Abstract
Swarming motility is suggested to be a social phenomenon that enables groups of bacteria to coordinately and rapidly move atop solid surfaces. This multicellular behavior, during which the apparently organized bacterial populations are embedded in an extracellular slime layer, has previously been linked with biofilm formation and virulence. Many population density-controlled activities involve the activation of complex signaling pathways using small diffusible molecules, also known as autoinducers. In Gram-negative bacteria, quorum sensing (QS) is achieved primarily by means of N-acylhomoserine lactones (AHLs). Here, we report on a dual function of AHL molecules in controlling swarming behavior of Rhizobium etli, the bacterial symbiotic partner of the common bean plant. The major swarming regulator of R. etli is the cinIR QS system, which is specifically activated in swarming cells by its cognate AHL and other long-chain AHLs. This signaling role of long-chain AHLs is required for high-level expression of the cin and rai QS systems. Besides this signaling function, the long-chain AHLs also have a direct role in surface movement of swarmer cells as these molecules possess significant surface activity and induce liquid flows, known as Marangoni flows, as a result of gradients in surface tension at biologically relevant concentrations. These results point to an as-yet-undisclosed direct role of long-chain AHL molecules as biosurfactants.
Keywords: cell–cell signaling, motility, quorum sensing
The recently proposed term “socio-microbiology” (1) summarizes in a cryptic way the ongoing research on different aspects of bacterial communities. Certain social phenomena like “quorum sensing” (QS) and “swarming” in several bacterial species are connected instead of operating separately (2). Cell–cell communication using N-acylhomoserine lactone (AHL) signals is one of the widespread and known mechanisms through which Gram-negative bacteria can communicate (3–5). AHL signals may be saturated or unsaturated, and the length of their acylgroup and the substituent may vary. The physiological processes regulated by AHLs in different bacterial species are diverse as reviewed in ref. 3. Often, the regulated genes are crucial to the colonization or infection of the eukaryotic hosts (6, 7). Local conditions on a microscopic scale (such as pH, enclosed environment, and diffusion characteristics) may affect signal molecule longevity, stability, and accumulation, which could be used to provide information in addition to population density (8).
Bacterial swarming is a flagella-induced movement in the presence of extracellular slime, the latter being a mixture of carbohydrates, proteins, peptides, surfactants, etc., by which the bacteria can spread over a surface (reviewed in refs. 9 and 10). This process and other forms of surface motility (reviewed in ref. 9) are found in many bacterial genera (11–15). Swarmer cells are often hyperflagellated, elongated, and multinucleated. These motile cells move in groups, colonizing the entire available surface. It has been suggested that glycolipid and/or lipopeptide biosurfactants thereby produced function as wetting agents (16). The migration front is preceded by a visible layer of slime-like extracellular material as observed, for example, for Burkholderia cepacia (12, 17). Within the matrix of extracellular polymeric material, the population densities are obviously extremely high (17). In support of this view, it has been demonstrated that QS is induced and AHLs are produced in swarming colonies (reviewed in ref. 2).
The Gram-negative nitrogen-fixing soil bacterium Rhizobium etli is the bacterial symbiotic partner of the common bean plant. R. etli CNPAF512 produces at least seven different QS signal molecules, produced by the cinIR and raiIR QS system. cinI and raiI code for the AHL synthases, and cinR and raiR code for the transcriptional regulators that bind the AHLs (18, 19). Recently, it was shown that both QS systems in R. etli are clearly responsive to distinct AHLs. Exogenously supplied long-chain AHLs C12-homoserine lactone (HSL) and C14-HSL activate the cinI fusion. Furthermore, the strongest induction occurs with the saturated long chain (slc) 3OH-(slc)-HSL, which is produced by CinI. In contrast to cinI, strong induction of the raiI-gusA fusion is obtained in the presence of OH,C8-HSL (unpublished results). For swarming migration to occur the cin QS system is required, as R. etli cin mutants are no longer able to move over this solid surface (2).
The original objectives of this research project were to elucidate the relationships between the above-mentioned social processes, QS and swarming, in R. etli. In the course of these studies, we discovered that AHLs have an additional role. This study specifically demonstrates that, in addition to a signaling function during swarming, long-chain AHL molecules also have a direct role as biosurfactants in promoting surface colonization.
Results
Endogenous Overproduction of CinI-Made 3OH-(slc)-HSL Restores cinR Mutant Swarming Behavior.
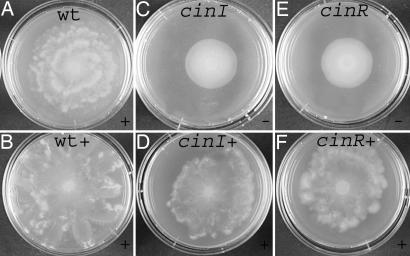
First, the role of the cinIR quorum system as the master regulator for R. etli swarming was investigated in macroscopic swarming experiments shown in Fig. 1. The typical pattern formation arising from swarming was observed in the WT colony (Fig. 1A). For the cinI mutant, swarming was absent (Fig. 1C). A constitutively induced, plasmid-borne cinI restores surface movement of the cinI mutant (Fig. 1D). Also, the same construct stimulates surface migration in the WT strain and induces the formation of pronounced extrusions along the colony border (Fig. 1 A and B). Unexpectedly, this constitutively induced, plasmid-borne cinI was also able to restore swarming of the cinR mutant (Fig. 1 E and F). It can be concluded that endogenous production of 3OH-(slc)-HSL stimulates surface movements of both cinI and cinR mutants indistinguishable from the WT (Fig. 1 A, D, and F). Although 3OH-(slc)-HSL is the natural CinI-made AHL, its signaling function is impaired in the cinR mutant as judged from the lack of cinI-gusA induction. These results are independent of the rai locus as endogenous production of 3OH-(slc)-HSL stimulates surface movements of the cinRraiR mutant and a raiI-gusA is not induced in cin mutants (data not shown). Also, this fusion is not expressed in cinR under free-living conditions by overproducing endogenous 3OH-(slc)-HSL. Moreover, introduction of the rai locus could not complement the cin mutants for surface migration (data not shown). Taken together, these data suggest a direct role for endogenous CinI-made 3OH-(slc)-HSL in swarming in addition to its known signaling function.
