Abstract
In the absence of added thiamine, Rhizobium leguminosarum bv. viciae strain 3841 does not grow in liquid medium and forms only “pin” colonies on agar plates, which contrasts with the good growth of Sinorhizobium meliloti 1021, Mesorhizobium loti 303099, and Rhizobium etli CFN42. These last three organisms have thiCOGE genes, which are essential for de novo thiamine synthesis. While R. leguminosarum bv. viciae 3841 lacks thiCOGE, it does have thiMED. Mutation of thiM prevented formation of pin colonies on agar plates lacking added thiamine, suggesting thiamine intermediates are normally present. The putative functions of ThiM, ThiE, and ThiD are 4-methyl-5-(β-hydroxyethyl) thiazole (THZ) kinase, thiamine phosphate pyrophosphorylase, and 4-amino-5-hydroxymethyl-2-methyl pyrimidine (HMP) kinase, respectively. This suggests that a salvage pathway operates in R. leguminosarum, and addition of HMP and THZ enabled growth at the same rate as that enabled by thiamine in strain 3841 but elicited no growth in the thiM mutant (RU2459). There is a putative thi box sequence immediately upstream of the thiM, and a gfp-mut3.1 fusion to it revealed the presence of a promoter that is strongly repressed by thiamine. Using fluorescent microscopy and quantitative reverse transcription-PCR, it was shown that thiM is expressed in the rhizosphere of vetch and pea plants, indicating limitation for thiamine. Pea plants infected by RU2459 were not impaired in nodulation or nitrogen fixation. However, colonization of the pea rhizosphere by the thiM mutant was impaired relative to that of the wild type. Overall, the results show that a thiamine salvage pathway operates to enable growth of Rhizobium leguminosarum in the rhizosphere, allowing its survival when thiamine is limiting.
Rhizobia form a species-specific symbiotic relationship with leguminous plants in which atmospheric N2 is reduced to NH3. To establish this symbiosis, bacteria must survive in the soil environment, competing with many organisms for nutrients. The availability of vitamins such as biotin, thiamine, and riboflavin in the rhizosphere limits the growth of Sinorhizobium meliloti (48). Auxotrophy for vitamins has also been described as the cause of ineffective nodule formation by some rhizobial strains, but effective module formation can be restored by adding the vitamins externally (46). Related to this, Mesorhizobium loti strains found in soil are often auxotrophic for the vitamins biotin, thiamine, and nicotinate and are converted to prototrophy by the acquisition of a symbiotic island (51).
Several metabolic processes, such as the synthesis of polyhydroxybutyrate and the excretion of amino acids and organic acids, are strongly affected by the absence of the vitamins biotin and thiamine (14). Soluble vitamins such as niacin, thiamine, riboflavin, pantothenic acid, and biotin are liberated from legume roots and are also produced at biologically active levels by many bacteria and fungi isolated from rhizosphere soil or the plant root surface (50). The production of these water-soluble vitamins by rhizospheric microorganisms such as Pseudomonas and Azospirillum spp. has been found to be related to the ability of these bacteria to enhance nitrogen fixation and the growth of legumes nodulated by Rhizobium (11, 42). External addition of biotin greatly enhances the bacterial growth and colonization of alfalfa roots by Sinorhizobium meliloti (15, 49). Overall, this suggests that vitamin supply may limit the growth of rhizobia in the rhizosphere and soil.
Thiamine (vitamin B1) is an essential cofactor required for carbohydrate and branched-chain amino acid metabolism. It is derived from thiamine monophosphate (TMP), the synthesis of which involves a complex multistep pathway (5). In the case of R. etli, the thiCOGE genes, present on the plasmid pRetCFN42b, have been shown to be essential for the de novo synthesis of thiamine (31). Several bacteria, including Escherichia coli and Bacillus subtilis, have a set of salvage kinases in addition to this method of de novo synthesis. These utilize dephosphorylated intermediates of the thiamine biosynthetic pathway present in the environment for TMP synthesis (30) (Fig. 1). The formation of TMP by one of these salvage pathways involves the condensation of two intermediates: 4-methyl-5-(β-hydroxyethyl) thiazole monophosphate (THZ-P) and 4-amino-5-hydroxymethyl-2-methyl pyrimidine pyrophosphate (HMP-PP) (34, 56). This condensation step is mediated by the ThiE protein (thiamine phosphate synthase). HMP-PP is derived from 4-amino-5-hydroxymethyl-2-methyl pyrimidine (HMP) by phosphorylation by the bifunctional HMP kinase/HMP monophosphate kinase, ThiD (43). THZ-P is derived from 4-methyl-5-(β-hydroxyethyl) thiazole (THZ) kinase by phosphorylation by the 4-methyl-5-(β-hydroxyethyl) thiazole kinase, ThiM (43).
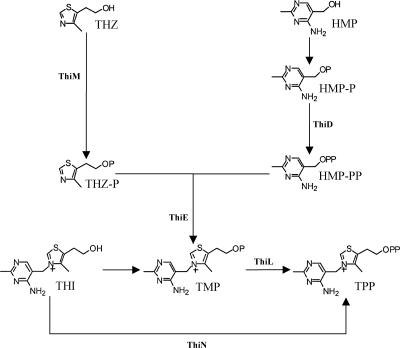
FIG. 1.
Proposed thiamine salvage pathway in R. leguminosarum bv. viciae 3841. THZ and HMP are intermediates in a salvage pathway. The conversion of thiamine (THI) to TPP may be catalyzed by ThiN (RL4610), which has 25% amino acid identity and 41% similarity to ThiN from Bacillus subtilis. The step from TMP to TPP is catalyzed by ThiL in E. coli but is undefined in R. leguminosarum. HMP-P, HMP monophosphate.
