Abstract
Fertility of spermatozoa depends on maintenance of the mitochondrial transmembrane potential (Δψm), which is generated by the electron-transport chain and regulated by an oxidation–reduction equilibrium of reactive oxygen intermediates, pyridine nucleotides, and glutathione (GSH). Here, we report that male mice lacking transaldolase (TAL)−/− are sterile because of defective forward motility. TAL−/− spermatozoa show loss of Δψm and mitochondrial membrane integrity because of diminished NADPH, NADH, and GSH. Mitochondria constitute major Ca2+ stores; thus, diminished mitochondrial mass accounts for reduced Ca2+ fluxing, defective forward motility, and infertility. Reduced forward progression of TAL-deficient spermatozoa is associated with diminished mitochondrial reactive oxygen intermediate production and Ca2+ levels, intracellular acidosis, and compensatory down-regulation of carbonic anhydrase IV and overexpression of CD38 and γ-glutamyl transferase. Microarray analyses of gene expression in the testis, caput, and cauda epididymidis of TAL+/+, TAL+/−, and TAL−/− littermates confirmed a dominant impact of TAL deficiency on late stages of sperm-cell development, affecting the electron-transport chain and GSH metabolism. Stimulation of de novo GSH synthesis by oral N-acetyl-cysteine normalized the low fertility rate of TAL+/− males without affecting the sterility of TAL−/− males. Whereas TAL−/− sperm failed to fertilize TAL+/+ oocytes in vitro, sterility of TAL−/− sperm was circumvented by intracytoplasmic sperm injection, indicating that TAL deficiency influenced the structure and function of mitochondria without compromising the nucleus and DNA integrity. Collectively, these data reveal an essential role of TAL in sperm-cell mitochondrial function and, thus, male fertility.
Forward motility and fertility of spermatozoa depend on production of reactive oxygen intermediates (ROIs) (1) and maintenance of the mitochondrial transmembrane potential (Δψm) (2, 3). Δψm is generated by the electron-transport chain and subject to regulation by an oxidation–reduction equilibrium of ROI, pyridine nucleotides (NADH/NAD + NADPH/NADP), and reduced glutathione (GSH) (4). In turn, NADPH, a reducing equivalent required for biosynthetic reactions and regeneration of GSH from its oxidized form, is produced by the pentose phosphate pathway (PPP) (5). The PPP was originally formulated based on metabolites and enzymes detected in yeast (6). Thus, PPP comprises two separate oxidative and nonoxidative phases. Enzymes of the oxidative phase, glucose 6-phosphate dehydrogenase (G6PD) and 6-phosphogluconate dehydrogenase, can generate both ribose 5-phosphate (R5P) and NADPH. Although enzymes of the nonoxidative phase, transketolase (TK) and transaldolase (TAL), can convert R5P into glucose 6-phosphate (G6P) for the oxidative phase, and, thus, indirectly, these enzymes can also contribute to the generation of NADPH, the significance of the nonoxidative branch is less well established. Certain organisms (7, 8) and mammalian tissues do not express TAL (9), and, significantly, the nonoxidative phase can be formulated without this enzyme (10); therefore, the overall reason for existence of TAL had not been established.
To investigate the role of TAL in the PPP and mammalian development in general, the genomic locus was inactivated in the mouse. Here, we report that TAL−/− mice develop normally; however, males are sterile because of functional and structural defects of mitochondria.
Results
Inactivation of the Mouse TAL (TAL-M) Gene Locus by Homologous Recombination.
We replaced a 1,116-bp EcoRI-PstI fragment harboring adjacent parts of exon 4 and intron 4 of the TAL-M locus with the neo gene (Fig. 7A, which is published as supporting information on the PNAS web site). This manipulation deleted amino acid residues 138–153, including lysine 142, which is essential for catalytic activity of TAL (11). The 129SvJ-derived TC1 ES cells heterozygous for the disrupted allele were microinjected into C57BL/6 blastocysts to generate chimeras. Chimeric males were mated with C57BL/6 females, and tail DNA of the offspring was tested for transmission of the targeted allele by PCR (Fig. 7B), Southern blot (Fig. 7C), and Western blot analyses (Fig. 7D). Among 692 pups born to heterozygote parents, the proportion of WT (168 of 692, 24.4%), heterozygote (355 of 692, 51.2%), and homozygote knockout genotypes (169 of 692, 24.4%) followed the expected Mendelian inheritance.
Complete Male Infertility in Homozygous and Partial Male Infertility in Heterozygous Knockout Mice.
Breeding pairs were set up between adult (>8 weeks of age) male and female mice born to different heterozygote parents. TAL+/− males had reduced fertility, and TAL−/− males were completely infertile (Fig. 1). Although vaginal plugs were observed, 15 TAL−/− males paired with WT females produced no pregnancy over 5 months. TAL−/− males displayed no obvious differences in weight and development. Weight and light-microscopic morphology of their reproductive organs (testes, seminal vesicles, prostate, and epididymis) did not differ from those of TAL+/− and TAL+/+ littermates. Sperm of all developmental stages were present in similar numbers in the testes and efferent ducts. Complete or partial TAL deficiency did not influence female fertility (Fig. 1).
