Abstract
Homologous integration of a foreign DNA segment into a chromosomal target sequence enables precise disruption or replacement of genes of interest and provides an effective means to analyze gene function. However, integration after transformation is predominantly nonhomologous in most species other than yeast. Here, we show that homologous integration in the filamentous fungus Neurospora requires the homologous-recombination proteins MEI-3 (yeast Rad51 homolog) and MUS-25 (yeast Rad54 homolog), whereas nonhomologous integration requires nonhomologous end-joining protein MUS-52 (yeast Ku80 homolog). Two additional minor integration pathways are present, one MEI-3-independent and homologous, the other MUS-52-independent and nonhomologous. Homologous and nonhomologous mechanisms compete when external DNA is integrated. In Neurospora, both nonhomologous integration pathways, MUS-52-dependent and MUS-52-independent, require MUS-53 (a homolog of human Lig4), which functions in the final step of nonhomologous end-joining. Because nonhomologous integration is eliminated in a LIG4-disrupted strain, integration occurs only at the targeted site in mus-53 mutants, making them an extremely efficient and safe host for gene targeting.
Keywords: homologous integration, LIG4, Neurospora crassa, gene targeting
DNA double-strand breaks (DSBs) are among the most detrimental DNA lesions. Two major recombination pathways have been identified for their repair (1). These pathways differ as to whether they require DNA sequence homology. Homologous recombination (HR) repairs DSBs by retrieving genetic information from an undamaged homolog, whereas nonhomologous end-joining (NHEJ) rejoins them by direct ligation of the strand ends without any requirement for sequence homology.
These repair mechanisms have been conserved through evolution and operate in a wide range of organisms. Proteins required for HR include Rad51, Rad52, Rad54, and RPA, whereas proteins which are involved in NHEJ include DNA-dependent protein kinase catalytic subunit (DNA-PKcs), the Ku70–Ku80 heterodimer, and the DNA ligase IV (Lig4)–Xrcc4 complex (2). DNA ligase IV is thought to be specific for NHEJ (3–5). The yeast Saccharomyces cerevisiae uses mainly the HR system for DSB repair. Therefore, in conventional gene targeting that occurs through the HR mechanism, S. cerevisiae shows a very high homologous integration (HI) rate. In contrast with yeast, many other organisms, including mammals, plants, and insects, seem to use NHEJ preferentially for DSB repair. As a result, exogenous DNA can be integrated anywhere in the chromosomes, even if it carries a long stretch of homologous sequence. Therefore, gene targeting is an inefficient method in most species other than yeast.
In humans, the technology of gene targeting through HR is regarded as a potential tool for gene therapy. Although various viral and nonviral vectors have been tried for gene targeting in mammalian cells, the introduced DNA was found to be integrated anywhere in the genome. In gene therapy it has become clear that random, nonhomologous integration (NHI) can activate protooncogenes in patients (6, 7). Therefore, attention is focused on what can be done to minimize the risks of insertional mutagenesis. However, the mechanism of nonhomologous integration, which probably involves the NHEJ pathway, has not yet been clarified.
The filamentous fungus Neurospora crassa has been used extensively for studying DNA repair. Genetic and molecular analyses of Neurospora mutants revealed that mei-3, mus-11, and mus-25 (homologs of S. cerevisiae RAD51, RAD52, and RAD54, respectively) are involved in HR (8–10). Our previous report (11) demonstrated that mus-51 and mus-52 (homologs of S. cerevisiae YKU70 and YKU80, which are homologs of human KU70 and KU80, respectively) are involved in NHEJ of N. crassa. We also showed that gene targeting rates in mus-51 and mus-52 were as high as 100%, compared with ∼20% in wild type. This finding was technically important because specific genes can be inactivated very easily if these strains are used as recipients for transformation. These strains have been used in the development of a high-throughput method for knocking out genes of unknown function in N. crassa (12). Highly efficient gene targeting has also been reported in KU-deficient mutants of Aspergillus nidulans, A. fumigatus, A. sojae, A. oryzae, and Cryptococcus neoformans (13–17), confirming that KU-deficient strains are excellent tools for gene targeting in filamentous fungi. NHEJ components other than KU70 and KU80 homologs have not been identified in filamentous fungi. These include homologs of LIG4 and XRCC4.
Here, to investigate the mechanism of gene targeting and chromosomal integration, we measured the chromosomal integration frequency and gene-targeting rate of exogenous DNA in Neurospora strains that are defective in HR and NHEJ. Our results suggest that chromosomal integration is achieved by four pathways: two involving MEI-3-dependent and MEI-3-independent HI and two involving MUS-52-dependent and MUS-52-independent NHI. Furthermore, the Neurospora LIG4-homolog, mus-53, was identified and characterized. Gene targeting in mus-53 was 100%, the same as in mus-51 and mus-52 mutants. However, unlike mus-51 and mus-52, the mus-53 mutant has a gene-targeting rate of 100% even if the DNA sequence homologous to the targeted is very short. We propose that MUS-53 is required for both the MUS-52-dependent and MUS-52-independent NHI pathways. These findings provide new insight into the chromosomal integration mechanisms. mus-53 also provides a highly efficient alternative to mus-51 or mus-52 for inactivating specific genes.
Results
Experimental System to Assay Efficiency.