Fig. 1.
Macroscopic observations of swarming in WT R. etli (A and B), cinI (C and D), and cinR mutant (E and F). Swarming of the WT strain (B), cinI (D), and cinR (F) in the presence of a constitutively induced, plasmid-borne cinI is shown (strains labeled with +). Diameter of the swarm plate is 6 cm. + corresponds to swarming; − corresponds to a negative swarming phenotype.
To verify this hypothesis, an extract containing the CinI-made 3OH-(slc)-HSL (×1 concentration) was supplied to swarm plates, and its ability to affect colony borders of the cinR mutant was investigated as shown in Fig. 2. Instead of a smooth colony pattern, supply of CinI-made 3OH-(slc)-HSL causes the appearance of extrusions along the cinR swarm colony border (Fig. 2 C and D). The extract's stimulating effect on the WT swarm colony diameter is shown in Fig. 2 A and B. Although the observed effect on cinR is less pronounced, especially with respect to the diameter of the colony, this subtle effect was observed in independent experiments several times. From control experiments showing a similar induction of cinI-gusA in the swarm colony atop of a plate and in a planktonic culture, both supplied with equal concentrations of 3OH-(slc)-HSL molecules, it was concluded that most of the 3OH-(slc)-HSL molecules supplied to the agar are freely available for the swarmers.
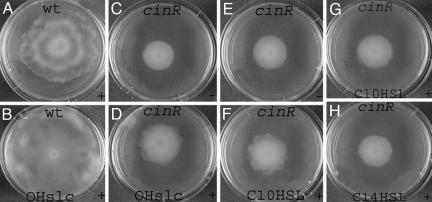
Fig. 2.
Macroscopic observations of swarming in WT R. etli (A and B) and cinR mutant (C–H) on plates containing synthetic AHLs. The plates are supplied with 3OH-(slc)-HSL (×1) (B and D), C10-HSL (100 μM, F; 25 μM, G), and C14-HSL (20 μM, H). For 3OH-(slc)-HSL extraction see Materials and Methods. For swarming, the solvent effect was studied in the presence of the corresponding amounts of acetonitrile (E) or when appropriate an extract from the strain devoid of the cin locus (C). The control plates never affected swarming positively or negatively in R. etli. X, the concentration factor of 3OH-(slc)-HSL compared with the supernatant concentration of the producer strain. Diameter of the swarm plate is 6 cm. + corresponds to swarming; − corresponds to a negative swarming phenotype.
Similarly as exogenously supplied 3OH-(slc)-HSL (Fig. 2D), synthetic molecules C10-HSL, C12-HSL, and C14-HSL were also able to slightly affect swarm colony borders of the cinR mutant in a concentration-dependent manner (10–100 μM). Representative concentrations of C10-HSL (Fig. 2 F and G) and C14-HSL (Fig. 2H) produced similar effects on swarming. Given the solubility of C14-HSL and restricted percentage of solvent allowed in the swarmer plates, we were unable to include higher concentrations for C14-HSL. In contrast, OH,C8-HSL or C6-HSL did not affect swarming positively in the cinR mutant (data not shown).
Extracellular Slime Surrounding the Swarm Cells Contains 3OH-(slc)-HSL.
To deduce the signal concentration available to the cells at the surface of the swarmer plate and inside of the bacterial slime layer, aqueous extracts were prepared from the WT, raiI, producing only 3OH-(slc)-HSL, and cinI swarm colonies. The cinI-inducing capacity of these extracts was assessed in a cinI planktonic culture (for an overview see Table 1).
Table 1.
Expression of cinI in planktonic cells
| Characteristic | Aqueous extracts from |
|
|---|---|---|
| Swarm colony | Liquid culture | |
| cinI-gusA | 1,174 (459) | 1,406 (374) |
| cfu/ml | 2.56 × 1010(1.15 × 1010) | 2.33 × 109 (0.7 × 109) |
Expression of cinI-gusAwas monitored in planktonic cinImutant R. etli. The cinI-inducing capacity of aqueous extracts from a swarm colony of WT cells (75-fold diluted) and stationary-phase culture of WT cells (5-fold diluted) was analyzed. The dilution factor is related to the initial volume of the isolated swarm colony and liquid culture, respectively. Values are the means of 10 (swarmers) and 5 (liquid culture) independent experiments. The standard deviation is indicated in parentheses.
The extract from the extracellular slime surrounding the WT swarmers strongly induced the cinI fusion [1,174 Miller units (MU); 75-fold diluted extract]. We anticipate that the concentration of 3OH-(slc)-HSL in the slime layer is at least 10-fold higher compared with the level reached in a stationary-phase planktonic culture (1,406 MU; 5-fold diluted). This higher concentration is in agreement with the higher concentration of WT swarmers compared with a planktonic culture (see Materials and Methods and Table 1).