Fig. 1.
Male infertility in TAL-deficient mice. Fifteen male and 15 female mice of each genotype were paired, with a single WT female or male per cage. Values indicate mean number ± SD of pups per breeding pair recorded for four generations. Based on comparison with TAL+/+ controls, P values <0.05 are shown.
Diminished Forward Motility and in Vitro Fertility of TAL-Deficient Spermatozoa.
The motility of spermatozoa was investigated by using a computer-assisted semen-analysis system. Although no significant differences were observed in total numbers and viability of spermatozoa, motility parameters, including progressive velocity (straight line velocity) and track speed were markedly reduced, whereas beat frequency of TAL−/− sperm was increased (Table 1). Tracings of video images revealed stiffened flagellar midpieces of TAL−/− sperm (Fig. 8, which is published as supporting information on the PNAS web site). We attempted to rescue infertility of TAL−/− sperm by in vitro fertilization. Sperm of four TAL−/− mice failed to fertilize oocytes from 8-week-old TAL+/+ females. By contrast, sperm of four control TAL+/+ littermates fertilized oocytes and resulted in development of blastocysts (Fig. 9, which is published as supporting information on the PNAS web site). Infertility of TAL−/− sperm was rescued by intracytoplasmic sperm injection (ICSI). Injection of sperm from the caput or cauda epididymidis of TAL−/− mice into oocytes led to blastocyst development (Table 2, which is published as supporting information on the PNAS web site). Blastocysts from TAL+/+ or TAL−/− sperm gave rise to similar numbers of pups, indicating that infertility of TAL−/− males was rescued by ICSI.
Table 1.
Motility analysis of spermatozoa from TAL+/+, TAL+/−, and TAL−/− mice
| Parameter | TAL+/+ | TAL+/− | TAL−/− |
|---|---|---|---|
| Motile, % | 45.3 ± 7.8 | 42.7 ± 7.9 (n.s.) | 24.6 ± 7.5 (0.004) |
| Path velocity, μm/s | 105.000 ± 8.9 | 72.6 ± 8.2 0.0183) | 37.6 ± 8.8 (0.0052) |
| Progressive velocity, μm/s | 51.3 ± 0.3 | 43.8 ± 0.6 (0.0070) | 33.0 ± 0.5 (0.0012) |
| Track speed, μm/s | 193.6 ± 17.9 | 139.8 ± 26.6 (0.0069) | 90.8 ± 36.6 (0.0026) |
| Beat frequency, Hz | 23.0 ± 1.2 | 27.6 ± 1.3 (0.0203) | 30.6 ± 1.9 (0.0074) |
Spermatozoa were isolated from cauda epididymidis, and 100–200 cells of each specimen were characterized by using an automated Hamilton–Thorne Research sperm analyzer. Data represent mean ± SE, based on analysis of six mice from each genotype. Sperm with progressive motility >11 μm/s were considered motile. P values (in parentheses) reflect comparison with TAL+/+ samples. n.s., not significant.
Loss of Δψm, Diminished Mitochondrial ROI Production, Reduced Cytoplasmic and Mitochondrial Ca2+ Levels, and Intracellular Acidification in TAL-Deficient Sperm Cells.
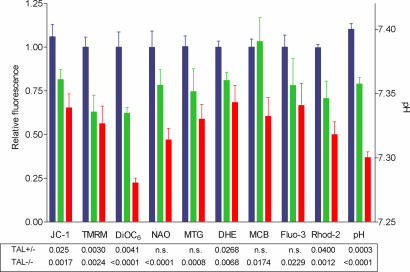
Mitochondria play crucial roles in spermatogenesis and sperm motility (12). Therefore, Δψm of TAL+/+, TAL+/−, and TAL−/− spermatozoa was comparatively analyzed by using potentiometric fluorescent dyes TMRM, DiOC6, and JC-1. Sperm viability was monitored by parallel staining with annexin V-PE or annexin V-FITC (13, 14) matched with emission spectra of potentiometric-, oxidation-, or Ca2+-sensitive fluorescent probes (15, 16). Although overall viability of TAL−/−, TAL+/−, or TAL+/+ spermatozoa was not different, Δψm of TAL−/− spermatozoa was profoundly diminished (Fig. 2 and Fig. 10, which is published as supporting information on the PNAS web site). Δψm of TAL+/− spermatozoa was reduced to a lesser extent. Mitochondrial mass in TAL−/− spermatozoa was diminished, as determined by NAO and MTG fluorescence. The extent of Δψm loss, as determined by DiOC6 fluorescence, exceeded the loss of mitochondrial mass detected by NAO and MTG fluorescence. Reduced JC-1 FL-2/FL-1 fluorescence also suggested loss of Δψm independent of mitochondrial mass.
Fig. 2.
Cumulative assessment of Δψm, mitochondrial mass, ROI, GSH, and cytoplasmic and mitochondrial Ca2+ levels in live spermatozoa from TAL+/+, TAL+/− and TAL−/− littermates. Results were expressed as relative fluorescence values with respect to those of TAL+/+ cells normalized at 1.0 on the left y axis. Absolute pH values based on SNARF-1 fluorescence of calibration standards are shown on the right y axis. Data present mean ± SE of eight independent experiments. P values under each column reflect comparison of TAL+/− (green) and TAL−/− (red) spermatozoa to TAL+/+ (blue) ones. n.s., not significant.
