Abstract
The presence of the envelope glycoprotein Env in HIV-1 virions is essential for infectivity. To date, the molecular mechanism by which Env is packaged into virions has been largely unknown. Here, we show that TIP47 (tail-interacting protein of 47 kDa), which has been shown to interact with Env, also binds the MA (matrix) domain of HIV-1 Gag protein and that these three proteins form a ternary complex. Mutations in Gag that abrogate interaction with TIP47 inhibit Env incorporation and virion infectivity as well as colocalization between Gag and Env. We also show that TIP47 silencing impairs Env incorporation and infectivity and abolishes coimmunoprecipitation of Gag with Env. In contrast, overexpression of TIP47 increases Env packaging. Last, we demonstrate that TIP47 can interact simultaneously with Env and Gag. Taken together, our results show that TIP47 is a cellular cofactor that plays an essential role in Env incorporation, allowing the encounter and the physical association between HIV-1 Gag and Env proteins during the viral assembly process.
Keywords: envelope incorporation, HIV-1 envelope glycoprotein, matrix domain, HIV-1 assembly, Gag precursor
The incorporation of the HIV-1 envelope glycoprotein Env into virions confers infectivity to HIV-1 particles, making it a key step in the viral life cycle. Env is synthesized as a precursor, gp160, which is cleaved to give the surface subunit SUgp120 and the transmembrane subunit TMgp41. This SUgp120/TMgp41 complex is then incorporated into budding virions. SUgp120 binds to the CD4 receptor of cells and then to the coreceptor, initiating HIV-1 entry, whereas TMgp41 induces the membrane fusion between viral and cellular lipid bilayers (1, 2).
Recent studies have elucidated most of the molecular mechanisms involved in the budding of HIV and other retroviruses (3). However, the mechanisms that govern the incorporation of Env into viral particles, conferring infectivity to these particles, remain largely unknown. Many studies suggest that the cytoplasmic domain (CD) of TMgp41 (TMgp41 CD) and the MA (matrix) domain of the Gag protein, a protein that is closely associated with the virion membrane, together with putative cellular cofactors, play a central role in this mechanism. Two models have been suggested for HIV-1 Env packaging. The first one, the “active packaging” model, proposes that a specific interaction occurs between Gag and Env proteins. This model is based on the observation that many deletions and substitutions in the MA domain and in the TMgp41 CD abolish the packaging of Env into Gag particles, whereas particle release remains unaffected (4–17). This model is also supported by an in vitro study that showed direct binding between HIV-1 MA and TMgp41 CD peptides (18) and by the fact that HIV-1 Env functions to drive basolateral budding of Gag in polarized cells (19). In the second model, the “passive packaging” model, Env is thought to be passively incorporated into nascent virions at the plasma membrane. HIV-1 Gag particles are able to accommodate heterologous Env proteins [such as those of murine leukemia virus or vesicular stomatitis virus (VSV)], and the TMgp41 CD has been found, in some cases, to be unnecessary for HIV-1 Env incorporation, supporting this passive model (6, 11, 20, 21).
We have recently shown that Env returns to the trans-Golgi network (TGN) after its internalization from the cell surface (22). This Env retrograde transport is mediated by a direct interaction between the cytoplasmic tail of Env and TIP47 (tail-interacting protein of 47 kDa), a protein previously described as essential for the endosome-to-TGN retrograde transport of mannose-6-phosphate receptors (23). The retrograde transport of Env to the TGN requires a Y802W803 diaromatic motif to be present in the TMgp41 CD. Mutations in this motif abolish the interaction of Env with TIP47, the targeting of Env to the TGN, and Env incorporation (22).
Here, we report that TIP47 acts as a linker between Gag and Env through its binding to MA and to the cytoplasmic tail of TMgp41, thus mediating Env incorporation. Both virion infectivity and Env incorporation are impaired by TIP47 silencing or by mutations abolishing TIP47–MA interaction. Moreover, the overexpression of TIP47 increases Env incorporation. Taken together, our results demonstrate that TIP47 is a cellular cofactor that plays an essential role in Env incorporation into HIV-1 virions by allowing the interaction between Gag and Env.
Results
Depletion of TIP47 in Producer Cells Abolishes Env Incorporation into Virions.
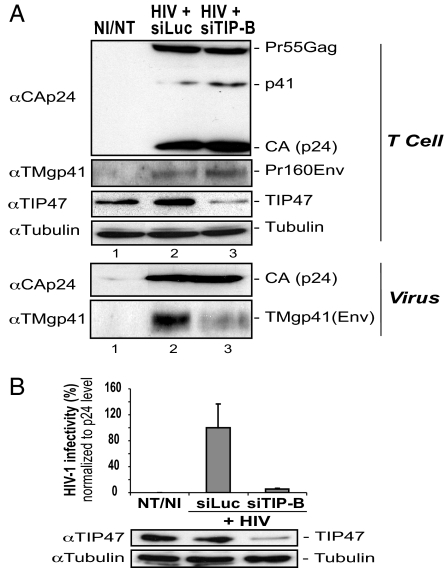
We investigated the role of cellular TIP47 in HIV-1 infectivity and in the Env incorporation process by infecting TIP47-depleted Jurkat T cells with HIV-1. Similar levels of HIV-1 Env TMgp41 subunit and Gag products (Pr55Gag, p41, and CAp24) were observed in producer T cells treated with siRNA against TIP47 (siTIP-B) or with siRNA against the GL2 luciferase sequence (siLuc) (24) used as a control (Fig. 1A Upper), indicating that TIP47 silencing did not influence the synthesis of intracellular Gag and Env proteins. Interestingly, the level of TMgp41 in virions produced from TIP47-depleted cells was much lower than in control virions (Fig. 1A Lower, lane 3 vs. lane 2), whereas similar levels of CAp24 (Gag protein) were found in all virus preparations (Fig. 1A Lower). Moreover, this Env incorporation defect was correlated with a 16-fold decrease in infectious titers (Fig. 1B). These results were confirmed in HeLa cells (compare lane 2 with lanes 3 and 4 in Fig. 8A Lower, which is published as supporting information on the PNAS web site). These effects were observed with two different siRNAs, siTIP-A and siTIP-B, and could be suppressed by reexpression of the HA-TIP47 construct, which is resistant to siRNA TIP-A. Transcomplementation of TIP47-depleted cells with HA-TIP47 restored both Env incorporation (Fig. 8A Lower, lane 5) and infectivity (Fig. 8C) of the produced virions.