We report here that the genome of Rhizobium leguminosarum genome lacks thiCOGE but has thiMED genes on plasmid pRL11JI (pRL110441-110443) (55). ThiMED catalyze a salvage pathway that operates under thiamine limitation in the rhizosphere. Some rhizobia exclusively use the de novo ThiCOGE pathway; others, such as strain 3841, use only the ThiMED salvage pathway, while R. etli strain CFN42 uses both.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
The bacterial strains and plasmids used in this study are detailed in Table 1. Rhizobium strains were grown at 28°C on either tryptone-yeast extract (TY) (6), standard acid minimal salts medium (AMS), or acid minimal salts agar (37) with 10 mM d-glucose and 10 mM ammonium chloride. The only exception was S. meliloti, for which the EDTA level in AMS was reduced to 1 μM. Antibiotics were used at the following concentrations (μg ml−1): streptomycin, 500; kanamycin, 20; tetracycline (Tet), 2 (in AMS) and 5 (in TY); gentamicin (Gm), 20; nyastatin, 50; and spectinomycin (Sp), 100. The concentrations of thiamine and its intermediate HMP (kindly provided by T. P. Begley, Cornell University, Ithaca NY) and 4-methyl-5-(β-hydroxyethyl) thiazole (Sigma-Aldrich) were 1 μg ml−1.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Description | Source or reference |
|---|---|---|
| Strains | ||
| E. coli TOP10 | F−mcrA Δ(mrr-hsdRMS-mcrBC)φ80dlacZΔM15 ΔlacX74 recA1 ara Δ139Δ(ara-leu)7697 galU galK rpsL (Strr) endA1 nupG | Invitrogen |
| E. coli DH5α | F− φ80dlacZΔM15 Δ(lacZYA-argF) U169 recA1 endA1 hsdR17(rK− mK+) phoA supE44 thi-1 gyrA96 relA1 | Invitrogen |
| R. leguminosarum bv. viciae | ||
| R. leguminosarum 3841 | Str derivative of R. leguminosarum bv. viciae strain 300 | 19 |
| R. leguminosarum VF39 | R. leguminosarum bv. viciae wild type, Smr | 39 |
| R. leguminosarum JI336 | R. leguminosarum bv. viciae | 13 |
| R. leguminosarum 248 | R. leguminosarum bv. viciae | 20 |
| R. leguminosarum RBL1309 | R. leguminosarum bv. viciae | 53 |
| R. leguminosarum WU235 | R. leguminosarum bv. viciae | 36 |
| R. leguminosarum strain 3855 | R. leguminosarum bv. viciae | 7 |
| R. leguminosarum 128C53 | R. leguminosarum bv. viciae | 44 |
| R. leguminosarum A34 | R. leguminosarum bv. viciae (formerly known as 8401/pRL1JI) | 12 |
| R. leguminosarum bv. trifolii | ||
| R. leguminosarum bv. W14-2 | R. leguminosarum bv. trifolii wild type, Smr | 4 |
| R. leguminosarum bv. RCR5 | R. leguminosarum bv. trifolii wild type | 18 |
| Rhizobium sp. strain NGR234 | Rifr derivative of NGR234 | 47 |
| M. loti MAFF303099 | Wild type | 21 |
| R. tropici CIAT899 | Wild-type R. tropici | 29 |
| S. meliloti 1021 | Wild type | 26 |
| S. meliloti RP254 | Bean isolate from Morocco | Mouhsine et al., unpublished |
| R.. etli CFN42 | Wild type contains 6 plasmids (p42a-p42f) | 41 |
| R.. etli CE3 | Str derivative of CFN42 | 33 |
| R. etli CFNX183 | R. etli CFN42 cured of pRetCFN42b | 8 |
| R. leguminosarum RU1302 | 3841 pRU504; Smr Gmr | 3 |
| R. leguminosarum RU2292 | 3841 pRU1515; Smr Gmr | This work |
| R. leguminosarum RU2295 | 3841 pRU1619 pthiM::egfp; Smr Gmr | This work |
| R. leguminosarum RU2459 | 3841 thiM::Ω; Smr Spr | This work |
| Plasmids | ||
| pOT2 | Promoter probe vector with promoterless gfp-UV; Gmr | 3 |
| pCR2.1TOPO | PCR product cloning vector; Apr Kmr | Invitrogen |
| pCR8/GW/TOPO | PCR product cloning vector; GW1 priming site, attL1 and attL2 sites for Gateway cloning; Spr | Invitrogen |
| pRU1097-D-TOPO | PCR product cloning vector; gfp-mut3.1 reporter gene; Gmr | This work |
| pGW1 | attR1 and attR2 entry vector for gateway cloning (pJQ200SK); Gmr | This work |
| pJQ200SK | pACYC derivative, P15A origin of replication; Gmr | 40 |
| pRK2013 | ColEI replicon with RK2 tra genes, helper plasmid used for mobilizing plasmids; Kmr | 16 |
| pHP45Ω | pBR322 derivative carrying ΩpHP45 replicon; Apr Spr | 38 |
| pHP45Ω | pBR322 derivative carrying ΩpHP45 replicon; Apr Tetr | 38 |
| pRK415-1 | Broad-host-range P-group cloning vector; Tetr | 23 |
| pRU504 | thiMED operon in pOT1identified by optical trapping | 3 |
| pRU1515 | Self-ligated pRU1097-D-TOPO; Gmr | This work |
| pRU1619 | p639/p640 PCR product in pRU1097-D-TOPO; Gmr | This work |
| pRU1734 | p639/p641 PCR product in pCR8/GW/TOPO; Spr | This work |
| pRU1738 | p753/p754 PCR product (thiME) in pCR2.1TOPO; Kmr | This work |
| pRU1752 | p754/p755 PCR product (thiMED) in pCR2.1TOPO; Kmr | This work |
| pRU1755 | pRU1734 with ligated ΩTet cassette into thiM gene via FspI digestion to generate thiM mutant; Spr Tetr | This work |
| pRU1774 | pGW1 carrying thiM gene with inserted Tet cassette; Gmr Tetr | This work |
| pRU1776 | pRU1774 with ΩTet cassette replaced with ΩSp cassette; Gmr Spr | This work |
| pRU1781 | thiME gene was cloned from pRU1738 as HindIII/XbaI into pRK415; Tetr | This work |
| pRU1782 | thiMED gene was cloned from pRU1752 as HindIII/XbaI into pRK415; Tetr | This work |
Genetic modification of bacterial strains.
All DNA cloning and analysis were performed as previously described (45). The DNA fragment containing the thiM promoter was PCR amplified using primers p639 and p640. PCRs were conducted in 50 μl, using 2.5 units of Pfu Turbo (Stratagene), 10 to 30 ng of genomic DNA, 1× PCR buffer (Stratagene), 0.2 mM deoxynucleoside triphosphates, and 1 μM primers. The cycling conditions were as follows: 1 cycle of 95°C for 2 min; 30 cycles of 95°C for 45 s, 57°C for 45 s, and 72°C for 2 min; and a final extension of 72°C for 10 min. The PCR product was cloned into the broad-host-range vector pRU1097 D-TOPO, resulting in plasmid pRU1619. Plasmid pRU1097 was custom adapted with topoisomerase by Invitrogen and was used according to their standard protocols for TOPO-adapted vectors.