Maintenance of Δψm is required for generation of ROI (17); thus, reduced Δψm may lead to diminished ROI production by TAL-deficient sperm cells. As shown in Fig. 2, ROI levels, monitored by dihydroethidium (hydroethidine, DHE) as well as by dihydrorhodamine and dichlorofluorescein (data not shown) (15), were diminished in TAL+/− and TAL−/− sperm. As monitored by monochlorobimane fluorescence, intracellular thiol content, largely composed of GSH, was diminished in TAL−/−, but not TAL+/−, spermatozoa.
Intracellular Ca2+ is essential for normal motility of sperm cells (18). As shown in Figs. 2 and 10, intracytosolic Ca2+ levels, as determined by Fluo-3 fluorescence and, in particular, mitochondrial Ca2+ levels, as determined by Rhod-2 fluorescence, were profoundly reduced, which may account for increased beat frequency and diminished forward motility of TAL−/− spermatozoa. Rhod-2, but not Fluo-3, fluorescence was also significantly reduced in TAL+/− spermatozoa, suggesting that lower mitochondrial Ca2+ levels may be primarily responsible for reduced fertility of TAL+/− males (Fig. 1). The acrosome reaction, a Ca2+-dependent process, was also reduced in TAL−/− sperm and, to a lesser extent, in TAL+/− cells (data not shown). With respect to WT cells, intracellular pH was reduced in TAL+/− and TAL−/− spermatozoa (Figs. 2 and 10).
Structural Abnormalities of Mitochondria in TAL−/− Spermatozoa.
Phase-contrast (data not shown) and bright-field microscopy showed no structural abnormalities in sperm cells isolated from cauda epididymidis of TAL+/− or TAL−/− mice. However, fluorescence microscopy revealed profoundly diminished DiOC6 staining of the mitochondrial midsection in TAL−/− spermatozoa and, to a lesser extent, in TAL+/− spermatozoa (Fig. 3A), in accordance with flow-cytometry studies (Fig. 2). Because NAO and MTG fluorescence also indicated an overall reduction of mitochondrial-membrane mass, the internal structure of the mitochondria was examined by EM. As expected (19), mitochondria were arranged in a cylinder-shaped sheath that wrapped around the axial outer dense fiber–axoneme complex of the midpiece (Fig. 3B). Although the outer mitochondrial membranes were appropriately retained, the internal membrane structure of the mitochondria was irregular, with disappearance of cristae-like folds and formation of electron-dense central clumps in TAL−/− spermatozoa (Fig. 3B). Similar changes were observed in sperm cells in the caput and cauda epididymidis but not in the testis of TAL−/− mice. The head of the spermatozoa was of normal size and shape, and the tail section exhibited the expected pattern of two central microtubules, surrounded by nine tubules and a set of outer dense fibers.
Fig. 3.
Loss of Δψm and structural changes in mitochondria of TAL-deficient sperm. (A) Fluorescence microscopy of spermatozoa from TAL+/+, TAL+/−, and TAL−/− mice. Cells were stained with DiOC6 (green) and DAPI (blue) to detect Δψm of mitochondria in the midsection and DNA in heads of sperm cells, respectively. BF, bright-field images. With respect to TAL+/+ sperm, Δψm was progressively diminished in TAL+/− and TAL−/− sperm. (Magnification, ×400.) (B) Transmission EM of cauda and caput epididymidis and testis from TAL+/+ and TAL−/− littermate mice. Original magnification is shown above images of cauda epididymidis. Mitochondria (M) are arranged in a cylinder-shaped sheath that wraps spirally around the axial outer dense fiber–axoneme complex of the midpiece (MP). No structural defects were observed in the head and tail section of TAL−/− spermatozoa. The head (H) was of normal size and shape. The axoneme complex (AC) of TAL−/− sperm showed the expected pattern of two central microtubules, surrounded by nine tubules, intact dynein arms, and a set of outer dense fibers. Although the outer mitochondrial membranes were appropriately retained, the internal membrane structure of mitochondria was irregular, with disappearance of cristae-like folds (white arrows) and formation of electron-dense central clumps in mitochondria of TAL−/− spermatozoa in cauda and caput epididymidis (black arrows).
Accumulation of Sedoheptulose 7-Phosphate (S7P) and Depletion of Pyridine Nucleotides in TAL-Deficient Tissues.