Similarly, extract from raiI mutant swarmers clearly induces the cinI fusion (5,274 MU; 25-fold diluted) to a high level in planktonic cells. As expected, extract from cinI swarmers does not induce the cinI fusion in a planktonic culture.
In addition, this same extract obtained from raiI mutant swarmer plates was supplied to fresh swarmer plates in the same concentration. Next, expression of cinI-gusA in the swarm colony atop of the plate was determined. The induction of the plasmid-encoded cinI fusion in this colony (2,139 MU) approaches that of planktonic cultures, indicating that most of the 3OH-(slc)-HSL molecules supplied to the agar are freely available for the swarmers. Extract from cinI mutant swarmers was not able to induce expression under the same conditions. Similarly, as described above, the swarming behavior of the cinR mutant was analyzed in the presence of increasing amounts of synthetic AHLs supplied to the swarm plates (10–100 μM). The induction of the cinI-gusA fusion was also analyzed in these swarmer cells grown in the presence of C12-HSL, C14-HSL, and 3OH-(slc)-HSL. Extrapolation of these values to free-living expressions show similar expression levels compared with those obtained in swarmer cells (data not shown), indicating that not all, but a large part, of the AHLs added to the agar plate are available in the swarmer biofilm at biologically relevant concentrations as indicated by the expression levels in swarmer cells.
Biosurfactant-Like Activity of 3OH-(slc)-HSL Produced by CinI in R. etli.
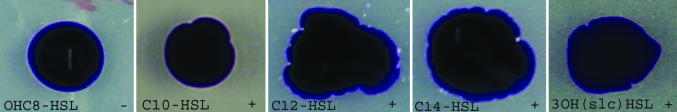
A first method for evaluating the surface activity of the AHL molecules was to monitor the spreading of water droplets on the surface of the agar plates when adding C6-HSL, OH,C8-HSL, C10-HSL, C12-HSL, C14-HSL, or 3OH-(slc)-HSL to the plate. For the pure water and C6-HSL, OH,C8-HSL a spherical droplet shape is observed. However, a clear effect of the long-chain AHLs on the edges of the spreading water droplet, indicative of biosurfactant-like activity, was observed (Fig. 3). This effect is similar to that observed on the edge of a swarming cinI and cinR colony grown in the presence of these AHLs. These observations support the hypothesis that 3OH-(slc)-HSL produced by R. etli CinI regulates swarming directly by a surface-active mechanism. It is generally well known that biosurfactants can also affect the contact angle between the liquid phase and the substrate. Direct measurements of the macroscopic contact angle of water droplets containing C12-HSL, C14-HSL, and 3OH-(slc)-HSL on silanized glass surfaces with varying wettability were performed. In the concentration range where the spreading of the water droplet is accompanied by extrusions (as in Fig. 3), the contact angles of the droplets containing AHLs did not differ from those obtained using water, within the measurement accuracy of the apparatus. Only at higher, but biologically less relevant, concentrations the contact angle was observed to decrease.
Fig. 3.
Spreading of a droplet of water (15 μl) on plates containing synthetic AHLs. Plates were supplied with (from left to right) OH,C8-HSL (100 μM), C10-HSL (100 μM), C12-HSL (50 μM), C14-HSL (20 μM), and 3OH-(slc)-HSL (×15). For photographing purposes, water was supplied with toluidine blue without interference. For 3OH-(slc)-HSL extraction see Materials and Methods. The control plates (acetonitrile or extract without 3OH-(slc)-HSL) never affected the water droplet positively or negatively. X, the concentration factor of 3OH-(slc)-HSL compared with the supernatant concentration of the producer strain. + corresponds to a positive effect.
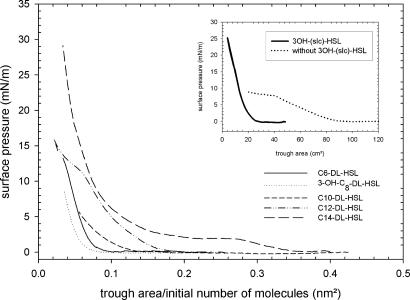
To demonstrate more clearly that AHLs are indeed biosurfactants at biologically relevant concentrations, the surface activity of the signaling molecules was determined by using a Langmuir trough and a Wilhelmy balance, which is a more sensitive method. Hereby, the AHLs are allowed to spread on a water surface. A strong increase of the surface pressure caused by compression is proof of a significant surface activity. Surface pressure-area isotherms of C10-HSL, C12-HSL, C14-HSL, OH,C8-HSL, C6-HSL, and 3OH-(slc)-HSL (concentrations see Materials and Methods) in Fig. 4 reveal a chain-length dependence of the surface activity. For C6-HSL, C10-HSL, and OH,C8-HSL, a monotonous increase of the surface pressure occurs only for very low values of trough area. This result indicates that these small-chain AHLs partially desorb from the interface at low values of trough area unlike biosurfactants. For the C12-HSL and C14-HSL molecules, the isotherms correspond to monolayers that are stable but undergo phase transitions. Similar behavior has been reported for many complex molecules such as, for example, fatty acids (20, 21). For 3OH-(slc)-HSL, secreted by the bacteria, the pressure-area isotherms are shown in Fig. 4 Inset. As the concentration of this molecule is not known, the data are reported as a function of the trough area (Fig. 4). The pressure-area isotherms of the long-chain AHLs are proof of significant surface activity. To better correlate these observations with other experiments, equilibrium surface pressure was determined when AHLs were injected in the subphase of the trough to give an overall concentration in the μM range (data not shown). Surface pressure increases with increasing bulk concentration and reaches a plateau above 4 μM. Along similar lines as the effect of long-chain AHLs on the swarming behavior of the cinR mutant (Figs. 1 and 2), the impact thereof on the spreading of a water droplet (Fig. 3), Fig. 4 clearly demonstrates significant surface activity of C10-HSL, C12-HSL, C14-HSL, and 3OH-(slc)-HSL, whereas the effect is weaker or even absent for C6-HSL and OH,C8-HSL.