Primers used in this study.
Primers used in this study were as follows: p639, CACCAACGACGAGTTCGGGGCGAT; p640, GAACCTTCCGCCTGTTCGAC; p641, AAGCACGTCAGGGCTCTCGT; p753, TCTAGAATGTCGCGGTGGTCAGTCAGCGC; p754, AAGCTTTTCGCCAAAGAGATCAAGCCGGG; p755, TCTAGAAGGTCTGGCACCTGTCACTCCTCC; p789, GTTCTCTTCGACATCGCGGACGGC; p790, GACCTTGAGGTTGATGCCGAGAAG; p827, CGCCTGCATGCCGTCGATCC; and p828, CGCCGCAAATGTCCTGCTCG.
Construction of a thiM mutant in strain 3841.
Primers p639 and p641 were used to PCR amplify the thiM region from R. leguminosarum 3841 genomic DNA, and the 2.4-kb PCR product was cloned into the vector pCR8-GW-TOPO (Invitrogen), producing plasmid pRU1734. An ΩTet cassette from pHP45 (38) was cloned into the FspI site of thiM in pRU1734 to produce pRU1755. The insert in pRU1755 containing the Ωtet cassette in thiM was Gateway cloned using Gateway LR clonase enzyme mix (Invitrogen) into pGW1, resulting in plasmid pRU1774. Plasmid pGW1 was made by inserting the Gateway cassette reading frame A into the SmaI site of pJQ200SK. To enable compatibility with subsequent plasmids used in complementation experiments, the ΩTet cassette in pRU1774 was replaced by an ΩSp cassette by SmaI digestion and religation to produce plasmid pRU1776. Plasmid pRU1776 was conjugated into strain 3841, and a thiM mutant was isolated by selecting for recombination using the sac mutagenesis strategy as previously described (24).
Primers p753/p754 and p754/p755 were used to amplify the thiME and thiMED genes from strain 3841. The PCR products were cloned into pCR2.1TOPO (pRU1738 and pRU1752, respectively), digested with HindIII/XbaI, and then cloned into pRK415-1, resulting in plasmids pRU1783 and pRU1784, respectively. All plasmids were conjugated into rhizobial strains by using pRK2013 as a helper plasmid to provide the transfer genes as previously described (35).
Measurement of reporter fusion activity.
Green fluorescent protein fluorescence was measured using a Tecan GENios fluorometer equipped with an excitation filter (485 nm) and an emission filter (510 nm). Cells of strain 3841 containing the plasmids pRU1515 (self-ligated vector) and pRU1619 (pthiM::egfp) were grown overnight in AMS supplemented with 10 mM glucose and 10 mM ammonium chloride and thiamine (1 μg/ml). The cells were harvested and washed in AMS three times to eliminate most entrained thiamine. Cells were then reinoculated into AMS (glucose, 10 mM; ammonium, 10 mM) with the following additions: none (control); thiamine; HMP; THZ; HMP and THZ; and HMP, THZ, and thiamine. Cells were taken at different time points, and the specific fluorescence was measured by dividing the fluorescence of the sample by the optical density at 590 nm of the culture.
Microscopy.
Plasmid pRU504 carrying the thiMED operon in pOT2 was previously isolated by optical trapping (3). Strain RU1302 (3841 pRU504) was used to study the expression of thiM in the rhizosphere. Microscopy was performed with a Carl Zeiss Axioskop 2.0 epifluorescence microscope with appropriate fluorescence sets. Images were captured using an Axiocam digital camera (22).
Plant growth and inoculation.
Vetch (Vicia sativa) seeds were surface sterilized in 95% ethanol for 30 seconds and then immersed in a solution of 2% sodium hypochlorite for 10 min. The seeds were washed extensively with sterile water and then allowed to germinate on sterile filter paper for 3 days in the dark. Plants were then placed on microscope slides overlaid with 0.75% agarose containing nitrogen-free rooting solution (35). Strain RU1302 was inoculated into the agarose at 107 CFU per plant, while control plants were inoculated with strain 3841 containing the vector (pOT2). Coverslips were placed over the agarose, and the microscope slides were inserted into 50-ml Falcon tubes with a few ml of nitrogen-free rooting solution at the bottom. The Falcon tubes were placed in a growth chamber (23°C, 16-h/8-h light/dark period). At 3 to 7 days postinoculation, the plant roots were observed for bacterial gfp expression.
Quantitative RT-PCR (Q-RT-PCR) of thiM.
For measurement of thiM expression in free-living R. leguminosarum 3841, bacteria were grown in 50 ml of AMS with glucose, ammonium, and thiamine (35) prior to being transferred into thiamine-free medium. For measurement of thiM expression in the rhizosphere, 7-day-old pea (Pisum sativum) seedlings grown in 25 ml of vermiculite in 50-ml sterile Falcon tubes were inoculated with 108 CFU of strain 3841 and grown at 23°C with a 16-h/8-h light/dark period. After 7 days of growth, the bacteria were harvested by adding sterile water (6 ml) plus RNAprotect (12 ml) to the roots, and this was mixed by vortexing for 30 s. The supernatant was filtered through four layers of sterile muslin cloth and spun down at 1,000 rpm in a Microfuge for 1 min at 4°C. The supernatant was further spun down at 8,000 rpm for 5 min to collect the bacteria. To isolate RNA, cells were resuspended in RNAprotect (RNA stabilization reagent) as described by the manufacturer (QIAGEN). RNA was isolated with an RNeasy Mini kit (QIAGEN), and contaminating DNA was removed by on-column treatment with RNase-free DNase (QIAGEN). RNA concentrations were determined with an Experion microfluidic RNA analyzer (Bio-Rad Laboratories). Reverse transcription-PCR (RT-PCR) was performed with a OneStep RT-PCR kit (QIAGEN) as recommended by the manufacturer, with 300 ng of the appropriate RNA sample and with mdh (malate dehydrogenase) serving as a reference gene. The data were analyzed by the relative quantification method (comparative cycle threshold method) to calculate the expression (n-fold) (9, 10).