To investigate the metabolic bases of mitochondrial dysfunction in TAL deficiency, phosphorylated sugar metabolites of the PPP and pyridine nucleotides were measured by HPLC. Complete TAL deficiency resulted in marked accumulation of S7P in testis of TAL−/− mice (Fig. 4A). S7P is a substrate of the forward reaction of TAL that generates glucose 6-phosphate for NADPH production by enzymes of the oxidative phase of the PPP (Fig. 11, which is published as supporting information on the PNAS web site). NADPH and NADH levels were diminished in TAL−/− and, to a lesser extent, in TAL+/− cauda epididymidis (Fig. 4B), suggesting a dominant role for TAL in the generation of these reducing equivalents by the PPP. NAD, ADP-ribose, AMP, cAMP, ADP, and ATP were diminished only in TAL−/− cauda epididymidis (Fig. 4B). Diminished NADH levels were consistent with the tendency to maintain NADPH at the expense of NADH by transhydrogenases (20). Depletion of ATP by 59.9% in TAL−/− (P = 0.0029), but not in TAL+/−, spermatozoa with respect to those TAL+/+ littermates was independently confirmed by using the luciferin–luciferase assay. ATP depletion may have resulted from the damage of mitochondria, which are estimated to provide as much as 90% of ATP in the sperm (21). However, sperm cells of mice lacking the sperm-specific isozyme of glyceraldehyde 3-phosphate dehydrogenase also show dysmotility and reduced ATP without mitochondrial damage (22). These findings suggest that mitochondrial ATP synthesis alone may not be responsible for sperm dysmotility. Indeed, in vitro addition of cell-permeable cAMP analogues cAMP-acetoxymethyl ester and dibutyryl cAMP (23), NADPH (1), or ATP, by using up to 500 μM each nucleotide, failed to enhance motility of TAL−/− or TAL+/− spermatozoa (data not shown).
Fig. 4.
HPLC analysis of PPP sugars and nucleotides. (A) HPLC analysis of S7P in testis of TAL+/+, TAL+/−, and TAL−/− mice. Chromatograms indicate electric charge (nanocoulomb) of sugar phosphates recorded on a pulse amperometric detector. S7P was positively identified by spiking TAL+/+ tissue extract with sugar phosphate standard (data not shown). Bar charts show comparative analysis of S7P levels in testis from age-matched TAL+/+, TAL+/−, and TAL−/− littermates. Data show mean ± SE of measurements from four mice per genotype. P values <0.05 reflect differences between genotypes. (B) Analysis of nucleotides in cauda epididymidis of TAL+/+, TAL+/−, and TAL−/− mice by HPLC. Chromatograms indicate UV absorbance (AU). Annotated peaks were positively identified by spiking TAL+/+ tissue extracts separately with each compound (data not shown). Bar charts show comparative analysis of nucleotide levels in tissues from age-matched TAL+/+, TAL+/−, and TAL−/− littermates. Data show mean ± SE of measurements from four mice per genotype. P values <0.05 are indicated.
TAL Deficiency-Induced Changes of Gene Expression Are Confined to the Cauda Epididymidis and Affect Mitochondrial Function, Ca2+ Signaling, and Pathways Involved in Sperm Motility.
To investigate the impact of TAL deficiency on signaling pathways involved in sperm motility, microarray and subsequent quantitative real-time RT-PCR analyses were performed on RNA from testis and caput and cauda epididymidis. Expression of 511 of 22,690 genes was increased or decreased by >2-fold in TAL+/− or TAL−/− tissues in comparison with TAL+/+ controls. As shown in the heat color diagrams (Fig. 12, which is published as supporting information on the PNAS web site), most genes altered by TAL deletion are differentially expressed between testis and caput and cauda epididymidis, suggesting involvement in sperm-cell maturation. The most profound changes were noted in cauda epididymidis of TAL−/− mice, where expression of 483 genes was increased or decreased by >2-fold. Similar changes affected only 84 and 4 genes in caput epididymidis and testis, respectively, of TAL−/− mice. Coordinate changes were noted in expression of genes of the PPP and interconnected metabolic pathways, the electron-transport chain, and other mitochondrial proteins, gene expression, apoptosis, adhesion and immune function, ion transport, GSH metabolism and redox signaling, nucleotide metabolism, Ca2+ signaling, and development and motility of spermatozoa (Fig. 12). With a focus on genes involved in sperm motility (24), quantitative real-time RT-PCR analyses confirmed altered expression of γ-glutamyl transpeptidase (GGT), GSH peroxidase, calmodulin, calmodulin-dependent cyclic nucleotide phosphodiesterase, Soggy1, sperm-specific lactate dehydrogenase, and carbonic anhydrase IV (CAIV). Changes in CAIV, CD38, and GGT expression may represent a complementary mechanism involved in male infertility of TAL−/− mice. Of note, CAIV has been implicated in regulating intracellular pH of spermatozoa (25, 26). Transcription of CAIV was increased 4.02-fold (P = 3.5 × 10−6) in TAL+/− cauda epididymidis, whereas it was undetectable in TAL−/− cauda epididymidis by real-time RT-PCR. As documented by Western blot analysis, CAIV protein levels were increased in TAL+/− cauda epididymidis by 82 ± 18% (P = 0.011) and profoundly reduced in cauda epididymidis of TAL−/− mice (−76 ± 3.5%, P < 0.001; Fig. 5A). In agreement with microarray data, expression of CD38 or NAD hydrolase was increased on the surface of TAL−/− spermatozoa (Fig. 5B). As a control (Fig. 10F), CD59 expression was reduced on the surface of TAL−/− spermatozoa (Fig. 5B).
Fig. 5.