Fig. 4.
Surface pressure-area isotherm for AHLs with hydrophobic chain lengths varying from C6 to C14. Subsequently, surface pressure-area isotherms were recorded (Langmuir trough with film balance and platinum Wilhelmy plate) by compressing the surface monolayer. (Inset) Shown are the isotherms for 3OH-(slc)-HSL. For 3OH-(slc)-HSL extraction see Materials and Methods. Control, extract from strain devoid of cin locus. Trough volume, 120 ml; synthetic AHLs, 200 μl, 0.5 mM; 3OH-(slc)-HSL, 120 μl, 75-fold concentrated compared with the supernatant concentration of the producer strain. The number of molecules initially spread per trough area is reported for synthetic AHLs.
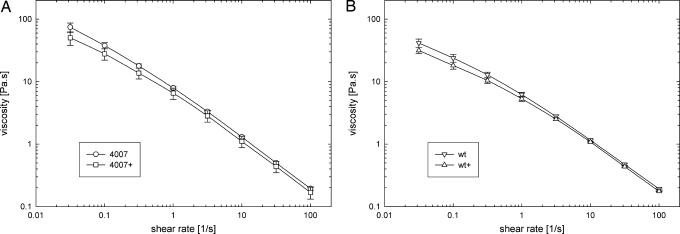
3OH-(slc)-HSL, Produced by CinI in R. etli, Decreases Viscosity of Swarmers' Slime.
The viscosity of the slime plays an important role in the motility of the bacteria and in controlling the swarming of the colony. The slime is a complex fluid, with a viscosity that will depend on the rate of deformation. The deformation rates involved in the process of swarming encompass several orders of magnitude. The movement of the bacteria through the slime is a rapid deformation: optical microscopy shows that the individual bacteria move at speeds of 10 μm to 1 mm/s (see Movie 1, which is published as supporting information on the PNAS web site), which given the film thickness leads to an estimate of the deformation rate of ≈10−2 to 10 s−1. In contrast, the velocity of the border of the swarming colony is much smaller, on the order of mm/hr near the edges of the film, and the relevant shear rate can be estimated to be of order 10−1 s−1. Therefore, the shear rate dependence of the slime and the effect of the AHLs on the flow curve was investigated over a wide range of shear rates. The flow curves, averaged over a number of sample loadings, of the WT and cinR mutant slime are shown in Fig. 5. Importantly, both for the WT and cinR, the viscosity of the slime decreases as the rate is increased, i.e., so-called shear thinning behavior is observed. The effect of endogenous CinI-made 3OH-(slc)-HSL on the cinR slime is shown in Fig. 5A. A small, but significant, decrease of the viscosity of the extracellular material containing excess 3OH-(slc)-HSL is observed for all shear rates tested (Fig. 5A). The low shear viscosity (left part of the curves in Fig. 5) is most sensitive to subtle changes in the interactions between the complex molecules contained within the slime when 3OH-(slc)-HSL is introduced. Increased production of CinI-made 3OH-(slc)-HSL reduces viscosity in the WT slime (Fig. 5B), similarly to what is observed for the cinR mutant. Yet, the effect of the AHLs on the overall viscosity curves is limited.
Fig. 5.
Viscosity as a function of shear rate for the extracellular slime produced during swarming by the cinR mutant (A) and WT R. etli (B). The deformation throughout the material was performed on a MCR-300 rheometer (Anton-Paar) equipped with a cone and plate geometry at 37°. The + corresponds to a constitutively induced, plasmid-borne cinI for studying the effect of endogenous 3OH-(slc)-HSL on the slime. The lines are drawn to guide the eye. Error bars are based on the standard deviation of at least three independent measurements on slime harvested from different swarm colonies.
Discussion
AHL molecules are widely known for their role as signal molecules in intercellular communication in bacterial populations. Here, we present evidence for a unique biological function of AHLs. Our data indicate that, in addition to a signaling role, AHLs carrying long acyl-side chains possess significant surface activity at biologically relevant concentrations. This property allows these molecules to function as biosurfactants. Other biological functions for oxo-AHLs have also been reported recently (22).