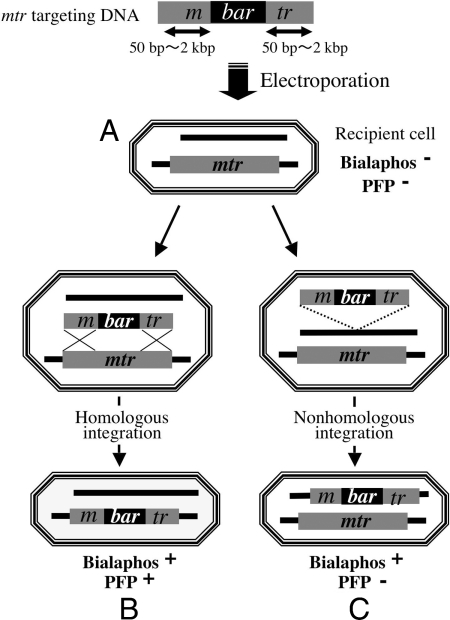
To study the mechanism of foreign DNA integration, we measured the targeting frequency in wild type, in HR-defective strains (mei-3, mus-11, and mus-25), and in the NHEJ-defective strain mus-52. Double mutants carrying mus-52 in combination with each of the HR-defective mutations were also tested to examine the detailed relationships between HR and NHEJ in chromosomal integration. The mtr gene on chromosome IV was selected as a target for gene disruption because mtr-defective mutants show resistance to the amino acid analog p-fluorophenylalanine (PFP) (18). If a DNA fragment including the bialaphos-resistance gene bar is integrated at the mtr locus, the strain should be resistant to both bialaphos and PFP. On the other hand, if the fragment is integrated at a site other than mtr, the strain should be resistant to bialaphos but not to PFP (Fig. 1). Transformation (chromosomal integration) frequencies were calculated as the number of bialaphos-resistant colonies per total recipient cell numbers. The HI rate (gene targeting) was calculated as the ratio of PFP-resistant transformants to bialaphos-resistant transformants. The NHI rate (nonhomologous chromosomal integration) is the ratio of PFP-sensitive transformants to bialaphos-resistant transformants. We constructed linear DNA fragments with long (2-kbp) or short (50-bp) sequences showing homology for the mtr region carried on each side of the bar gene. These fragments were introduced by electroporation into recipient strains with different genetic backgrounds.
Fig. 1.
Scheme of experiments to assay chromosomal integration and targeting efficiency. The black box represents the selectable marker, the bialaphos-resistance gene bar. The gray box shows the mtr gene. The targeting vector was constructed by replacing part of the mtr ORF with bar. The mtr targeting DNA was flanked by homologous sequences ranging from 50 bp to 2 kbp. Targeting DNA was introduced by electroporation into recipient cells, which are sensitive to both bialaphos and PFP (A). Integration into mtr by HI results in resistance to both bialaphos and PFP (B). NHI produced transformants resistant to bialaphos but sensitive to PFP, because the endogenous mtr gene is intact and the mtr mutation is recessive (C). Integration efficiency was calculated as number of bialaphos-resistant cells per total recipient cell number [(B + C)/A]. The targeting (HI) rate of mtr was calculated as the ratio of PFP-resistant transformants to bialaphos-resistant transformants [B/(B + C)], and the NHI rate was calculated as the ratio of PFP-sensitive transformants to bialaphos-resistant transformants [C/(B + C)].
Four Independent Pathways for Integrating Foreign DNA into N. crassa Chromosomes.
The HI rate was 23.33% in wild-type but 0% in HR-defective strains, including mei-3, mus-11, and mus-25 (Table 1). This result clearly demonstrates that homology-dependent integration depends on a functional HR mechanism. On the other hand, as shown in ref. 11, HI was 100% and NHI was 0% in mus-52, suggesting that all NHI events depend on MUS-52 when foreign DNA carrying 2-kbp homology is introduced into wild type. Transformation frequency in mus-52 (3.81 ± 0.39 × 10−6) was almost the same as in wild type (4.07 ± 0.67 × 10−6). However, in the NHI-defective mutant, homology-dependent integration events increased 4-fold over wild type. Conversely, the NHI frequency in HR-defective mutants, in which HI could not occur, increased significantly compared with wild type. These results suggest that pathways of HI and NHI compete against one another in wild-type cells.
Table 1.
Integration frequency of an exogenous DNA fragment that has a 2-kbp mtr homologous sequence flanking the bar gene on both sides
| Strain | S. cerevisiae homolog | TF ± SE,*×10−6 | Relative value of TF† | HI,‡ % | HI frequency,§ ×10−6 | Relative value of HI frequency¶ | NHI,‖ % | NHI frequency,** ×10−6 | Relative value of NHI frequency†† |
|---|---|---|---|---|---|---|---|---|---|
| Wild type | 4.07 ± 0.67 | 1 | 23.33 | 0.95 | 1 | 76.67 | 3.12 | 1 | |
| mus-52 | YKU80 | 3.81 ± 0.39 | 0.94 | 100 | 3.81 | 4.01 | 0 | 0 | 0 |
| mus-11 | RAD52 | 27.33 ± 6.33 | 6.71 | 0 | 0 | 0 | 100 | 27.33 | 8.76 |
| mei-3 | RAD51 | 29.83 ± 3.94 | 7.33 | 0 | 0 | 0 | 100 | 29.83 | 9.56 |
| mus-25 | RAD54 | 15.17 ± 2.47 | 3.73 | 0 | 0 | 0 | 100 | 15.17 | 4.86 |
| mus-52 mus-11 | YKU80 RAD52 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| mus-52 mei-3 | YKU80 RAD51 | 0.32 ± 0.04 | 0.08 | 96.26 | 0.31 | 0.33 | 3.74 | 0.01 | 0.003 |
| mus-52 mus-25 | YKU80 RAD54 | 0.08 ± 0.03 | 0.02 | 88.9 | 0.07 | 0.07 | 11.1 | 0.009 | 0.003 |
TF, transformation frequency.