Acetylene reduction and dry weight determination.
Acetylene reduction was determined for plants incubated in 95% air and 5% acetylene for 1 h in 250-ml Schott bottles (2). For determination of plant dry weight, the shoot was removed from the root and dried in at 70°C in a dry-heat incubator for 3 days before being weighed.
Plant assays.
For nodulation competition experiments, pea plants were grown in sterile vermiculite (250-ml flasks) and watered with sterile nitrogen-free rooting solution as described previously (35). An inoculum of 106 CFU, confirmed by plate count, was applied to each plant. Plants were harvested 4 weeks postinoculation. To determine nodule occupancy, 120 nodules from 12 plants were surface sterilized as previously described (37), crushed, and plated on TY medium and then on TY medium containing either streptomycin by itself or streptomycin plus spectinomycin.
To determine rhizosphere colonization levels, bacteria were inoculated onto pea seedlings as described above for Q-RT-PCR. Strains 3841 and RU2459 were inoculated at the following CFU ratios: 1,000:0, 0:1,000, 1,000:1,000, 10,000:1,000, and 1,000:10,000. After 7 days of growth, sterile phosphate-buffered saline (20 ml) was added to roots in the vermiculite and vortexed for 30 s. Bacteria were serially diluted and plate counted on TY medium containing either streptomycin and nyastatin or streptomycin, nyastatin, and spectinomycin, giving the total number of viable rhizosphere- and root-associated bacteria.
Bioinformatic analysis of the thi box.
The presence of thi box riboswitches and RNA secondary structure in the thiM gene was analyzed using the RibEx website (1).
RESULTS
Growth of rhizobia on minimal medium with and without thiamine.
R. leguminosarum bv. viciae strain 3841 did not grow without thiamine either in liquid minimal medium or on medium solidified with agarose. However, it formed “pin” colonies on agar-solidified minimal medium in the absence of added thiamine (Table 2). These data are consistent with the absence of a complete thiCOGE biosynthetic operon in the genome sequence of strain 3841 (55). A gfp-UV fusion to the thiE gene from strain 3841 had previously been isolated from the rhizosphere of peas (3), and therefore we could use bioinformatic analysis of the region surrounding thiE to identify three genes, thiMED, which are present on plasmid pRL11JI.
TABLE 2.
Growth of Rhizobium strains in the presence and absence of thiamine and salvage intermediates
| Straina | Growth on minimal medium withb:
|
||||
|---|---|---|---|---|---|
| Thiamine | No addition | HMP | THZ | HMP and THZ | |
| R. leguminosarum 3841 | ++ | + | + | + | ++ |
| R. leguminosarum VF39 | ++ | + | + | + | ++ |
| R. leguminosarum RBL1309 | ++ | + | + | + | ++ |
| R. leguminosarum 3855 | ++ | ++ | ND | ND | ND |
| R. leguminosarum WU235 | ++ | ++ | ND | ND | ND |
| R. leguminosarum A34 | ++ | ++ | ND | ND | ND |
| R. leguminosarum 248 | ++ | ++ | ND | ND | ND |
| R. leguminosarum JI336 | ++ | ++ | ND | ND | ND |
| R. leguminosarum 128C53 | ++ | ++ | ND | ND | ND |
| R. leguminosarum bv. trifolii RCR5 | ++ | ++ | ND | ND | ND |
| R. leguminosarum bv. trifolii W14-2 | ++ | ++ | ND | ND | ND |
| RU2459 (thiM mutant) | ++ | − | − | − | − |
| RU2459 (pRU1781) | ++ | + | + | + | ++ |
| RU2459 (pRU1782) | ++ | + | + | + | ++ |
| R. etli CFN42 | ++ | ++ | ND | ND | ND |
| R. etli CFNX183 | ++ | + | + | + | ++ |
| R. etli CE3 | ++ | ++ | ND | ND | ND |
| R. tropici CIAT899 | ++ | ++ | ND | ND | ND |
| M. loti MAFF 303099 | ++ | ++ | ND | ND | ND |
| S. meliloti 1021 | ++ | ++ | ND | ND | ND |
| S. meliloti RP254 | ++ | ++ | ND | ND | ND |
| Rhizobium sp. strain NGR234 | ++ | ++ | ND | ND | ND |
Plasmids pRU1781 and pRU1782 contain thiME and thiMED, respectively.
Growth on minimal medium was scored as follows: ++, good growth; +, pin colony formation; −, no growth; ND, not determined.
BLAST analysis was used to determine the distribution of the thiMED and thiCOGE genes in different sequenced Rhizobium strains (Mesorhizobium loti, S. meliloti, Bradyrhizobium japonicum, R. leguminosarum, R. etli, and Agrobacterium tumefaciens). Only R. leguminosarum 3841 and its close relative R. etli CFN42 have the thiMED genes. The ThiMED proteins from R. etli and R. leguminosarum 3841 have 95%, 92%, and 91% identity, respectively (17). Excepting R. leguminosarum 3841, all species have thiCOGE. R. etli CFN42 is unusual in that the genes for both the de novo thiamine synthesis pathway (thiCOGE) and the putative salvage pathway (thiMED) are present on plasmids pRetCFN42b and pRetCFN42e, respectively (17). To test whether the presence of thiCOGE enables rhizobial strains to grow in the absence of added thiamine, the sequenced strains R. etli CFN42, M. loti MAFF 303099, and S. meliloti 1021, as well as several other common laboratory strains, were grown in the presence and the absence of thiamine (Table 2). Three out of 11 strains of R. leguminosarum (3841, VF39, and RBL1309) did not grow well in the absence of added thiamine. All of the other rhizobia tested grew well in the absence of added thiamine, implying they have a thiCOGE operon or the equivalent.