Traditionally, biosurfactants were categorized into one of the following classes: glycolipids, lipoproteins, phospholipids, fatty acid salts, and polymeric biosurfactants. The importance of biosurfactants includes the role of surfactin for the erection of Bacillus fruiting bodies (23) and serrawettin in Serratia swarming (10). Furthermore, roles for the LPS O-antigen in Proteus mirabilis, Salmonella enterica Serovar Typhimurium, and Serratia marcescens swarming (24–26) and for a capsular polysaccharide in enhancing medium surface fluidity during Pr. mirabilis population migration and in influencing cell–cell interactions was previously reported (27). Remarkable is the role of small hydrophobic proteins, SapB or the hydrophobin SC3, in the formation of Streptomyces or fungal aerial mycelia (28). QS-regulated biosurfactant production via AHLs with side chains from four to six carbons has been demonstrated in Serratia liquefaciens (10), Pseudomonas aeruginosa (29, 30), and possibly B. cepacia (17). Moreover, in these bacteria the link between the biosurfactant production and swarming has also been shown. Recently, it has been suggested that the precursor of rhamnolipids, 3-(3-hydroxyalkanoyloxy)alkanoic acid, is required for surface wettability and rhamnolipids for modulating Ps. aeruginosa swarming behavior (29, 31). Our results add a class of biosurfactant molecules to this list. The chain-length and concentration dependence of the surface activity of the AHLs was derived from the free energy per unit of surface by using a Langmuir trough (20, 21). For the long-chain AHLs, the pressure-area isotherms give clear indications of significant biosurfactant activity at biologically relevant concentrations.
The complex shape of the swarming colonies suggests that the role of surface activity in controlling swarming is caused by so-called Marangoni effects (32). Concentration gradients of biosurfactants may give rise to convection phenomena resulting from gradients in surface tension (32). This requirement for concentration gradients of AHLs explains the observed differences between in situ-produced 3OH-(slc)-HSL and exogenously supplied AHLs (compare Figs. 1 and 2). In spreading of thin surfactant films on a water film, these Marangoni stresses are responsible for the occurrence of a characteristic fingering pattern (33) that strongly resembles the edges of a swarm colony. It should be pointed out that other explanations for this fingering pattern based on an inhomogeneous distribution of nutrients have been proposed (34). This mechanism is contradicted by the observation that the swarm edges are smooth in the cinR colony. It should be pointed out that the experimentally measured decrease in macroscopic contact angle is too small for pure wetting properties to play an important role during swarming of an R. etli colony; moreover, this would lead to a homogeneous spreading of the extracellular material (35). Biosurfactant-induced reduction of the slime viscosity as observed in R. etli could also promote swarming. The effect of 3OH-(slc)-HSL on the flow curve is, however, limited.
To assess the plausibility of the Marangoni effect as the driving force for swarming, the dimensionless groups involved were estimated. Matar and Troian (33) presented an analysis of the spreading of a thin film with a stepwise concentration profile of surfactant at the air liquid interface and showed the characteristic spreading speed is given by U = εΠ/η, with ε the film thickness over diameter, Π the surface pressure, and η the viscosity of the film. With microscopy, the velocity of the swarming front was measured to be on the order of 0.5 μm/s. At the edge of the swarm the characteristic film thickness is comparable to the physical size of the bacteria (≈1 μm). At the shear rates obtained in the film, Fig. 5 allows for a reliable estimate of the viscosity of the slime of 10 Pa·s. Assuming a characteristic film diameter of 1 cm the required surface pressure can be estimated to be 50 mN/m, which is the same order of magnitude as excess pressures shown in Fig. 4 for the long-chain AHLs. The existence of a concentration gradient in biosurfactant is plausible when the bacteria cannot fully penetrate to the edge of the extracellular slime. At the edges, the film thickness is too small for the bacteria to reside because of their physical size. The experimentally observed spreading speed is hence consistent with the one expected for Marangoni flows for the surface pressures, thickness, and viscosities that have been observed. A second condition to be fulfilled is that local concentration gradients are sustainable. Gradients in biosurfactant concentration can exist if Marangoni effects dominate surface diffusion. In this end, the modified surface Péclet number Pes = Πh/ηD, with h the film thickness and D the surface diffusivity of the biosurfactant, must be sufficiently large (35). Typical values for D for small amphiphiles at the air water interface is 10−7 cm2/s (36), which combined with the values for film thickness, surface pressure, and viscosity, results in Pes > 106, indicating that concentration gradients can indeed exist. It should be pointed out that the analysis of Matar and Troian (33) is limited to Newtonian liquids, whereas in the present case the shear thinning profile of the viscosity (Fig. 5) of the matrix for R. etli swarming will amplify the Marangoni effect.
Our data indicate that 3OH-(slc)-HSL, produced by CinI, shows biosurfactant-like activity at biologically relevant concentrations and directly promotes surface migration in R. etli independently from specific target genes. A high population density likely needs to be reached to obtain a sufficient concentration of AHL biosurfactant in the extracellular medium, linking this system to other QS-regulated phenomena. Considering these findings, it might be appropriate to re-evaluate the function of AHLs with long acyl-side chains with respect to a direct role affecting the surface tension of liquids and presence of Marangoni effects.
Materials and Methods
Bacterial Strains and Culture Conditions.
R. etli strains include WT CNPAF512 (37), a cinI mutant (FAJ4006) (19), a cinR mutant (FAJ4007) (19), and a raiI mutant (FAJ1328) (18). A cinRraiR mutant (CMPG8299) was constructed. For this, the Km cassette (pHP45Ω) was inserted into the NdeI site of pFAJ1327 (18) containing raiR after blunting of the fragments, yielding plasmid pCMPG8299. This sacB suicide vector was used to introduce the raiR mutant allele into FAJ4007. The reporter fusions used are cinI-gusA (pFAJ4014) (19) and raiI-gusA (pFAJ1458) (E. Luyten, personal communication). A plasmid-borne PnptII–cinI fusion was constructed in the vector pFAJ1709 (38). For this, the 1.4-kbp HindIII–BamHI fragment of pFAJ4004 containing cinI (19) was cloned into the HpaI site of pFAJ1709 after blunting of the fragments, yielding plasmid pCMPG8798. Plasmids were introduced into CNPAF512 by conjugation (19). For heterologous expression in Agrobacterium tumefaciens, R. etli cinIR was cloned into a pPZP200 derivative (pFAJ4013) (19).