*Frequency was calculated as the number of bialaphos-resistant colonies per total cell number. Averages and standard errors are from more than three independent experiments.
†Relative value of transformation frequency was calculated as the transformation frequency in the mutant divided by the transformation frequency in the wild type.
‡HI (%) = (the number of PFP-resistant colonies/the number of bialaphos-resistant colonies) × 100.
§HI frequency = (the number of PFP-resistant colonies/the number of bialaphos-resisrant colonies) × transformation frequency.
¶Relative value of HI frequency was calculated as the HI frequency in the mutant divided by the HI frequency in the wild type.
‖NHI (%) = (the number of PFP-sensitive colonies/the number of bialaphos resistant colonies) × 100.
**NHI frequency = (the number of PFP-sensitive colonies/the number of bialaphos-resistant colonies) × transformation frequency.
††Relative value of NHI frequency was calculated as the NHI frequency in the mutant divided by the NHI frequency in the wild type.
To further investigate the relationship between HI and NHI in the chromosome integration pathway, we examined the transformation frequency of the mus-52 mus-11 double mutant, in which both NHEJ and HR are inactive. No transformants appeared, indicating that foreign DNA carrying 2 kbp of homology was not integrated in this double mutant (Table 1) and that the RAD52 homolog mus-11 and the YKU80 homolog mus-52 are absolutely required for integration of foreign DNA carrying 2 kbp of homology.
On the other hand, a small number of bialaphos-resistant colonies were still found in the mus-52 mei-3 and the mus-52 mus-25 double mutants, which are also defective in the functions of both HR and NHEJ. The majority of those colonies (96.26 and 88.9%, respectively) were resistant to PFP, meaning that they were derived from HI although the recipient strains were defective in HR. We also confirmed that bar was integrated at the mtr locus using PCR (data not shown). This finding suggests that a second HI pathway is activated when the two major chromosomal integration pathways, MUS-52-dependent NHI and MEI-3-dependent HI, are blocked. This putative second HI pathway must depend on MUS-11, because DNA fragments were not integrated into the chromosome at all in the mus-52 mus-11 double mutant. Furthermore, transformation by NHI also occurred at a very low level in mus-52 mei-3 and mus-52 mus-25 (0.01 × 10−6 and 0.009 × 10−6, respectively), suggesting the existence of a MUS-11-dependent but MUS-52-independent second NHI pathway for chromosomal integration.
With 50-bp homology (Table 2), the HI rate in wild type was extremely low (0.18%), indicating that 50 bp of homology is not enough for effective homology searching and targeting. HI was not increased even in mus-52. However, a low rate of NHI was detected in mus-52, which is defective in NHEJ function. This finding supports the existence of MUS-52-independent NHI. A similar effect was seen in mus-52 mei-3 and mus-52 mus-25 double mutants. We conclude that MUS-11 and MUS-52 are key regulators of integration in Neurospora and that there are four subpathways: two major pathways, one of which is MEI-3-dependent, governing HI, the other MUS-52-dependent, governing NHI, and two minor “back-up” pathways, one MEI-3-independent HI and the other MUS-52-independent NHI.
Table 2.
Integration frequency of an exogenous DNA fragment that has 50-bp mtr homologous sequence flanking the bar gene on both sides
| Strain | S. cerevisiae homolog | TF ± SE,* ×10−6 | Relative value of TF† | HI,‡ % | HI frequency,§ × 10−6 | Relative value of HI frequency¶ | NHI,‖ % | NHI frequency, ** ×10−6 | Relative value of NHI frequency†† |
|---|---|---|---|---|---|---|---|---|---|
| Wild type | 17.25 ± 1.91 | 1 | 0.18 | 0.03 | 1 | 99.82 | 17.22 | 1 | |
| mus-52 | YKU80 | 0.03 ± 0.01 | 0.0017 | 0 | 0 | 0 | 100 | 0.03 | 0.0017 |
| mus-11 | RAD52 | 125.2 ± 25.75 | 7.26 | 0 | 0 | 0 | 100 | 125.2 | 7.27 |
| mei-3 | RAD51 | 128.33 ± 15.9 | 7.44 | 0 | 0 | 0 | 100 | 128.33 | 7.45 |
| mus-25 | RAD54 | 124.4 ± 21.11 | 7.21 | 0 | 0 | 0 | 100 | 124.4 | 7.22 |
| mus-52 mus-11 | YKU80 RAD52 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| mus-52 mei-3 | YKU80 RAD51 | 0.0025 ± 0.0025 | 0.00014 | 0 | 0 | 0 | 100 | 0.0025 | 0.00015 |
| mus-52 mus-25 | YKU80 RAD54 | 0.005 ± 0.0022 | 0.00029 | 0 | 0 | 0 | 100 | 0.005 | 0.00029 |
TF, transformation frequency.