Fig. 1.
TIP47 depletion decreases Env incorporation and HIV-1 infectivity. (A) Jurkat T cells were electroporated with the indicated siRNA and infected 48 h later with VSV-G-pseudotyped HIV-1 virus stock. Cell (Upper) and virion (Lower) lysates were analyzed by Western blotting with anti-CAp24, anti-TMgp41, anti-TIP47, and anti-tubulin. NI/NT, noninfected/nontransfected cells. (B) Supernatants of TIP47-depleted Jurkat T cells were used to infect HeLa P4-2 indicator cells. HIV-1 infectivity corresponded to the ratio of the titer of the produced virus to the quantity of p24 detected in the supernatant. The data are representative of three experiments performed in duplicate.
Taken together, these results demonstrate that TIP47 is essential for Env incorporation into virions. These data were corroborated by the fact that depletion of TIP47 strongly affected the spreading of HIV-1 in HeLa P4-2 and Jurkat T cell cultures (data not shown).
Overexpression of TIP47 Enhances Env Incorporation into Virions.
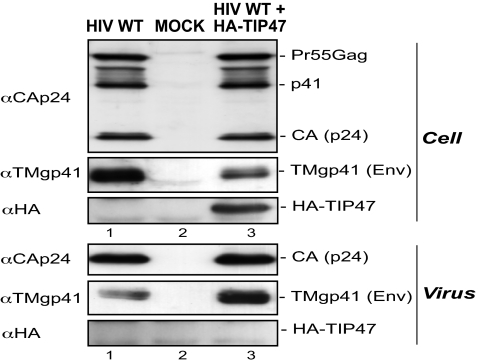
Next, we investigated whether overexpression of TIP47 could increase the amount of Env that is incorporated into virions. We produced HIV-1 from HeLa cells transfected with HIV-1 proviral DNA and a vector expressing HA-TIP47. We detected similar levels of Gag protein in cells overexpressing HA-TIP47 and in control cells (Fig. 2 Upper, lane 3 vs. lane 1). In contrast, the level of TMgp41 was three times lower in cells overexpressing HA-TIP47 (Fig. 2 Upper, lane 3 vs. lane 1). This decrease in producer cells correlated with an increase in the level of TMgp41 incorporated into virions, which was estimated as being 3.3-fold higher than in viruses produced by control cells (Fig. 2 Lower, lane 3 vs. lane 1), whereas the level of CAp24 was similar in both types of virions. These data show that overexpression of TIP47 enhances Env incorporation into virions. Interestingly, HIV-1 infectivity was not modified by the 3-fold increase of viral Env content (Fig. 8D).
Fig. 2.
Overexpression of TIP47 enhances Env incorporation. Cell (Upper) and virion (Lower) lysates were prepared from HeLa cells transfected with WT HIV-1 proviral DNA (lanes 1 and 3), pcDNA3 (lane 1), and pAS1B-HA-TIP47 (lane 3). The amount of Gag (Pr55Gag, p41, and CAp24), Env (TMgp41), and HA-TIP47 was analyzed by Western blotting with anti-CAp24, anti-TMgp41, and anti-HA antibodies. NT, nontransfected cells. These results are representative of three independent experiments.
Moreover, we could detect neither TIP47 (data not shown) nor HA-TIP47 in virions produced from cells overexpressing HA-TIP47 (Fig. 2 Lower, lane 3) despite the high level of the overexpressed protein. Thus, either TIP47 is not incorporated into particles despite its role in Env incorporation or it is incorporated at a level below the threshold of detection by anti-TIP or anti-HA antibodies.
MAp18 Interacts with TIP47.
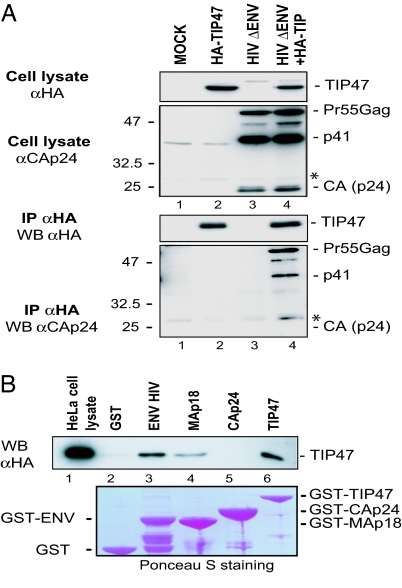
Because TIP47 is required for producing infectious HIV-1 virions and interacts with Env through a Y802W803 motif located in the TMgp41 tail (22), we wondered whether TIP47 directly connects Env to Gag, thereby promoting Env incorporation into Gag particles. Therefore, we carried out coimmunoprecipitation assays between Gag protein and TIP47. HA-TIP47 efficiently coprecipitated the Pr55Gag precursor as well as the p41 intermediate cleavage product [corresponding to the MA domain linked to the capsid (CA) domain; Fig. 3A Lower, lane 4]. Interestingly, the CA domain, which is abundant in transfected HeLa cell lysates, was not coprecipitated with HA-TIP47. Thus, these data show that Gag binds to TIP47 and suggesting that the Gag–TIP47 interaction may require the MA domain that is present in both Pr55Gag and p41.