Rhizobium was grown at 30°C in trypton yeast extract (19) or yeast extract mannitol medium (39). A. tumefaciens NT1 was grown in agrobacterium medium at 28°C (19). When appropriate, the media were supplemented with 30 μg/ml nalidixic acid, 60 μg/ml kanamycin or neomycin, and 1 μg/ml tetracycline for R. etli and with 300 μg/ml streptomycin and 100 μg/ml spectinomycin for A. tumefaciens.
Motility Assays.
For swarming, yeast extract mannitol (containing 0.4 g/liter MgSO4·7H2O) soft agar plates (0.75%; 60/15 mm, Greiner Bio-One, Kremsmünster, Austria) were dried for 5 h (16°C) and spot-inoculated centrally on the surface with the appropriate R. etli WT or mutant strain (1.2 μl of overnight culture brought at OD595 0.7). The plates were incubated for 3 days at 30°C. Restoration of the swarming behavior was analyzed on swarmer plates containing either synthetic AHLs (10–100 μM) or a filter-sterilized aqueous extract of a swarming colony (25-fold diluted). The latter extract was prepared as follows: a swarm colony (containing the cells and extracellular slime from one swarm plate) was scraped off and collected in 1 ml of MilliQ water and subsequently centrifuged (10 min, 3,300 × g). To correlate AHL production on the plate with the number of bacteria present, cells and extracellular slime from one plate was scraped off, the weight was determined and adjusted to 1 ml with 10 mM MgSO4, and cfus were determined. From the weight of the collected slime, the volume was calculated (mean weight 0.1085 g/0.1 ml slime). A total of 10-fold more bacteria were detected on a swarm plate (2.56 1010 cfu/ml; n = 10) compared with a stationary phase R. etli culture (2.33 109 cfu/ml; n = 5). cinI-gusA induction was used as a tool to quantify 3OH-(slc)-HSL production in the same extract from WT swarmers (1,174 MU; SD 459; n = 10; 75-fold diluted extract) and planktonic bacteria (1,406 MU; SD 374; n = 5; 5-fold diluted). Macroscopic observation of swarming was done with a digital camera (Sony, Tokyo, Japan). Light microscopic photographs were taken with a Leica (Deerfield, IL) microscope (MZ FLIII) equipped with a SPOT RT Slider camera (Diagnostic Instruments, Sterling Heights, MI) (ImagePro software).
Synthetic AHLs.
C6-DL-HSL, C10-DL-HSL, C12-DL-HSL, and C14-DL-HSL were from Sigma-Aldrich (St. Louis, MO). 3OH,C8-DL-HSL was synthesized as reported (40, 41) except that the hydroxylated molecule was extracted with dichloromethane/water (1:1). Stocks (10 and 50 mM) were in acetonitrile.
Extraction and Detection of QS Signal Molecules.
Dichloromethane (containing 1.5 ml of HAc/liter) extract from 1 liter of a stationary-phase culture of R. etli (in AMS mannitol) or Agrobacterium transformants [production of 3OH-(slc)-HSL] in agrobacterium medium at 30°C was prepared and the AHLs were detected, essentially as described (19).
Expression Analysis.
For expression tests in planktonic cells, an overnight culture of R. etli was diluted 40-fold in microtiter dishes sealed with a breathable membrane (Greiner Bio-One) and incubated for 40 h in the presence of either synthetic (0.5 nM-0.5 mM) or purified AHLs, or a filter-sterilized aqueous extract of a swarming colony. Alternatively, a swarm colony (containing ≈0.1–0.2 ml of cells and extracellular slime from one swarm plate) was scraped off and collected in 1 ml of 10 mM MgSO4, after which expression was analyzed. The swarmer colony was grown on agar exogenously supplied with various concentrations of AHLs (0–100 μM). Quantitative analysis of GusA activity was carried out in microtiter plates with p-nitrophenyl-β-d-glucuronide as the substrate by the method of Miller (42). GusA activity was examined in a VERSAmax microplate reader (Molecular Devices, Sunnyvale, CA) (37).
Surface-Activity Assay.
The surface pressure is measured by using a Langmuir trough equipped with a film balance and a Platinum Wilhelmy plate (KSV Instruments, Helsinki, Finland) (20). Typically, the surface pressure is reported, which is defined as Π0 = γ0 − γ, where γ is the surface tension of the surface covered with surface-active components and γ0 is the surface tension of the surfactant-free interface. In the experiments, the trough was filled with water and the AHL to be tested was injected onto the air-water interface [200 μl of 0.5 mM AHL or 120 μl of 75-fold concentrated 3OH-(slc)-HSL, compared with the supernatant concentration of the producer strain; trough volume 120 ml]. Subsequently and during compression, adsorption equilibrium between bulk and surface sets in. Subsequently, surface pressure-area isotherms were recorded by compressing and expanding the surface monolayer. To compare the isotherms, the number of molecules initially spread per trough area was reported. In adsorption equilibrium experiments the AHLs were injected in the subphase of the trough to give an overall concentration of 0–6 μM, and for each concentration the equilibrium surface pressure was measured.