Since strain 3841 formed only pin colonies on agar medium lacking thiamine, one possibility is that thiMED constitutes a salvage pathway that uses intermediates in thiamine biosynthesis normally present in agar and presumably in the soil environment. The putative reactions catalyzed by ThiM, the 4-methyl-5-(β-hydroxyethyl) thiazole kinase, by ThiE, the thiamine phosphate synthase, and by ThiD, the HMP kinase, suggest that HMP and THZ might be the intermediates used (Fig. 1). When added alone, neither HMP nor THZ rescued growth of strain 3841; however, when these were added together, the wild-type strain grew as well as it did when thiamine was added (Table 2). Strains VF39 and RBL1309, which do not grow in the absence of thiamine, also grew well on HMP and THZ (Table 2). To determine if the pin colony growth seen on solid medium results from the operation of the salvage pathway, a thiM mutant (RU2459) was made by the insertion of an omega interposon into thiM of strain 3841. Strain RU2459 no longer formed pin colonies on agar plates and, as expected, was unable to grow in the presence of HMP and THZ but could use thiamine (Table 2). Strain RU2459 was complemented for growth on HMP and THZ with plasmids containing thiME (pRU1781) and thiMED (pRU1782) (Table 2). These results indicate that a salvage pathway for condensation of HMP and THZ to thiamine, using ThiM, ThiE, and ThiD, operates in R. leguminosarum 3841. In addition, the thiM gene could be amplified by PCR from genomic DNA of VF39 and RBL1309 (data not shown), indicating they also have this pathway.
In order to check whether the thiMED genes in R. etli CFN42 confer on it a putative salvage pathway, strain CFNX183, a derivative of CFN42 lacking plasmid pRetCFN42b, which contains the thiCOGE genes, was grown on various media. It formed pin colonies when grown on media lacking thiamine but grew well on the intermediates HMP and THZ, which are used by the ThiMED salvage pathway (Table 2). This suggests that the ThiMED salvage pathway operates in R. etli.
Regulation of the thiM promoter.
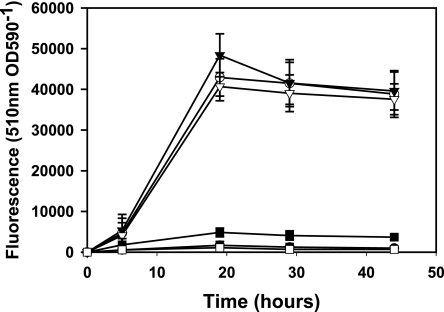
It has been shown that a gfp-UV fusion to thiE is expressed under thiamine limitation and in the rhizosphere of peas (3). However, this fusion contains 1.8 kb of DNA upstream of thiE, which contains thiM and a gene coding for a hypothetical protein. To see if the thiMED genes are regulated from a promoter immediately upstream of thiM, an 889-bp fragment of this region was fused to gfp-mut3.1 in plasmid pRU1097, producing pRU1619. Strains RU2292 (strain 3841 containing pRU1515, which is self-ligated pRU1097) and RU2295 (strain 3841 containing pRU1619, i.e., pthiM::gfp-mut3.1) were grown in the presence of thiamine overnight and then transferred into AMS containing various combinations of thiamine, HMP, and THZ (Fig. 2). The expression of thiM::gfp-mut3.1 was very low when thiamine was present but high in growth medium from which thiamine was absent. Together, but not alone, the thiamine intermediates HMP and THZ also repressed the thiM promoter. These data confirm that there is a thiamine-repressible promoter immediately upstream of thiM. Furthermore, immediately upstream of thiM in both R. leguminosarum 3841 and R. etli CFN42 there is a thi box consisting of three thiamine pyrophosphate [TPP] riboswitches named 1, 2, and 3, which have 65, 63, and 61% identity, respectively, to the characterized R. etli thiC TPP riboswitch subregions.
FIG. 2.
Expression of pthiM::gfp-mut3.1 on different thiamine intermediates. Symbols: •, thiamine; ○, no addition; ▾, HMP; ▿, THZ; ▪, HMP and THZ; □, HMP, THZ, and thiamine. OD590, optical density at 590 nm.
Complementation of a thiM mutant (RU2459) with a plasmid containing thiME may indicate that there is another promoter downstream of thiM. However, while the omega interposons do have strong transcriptional terminators, their insertion may generate outwardly directed promoters which complicate such analysis. RT-PCR products were also obtained for thiM, thiE, and thiD and for primer pairs spanning thiME and thiED, with RNA obtained from thiamine-limited cultures but not from those with an excess of thiamine (data not shown). This indicates the presence of an operon spanning thiMED, with a promoter upstream of thiM, although it does not preclude the presence of other promoters.
Quantitation by Q-RT-PCR and rhizosphere visualization.
Quantitative RT-PCR was used to measure the mRNA expression levels of strain 3841 thiM in the rhizosphere as well as in thiamine-starved cells grown in culture. In cultured bacteria, thiamine starvation resulted in a 16.5 ± 2.1 (mean ± standard error of the mean [SEM])-fold increase in expression (n = 3). Likewise, bacteria isolated from the rhizosphere had a 20.8 ± 9.0 (mean ± SEM)-fold increase in thiM expression (n = 3) relative to laboratory cells grown on excess thiamine. This suggests that thiamine is present at limiting concentrations in the pea rhizosphere.
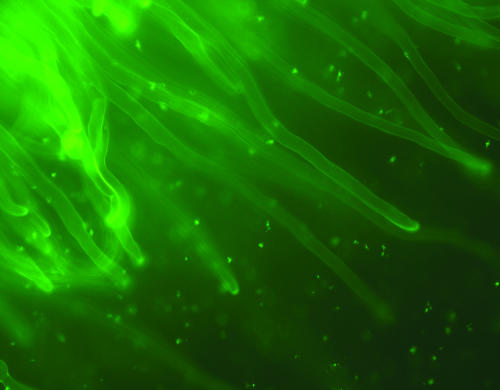
The previously isolated thiME::gfp-UV fusion (pRU504) was conjugated into strain 3841 and inoculated in the vetch plants to directly visualize expression in the rhizosphere (Fig. 3). It can be seen that the expression of the fusion was high in the rhizosphere, confirming the Q-RT-PCR results.
FIG. 3.
Expression of the thiME fusion (pRU504) in the rhizosphere of vetch. Fluorescent bacteria can be seen throughout the rhizosphere. No fluorescent bacteria were seen in the absence of the thiME promoter in the parent plasmid pOT1.
Plant properties of the thiM mutant and the wild type.
The wild type and the thiM mutant (RU2459) reduced acetylene at 2.1 ± 0.4 (mean ± SEM) (n = 6) and 1.9 ± 0.4 (mean ± SEM) (n = 6) μmol ethylene per plant per hour, respectively. The dry weights of plants inoculated with either the wild type or the thiM mutant and harvested at 6 weeks were 1.40 g ± 0.24 g (mean ± SEM) (n = 6) and 1.61 g ± 0.40 g (mean ± SEM) (n = 6), respectively. None of these results are significantly different in t tests, indicating that the mutation in thiM has no significant effect on nitrogen fixation.