*Frequency was calculated as the number of bialaphos-resistant colonies per total cell number. Averages and standard errors are from more than three independent experiments.
†Relative value of transformation frequency was calculated as the transformation frequency in the mutant divided by the transformation frequency in the wild type.
‡HI (%) = (the number of PFP-resistant colonies/the number of bialaphos-resistant colonies) × 100.
§HI frequency = (the number of PFP-resistant colonies/the number of bialaphos-resisrant colonies) × transformation frequency.
¶Relative value of HI frequency was calculated as the HI frequency in the mutant divided by the HI frequency in the wild type.
‖NHI (%) = (the number of PFP-sensitive colonies/the number of bialaphos resistant colonies) × 100.
**NHI frequency = (the number of PFP-sensitive colonies/the number of bialaphos-resistant colonies) × transformation frequency.
††Relative value of NHI frequency was calculated as the NHI frequency in the mutant divided by the NHI frequency in the wild type.
Characterization of the Neurospora LIG4 Homolog, mus-53.
To further study the mechanism of NHI by NHEJ, we searched the Neurospora genome database (www.broad.mit.edu/annotation/fungi/neurospora/) to find an ortholog of human LIG4. A candidate was identified that encodes a 1,046-aa polypeptide which shows 25% identity and 38% similarity to human Lig4. Furthermore, this protein has two tandem BRCA1 C-terminal domains at the C terminus, which are conserved in human Lig4 (19) and S. cerevisiae Dnl4/Lig4 (20). The BRCA1 C-terminal domains of DNA ligase IV in human and yeast are essential for binding to another NHEJ protein, Xrcc4 or Lif1, respectively (21, 22).
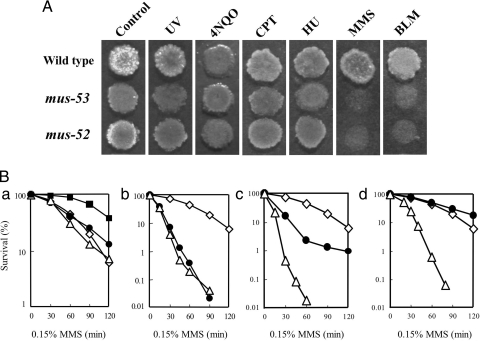
To investigate its function in Neurospora, we used gene replacement to disrupt the LIG4 homolog as described in Materials and Methods. Mutagen sensitivity of the disrupted mutant was analyzed by spot test (Fig. 2A). The mutant showed mild sensitivity to methyl methanesulfonate (MMS) and bleomycin (BLM) but was not sensitive to UV or to other chemical agents. Following the established rules of genetic nomenclature in Neurospora (23), this gene was named mus-53. The gene maps near leu-1 in linkage group IIIR. Apical growth of mus-53 in race tubes was normal, and no morphological abnormalities were observed (data not shown). Homozygous crosses were fertile, with normal asci and ascospores.
Fig. 2.
Sensitivity and epistasis analysis of mus-53. (A) Sensitivity of wild type, mus-52, and mus-53 to UV and chemical agents. Conidial suspension was spotted on the agar surface of plates containing 4-nitroquinoline 1-oxide (4NQO, 75 ng/ml), camptothecin (CPT, 0.4 μg/ml), hydroxyurea (HU, 1.5 mg/ml), MMS (0.175 μl/ml), and bleomycin (BLM, 5 μg/ml). Cells were UV-irradiated at 375 J/m2. (B) Sensitivity to MMS of double mutants compared with their parental single mutants. A conidial suspension was treated with MMS (1.5 μl/ml) for the indicated time. (a) Wild type (filled squares), mus-52 (filled circles), mus-53 (open diamonds), and mus-52 mus-53 (open triangles). (b) mus-53 (open diamonds), uvs-6 (filled circles), and mus-53 uvs-6 (open triangles). (c) mus-11 (filled circles), mus-53 (open diamonds), and mus-11 mus-53 (open triangles). (d) mei-3 (filled circles), mus-53 (open diamonds), and mei-3 mus-53 (open triangles).
Epistatic relationships between mus-53 and other recombination repair genes were examined using double mutants with uvs-6, mei-3, mus-11, and mus-52, and determining the sensitivity of each strain to MMS (Fig. 2B). The uvs-6 mutation (yeast RAD50) is epistatic to mus-53. In contrast, both the mus-53 mei-3 and the mus-53 mus-11 double mutants were more sensitive than either parental single mutant. Sensitivity of the mus-53 mus-52 double mutant was identical to that of the parental mus-53 strain. mus-53 therefore belongs to the NHEJ group of recombination repair mutants and not to the HR group.
Highly Efficient Gene Targeting Is Observed in mus-53.
Gene targeting was used to determine whether the gene targeting frequency was increased in mus-53 as well as in mus-52. With 2-kbp homology, the targeting frequency was 100% in mus-53, the same as in mus-52 (Table 3). However, transformation was five times less frequent than in wild type (0.85 ± 0.08 × 10−6 vs. 4.07 ± 0.67 × 10−6), and this low transformation frequency was suppressed by mus-52. The low transformation frequency in mus-53 might be explained if the repair pathway cannot subsequently be changed from NHI to HI once MUS-52 proteins start to work. This result suggests that MUS-53 acts later than MUS-52 during gene integration. On the other hand, when foreign DNA with only 50-bp homology was introduced into mus-53, no transformants appeared. We hypothesize that MUS-53 is essential for integration of foreign DNA with short homology.