Fig. 3.
TIP47 interacts with MAp18. (A) Coimmunoprecipitation between Gag proteins and TIP47. HeLa cells were transfected with pcDNA3 (lane 1), pcDNA3 and pAS1B-HA-TIP47 (lane 2), pcDNA3 and HIV ΔENV proviral DNA (lane 3), or pAS1B-HA-TIP47 and HIV ΔENV proviral DNA (lane 4). Cell lysates were precipitated with anti-HA mAb. Crude lysates (Upper) and precipitated proteins (Lower) were detected with rabbit anti-CAp24 and anti-HA HRP mAbs. The nonspecific bands are marked with an asterisk. (B) Binding of MAp18 to TIP47 in a cell lysate-binding assay. HeLa cell lysates producing HA-TIP47 were incubated with equal amounts (5 μg) of purified GST (lane 2), GST fused to the CD of HIV-1 TMgp41 (ENV HIV) (lane 3), MAp18 (lane 4), CAp24 (lane 5), or TIP47 (lane 6). TIP47 binding was analyzed by Western blotting with an anti-HA mAb. Crude lysates (2 × 105 cells) were run as a control (lane 1).
We then used an in vitro TIP47-binding assay to characterize the Gag precursor domain that is involved in the Gag–TIP47 interaction (Fig. 3B). HA-TIP47 bound to MA (Fig. 3B, lane 4) but not to GST alone (lane 2) or to CA (lane 5). HA-TIP47 also interacts with itself (Fig. 3B, TIP47, lane 6) and with the TMgp41 CD (Env HIV, lane 3), as shown in refs. 22 and 25. We confirmed the interaction between the MA domain and TIP47 by using a yeast two-hybrid assay (Fig. 9A, which is published as supporting information on the PNAS web site). Only the MA-containing constructs, MA and MA-CA, bound efficiently to TIP47. These results demonstrate that TIP47 physically interacts with Gag through the MA domain.
Residues 5–16 of the MA Domain Are Required for Binding to TIP47.
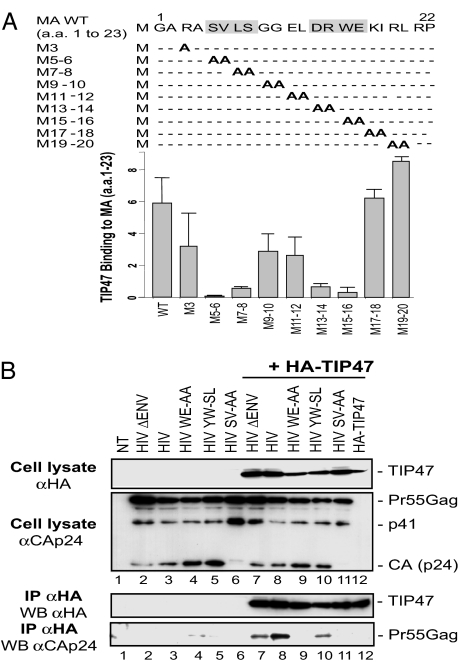
We used several C-terminal-deleted constructs of MA in yeast two-hybrid assays to determine the MA region that is required for the MA–TIP47 interaction. These deletion studies showed that the first 23 aa of the MA domain are sufficient to mediate the interaction with TIP47 (Fig. 9B). A quantitative two-hybrid assay to measure TIP47 binding to MA1–23 mutants showed that amino acids 5–16 of MA were involved in the MA–TIP47 interaction. Specifically, the peptide fragments S5-VL-S8 and D13-RW-E16 appeared to be essential for the MA–TIP47 interaction (Fig. 4A).
Fig. 4.
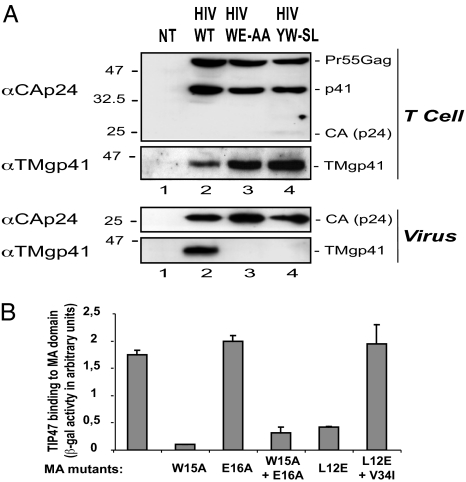
Amino acid residues 5–16 of MAp18 are required for binding to TIP47. (A) Mapping of the region involved in TIP47 binding. Yeast L40 reporter strains containing the β-Gal LexA-inducible gene were cotransformed, and the interaction between WT or mutated MA1–23 and TIP47 was evaluated by β-gal activity in a liquid culture assay. Results are representative of three independent experiments. (B) Coimmunoprecipitation between MA mutants and TIP47. HeLa cells were transfected with pcDNA3 (lanes 1–6) or pAS1B-HA-TIP47 (lanes 7–12) and HIV ΔENV (lanes 2 and 7), HIV WT (lanes 3 and 8), HIV W15E16-AA (lanes 4 and 9), HIV Y802W803-SL (lanes 5 and 10), HIV S5V6-AA (lanes 6 and 11), or pcDNA3 (lanes 1 and 12). Cell lysates were precipitated with anti-HA mAbs. Crude lysates (Upper) and precipitated proteins (Lower) were detected with rabbit anti-CAp24 and anti-HA HRP mAbs.