Surface activity was also investigated by means of contact angle measurements. For this, water droplets with AHLs [0.5 nM-100 μM C14-HSL or 250 μM C12-HSL; 1- to 10-fold concentrated 3OH-(slc)-HSL compared with the supernatant concentration of the producer strain] were deposited on hydrophobized glass slides. The slides were rendered hydrophobic by using a solution of dimethyldichlorosilane in heptane (Fluka, Buchs, Switzerland). By controlling the reaction time with the silanization solution, substrates with different water contact angles could be realized. Contact angles were measured with a KSV CAM 200 (KSV Instruments) by recording the droplet shape and fitting this to the Young-Laplace equation (35).
Rheological Properties of the Extracellular Slime.
The swarmer cells together with the extracellular slime were scraped off with the backside of a spoon and collected from the colony borders of three swarm plates after which viscosity was determined as a function of the shear rate. To avoid taking material from the center of the swarm plate, the central region (diameter 1 cm) was carefully removed with a plastic cylinder before isolation was started. The rheological measurements on the extracellular slime were performed on a MCR-300 rheometer (Anton-Paar, Graz, Austria) equipped with a cone and plate geometry to ensure a homogeneous deformation throughout the material. Temperature was controlled to 37.0°C with the help of a Peltier element attached to the lower plate, and the system was covered with a Peltier hood. The atmosphere inside the hood was kept in constant contact with a saturated KNO3 solution in water to create constant humidity conditions. In such a way evaporation of water from the free surface of the extracellular slime between the cone and plate could be minimized.
Supplementary Material
Acknowledgments
We thank J. Desair and D. Bachaspatimayum for technical assistance. This work was supported by Geconcerteerde Onderzoeksacties Grant GOA/2003/09, Postdoctoraal Mandaat Onderzoeksfonds Katholieke Universiteit Leuven Grant PDM/04/182, and Fonds Wetenschappelijk Onderzoek–Vlaanderen Research Project G.0287.04.
Abbreviations
- QS
quorum sensing
- AHL
acylhomoserine lactone
- HSL
homoserine lactone
- slc
saturated long chain
- MU
Miller units.
Footnotes
The authors declare no conflict of interest.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Parsek MR, Greenberg EP. Trends Microbiol. 2005;13:27–33. doi: 10.1016/j.tim.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 2.Daniels R, Vanderleyden J, Michiels J. FEMS Microbiol Rev. 2004;28:261–289. doi: 10.1016/j.femsre.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 3.Whitehead NA, Barnard AM, Slater H, Simpson NJ, Salmond GP. FEMS Microbiol Rev. 2001;25:365–404. doi: 10.1111/j.1574-6976.2001.tb00583.x. [DOI] [PubMed] [Google Scholar]
- 4.Fuqua C, Greenberg EP. Nat Rev Mol Cell Biol. 2002;3:685–695. doi: 10.1038/nrm907. [DOI] [PubMed] [Google Scholar]
- 5.Henke JM, Bassler BL. Trends Cell Biol. 2004;14:648–656. doi: 10.1016/j.tcb.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 6.Parsek MR, Greenberg EP. Proc Natl Acad Sci USA. 2000;97:8789–8793. doi: 10.1073/pnas.97.16.8789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mathesius U, Mulders S, Gao M, Teplitski M, Caetano-Anolles G, Rolfe BG, Bauer WD. Proc Natl Acad Sci USA. 2003;100:1444–1449. doi: 10.1073/pnas.262672599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Newton JA, Fray RG. Cell Microbiol. 2004;6:213–224. doi: 10.1111/j.1462-5822.2004.00362.x. [DOI] [PubMed] [Google Scholar]
- 9.Harshey RM. Annu Rev Microbiol. 2003;57:249–273. doi: 10.1146/annurev.micro.57.030502.091014. [DOI] [PubMed] [Google Scholar]
- 10.Eberl L, Molin S, Givskov M. J Bacteriol. 1999;181:1703–1712. doi: 10.1128/jb.181.6.1703-1712.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harshey RM. Mol Microbiol. 1994;13:389–394. doi: 10.1111/j.1365-2958.1994.tb00433.x. [DOI] [PubMed] [Google Scholar]
- 12.Fraser GM, Hughes C. Curr Opin Microbiol. 1999;2:630–635. doi: 10.1016/s1369-5274(99)00033-8. [DOI] [PubMed] [Google Scholar]
- 13.Senesi S, Celandroni F, Salvetti S, Beecher DJ, Wong AC, Ghelardi E. Microbiology. 2002;148:1785–1794. doi: 10.1099/00221287-148-6-1785. [DOI] [PubMed] [Google Scholar]
- 14.Soto MJ, Fernandez-Pascual M, Sanjuan J, Olivares J. Mol Microbiol. 2002;43:371–382. doi: 10.1046/j.1365-2958.2002.02749.x. [DOI] [PubMed] [Google Scholar]