Table 3.
Integration frequency of an exogenous DNA fragment that has a 2-kbp (bold) or 50-bp (italic) mtr homologous sequence on both sides of bar gene in the mus-53 mutant
| Strain | S. cerevisiae homolog | TF ± SE,*×10−6 | Relative value of TF† | HI,‡ % | HI frequency,§ ×10−6 | Relative value of HI frequency¶ | NHI,‖ % | NHI frequency,** ×10−6 | Relative value of NHI frequency†† |
|---|---|---|---|---|---|---|---|---|---|
| Wild type | 4.07 ± 0.67 | 1 | 23.33 | 0.95 | 1 | 76.67 | 3.12 | 1 | |
| 17.25 ± 1.91 | 1 | 0.18 | 0.03 | 1 | 99.82 | 17.22 | 1 | ||
| mus-52 | YKU80 | 3.81 ± 0.39 | 0.94 | 100 | 3.81 | 4.01 | 0 | 0 | 0 |
| 0.03 ± 0.01 | 0.0017 | 0 | 0 | 0 | 100 | 0.03 | 0.0017 | ||
| mus-53 | LIG4 | 0.85 ± 0.08 | 0.21 | 100 | 0.85 | 0.89 | 0 | 0 | 0 |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| mus-52 mus-53 | YKU80 LIG4 | 4.37 ± 0.29 | 1.07 | 100 | 4.37 | 4.6 | 0 | 0 | 0 |
TF, transformation frequency. See Fig. 3 for the effect of intermediate-sized flanks.
*Frequency was calculated as the number of bialaphos-resistant colonies per total cell number. Averages and standard errors are from more than three independent experiments.
†Relative value of transformation frequency was calculated as the transformation frequency in the mutant divided by the transformation frequency in the wild type.
‡HI (%) = (the number of PFP-resistant colonies/the number of bialaphos-resistant colonies) × 100.
§HI frequency = (the number of PFP-resistant colonies/the number of bialaphos-resisrant colonies) × transformation frequency.
¶Relative value of HI frequency was calculated as the HI frequency in the mutant divided by the HI frequency in the wild type.
‖NHI (%) = (the number of PFP-sensitive colonies/the number of bialaphos resistant colonies) × 100.
**NHI frequency = (the number of PFP-sensitive colonies/the number of bialaphos-resistant colonies) × transformation frequency.
††Relative value of NHI frequency was calculated as the NHI frequency in the mutant divided by the NHI frequency in the wild type.
MUS-53 Is a Key Regulator of NHI.
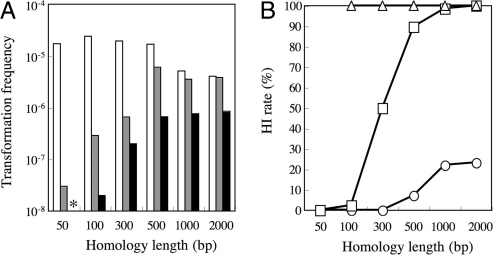
To test whether our hypothesis is correct, 2-kbp, 1-kbp, 500-bp, 300-bp, 100-bp, and 50-bp lengths of the mtr gene were attached on both sides of the bar gene and transformed into wild type, mus-52, and mus-53. Transformation frequency and the rate of gene targeting were assayed (Fig. 3). In wild type, the gene-targeting rate was low when the homology was short. HI was rare even with a homology length of 300 bp. In contrast, the gene-targeting rate in mus-52 was ≈50% at 300-bp homology length, increasing to 100% with homology >1 kbp. In mus-52, both transformation frequency and gene-targeting rate were low when the homology was short, perhaps because the MUS-52-independent NHI pathway was activated by the short homology even though integration could not occur via HR. Transformation frequency was lower in mus-53 than in mus-52 when homology was short. In mus-53, surprisingly, gene targeting was 100% even with 100-bp homology, but no transformants appeared with 50-bp homology. This means that 50-bp homology is not enough for gene targeting even in mus-53.
Fig. 3.
Homology-dependent transformation frequency and HI in wild type, mus-52, and mus-53. Transformation frequency and HI were measured according to the strategy shown in Fig. 1. (A) Open bars, gray bars, and filled bars indicate transformation frequency in wild type, mus-52, and mus-53 as recipients. (B) Open circles, open squares, and open triangles indicate rates of HI in wild type, mus-52, and mus-53, respectively. The asterisk indicates that there was no mtr-resistant transformant.
We conclude that MUS-53 function is required for all NHI pathways, both MUS-52-dependent and MUS-52-independent. Accordingly, DNA introduced into mus-53 is integrated only at the target site.
Discussion
We demonstrated previously that gene-targeting efficiency becomes 100% in Neurospora mutants mus-51 or mus-52, which are defective in end-joining (11). The first step in non-HR repair of DSBs is binding of Ku70 and Ku80 heterodimers to broken DNA ends. After the DNA ends are processed by Ku proteins, they are joined by the Lig4–Xrcc4 complex. Genetic characterization of mus-53, which is the Neurospora ortholog of human LIG4, indicated that Lig4 plays a significant role in NHI.
