304:435–438. doi: 10.1126/science.1097196. [DOI] [PubMed] [Google Scholar]
- 25.Andree HA, Reutelingsperger CP, Hauptmann R, Hemker HC, Hermens WT, Willems GM. J Biol Chem. 1990;265:4923–4928. [PubMed] [Google Scholar]
- 26.Blackwood RA, Ernst JD. Biochem J. 1990;266:195–200. doi: 10.1042/bj2660195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Campos B, Mo YD, Mealy TR, Li CW, Swairjo MA, Balch C, Head JF, Retzinger G, Dedman JR, Seaton BA. Biochemistry. 1998;37:8004–8010. doi: 10.1021/bi973142n. [DOI] [PubMed] [Google Scholar]
- 28.Raynal P, Pollard HB. Biochim Biophys Acta. 1994;1197:63–93. doi: 10.1016/0304-4157(94)90019-1. [DOI] [PubMed] [Google Scholar]
- 29.Schlaepfer DD, Mehlman T, Burgess WH, Haigler HT. Proc Natl Acad Sci USA. 1987;84:6078–6082. doi: 10.1073/pnas.84.17.6078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chernomordik L, Kozlov MM, Zimmerberg J. J Membr Biol. 1995;146:1–14. doi: 10.1007/BF00232676. [DOI] [PubMed] [Google Scholar]
- 31.Chernomordik LV, Kozlov MM. Annu Rev Biochem. 2003;72:175–207. doi: 10.1146/annurev.biochem.72.121801.161504. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.