Effect of TAL deficiency on expression of CAIV, CD38, and GGT. (A) Western blot analysis of CAIV expression in testis and caput and cauda epididymidis of TAL+/+, TAL+/−, and TAL−/− littermates. Blots were first developed with rabbit antibody to CAIV then reexposed to antibodies to TAL and actin. By using automated densitometry, CAIV expression levels in each tissue were normalized to actin levels and compared with TAL+/+ cells set at 1.0. (B) Expression of CD38 on the surface of sperm cells from TAL+/+, TAL+/−, and TAL−/− littermates gated on live cells based on forward scatter and side scatter. Antibody to CD59 was used as a control. Histograms of TAL+/− and TAL−/− spermatozoa (shaded curves) are overlayed on those from TAL+/+ littermate controls (open curves). Data represent analysis of five experiments. (C) Activity of TAL, TK, G6PD (left y axis), and GGT (right y axis) in cauda epididymidis of TAL+/+, TAL+/−, and TAL−/− mice. Data represent mean ± SD of four sets of TAL+/+, TAL+/−, and TAL−/− littermates. ∗, P < 0.05; ∗∗, P < 0.01; ∗∗∗, P < 0.0001.
TAL protein and activity levels were halved in TAL+/− testis, epididymis, and spermatozoa, and they were undetectable in TAL−/− tissues (Fig. 5C). Activities of PPP enzymes G6PD and TK were unchanged in these TAL+/− and TAL−/− tissues. GSH was reduced in TAL−/− spermatozoa (Fig. 2), indicating that profound NADPH depletion affected regeneration of GSH from its oxidized form, GSSG. Whereas NADPH was reduced, GSH levels were normal in TAL+/− spermatozoa, suggesting compensatory changes in de novo synthesis or salvage of GSH. Activity of GGT, an ectoenzyme involved in GSH salvage, was increased in both TAL+/− and TAL−/− cauda epididymidis (Fig. 5C).
Interestingly, both male and female GGT-deficient mice are infertile, and their fertility can be restored by stimulation of de novo GSH synthesis with N-acetyl cysteine (NAC) (27). After weaning at 3 weeks of age, 10 g/liter NAC was added to the drinking water of male littermates. At the age of 3 months, breeding pairs were set up between TAL+/+, TAL+/−, or TAL−/− male littermates, six of each genotype, and age-matched TAL +/− female littermates and given NAC in the drinking water. Oral NAC treatment normalized the Δψm, mitochondrial mass, mitochondrial and cytosolic Ca2+ levels, and ROI production of TAL+/− sperm (data not shown). Whereas TAL−/− males produced no pregnancies, TAL+/+ and TAL+/− males produced 15 ± 1.9 and 16 ± 2.9 pups, respectively, over a period of 60 days. Thus, NAC normalized the fertility of TAL+/− males without affecting the sterility of TAL−/− males.
Discussion
The present data show that TAL plays a critical role in male fertility through the maintenance of the Δψm and structural integrity. TAL is an enzyme of the nonoxidative phase of the PPP, which generates two major products, (i) NADPH for biosynthetic reactions and maintenance of a reducing environment and (ii) ribose 5-phosphate for synthesis of nucleotides (Fig. 11). The medical importance of PPP was first appreciated when partial G6PD deficiency was associated with hemolytic anemia (28). G6PD has been uniformly regarded as the rate-controlling enzyme of the PPP (5). Targeted disruption of the G6PD gene in mouse ES cells showed a complete lack of survival of homozygous knockout cells (29). Likewise, mouse embryos with complete TK deficiency were not viable. TK+/− mice exhibited general growth retardation (−35%) and a similar reduction in gonadal mass and the number of pups born to TK+/− females (30). These results indicated that G6PD and TK play essential housekeeping roles in cellular metabolism (29). By contrast, TAL+/− or TAL−/− mice developed normally and exhibited no reduction in weight or lifespan at 1 year of age.
The role of TAL in the functioning of the PPP and metabolism in general has been poorly understood. The PPP has been formulated with and without TAL, and, indeed, organisms lacking TAL have been documented (7, 8). Enzymatic activity (31–37) and expression of TAL is regulated in a tissue-specific manner (9, 38–40). TAL catalyzes the transfer of a three-carbon fragment, corresponding to dihydroxyacetone, from S7P and fructose 6-phosphate to d-glyceraldehyde 3-phosphate and d-erythrose 4-phosphate in the forward and reverse reactions, respectively (Fig. 11). In the absence of TAL, S7P, a substrate of the forward TAL reaction accumulated >7-fold in testis, urine, and liver (data not shown) from TAL−/−, but not TAL+/−, mice. Accumulation of S7P is compatible with a failure to recycle ribose 5-phosphate into glucose 6-phosphate through the nonoxidative phase of the PPP, thus, reducing NADPH production by G6PD and 6-phosphogluconate dehydrogenase (Fig. 11). Indeed, NAPDH levels were reduced in cauda epididymidis of TAL+/− or TAL−/− mice (Fig. 4B).