We then introduced mutations on residues S5V6 and W15E16 in the HIV-1 proviral DNA to analyze their involvement in the MA–TIP47 interaction in infected cells. Pr55Gag coimmunoprecipitated with HA-TIP47 in cells expressing the Gag precursor alone (Fig. 4B Lower, HIV ΔEnv, lane 7), WT HIV-1 (HIV, lane 8), or Env Y802W803-SL-mutated HIV-1 [a virus having an envelope glycoprotein that cannot interact with TIP47 (22), lane 10]. In contrast, we observed no association of Gag with HA-TIP47 in cells expressing HIV-1 mutated in residues S5V6 (Fig. 4B Lower, lane 11) or W15E16 (lane 9) of the MA domain. Interestingly, we could not detect the CA product in HIV S5V6-AA cell lysates (Fig. 4B Upper, αCAp24, lanes 6 and 11), showing that the S5V6-AA mutation blocked the intracellular maturation of Pr55Gag, whereas the W15E16-AA MA mutation had no effect on the Pr55Gag maturation (αCAp24, lanes 4 and 9). A similar maturation defect has been previously described in viruses bearing S5-I or V6-R mutations in the MA domain (26), mutations that remove, respectively, a critical residue for the MA myristoylation signal or for Gag-membrane binding. These results show that amino acids 5–16 of MA, which include residues W15E16, are required for TIP47 binding.
The MA W15E16-AA Mutation Results in a Defect in Env Incorporation.
We then checked Env incorporation of HIV-1 virus bearing the MA W15E16-AA mutation to correlate the absence of the Gag–TIP47 interaction with a defect in Env packaging. The same level of intracellular expression and processing of Gag was observed in Jurkat T cells producing WT or mutated HIV-1 viruses, whereas Env protein level was higher in cells producing mutated viruses (Fig. 5A Upper). We detected the same level of CAp24 in all virions but a barely detectable level of Env TMgp41 protein in the MA W15E16-AA or Env Y802W803-SL mutant virions (22) (Fig. 5A Lower, lanes 3 and 4 vs. lane 2). Similar results were observed in HeLa cells, indicating that mutations blocking Gag–TIP47 or Env–TIP47 interactions reduced Env incorporation into virions and thus diminished virion infectivity (see Fig. 10 C and D, which is published as supporting information on the PNAS web site). Moreover, the MA W15E16-AA HIV-1 mutant, as well as the Env Y802W803-SL HIV-1 mutant, failed to replicate in T cells, whereas the HIV-1 WT virus productively infected these cells (Fig. 10 A and B).
Fig. 5.
Impairment of MA–TIP47 binding blocks Env incorporation. (A) Env incorporation into WT, MA W15E16-AA, and Env Y802W803-SL mutant virions produced by Jurkat T cells. Cell (Upper) and virion (Lower) lysates were prepared from Jurkat T cells infected with WT, MA W15E16-AA, and Env Y802W803-SL viruses pseudotyped with VSV-G. The amount of Gag (Pr55Gag and CAp24) and Env (TMgp41) proteins was analyzed by Western blotting with anti-CAp24 and anti-TMgp41 mAbs. NT, nontransfected cells. The results are representative of duplicate experiments. (B) Interaction of mutated MAp18 with TIP47. Yeast L40 reporter strains containing the β-Gal LexA-inducible gene were cotransformed, and the interaction between WT or mutated MA1–132 and TIP47 was evaluated by β-gal activity in a liquid culture assay. Data are representative of three independent experiments.
It was previously reported that a single amino acid substitution on residue W15 (indicated as W16 in ref. 17) or on residue L12 (11) blocks Env incorporation. Therefore, we tested whether these mutations or MA E16A single mutation still binds TIP47. Like the W15E16-AA mutant, the MA L12E and the MA W15A mutants had reduced binding capacity to TIP47 (Fig. 5B). However, the MA E16A mutation was still capable of binding TIP47. Interestingly, appearance of a second-site revertant mutation on the V34I residue in the MA L12E domain led to a MA L12E-V34I mutant, which compensated the Env incorporation defect (6) and restored the interaction between TIP47 and the MA domain (Fig. 5B).
Taken together, these results point out the requirement of MA–TIP47 binding for Env incorporation.
TIP47 Associates the MA Domain to TMgp41.
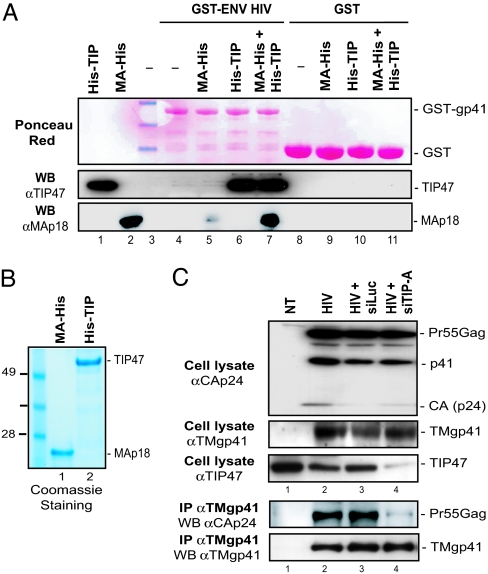
TIP47 can interact with both the Env TMgp41 subunit and the MA domain of Gag. Therefore, to demonstrate that TIP47 can act as a “bridge” between Gag and Env, we performed in vitro binding studies with the three purified partners: TMgp41 CD (Fig. 6A, see the Ponceau red staining of GST-ENV HIV), TIP47, and the MA domain of Gag (see the purity of recombinant His-TIP47 and MA-His in Fig. 6B). The TMgp41 CD fused to GST (GST-ENV HIV) was able to bind quantitatively to MA only in the presence of recombinant TIP47 (Fig. 6A Bottom, compare lanes 5 and 7). Interestingly, TIP47 interacted with GST-ENV HIV in the absence of MA (Fig. 6A Middle, lane 6) as efficiently as in the presence of MA (lane 7). This experiment establishes that recombinant TIP47 binds both Env and Gag simultaneously (Fig. 6A, lane 7) and suggests that the formation of the ternary complex does not require the contribution of other cellular or viral proteins.