- 15.Ghelardi E, Celandroni F, Salvetti S, Beecher DJ, Gominet M, Lereclus D, Wong AC, Senesi S. J Bacteriol. 2002;184:6424–6433. doi: 10.1128/JB.184.23.6424-6433.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ron EZ, Rosenberg E. Environ Microbiol. 2001;3:229–236. doi: 10.1046/j.1462-2920.2001.00190.x. [DOI] [PubMed] [Google Scholar]
- 17.Huber B, Riedel K, Hentzer M, Heydorn A, Gotschlich A, Givskov M, Molin S, Eberl L. Microbiology. 2001;147:2517–2528. doi: 10.1099/00221287-147-9-2517. [DOI] [PubMed] [Google Scholar]
- 18.Rosemeyer V, Michiels J, Verreth C, Vanderleyden J. J Bacteriol. 1998;180:815–821. doi: 10.1128/jb.180.4.815-821.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daniels R, De Vos DE, Desair J, Raedschelders G, Luyten E, Rosemeyer V, Verreth C, Schoeters E, Vanderleyden J, Michiels J. J Biol Chem. 2002;277:462–468. doi: 10.1074/jbc.M106655200. [DOI] [PubMed] [Google Scholar]
- 20.Kaganer VM, Mohwald H, Dutta P. Rev Mod Phys. 1999;71:779–819. [Google Scholar]
- 21.McConnell HM. Annu Rev Phys Chem. 1991;42:171–195. [Google Scholar]
- 22.Kaufmann GF, Sartorio R, Lee SH, Rogers CJ, Meijler MM, Moss JA, Clapham B, Brogan AP, Dickerson TJ, Janda KD. Proc Natl Acad Sci USA. 2005;102:309–314. doi: 10.1073/pnas.0408639102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Branda SS, Gonzalez-Pastor JE, Dervyn E, Ehrlich SD, Losick R, Kolter R. J Bacteriol. 2004;186:3970–3979. doi: 10.1128/JB.186.12.3970-3979.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Belas MR, Goldman M, Ashliman K. J Bacteriol. 1995;177:823–828. doi: 10.1128/jb.177.3.823-828.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Toguchi A, Siano M, Burkart M, Harshey RM. J Bacteriol. 2000;182:6308–6321. doi: 10.1128/jb.182.22.6308-6321.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Izquierdo L, Abitiu N, Coderch N, Hita B, Merino S, Gavin R, Tomas JM, Regue M. Microbiology. 2002;148:3485–3496. doi: 10.1099/00221287-148-11-3485. [DOI] [PubMed] [Google Scholar]
- 27.Rahman MM, Guard-Petter J, Asokan K, Hughes C, Carlson RW. J Biol Chem. 1999;274:22993–22998. doi: 10.1074/jbc.274.33.22993. [DOI] [PubMed] [Google Scholar]
- 28.Tillotson RD, Wosten HA, Richter M, Willey JM. Mol Microbiol. 1998;30:595–602. doi: 10.1046/j.1365-2958.1998.01093.x. [DOI] [PubMed] [Google Scholar]
- 29.Deziel E, Lepine F, Milot S, Villemur R. Microbiology. 2003;149:2005–2013. doi: 10.1099/mic.0.26154-0. [DOI] [PubMed] [Google Scholar]
- 30.Kohler T, Curty LK, Barja F, Van Delden C, Pechere JC. J Bacteriol. 2000;182:5990–5996. doi: 10.1128/jb.182.21.5990-5996.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caiazza NC, Shanks RM, O'Toole GA. J Bacteriol. 2005;187:7351–7361. doi: 10.1128/JB.187.21.7351-7361.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Edwards DA, Brenner H, Wasan DT. Interfacial Transport Processes and Rheology. Boston: Butterworth–Heinemann; 1991. [Google Scholar]
- 33.Matar OK, Troian SM. Phys Fluids. 1999;11:3232–3246. [Google Scholar]
- 34.Zorzano MP, Hochberg D, Cuevas MT, Gomez-Gomez JM. Phys Rev E Stat Nonlin Soft Matter Phys. 2005;71:031908. doi: 10.1103/PhysRevE.71.031908. [DOI] [PubMed] [Google Scholar]
- 35.Adamson AW, Gast A. Physical Chemistry of Surfaces. New York: Wiley; 1997. [Google Scholar]
- 36.Kang YS, Majda M. J Phys Chem B. 2000;104:2082–2089. [Google Scholar]
- 37.Michiels J, Van Soom T, D'hooghe I, Dombrecht B, Benhassine T, de Wilde P, Vanderleyden J. J Bacteriol. 1998;180:1729–1740. doi: 10.1128/jb.180.7.1729-1740.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dombrecht B, Vanderleyden J, Michiels J. Mol Plant–Microbe Interact. 2001;14:426–430. doi: 10.1094/MPMI.2001.14.3.426. [DOI] [PubMed] [Google Scholar]
- 39.Vincent JM. A Manual for the Practical Study of the Root-Nodule Bacteria. Oxford: Blackwell; 1970. [Google Scholar]
- 40.Chhabra SR, Stead P, Bainton NJ, Salmond GP, Stewart GS, Williams P, Bycroft BW. J Antibiot (Tokyo) 1993;46:441–454. doi: 10.7164/antibiotics.46.441. [DOI] [PubMed] [Google Scholar]
- 41.Chhabra SR, Harty C, Hooi DS, Daykin M, Williams P, Telford G, Pritchard DI, Bycroft BW. J Med Chem. 2003;46:97–104. doi: 10.1021/jm020909n. [DOI] [PubMed] [Google Scholar]
- 42.Miller YH. Experiments in Molecular Genetics. Plainview, NY: Cold Spring Harbor Lab Press; 1972. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.