The fact that there is no effect of mutation of thiM on the ability of R. leguminosarum to nodulate peas and fix nitrogen suggests that the supply of thiamine by the plant to bacteroids and bacteria in infection threads is not limiting. However, the competitive ability of R. leguminosarum in the rhizosphere, where thiamine is limiting, may be altered. In order to determine if there is an effect on the nodulation competitiveness of the thiM mutant, a large inoculum (106 cells of thiM and wild-type strains) was placed onto pea seedlings. At 4 weeks postinoculation, 10 nodules from each of 12 plants were picked randomly and checked for occupation by bacteria. The results indicated that the thiM mutant was at a modest, but significant, competitive disadvantage relative to the wild type, occupying 35% ± 8% (means ± SEM) of nodules on each plant (t test; P < 0.05).
Competition between the wild type and the thiM mutant for rhizosphere colonization.
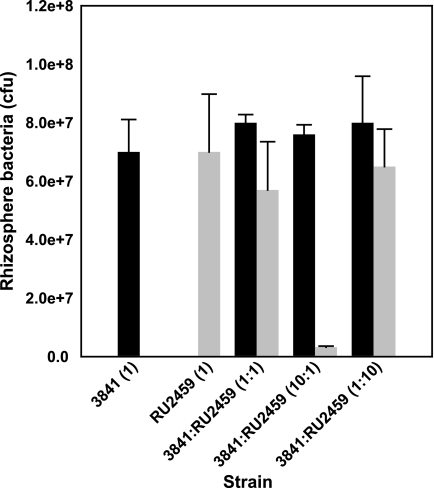
Competition between the thiM mutant and the wild type for growth in the pea rhizosphere was measured by inoculating a low number of bacteria into the pea rhizosphere (103 to 104 bacteria per seedling) and determining total bacteria after 7 days. When the mutant and the wild type were inoculated alone into a sterile rhizosphere, almost identical numbers of bacteria were recovered after 7 days (Fig. 4). However, when these strains were inoculated together, the thiM mutant was at a slight, but significant, disadvantage compared to the wild type (t test; P < 0.05). Even when strain RU2459 was inoculated at a 10-fold excess over the wild type, it still accounted for only 18% of bacteria recovered (Fig. 4). The ability of the thiM mutant to grow in a sterile rhizosphere and to nodulate and fix nitrogen on peas shows that thiamine must be released by pea roots. At higher cell densities, however, the competition for thiamine in the rhizosphere presumably becomes more acute, and the presence of a thiamine salvage pathway becomes increasingly important.
FIG. 4.
Competition of the wild type (3841) (black bars) and the thiM mutant (RU2459) (gray bars) in sterile rhizospheres. Inoculation ratios are given on the x axis, with 1 corresponding to 1,000 CFU. Bacterial numbers recovered from 12 plants (mean ± SEM) are shown.
DISCUSSION
In this paper, we report that the biosynthesis of thiamine in R. leguminosarum bv. viciae strain 3841 is mediated by a salvage pathway requiring ThiMED. In medium solidified with agar without thiamine, strain 3841 formed pin colonies, while the presence of the salvage intermediates HMP and THZ allowed normal growth. The thiM mutant (RU2459) did not grow without the addition of thiamine. Complementing plasmids containing the thiM gene restored growth on HMP and thiazole. This indicates that, as expected, ThiMED are 4-methyl-5-(β-hydroxyethyl) thiazole kinase, thiamine phosphate synthase, and 4-amino-5-hydroxymethyl-2-methyl pyrimidine kinase. In the sequenced rhizobia S. meliloti 1021 and M. loti MAFF303099, the presence of the ThiCOGE pathway presumably mediates the de novo synthesis of thiamine, while R. etli CFN42 has both this and the ThiMED salvage pathway. Three out of 11 tested strains of R. leguminosarum will grow only with added thiamine or HMP and THZ, indicating dependence on the ThiMED pathway, but the other strains are capable of growing in the absence of added thiamine. Thus, it is common, but not universal, for strains of R. leguminosarum to use the ThiMED pathway for thiamine synthesis. It is noteworthy that the thiMED genes are on plasmid pRL11JI in R. leguminosarum 3841 and R. etli has both the thiCOGE and thiMED genes on plasmids pRetCFN42b and pRetCFN42e, respectively, while S. meliloti has the thiCOGE genes on pSymB. Thus, rhizobia often have genes for thiamine biosynthesis on plasmids.
An intriguing example of how vitamins can limit the growth of rhizobia in the environment is found in Mesorhizobium sp. strain R7A, in which genes for symbiosis and for biotin, thiamine, and nicotinate biosynthesis are found on a chromosomal (symbiosis) island. Strains found in the soil lack the symbiotic island and are auxotrophic for all three vitamins (or just for thiamine and biotin in some cases), but transfer of the symbiotic island restores prototrophy (51). Overall, it appears to be common for the growth of rhizobia in soil to be limited by the rate at which vitamins such as biotin and thiamine can be synthesized; their growth may be arrested until they are available, perhaps in the rhizosphere.
It is interesting that some rhizobia possess a full pathway for de novo thiamine biosynthesis and others, such as strain 3841, possess only a salvage pathway. The use of a thiE gfp-UV biosensor as well as Q-RT-PCR confirmed that the thiMED genes are expressed in the rhizosphere, indicating that limiting levels of thiamine are present. Such a limitation suggests that the ability to synthesize thiamine either de novo or via the ThiMED salvage pathway should be important for growth in the rhizosphere. Consistent with this, a thiM mutant was shown to be at a competitive disadvantage for growth in the rhizosphere and for nodulation (Fig. 4). We have not attempted to address the experimentally difficult question of whether the thiMED genes might be required for long-term survival in soil. However, the example of acquisition of de novo thiamine biosynthesis, via a symbiosis island, by strains of M. loti that exist in the soil without this capacity highlights this issue (51). Can such strains exist without the ability to make any thiamine, or do they possess salvage pathways such as ThiMED?