Fig. 6.
TIP47 associates the MA domain to TMgp41. (A) Binding of the MA domain to the TMgp41 CD in the presence of TIP47. Purified His-TIP47 (3 μg, lanes 6, 7, 10, and 11) and MA-His (3 μg, lanes 5, 7, 9, and 11) were incubated with purified GST (lanes 8–11) or GST fused to the CD of HIV-1 TMgp41 (ENV HIV) (lanes 4–7). MA binding and TIP47 binding were analyzed by Western blotting with anti-MA and anti-TIP47 antibodies, respectively. Purified His-TIP47 (500 ng, lane 1) and MA-His (500 ng, lane 2) were run as controls. (B) Coomassie blue staining of purified MA-His (3 μg, lane 1) and His-TIP47 (4.8 μg, lane 2) proteins. (C) Reduction of Gag-Env coimmunoprecipitation by TIP47 silencing. HeLa P4-2 cells were transfected twice with HIV proviral DNA and TIP-A siRNA (lane 4) or control siRNA (Luc, lane 3). Cell lysates were precipitated with anti-TMgp41 (41A) mAb. Crude lysates (Upper) and precipitated proteins (Lower) were detected with rabbit anti-CAp24, anti-TMgp41 (2F5), and anti-TIP47 antibodies.
Then, we examined the effect of TIP47 depletion on the binding of Env to Gag in HeLa cells transfected with HIV-1 proviral DNA. Gag efficiently coimmunoprecipitated with TMgp41 in untreated cells (Fig. 6C, lane 2) or in cells treated with Luc siRNA (lane 3), whereas in TIP47-depleted cells, the association of Gag to TMgp41 was considerably reduced (Fig. 6C Lower, lanes 2 and 3 vs. lane 4). These results demonstrate that TIP47 expression is required for the association of Gag with the TMgp41 subunit of Env in virion-producing cells.
Finally, we examined the colocalization of Gag, Env, and CD63, a cellular marker of the late endosomal compartment, in WT or mutant HIV-infected HeLa cells and in HIV-1-infected cells treated with TIP-B siRNA (see Fig. 11A, which is published as supporting information on the PNAS web site). In WT HIV-1-infected cells, Gag and Env colocalized as yellow dots in patches at the cell surface (Fig. 11A a–d). No colocalization was observed among Gag, Env, and CD63 simultaneously (Fig. 11A d). Impairment of the Gag–TIP47 interaction by MA W15E16-AA mutation (Fig. 11A e–h) or TIP47 silencing (Fig. 11A i–l) resulted in the loss of colocalization between Gag and Env and did not induce significant modification of intracellular Gag localization (Fig. 11A b, f, and j). Interestingly, we observed partial localization among Gag, Env, and endogenous TIP47 in some spots in HeLa cells infected with HIV-1 (see Fig. 11B). Taken together, these results suggest that TIP47 mediates a physical link between Gag and Env to ensure their intracellular encounter during viral assembly process.
TIP47 Is Required Specifically for HIV-1 Full-Length Env Incorporation.
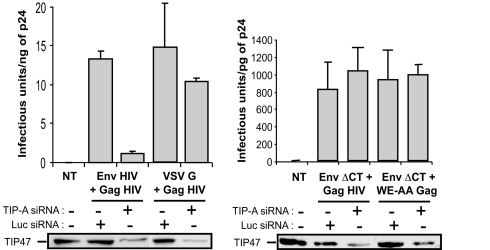
We then examined the effect of TIP47 depletion on the infectious titers of HIV Gag particles pseudotyped with HIV-1 cytoplasmic tailless Env or with the VSV G heterologous envelope (Fig. 7). As predicted, TIP47 depletion affected the pseudotyping of WT HIV-1 Env into HIV-1 Gag particles, thus decreasing by 10-fold the particle infectivity. In contrast, TIP47 silencing did not significantly modify the infectivity of WT HIV-1 Gag particles pseudotyped with the VSV G envelope or the infectivity of either WT or W15E16-mutated HIV-1 Gag particles pseudotyped with a cytoplasmic tailless HIV-1 Env. Thus, our data show that the incorporation of full-length Env into Gag particles is TIP47-dependent, whereas the mechanism of incorporation of the VSV G heterologous envelope or artificial cytoplasmic tailless HIV-1 Env is independent of TIP47.
Fig. 7.
Infectivity of the pseudotyped HIV-1 core. TIP47-depleted HeLa cells were cotransfected with the indicated Gag and envelope vectors. Supernatants were used to infect HeLa P4-2 indicator cells. Infectivity corresponded to the ratio of the titer of the produced virus to the quantity of p24 detected in the supernatant. The data are representative of three experiments performed in duplicate.
Discussion
In this study, we show that the expression of TIP47 is essential for incorporation of the full-length HIV-1 envelope glycoprotein into Gag particles to confer full infectivity to budding HIV-1 virions. This conclusion is supported by the following results: (i) TIP47 silencing leads to impaired Env incorporation into viral particles and virion infectivity, (ii) overexpression of TIP47 causes a significant increase in Env incorporation, and (iii) TIP47 is essential for the incorporation of full-length HIV-1 Env, with its unusually long cytoplasmic tail, but is dispensable for the incorporation of HIV-1 Env, with a truncated cytoplasmic tail, or of the VSV G envelope.