Targeting experiments showed distinct differences between mus-52 and mus-53. In mus-52, transformation frequency was almost the same as in wild type, but in mus-53 it was 4–5 times lower, as expected if HI frequency per total transformants is the same in mus-53 as in the wild type, and the only transformants appearing on the selection plate are from HI. In a mutant defective in MUS-53 protein, all integrations are processed by the HR pathway.
Use of mus-53 offers a distinct advantage when the transforming DNA has limited homology with the recipient sequence. When DNA with <500-bp homology is introduced into mus-52, HI decreases and NHI increases as homology length becomes shorter. In mus-53, however, transformants are exclusively from HI, even in experiments with short homology. This result is important because it means that with mus-53 as recipient, all transformants will be from HI and none will be from NHI. The host is thus protected from NHI, and there is no need for further tedious experiments to determine whether DNA fragments are integrated ectopically. Although targeting has great promise for gene therapy, NHI has been a serious problem because it may activate protooncogenes and result in cancer. The system described here may provide a solution.
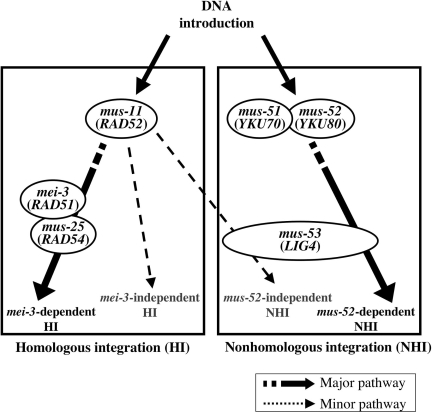
Four targeting patterns were observed in targeting experiments using three HR-deficient strains (mei-3, mus-11, and mus-25) and two NHEJ-deficient strains (mus-52 and mus-53) (Fig. 4). Major transformation pathways are MEI-3-dependent HI and MUS-52-dependent NHI, whereas minor pathways are MUS-11-dependent but MEI-3-independent HI and MUS-11-dependent but MUS-52-independent NHI. MUS-51 and MUS-52 interact physically and are concerned in the same function (data not shown). The fact that no transformants appeared in the mus-11 mus-52 double mutant means that only these two chromosomal-integration pathways are present.
Fig. 4.
Pathways for integration of exogenous DNA into chromosomal DNA. Exogenous DNA is integrated into the chromosomes by two major pathways, mus-11-dependent and mus-52-dependent. Downstream of these two chromosomal integration pathways is achieved by four pathways: two involving mei-3-dependent and mei-3-independent HI and two involving mus-52-dependent and mus-52-independent non-HI. Both nonhomologous integration pathways, mus-52-dependent and mus-52-independent, require mus-53. The genes in parentheses represent S. cerevisiae homologs.
In yeast, Rad51-dependent HR and Ku-Lig4-dependent NHEJ are major DSB repair pathways. At least three additional pathways have been reported: Ku-independent DSB rejoining, Rad52-dependent single strand annealing (SSA), and Rad52-dependent end-joining (24–26). We anticipate that these pathways exist and function in the minor integration pathway in Neurospora.
NHEJ-independent DSB rejoining has also been reported in mammals (27). However, the relationship between DSB repair and gene targeting is not clear. In the present study, we used Neurospora to demonstrate the importance of using a Lig4-deficient strain for gene targeting. Our results may provide a pathway for developing gene-targeting methods in higher eukaryotes.
Materials and Methods
Strains and Plasmids.
Table 4 shows the N. crassa strains used in these experiments. C1-T10-34A, C1-T10-28a (28), and 74-OR31-16A (29) are wild-type strains. The mus-53::Hygr strain was generated by gene replacement as described (11); to make a targeting vector, 1.3-kbp EcoRV DNA of the central region of the N. crassa LIG4 homolog, mus-53 (NCU06264.2), was replaced by a 1.5-kbp length of the hygromycin-resistance gene (Hygr). The vector was introduced into wild-type 74-OR31-16A. Hygromycin-resistant transformants were isolated, and it was confirmed by PCR and Southern blotting whether a mus-53 gene was disrupted correctly in those strains. The transformants were then backcrossed to wild-type C1-T10-28a to make the genetic background homogeneous. Strains KZM6220A (FGSC10139A) and KZM6224a (FGSC10140a) were used as mus-53A and mus-53a standards. Escherichia coli strains DH1 and XL-1 Blue were used for amplification of plasmids. Plasmids pUC19 (Stratagene, La Jolla, CA) and pGEM-T Easy (Promega, Madison, WI) were generally used for construction of new vectors. pBARGEM7-1 (30) carrying bar and pCSN43 (31) carrying Hygr were obtained from the Fungal Genetics Stock Center (University of Missouri, Kansas City, MO).
Table 4.