No thiamine-regulatory proteins have been found in bacteria (25, 52). Instead, the corresponding genes have been found to be regulated by riboswitches in different bacteria, including R. etli (28, 32). TPP directly regulates the expression of the thiamine biosynthesis genes by a novel mechanism involving the formation of a riboswitch. TPP interacts with the nascent mRNA message at a cis-acting region within the 5′ leader, called the thi box, to form a secondary structure that allows the formation of a transcription terminator (27, 28, 32, 54). The presence of a thi box upstream of thiM indicates there is a riboswitch regulating the thiMED genes, just as there is one regulating the de novo thiCOGE thiamine biosynthetic operon in R. etli. This is consistent with the repression of the thiMED genes in cultures grown on excess thiamine.
Competition experiments suggest that the thiMED salvage pathway will become increasingly important as bacteria increase in number and thereby begin to compete for limiting quantities of thiamine in the rhizosphere. Key questions that we cannot yet answer include what levels of intermediates such as HMP and THZ are in soil and whether they are released at higher levels by plant roots. Overall, R. leguminosarum 3841, in common with most rhizobia, appears to adopt a survival strategy in soil, where vitamins will limit its growth and cause it to rely on a plant host for provision of these factors in the rhizosphere.
Acknowledgments
This work was funded by the Biotechnology and Biological Sciences Research Council UK.
We thank T. P. Begley, Cornell University, Ithaca, NY, for providing HMP.
REFERENCES
- 1.Abreu-Goodger, C., and E. Merino. 2005. RibEx: a web server for locating riboswitches and other conserved bacterial regulatory elements. Nucleic Acids Res. 33:W690-W692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allaway, D., E. Lodwig, L. A. Crompton, M. Wood, T. R. Parsons, T. Wheeler, and P. S. Poole. 2000. Identification of alanine dehydrogenase and its role in mixed secretion of ammonium and alanine by pea bacteroids. Mol. Microbiol. 36:508-515. [DOI] [PubMed] [Google Scholar]
- 3.Allaway, D., N. A. Schofield, M. E. Leonard, L. Gilardoni, T. M. Finan, and P. S. Poole. 2001. Use of differential fluorescence induction and optical trapping to isolate environmentally induced genes. Environ. Microbiol. 3:397-406. [DOI] [PubMed] [Google Scholar]
- 4.Baldani, J. I., R. W. Weaver, M. F. Hynes, and B. D. Eardly. 1992. Utilization of carbon substrates, electrophoretic enzyme patterns, and symbiotic performance of plasmid cured clover rhizobia. Appl. Environ. Microbiol. 58:2308-2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Begley, T. P., D. M. Downs, S. E. Ealick, F. W. McLafferty, A. P. Van Loon, S. Taylor, N. Campobasso, H. J. Chiu, C. Kinsland, J. J. Reddick, and J. Xi. 1999. Thiamin biosynthesis in prokaryotes. Arch. Microbiol. 171:293-300. [DOI] [PubMed] [Google Scholar]
- 6.Beringer, J. E. 1974. R factor transfer in Rhizobium leguminosarum. J. Gen. Microbiol. 84:188-198. [DOI] [PubMed] [Google Scholar]
- 7.Brewin, N. J., E. A. Wood, A. W. B. Johnston, N. J. Dibb, and G. Hombrecher. 1982. Recombinant nodulation plasmids in Rhizobium leguminosarum. J. Gen. Microbiol. 128:1817-1827. [Google Scholar]
- 8.Brom, S., A. G. Delossantos, T. Stepkowsky, M. Flores, G. Dávila, D. Romero, and R. Palacios. 1992. Different plasmids of Rhizobium leguminosarum bv. phaseoli are required for optimal symbiotic performance. J. Bacteriol. 174:5183-5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bustin, S. A. 2000. Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J. Mol. Endocrinol. 25:169-193. [DOI] [PubMed] [Google Scholar]
- 10.Bustin, S. A. 2002. Quantification of mRNA using real-time reverse transcription PCR (RT-PCR): trends and problems. J. Mol. Endocrinol. 29:23-39. [DOI] [PubMed] [Google Scholar]
- 11.Derylo, M., and A. Skorupska. 1993. Enhancement of symbiotic nitrogen fixation by vitamin-secreting fluorescent Pseudomonas. Plant Soil 154:211-217. [Google Scholar]
- 12.Downie, J. A., G. Hombrecher, Q. S. Ma, C. D. Knight, B. Wells, and A. W. B. Johnston. 1983. Cloned nodulation genes of Rhizobium leguminosarum determine host range specificity. Mol. Gen. Genet. 190:359-365. [Google Scholar]
- 13.Dye, M. 1981. Rothamsted collection of Rhizobium: catalogue of strains, 3rd ed. Rothamsted Experimental Station, Harpenden, United Kingdom.