We also demonstrate that TIP47 binds to both the MA domain of Gag and the cytoplasmic tail of TMgp41 (TMgp41 CD), supporting a model in which TIP47 is essential for the association of these two crucial viral components to ensure Env incorporation into Gag particles. This model is supported by the fact that (i) mutations within the MA domain or the TMgp41 CD that inhibit their binding to TIP47 lead to impaired Env incorporation, (ii) Gag does not coimmunoprecipitate with Env in the absence of TIP47, (iii) TIP47 interacts simultaneously with both the TMgp41 CD and the MA domain, and (iv) the colocalization between Gag and Env requires TIP47 expression and MA–TIP47 interaction.
Previous evidence suggested a possible role for the MA domain of Gag in Env incorporation. Several mutations in the N-terminal region of MA were described as being responsible for the production of noninfectious virus by impairing Env incorporation (4–16). However, the molecular mechanism of such a defect was not elucidated. Here, we identified a mutation (W15E16-AA) in MA that results not only in defective Env incorporation but also in reduced binding to TIP47. We found that amino acid residues 5–16 of MA, a region containing the H1 helix of MA (residues 11–19; ref. 27), previously implicated in the interaction with the AP3 adaptor (28), are required for the binding to TIP47. In particular, we showed that the residues L12 and W15 are necessary for TIP47–MA interaction. Interestingly, some of these residues (L7S8, L12, W15E16K17, and W15) have been reported as being critical for Env incorporation (4–17), and the second-site revertant mutant of MA, L12E-V34I, which recovers normal Env incorporation and infectivity (6), fully recovers TIP47 binding. These data show a strong correlation between the ability of MA to bind to TIP47 and its ability to incorporate Env into Gag particles and suggests that other regions of MA might also participate in the MA–TIP47 interaction. One cannot exclude that the Env incorporation defect observed with MA mutants may reflect the failure of the Gag–TIP47 interaction, as well as local or global MA misfolding and/or modification of myristyl switching.
The involvement of cellular cofactors in Env incorporation into HIV-1 Gag particles has been suspected for many years (11, 16). In this study, we identify TIP47 as a cellular cofactor that is essential in Env incorporation. TIP47 protein was initially identified as a partner of the mannose-6-phosphate receptors (MPRs) required for the endosome-to-TGN transport of MPR (23, 29) and that of HIV-1 Env (22). TIP47 also binds directly to active Rab9 GTPase (30), whose silencing resulted in a defect in HIV-1 replication (31). TIP47 has been described as a cytosolic and endosomal membrane-bound protein (23), but a fraction was also found at the surface of lipid storage droplets (32–34) and at the P face of the plasma membrane (35). To date, we do not know which fraction of TIP47 is required for Env incorporation, but a close association of TIP47 with a cellular membrane is presumably required for its function in Env packaging.
Our functional and biochemical studies support the model that TIP47 acts in Env packaging by facilitating an association between Gag and Env. Indeed, our in vitro binding assay with the three purified proteins demonstrates that TIP47 can bind both Env and Gag simultaneously, without the contribution of other cellular or viral proteins. However, it has been proposed that a direct interaction between MA and TMgp41 promotes Env packaging into virions (18), which was demonstrated by a GST pull-down assay. But it is highly likely that the binding between the MA and the GST-TMgp41 CD observed in this study involved TIP47 molecules present in the lysate of the MA-transfected cells.
In addition to its role as a “connector,” TIP47 could also participate in Gag and Env trafficking throughout the intracellular compartments, allowing them to be in the right compartment for their encounter. We previously showed that TIP47 plays a major role in Env retrograde transport (22); however, we did not observed a role for TIP47 in proper Gag localization (see Fig. 11). Impairment of Gag–TIP47 interaction by TIP47 silencing or by mutation in MA modified neither Gag intracellular localization nor Gag budding at the cell surface, suggesting that the major function of TIP47 in HIV-1-infected cells is to physically link Gag and Env together during the viral assembly process.
The cellular location hosting the initial encounter of Gag, Env, and TIP47 is unknown. We observed mainly the colocalization of MA and Env at the plasma membrane, presumably representing the final stage of viral morphogenesis. The involvement of TIP47 in Env retrograde transport from late endosomes to the TGN raises the possibility that Gag could meet Env and TIP47 on endosomal compartments. This association could redirect this complex from the retrograde pathway to the plasma membrane. Further studies will be necessary to determine whether these interactions take place before or after recruitment of the ESCRT (endosomal sorting complex required for transport) machinery by Gag for HIV-1 budding (36–38).
In conclusion, we have identified TIP47 as a cellular cofactor that is involved in the incorporation of full-length HIV-1 Env into HIV-1 Gag particles. This function involves the interaction between TIP47 and two essential viral proteins, Gag and Env, making TIP47 indispensable for HIV-1 Env incorporation and HIV-1 infectivity. These findings may provide insight into the development of innovative strategies for impairing the generation of infectious viruses and the spreading of HIV-1.
Materials and Methods
Incorporation Assays.
Jurkat T cells (2 × 106) resuspended in phosphate buffer-sucrose were electroporated with 5 μl of a 1 mM solution of siRNA TIP-B (positions 495–512, 5′-ggacacgguggccacccaatt-3′) or control siRNA Luc (positions 153–173, 5′-cguacgcggaauacuucga-3′) with a Gene Pulser II electroporator with an RF module (Bio-Rad, Hercules, CA) (100 V; modulation, 50%; 25 kHz; 10 bursts of 2 ms; burst interval, 100 ms). Forty-eight hours later, cells were infected with WT HIV-1 HXB2R pseudotyped with VSV-G at a multiplicity of infection of 1. Eight hours later, cells were washed and placed in the presence of AMD3100, a CXCR4 inhibitor. The supernatants were collected 48 h later, 0.45-μm-filtered, quantified for HIV-1 p24 antigen, and ultracentrifuged at 150,000 × g for 1 h 20 min. Cell lysates and pelleted viruses were subjected to Western blotting with anti-p24 (ARP366, National Institute for Biological Standards and Control, Hertfordshire, U.K.), rabbit anti-TIP47, anti-TMgp41 (2F5, National Institutes of Health, Bethesda, MD), and anti-tubulin (DM1A, Sigma, Lyon, France) antibodies. For infectivity assay in T cells, the supernatants without AMD3100 were collected 24 h after infection.