Strains of Neurospora crassa used in this study
| Strain/FGSC number | Genotype | Source/reference |
|---|---|---|
| C1-T10-37A | A | Laboratory stock |
| C1-T10-28a | A | Laboratory stock |
| 74-OR31-16A | A al-2 pan-2 cot-1 | (29) |
| 54yo-828-3 | A mus-52::Hygr | (11) |
| 54yo-828-4 | a mus-52::Hygr | (11) |
| FGSC6409A | A mus-11 | FGSC |
| FGSC2764A | A mei-3 | FGSC |
| FGSC6425a | a mus-25 | FGSC |
| 54yo-8m11-4 | A mus-52::Hygr mus-11 | This study |
| 54yo-8m3-17 | A mus-52::Hygr mei-3 | (11) |
| 54yo-8m25-13 | A mus-52::Hygr mus-25 | This study |
| FGSC10139A | A mus-53::Hygr | This study |
| FGSC10140a | a mus-53::Hygr | This study |
| ICBC480A | A mus-52::Hygr mus-53::Hygr al-2 | This study |
| 74OR-270-104a | a uvs-6 al-2 pan-2 cot-1 | Laboratory stock |
| ICBC461a | a mus-53::Hygr uvs-6 al-2 pan-2 | This study |
| ICBC422a | a mus-53::Hygr mus-11 pan-2 | This study |
| ICBC436A | A mus-53::Hygr mei-3 pan-2 | This study |
FGSC, Fungal Genetics Stock Center.
General Genetic Manipulation in N. crassa.
Genetic analysis was carried out as described by Davis and de Serres (32).
Electroporation.
Electroporation was used for introduction of exogenous DNA into recipient cells as described (11).
Determination of Transformation Frequency and Gene Targeting Rate.
To measure integration frequency and the rate of targeting to the mtr locus, vector pGS1-2KR (9.5 kbp) was constructed as follows: A DNA fragment of mtr was generated by PCR using N. crassa genomic DNA as a template and the two primers: mtr-5′ (5′-GAAACGACGGGATGTGAGAT-3′) and mtr-3′ (5′-GATAATGAGGTAGCAGGAGC-3′). PCR cycling was carried out with the Expand High-Fidelity PCR system (Roche Diagnostics, Indianapolis, IN) following the manufacturer's protocol. The PCR product was integrated into pGEM-T Easy (Promega) to make pGEMMTR. Then it was digested with blunting enzyme MscI to delete a ∼1-kbp fragment containing the promoter and part of the mtr ORF. A 2.7-kbp DNA fragment containing the bialaphos resistance gene bar was cut out from pBARKS1 with blunting enzymes ScaI and SmaI and inserted into MscI-digested pGEMMTR to produce pGS1-2KR, which carries 2 kbp of the mtr gene on both sides of bar. We also constructed pGS1-1KR, pGS1-500R, pGS1-300R, pGS1-100R, and pGS1-50R, which have 1 kbp, 500 bp, 300 bp, 100 bp, or 50 bp of mtr on both sides of bar, respectively. Digestion of these plasmids with EcoRI produces 6.7-kbp, 4.7-kbp, 3.7-kbp, 3.3-kbp, 2.9-kbp, and 2.8-kbp linear fragments carrying 2 kbp, 1 kbp, 500 bp, 300 bp, 100 bp, or 50 bp of mtr on both sides of bar, respectively. These fragments were introduced into various host strains by electroporation. Conidial suspensions were then plated on medium containing bialaphos (200 μg/ml) and incubated at 30° for 3 days. Transformants resistant to bialaphos were isolated and tested by spot tests for resistance to PFP (20 μg/ml) and to bialaphos. To confirm whether transformants included products of HI, PCR with two primer sets was performed as described (11).
Mutagen Sensitivity.
Sensitivity was investigated by spotting a conidial suspension on agar medium containing the chemical agent (33). Sensitivity to MMS was determined as described (34).
Acknowledgments
This work was supported by Rational Evolutionary Design of Advanced Biomolecules, Saitama Prefecture Collaboration of Regional Entities for the Advancement of Technological Excellence, Japan Science and Technology Agency.
Abbreviations
- HI
homologous integration
- NHI
nonhomologous integration
- HR
homologous recombination
- NHEJ
nonhomologous end-joining
- DSBs
DNA double-strand breaks
- MMS
methyl methanesulfonate
- PFP
p-fluorophenylalanine.
Footnotes
The authors declare no conflict of interest.
This paper was submitted directly (Track II) to the PNAS office.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AB261106).