Male infertility affects as much as 10% of the adult population. Most cases are characterized by a reduced or absent movement of spermatozoa of unknown etiology (41). The present data reveal a critical role for TAL in NADPH production and maintenance of Δψm and motility of spermatozoa. Whereas TAL−/− sperm failed to fertilize TAL+/+ oocytes in vitro, the fertility of TAL−/− sperm was rescued by ICSI. These results indicated that TAL deficiency selectively impacted the structure and function of mitochondria without affecting the nucleus and DNA integrity. Thus, this study clearly identifies TAL deficiency as a cause of sperm dysmotility and male infertility.
Materials and Methods
Development of TAL-Deficient Mice.
Cloning of the mouse TAL (TALDO1) genomic locus, construction of the TALDO1 targeting vector, homologous recombination and generation of germ-line chimeras, and testing of WT (TAL+/+), heterozygote (TAL+/−), and homozygote (TAL−/−) offspring by PCR, Southern blot hybridization, and Western blot analysis are described in Supporting Methods, which is published as supporting information on the PNAS web site.
Microarray and Quantitative Real-Time Reverse-Transcriptase-Mediated PCR Analysis of Gene Expression.
See Supporting Methods.
Enzyme Activity Assays.
TAL, TK, G6PD, and GGT activities were determined as described (42, 43).
Assessment of Fertility.
Littermate males (>8 weeks of age) were separated and paired with females (>8 weeks of age) for 5 months or more. The number of mice achieving pregnancy and the number of offspring from each breeding pair and pregnancy were recorded.
Sperm-Motility Analysis.
The cauda epididymidis was excised, placed into 5 ml of Sperm Washing Medium (Irvine Scientific, Santa Ana, CA), and minced, and dispersed sperm cells were separated from solid tissue remnants by passing through a 70-μm cell strainer (Becton Dickinson, Franklin Lakes, NJ). Sperm movement was quantified within 30 min after removal from animals by a computer-assisted semen-analysis system (IVOS version 12, Hamilton–Thorne Research, Beverly, MA).
Flow Cytometry.
Δψm, mitochondrial mass, ROI production, GSH, cytoplasmic and mitochondrial Ca2+ level, intracellular pH, and acrosome reaction were analyzed by flow cytometry, as described in the Supporting Methods.
HPLC and Mass Spectrometry.
Measurement of PPP sugar metabolites and nucleotides by HPLC and tandem mass spectrometry is described in Supporting Methods.
ATP Measurement by Chemiluminescence.
Intracellular ATP levels were determined by using the luciferin–luciferase method (44).
Video Microscopy.
Motility of live spermatozoa was observed on a Zeiss Axioskop microscope under stroboscopic dark-field illumination at flash rates between 50 and 150 Hz and recorded with a Sony (Tokyo, Japan) AVC-D7 video camera (Movie 1, which is published as supporting information on the PNAS web site).
Immunofluorescence Microscopy.
For fluorescence microscopy, sperm cells were resuspended in Sperm Refrigeration Medium (Irvine Scientific) and stained with 40 nM DiOC6 (green) and 1 μM DAPI (blue) to detect Δψm of mitochondria in the midsection and DNA in heads of sperm cells. Specimen were examined with a Zeiss Axioskop 2 microscope equipped with a Hamamatsu (Hamamatsu City, Japan) OCRA-ER digital camera.
EM.
Freshly excised tissues were fixed overnight in PBS with 2.5% glutaraldehyde, postfixed in 1% OsO4, dehydrated in graded ethanol series, infiltrated with propylene oxide, and embedded in Araldite 502 epoxy resin. Ultrathin sections were stained with uranyl acetate and Reynold's lead citrate before examination under a Tecnai BioTWIN 12 transmission electron microscope (FEI, Hillsboro, OR).
In Vitro Fertilization (IVF) and ICSI.
IVF (45) and ISCI were performed, as described in the Supporting Methods (46–48).
Statistics.
Results were analyzed by Student's t test or Mann–Whitney rank-sum test for nonparametric data. Correlation was measured by using Pearson's correlation coefficient. Changes were considered significant at P < 0.05.
Supplementary Material
Acknowledgments
We thank Dr. Paul Phillips for continued encouragement and support; Drs. Steven Grassl, Michael Lieberman, David Mitchell, and Gary Olson for helpful discussions; Dr. William Sly (St. Louis University, St. Louis, MO) for providing antibody to carbonic anhydrase; and Michael Hillman for technical assistance. This work was supported by National Institutes of Health Grant R01 DK49221.
Abbreviations
- CAIV
carbonic anhydrase IV
- Δψm
mitochondrial transmembrane potential
- G6PD
glucose 6-phosphate dehydrogenase
- GGT
γ-glutamyl transpeptidase
- GSH
glutathione
- ICSI
intracytoplasmic sperm injection
- NAC
N-acetyl cysteine
- PPP
pentose phosphate pathway
- ROI
reactive oxygen intermediate
- S7P
sedoheptulose 7-phosphate
- TAL
transaldolase
- TK
transketolase.
Footnotes
The authors declare no conflict of interest.
This paper was submitted directly (Track II) to the PNAS office.
Data deposition: The sequence reported in the paper has been deposited in the GenBank database (accession no. AY466103).