Incorporation assays in Jurkat T cells with HXB2R MA W15E16-AA or Env Y802W803-SL mutants were carried out as described in ref. 22.
For incorporation assays in the presence of overexpressed TIP47, HeLa cells were transfected with 4 μg of HXB2R WT proviral DNA and 4 μg of either pAS1B-HA-TIP47 or pAS1B vectors by using Lipofectamine 2000 reagent (Invitrogen, Cergy-Pontoise, France). Forty-eight hours later, cells and supernatants were treated as described above.
Infectivity Assays, Two-Hybrid Assays, and TIP47-Binding Assays.
These assays are described in Supporting Materials and Methods, which is published as supporting information on the PNAS web site.
Coimmunoprecipitation Experiments.
We generated MA S5V6-AA and MA W15E16-AA mutant HIV-1 proviral clones by site-directed mutagenesis on the NarI-SpeI fragment (nucleotides 655-1524) from HXB2R. We transfected, using the Lipofectamine Plus reagent (Invitrogen), 2 × 105 HeLa cells with 1 μg of either the pAS1B-HA-TIP47 or pAS1B vectors and 1 μg of one of the following proviral DNAs: HXB2R WT, HXB2R ΔENV (a virus that cannot express Env products; provided by F. Mammano, INSERM, Paris, France), pMA243 (a virus that cannot express Gag products; provided by M. Alizon, INSERM), HXB2R Env Y802W803-SL (22) (a virus with mutated Y802W803 Env residues), HXB2R MA S5V6-AA, or HXB2R MA W15E16-AA. Forty-eight hours later, cells were harvested and lysed in lysis buffer (1% Triton X-100/150 mM NaCl/10 mM Tris·HCl, pH 7.5/0.1% deoxycholate/1 mM EDTA). The cell lysates were incubated with protein G-Sepharose beads and with anti-HA (12CA5, Roche, Basel, Switzerland) or anti-TMgp41 (41A, Hybridolabs, Paris, France) antibodies and then washed six times with the lysis buffer. Bound cellular proteins were subjected to Western blotting using rabbit anti-CAp24 (National Institutes of Health), human anti-TMgp41 (2F5), and rat anti-HA (3F10) antibodies.
For coimmunoprecipitation with siRNA against TIP47, 2 × 105 HeLa cells were transfected with 30 nM TIP-A (positions 15–33, 5′-tgaagtcgcggccgctgtt-3′) or control Luc siRNA and either pcDNA3 or HXB2R WT proviral DNA by using Lipofectamine Plus reagent. After 24 h, the cells were transfected again with 30 nM siRNA by using Oligofectamine reagent (Invitrogen). The cells were lysed 48 h later and immunoprecipitated with anti-TMgp41 (41A) antibodies as described above.
Supplementary Material
Acknowledgments
We thank F. Margottin-Goguet, E. Bertrand, and S. Emiliani for helpful discussions; S. Pfeffer (Stanford University, Stanford, CA), M. Thali (University of Vermont, Burlington, VT), E. Freed (National Cancer Institute, Frederick, MD), F. Mammano, D. Burton (La Jolla, CA), P. Parren, M. Alizon, the National Institute for Biological Standards and Control, and the National Institutes of Health for kindly providing reagents; and N. Kubat and M. Maroun for critical reading. This work was supported by fellowships from the Ministère de l'Education Nationale, de la Recherche, et de la Technologie (to S.L.-V.), Agence Nationale de Recherches sur le SIDA (ANRS) (to G.C.), SIDACTION (to G.B.), and grants from ANRS, SIDACTION, Fondation pour la Recherche Médicale, and the European Sixth Framework Program for Research and Development through Targeting Replication and Integration of HIV Project (TRIoH) LSHB-CT-2003-503.
Abbreviations
- MA
matrix
- CA
capsid
- CD
cytoplasmic domain
- TGN
trans-Golgi network
- VSV
vesicular stomatitis virus.