References
- 1.Haber J. Trends Genet. 2000;16:259–264. doi: 10.1016/s0168-9525(00)02022-9. [DOI] [PubMed] [Google Scholar]
- 2.Critchlow SE, Jackson SP. Trends Biochem Sci. 1998;23:394–398. doi: 10.1016/s0968-0004(98)01284-5. [DOI] [PubMed] [Google Scholar]
- 3.Teo S-H., Jackson SP. EMBO J. 1997;16:4788–4795. doi: 10.1093/emboj/16.15.4788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adachi N, Ishino T, Ishii Y, Takeda S, Koyama H. Proc Natl Acad Sci USA. 2001;98:12109–12113. doi: 10.1073/pnas.201271098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daley JM, Palmbos PL, Wu D, Wilson TE. Annu Rev Genet. 2005;39:431–451. doi: 10.1146/annurev.genet.39.073003.113340. [DOI] [PubMed] [Google Scholar]
- 6.Check E. Nature. 2002;420:116–118. doi: 10.1038/420116a. [DOI] [PubMed] [Google Scholar]
- 7.Hacein-Bey-Abina S, Von Kalle C, Schmidt M, McCormack MP, Wulffraat N, Leboulch P, Lim A, Osborne CS, Pawliuk R, Morillon E, et al. Science. 2003;302:415–419. doi: 10.1126/science.1088547. [DOI] [PubMed] [Google Scholar]
- 8.Hatakeyama S, Ishii C, Inoue H. Mol Gen Genet. 1995;249:439–446. doi: 10.1007/BF00287106. [DOI] [PubMed] [Google Scholar]
- 9.Sakuraba Y, Schroeder AL, Ishii C, Inoue H. Mol Gen Genet. 2000;264:392–401. doi: 10.1007/s004380000342. [DOI] [PubMed] [Google Scholar]
- 10.Handa N, Noguchi Y, Sakuraba Y, Ballario P, Macino G, Fujimoto N, Ishii C, Inoue H. Mol Gen Genet. 2000;264:154–163. doi: 10.1007/s004380000303. [DOI] [PubMed] [Google Scholar]
- 11.Ninomiya Y, Suzuki K, Ishii C, Inoue H. Proc Natl Acad Sci USA. 2004;101:12248–12253. doi: 10.1073/pnas.0402780101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colot HV, Park G, Turner GE, Ringelberg C, Crew CM, Litvinkova L, Weiss RL, Borkovich KA, Dunlap JC. Proc Natl Acad Sci USA. 2006;103:10352–10357. doi: 10.1073/pnas.0601456103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nayak T, Szewczyk E, Oakley CE, Osmani A, Ukil L, Murray SL, Hynes MJ, Osmani SA, Oakley BR. Genetics. 2006;172:1557–1566. doi: 10.1534/genetics.105.052563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.da Silva Ferreira ME, Kress MR, Savoldi M, Goldman MH, Hartl A, Heinekamp T, Brakhage AA, Goldman GH. Eukaryotic Cell. 2006;5:207–211. doi: 10.1128/EC.5.1.207-211.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krappmann S, Sasse C, Brous GH. Eukaryotic Cell. 2006;5:212–215. doi: 10.1128/EC.5.1.212-215.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takahashi T, Masuda T, Koyama Y. Mol Genet Genomics. 2006;275:460–470. doi: 10.1007/s00438-006-0104-1. [DOI] [PubMed] [Google Scholar]
- 17.Goins CL, Gerik KJ, Lodge JK. Fungal Genet Biol. 2006;43:531–544. doi: 10.1016/j.fgb.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 18.DeBusk RM, Debusk AG. J Bacteriol. 1980;143:188–197. doi: 10.1128/jb.143.1.188-197.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grawunder U, Wilm M, Wu X, Kulesza P, Wilson TE, Mann M, Lieber MR. Nature. 1997;388:428–429. doi: 10.1038/41358. [DOI] [PubMed] [Google Scholar]
- 20.Wilson TE, Grawunder U, Lieber MR. Nature. 1997;388:495–498. doi: 10.1038/41365. [DOI] [PubMed] [Google Scholar]
- 21.Dore AS, Furnham N, Davies OR, Sibanda BL, Chirgadze DY, Jakson SP, Pellegrini L, Blundell TL. DNA Repair. 2006;7:362–368. doi: 10.1016/j.dnarep.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 22.Critchlow SE, Bowater RP, Jackson SP. Curr Biol. 1997;7:588–598. doi: 10.1016/s0960-9822(06)00258-2. [DOI] [PubMed] [Google Scholar]
- 23.Perkins DD, Radford A, Sachs MS. The Neurospora Compendium. New York: Academic; 2001. [Google Scholar]
- 24.Ma JL, Kim EM, Haber JE, Lee SE. Mol Cell Biol. 2003;23:8820–8828. doi: 10.1128/MCB.23.23.8820-8828.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu X, Gabriel A. Genetics. 2003;163:843–856. doi: 10.1093/genetics/163.3.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Daley JM, Wilson TE. Mol Cell Biol. 2005;25:896–906. doi: 10.1128/MCB.25.3.896-906.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang H, Perrault AR, Takeda Y, Qin W, Wang H, Iliakis G. Nucleic Acids Res. 2003;31:5377–5388. doi: 10.1093/nar/gkg728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tamaru H, Inoue H. J Bacteriol. 1989;171:6288–6293. doi: 10.1128/jb.171.11.6288-6293.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Serres FJ, Inoue H, Schupbach ME. Mutation Res. 1980;71:53–65. doi: 10.1016/0027-5107(80)90006-8. [DOI] [PubMed] [Google Scholar]
- 30.Pall ML, Brunelli JP. Fungal Genet Newslett. 1993;40:59–62. [Google Scholar]
- 31.Staben C, Jensen B, Singer M, Pollock J, Schechman M, Kinsey J, Selker E. Fungal Genet Newslett. 1989;36:79–81. [Google Scholar]
- 32.Davis RH, de Serres FJ. Methods Enzymol. 1970;17:79–143. [Google Scholar]
- 33.Kato A, Akamatsu Y, Sakuraba Y, Inoue H. Curr Genet. 2004;45:37–44. doi: 10.1007/s00294-003-0459-3. [DOI] [PubMed] [Google Scholar]
- 34.Inoue H, Ishii C. Mutation Res. 1984;125:185–194. doi: 10.1016/0027-5107(84)90068-x. [DOI] [PubMed] [Google Scholar]