References
- 1.Fisher HM, Aitken RJ. J Exp Zool. 1997;277:390–400. doi: 10.1002/(sici)1097-010x(19970401)277:5<390::aid-jez5>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 2.Marchetti C, Obert G, Deffosez A, Formstecher P, Marchetti P. Hum Reprod. 2002;17:1257–1265. doi: 10.1093/humrep/17.5.1257. [DOI] [PubMed] [Google Scholar]
- 3.Marchetti C, Jouy N, Leroy-Martin B, Defossez A, Formstecher P, Marchetti P. Hum Reprod. 2004;19:2267–2276. doi: 10.1093/humrep/deh416. [DOI] [PubMed] [Google Scholar]
- 4.Perl A, Gergely P, Jr, Puskas F, Banki K. Antiox Redox Signal. 2002;4:427–443. doi: 10.1089/15230860260196227. [DOI] [PubMed] [Google Scholar]
- 5.Mayes PA. In: Harper's Biochemistry. Murray RK, Granner DK, Mayes PA, Rodwell VW, editors. Norwalk, CT: Appleton & Lange; 1993. pp. 201–211. [Google Scholar]
- 6.Horecker BL, Mehler AH. Annu Rev Biochem. 1955;24:207–274. doi: 10.1146/annurev.bi.24.070155.001231. [DOI] [PubMed] [Google Scholar]
- 7.Susskind BM, Warren LG, Reeves RE. Biochem J. 1982;204:191–199. doi: 10.1042/bj2040191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feldmann SD, Sahm H, Sprenger GA. Appl Microbiol Biotechnol. 1992;38:354–361. [Google Scholar]
- 9.Grossman CE, Qian Y, Banki K, Perl A. J Biol Chem. 2004;279:12190–12205. doi: 10.1074/jbc.M307039200. [DOI] [PubMed] [Google Scholar]
- 10.Wood T. FEBS Lett. 1972;25:153–155. doi: 10.1016/0014-5793(72)80474-5. [DOI] [PubMed] [Google Scholar]
- 11.Banki K, Perl A. FEBS Lett. 1996;378:161–165. doi: 10.1016/0014-5793(95)01446-2. [DOI] [PubMed] [Google Scholar]
- 12.Bourgeron T. In: The Genetic Basis of Male Infertility. McElreavey K, editor. Berlin; Heildelberg: Springer; 2000. pp. 187–210. [Google Scholar]
- 13.Paasch U, Sharma RK, Gupta AK, Grunewald S, Mascha EJ, Thomas AJ, Jr, Glander HJ, Agarwal A. Biol Reprod. 2004;71:1828–1837. doi: 10.1095/biolreprod.103.025627. [DOI] [PubMed] [Google Scholar]
- 14.Maeda Y, Shiratsuchi A, Namiki M, Nakanishi Y. Cell Death Differ. 2002;9:742–749. doi: 10.1038/sj.cdd.4401046. [DOI] [PubMed] [Google Scholar]
- 15.Banki K, Hutter E, Gonchoroff N, Perl A. J Immunol. 1999;162:1466–1479. [PMC free article] [PubMed] [Google Scholar]
- 16.Nagy G, Koncz A, Perl A. J Immunol. 2003;171:5188–5197. doi: 10.4049/jimmunol.171.10.5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stryer L. Biochemistry. New York: Freeman; 1988. [Google Scholar]
- 18.Suarez SS, Varosi SM, Dai X. Proc Natl Acad Sci USA. 1993;90:4660–4664. doi: 10.1073/pnas.90.10.4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olson GE, Winfrey VP. Mol Reprod Dev. 1992;33:89–98. doi: 10.1002/mrd.1080330113. [DOI] [PubMed] [Google Scholar]
- 20.Rydstrom J. Biochim Biophys Acta. 1977;463:155–184. doi: 10.1016/0304-4173(77)90007-6. [DOI] [PubMed] [Google Scholar]
- 21.Meinhardt A, Wilhelm B, Seitz J. Hum Reprod Update. 1999;5:108–119. doi: 10.1093/humupd/5.2.108. [DOI] [PubMed] [Google Scholar]
- 22.Miki K, Qu W, Goulding EH, Willis WD, Bunch DO, Strader LF, Perreault SD, Eddy EM, O'Brien DA. Proc Natl Acad Sci USA. 2004;101:16501–16506. doi: 10.1073/pnas.0407708101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Esposito G, Jaiswal BS, Xie F, Krajnc-Franken MAM, Robben TJAA, Strik AM, Kuil C, Philipsen RLA, van Duin M, Conti M, et al. Proc Natl Acad Sci USA. 2004;101:2993–2998. doi: 10.1073/pnas.0400050101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matzuk MM, Lamb DJ. Nat Cell Biol. 2002;4:S33–S40. doi: 10.1038/ncb-nm-fertilityS41. [DOI] [PubMed] [Google Scholar]
- 25.Kaunisto K, Fleming RE, Kneer J, Sly WS, Rajaniemi H. Biol Reprod. 1999;61:1521–1526. doi: 10.1095/biolreprod61.6.1521. [DOI] [PubMed] [Google Scholar]