Footnotes
The authors declare no conflict of interest.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Hunter E, Swanstrom R. Curr Top Microbiol Immunol. 1990;157:187–253. doi: 10.1007/978-3-642-75218-6_7. [DOI] [PubMed] [Google Scholar]
- 2.Coffin JM, Hughes SH, Varmus HE. Retroviruses. Cold Spring Harbor NY: Cold Spring Harbor Lab Press; 1997. [PubMed] [Google Scholar]
- 3.Morita E, Sundquist WI. Annu Rev Cell Dev Biol. 2004;20:395–425. doi: 10.1146/annurev.cellbio.20.010403.102350. [DOI] [PubMed] [Google Scholar]
- 4.Dorfman T, Mammano F, Haseltine WA, Gottlinger HG. J Virol. 1994;68:1689–1696. doi: 10.1128/jvi.68.3.1689-1696.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu X, Yuan X, Matsuda Z, Lee TH, Essex M. J Virol. 1992;66:4966–4971. doi: 10.1128/jvi.66.8.4966-4971.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freed EO, Martin MA. J Virol. 1996;70:341–351. doi: 10.1128/jvi.70.1.341-351.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang CT, Zhang Y, Mc Dermott J, Barklis E. J Virol. 1993;67:7067–7076. doi: 10.1128/jvi.67.12.7067-7076.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mammano F, Kondo E, Sodroski J, Bukovsky A, Gottlinger HG. J Virol. 1995;69:3824–3830. doi: 10.1128/jvi.69.6.3824-3830.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buchschacher GL, Jr, Freed EO, Panganiban AT. J Virol. 1995;69:1344–1348. doi: 10.1128/jvi.69.2.1344-1348.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ono A, Huang M, Freed EO. J Virol. 1997;71:4409–4418. doi: 10.1128/jvi.71.6.4409-4418.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freed EO, Martin MA. J Virol. 1995;69:1984–1989. doi: 10.1128/jvi.69.3.1984-1989.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dubay JW, Roberts SJ, Hahn BH, Hunter E. J Virol. 1992;66:6616–6625. doi: 10.1128/jvi.66.11.6616-6625.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akari H, Fukumori T, Adachi A. J Virol. 2000;74:4891–4893. doi: 10.1128/jvi.74.10.4891-4893.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Piller SC, Dubay JW, Derdeyn CA, Hunter E. J Virol. 2000;74:11717–11723. doi: 10.1128/jvi.74.24.11717-11723.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murakami T, Freed EO. J Virol. 2000;74:3548–3554. doi: 10.1128/jvi.74.8.3548-3554.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murakami T, Freed EO. Proc Natl Acad Sci USA. 2000;97:343–348. doi: 10.1073/pnas.97.1.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paillart JC, Gottlinger HG. J Virol. 1999;73:2604–2612. doi: 10.1128/jvi.73.4.2604-2612.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cosson P. EMBO J. 1996;15:5783–5788. [PMC free article] [PubMed] [Google Scholar]
- 19.Lodge R, Lalonde JP, Lemay G, Cohen EA. EMBO J. 1997;16:695–705. doi: 10.1093/emboj/16.4.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Landau NR, Page KA, Littman DR. J Virol. 1991;65:162–169. doi: 10.1128/jvi.65.1.162-169.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gabuzda DH, Lever A, Terwilliger E, Sodroski J. J Virol. 1992;66:3306–3315. doi: 10.1128/jvi.66.6.3306-3315.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blot G, Janvier K, Le Panse S, Benarous R, Berlioz-Torrent C. J Virol. 2003;77:6931–6945. doi: 10.1128/JVI.77.12.6931-6945.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diaz E, Pfeffer SR. Cell. 1998;93:433–443. doi: 10.1016/s0092-8674(00)81171-x. [DOI] [PubMed] [Google Scholar]
- 24.Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Nature. 2001;411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- 25.Sincock PM, Ganley IG, Krise JP, Diederichs S, Sivars U, O'Connor B, Ding L, Pfeffer SR. Traffic. 2003;4:18–25. doi: 10.1034/j.1600-0854.2003.40104.x. [DOI] [PubMed] [Google Scholar]
- 26.Ono A, Freed EO. J Virol. 1999;73:4136–4144. doi: 10.1128/jvi.73.5.4136-4144.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hill CP, Worthylake D, Bancroft DP, Christensen AM, Sundquist WI. Proc Natl Acad Sci USA. 1996;93:3099–3104. doi: 10.1073/pnas.93.7.3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dong X, Li H, Derdowski A, Ding L, Burnett A, Chen X, Peters TR, Dermody TS, Woodruff E, Wang JJ, Spearman P. Cell. 2005;120:663–674. doi: 10.1016/j.cell.2004.12.023. [DOI] [PubMed] [Google Scholar]
- 29.Orsel JG, Sincock PM, Krise JP, Pfeffer SR. Proc Natl Acad Sci USA. 2000;97:9047–9051. doi: 10.1073/pnas.160251397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carroll KS, Hanna J, Simon I, Krise J, Barbero P, Pfeffer SR. Science. 2001;292:1373–1376. doi: 10.1126/science.1056791. [DOI] [PubMed] [Google Scholar]
- 31.Murray JL, Mavrakis M, Mc Donald NJ, Yilla M, Sheng J, Bellini WJ, Zhao L, Le Doux JM, Shaw MW, Luo CC, et al. J Virol. 2005;79:11742–11751. doi: 10.1128/JVI.79.18.11742-11751.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wolins NE, Rubin B, Brasaemle DL. J Biol Chem. 2001;276:5101–5108. doi: 10.1074/jbc.M006775200. [DOI] [PubMed] [Google Scholar]
- 33.Miura S, Gan JW, Brzostowski J, Parisi MJ, Schultz CJ, Londos C, Oliver B, Kimmel AR. J Biol Chem. 2002;277:32253–32257. doi: 10.1074/jbc.M204410200. [DOI] [PubMed] [Google Scholar]
- 34.Brasaemle DL, Dolios G, Shapiro L, Wang R. J Biol Chem. 2004;279:46835–46842. doi: 10.1074/jbc.M409340200. [DOI] [PubMed] [Google Scholar]
- 35.Robenek H, Robenek MJ, Buers I, Lorkowski S, Hofnagel O, Troyer D, Severs NJ. J Biol Chem. 2005;280:26330–26338. doi: 10.1074/jbc.M413312200. [DOI] [PubMed] [Google Scholar]
- 36.Garrus JE, von Schwedler UK, Pornillos OW, Morham SG, Zavitz KH, Wang HE, Wettstein DA, Stray KM, Cote M, Rich RL, et al. Cell. 2001;107:55–65. doi: 10.1016/s0092-8674(01)00506-2. [DOI] [PubMed] [Google Scholar]
- 37.Strack B, Calistri A, Craig S, Popova E, Gottlinger HG. Cell. 2003;114:689–699. doi: 10.1016/s0092-8674(03)00653-6. [DOI] [PubMed] [Google Scholar]
- 38.von Schwedler UK, Stuchell M, Muller B, Ward DM, Chung HY, Morita E, Wang HE, Davis T, He GP, Cimbora DM, et al. Cell. 2003;114:701–713. doi: 10.1016/s0092-8674(03)00714-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.