- 26.Ekstedt E, Holm L, Ridderstrale Y. J Mol Histol. 2004;35:167–173. doi: 10.1023/b:hijo.0000023387.02793.af. [DOI] [PubMed] [Google Scholar]
- 27.Kumar TR, Wiseman AL, Kala G, Kala SV, Matzuk MM, Lieberman MW. Endocrinology. 2000;141:4270–4277. doi: 10.1210/endo.141.11.7760. [DOI] [PubMed] [Google Scholar]
- 28.Cooper RA, Bunn HF. In: Harrison's Principles of Internal Medicine. Wilson JD, Braunwald E, Isselbacher KJ, Petersdorf RG, Martin JB, Fauci AS, Root RK, editors. New York: McGraw–Hill; 1991. pp. 1531–1543. [Google Scholar]
- 29.Pandolfi PP, Sonati F, Rivi R, Mason P, Grosveld F. EMBO J. 1995;14:5209–5215. doi: 10.1002/j.1460-2075.1995.tb00205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu ZP, Wawrousek EF, Piatigorsky J. Mol Cell Biol. 2002;22:6142–6147. doi: 10.1128/MCB.22.17.6142-6147.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Novello F, McLean P. Biochem J. 1968;107:775–791. doi: 10.1042/bj1070775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heinrich PC, Morris HP, Weber G. Cancer Res. 1976;36:3189–3197. [PubMed] [Google Scholar]
- 33.Severin SE, Stepanova NG. Adv Enzyme Regul. 1981;19:235–255. doi: 10.1016/0065-2571(81)90018-2. [DOI] [PubMed] [Google Scholar]
- 34.Wood T. The Pentose Phosphate Pathway. New York: Academic; 1985. [Google Scholar]
- 35.James HM, Williams SG, Bais R, Rofe AM, Edwards JB, Conyers RAJ. Int J Vitam Nutr Res. 1985;28(Suppl):29–46. [PubMed] [Google Scholar]
- 36.Kauffman FC, Harkonen MHA. J Neurochem. 1977;28:745–750. doi: 10.1111/j.1471-4159.1977.tb10622.x. [DOI] [PubMed] [Google Scholar]
- 37.Hothersall JS, Baquer NZ, McLean P. Enzyme. 1982;27:259–267. doi: 10.1159/000459058. [DOI] [PubMed] [Google Scholar]
- 38.Banki K, Colombo E, Sia F, Halladay D, Mattson D, Tatum A, Massa P, Phillips PE, Perl A. J Exp Med. 1994;180:1649–1663. doi: 10.1084/jem.180.5.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baquer NZ, Hothersall JS, McLean P, Greenbaum AL. Dev Med Child Neurol. 1977;19:81–104. doi: 10.1111/j.1469-8749.1977.tb08027.x. [DOI] [PubMed] [Google Scholar]
- 40.Thom E, Mohlman T, Quick WP, Camara B, Neuhaus HE. Planta. 1998;204:226–233. [Google Scholar]
- 41.Acacio BD, Gottfried T, Israel R, Sokol RZ. Fertil Steril. 2000;73:595–597. doi: 10.1016/s0015-0282(99)00591-9. [DOI] [PubMed] [Google Scholar]
- 42.Banki K, Hutter E, Colombo E, Gonchoroff NJ, Perl A. J Biol Chem. 1996;271:32994–33001. doi: 10.1074/jbc.271.51.32994. [DOI] [PubMed] [Google Scholar]
- 43.Lachaise F, Martin G, Drougard C, Perl A, Vuillaume M, Wegnez M, Sarasin A, Daya-Grosjean L. Free Radical Biol Med. 2001;30:1365–1373. doi: 10.1016/s0891-5849(01)00532-9. [DOI] [PubMed] [Google Scholar]
- 44.Gergely PJ, Niland B, Gonchoroff N, Pullmann R, Jr, Phillips PE, Perl A. J Immunol. 2002;169:1092–1101. doi: 10.4049/jimmunol.169.2.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Summers MC, Bhatnagar PR, Lawitts JA, Biggers JD. Biol Reprod. 1995;53:431–437. doi: 10.1095/biolreprod53.2.431. [DOI] [PubMed] [Google Scholar]
- 46.Kimura Y, Yanagimachi R. Biol Reprod. 1995;52:709–720. doi: 10.1095/biolreprod52.4.709. [DOI] [PubMed] [Google Scholar]
- 47.Shirley CR, Hayashi S, Mounsey S, Yanagimachi R, Meistrich ML. Biol Reprod. 2004;71:1220–1229. doi: 10.1095/biolreprod.104.029363. [DOI] [PubMed] [Google Scholar]
- 48.Li MW, McGinnis L, Zhu L, Lawitts J, Biggers J, Lloyd KC. Comp Med. 2003;53:265–269. [PubMed] [Google Scholar]
- 49.Masters BA, Kelly EJ, Quaife CJ, Brinster RL, Palmiter RD. Proc Natl Acad Sci USA. 1994;91:584–588. doi: 10.1073/pnas.91.2.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Banki K, Halladay D, Perl A. J Biol Chem. 1994;269:2847–2851. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.