- 14.Encarnación, S., M. Dunn, K. Willms, and J. Mora. 1995. Fermentative and aerobic metabolism in Rhizobium etli. J. Bacteriol. 177:3058-3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Entcheva, P., D. A. Phillips, and W. R. Streit. 2002. Functional analysis of Sinorhizobium meliloti genes involved in biotin synthesis and transport. Appl. Environ. Microbiol. 68:2843-2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Figurski, D. H., and D. R. Helinski. 1979. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc. Natl. Acad. Sci. USA 76:1648-1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.González, V., R. I. Santamaría, P. Bustos, I. Hernández-González, A. Medrano-Soto, G. Moreno-Hagelsieb, S. C. Janga, M. A. Ramírez, V. Jiménez-Jacinto, J. Collado-Vides, and G. Dávila. 2006. The partitioned Rhizobium etli genome: genetic and metabolic redundancy in seven interacting replicons. Proc. Natl. Acad. Sci. USA 103:3834-3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hooykaas, P. J. J., A. A. N. Van Brussel, H. den Dulk-Ras, G. M. S. van Slogteren, and R. A. Schilperoort. 1981. Sym plasmid of Rhizobium trifolii expressed in different rhizobial species and Agrobacterium tumefaciens. Nature 291:351-353. [Google Scholar]
- 19.Johnston, A. W. B., and J. E. Beringer. 1975. Identification of the Rhizobium strains in pea root nodules using genetic markers. J. Gen. Microbiol. 87:343-350. [DOI] [PubMed] [Google Scholar]
- 20.Josey, D. P., J. L. Beynon, A. W. B. Johnston, and J. E. Beringer. 1979. Strain identification in Rhizobium leguminosarum using intrinsic antibiotic resistance. J. Appl. Bacteriol. 46:343-350. [Google Scholar]
- 21.Kaneko, T., Y. Nakamura, S. Sato, E. Asamizu, T. Kato, S. Sasamoto, A. Watanabe, K. Idesawa, A. Ishikawa, K. Kawashima, T. Kimura, Y. Kishida, C. Kiyokawa, M. Kohara, M. Matsumoto, A. Matsuno, Y. Mochizuki, S. Nakayama, N. Nakazaki, S. Shimpo, M. Sugimoto, C. Takeuchi, M. Yamada, and S. Tabata. 2000. Complete genome structure of the nitrogen-fixing symbiotic bacterium Mesorhizobium loti. DNA Res. 7:331-338. [DOI] [PubMed] [Google Scholar]
- 22.Karunakaran, R., T. H. Mauchline, A. H. F. Hosie, and P. S. Poole. 2005. A family of promoter probe vectors incorporating autofluorescent and chromogenic reporter proteins for studying gene expression in Gram-negative bacteria. Microbiology 151:3249-3256. [DOI] [PubMed] [Google Scholar]
- 23.Keen, N. T., S. Tamaki, D. Kobayashi, and D. Trollinger. 1988. Improved broad-host-range plasmids for DNA cloning in Gram-negative bacteria. Gene 70:191-197. [DOI] [PubMed] [Google Scholar]
- 24.Kumar, S., A. Bourdes, and P. S. Poole. 2005. De novo alanine synthesis by bacteroids of Mesorhizobium loti is not required for nitrogen transfer in the determinate nodules of Lotus corniculatus. J. Bacteriol. 187:5493-5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lawhorn, B. G., S. Y. Gerdes, and T. P. Begley. 2004. A genetic screen for the identification of thiamin metabolic genes. J. Biol. Chem. 279:43555-43559. [DOI] [PubMed] [Google Scholar]
- 26.Leigh, J. A., E. R. Signer, and G. C. Walker. 1985. Exopolysaccharide-deficient mutants of Rhizobium meliloti that form ineffective nodules. Proc. Natl. Acad. Sci. USA 82:6231-6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lesnik, E. A., G. B. Fogel, D. Weekes, T. J. Henderson, H. B. Levene, R. Sampath, and D. J. Ecker. 2005. Identification of conserved regulatory RNA structures in prokaryotic metabolic pathway genes. Biosystems 80:145-154. [DOI] [PubMed] [Google Scholar]
- 28.Mandal, M., B. Boese, J. Barrick, W. Winkler, and R. Breaker. 2003. Riboswitches control fundamental biochemical pathways in Bacillus subtilis and other bacteria. Cell 112:577-586. [DOI] [PubMed] [Google Scholar]
- 29.Martínez-Romero, E., L. Segovia, F. M. Mercante, A. A. Franco, P. Graham, and M. A. Pardo. 1991. Rhizobium tropici, a novel species nodulating Phaseolus vulgaris L. beans and Leucaena sp. trees. Int. J. Syst. Bacteriol. 41:417-426. [DOI] [PubMed] [Google Scholar]
- 30.Melnick, J., E. Lis, J. H. Park, C. Kinsland, H. Mori, T. Baba, J. Perkins, G. Schyns, O. Vassieva, A. Osterman, and T. P. Begley. 2004. Identification of the two missing bacterial genes involved in thiamine salvage: thiamine pyrophosphokinase and thiamine kinase. J. Bacteriol. 186:3660-3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miranda-Ríos, J., C. Morera, H. Taboada, A. Dávalos, S. Encarnacion, J. Mora, and M. Soberón. 1997. Expression of thiamin biosynthetic genes (thiCOGE) and production of symbiotic terminal oxidase cbb3 in Rhizobium etli. J. Bacteriol. 179:6887-6893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miranda-Ríos, J., M. Navarro, and M. Soberón. 2001. A conserved RNA structure (thi box) is involved in regulation of thiamin biosynthetic gene expression in bacteria. Proc. Natl. Acad. Sci. USA 98:9736-9741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Noel, K. D., A. Sanchez, L. Fernandez, J. Leemans, and M. A. Cevallos. 1984. Rhizobium phaseoli symbiotic mutants with transposon Tn5 insertions. J. Bacteriol. 158:148-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peterson, L. A., and D. M. Downs. 1997. Identification and characterization of an operon in Salmonella typhimurium involved in thiamine biosynthesis. J. Bacteriol. 179:4894-4900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Poole, P. S., A. Blyth, C. J. Reid, and K. Walters. 1994. myo-Inositol catabolism and catabolite regulation in Rhizobium leguminosarum bv viciae. Microbiology 140:2787-2795. [Google Scholar]
- 36.Poole, P. S., M. J. Dilworth, and A. R. Glenn. 1984. Acquisition of aspartase activity in Rhizobium leguminosarum WU235. J. Gen. Microbiol. 130:881-886. [Google Scholar]
- 37.Poole, P. S., N. A. Schofield, C. J. Reid, E. M. Drew, and D. L. Walshaw. 1994. Identification of chromosomal genes located downstream of dctD that affect the requirement for calcium and the lipopolysaccharide layer of Rhizobium leguminosarum. Microbiology 140:2797-2809. [DOI] [PubMed] [Google Scholar]
- 38.Prentki, P., and H. M. Krisch. 1984. In vitro insertional mutagenesis with a selectable DNA fragment. Gene 29:303-313. [DOI] [PubMed] [Google Scholar]
- 39.Priefer, U. B. 1989. Genes involved in lipopolysaccharide production and symbiosis are clustered on the chromosome of Rhizobium leguminosarum biovar viciae VF39. J. Bacteriol. 171:6161-6168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Quandt, J., and M. F. Hynes. 1993. Versatile suicide vectors which allow direct selection for gene replacement in Gram-negative bacteria. Gene 127:15-21. [DOI] [PubMed] [Google Scholar]
- 41.Quinto, C., H. Delavega, M. Flores, L. Fernandez, T. Ballado, G. Soberón, and R. Palacios. 1982. Reiteration of nitrogen-fixation gene sequences in Rhizobium phaseoli. Nature 299:724-726. [Google Scholar]
- 42.Rodelas, B., J. Gonzalez-Lopez, V. Salmeron, C. Pozo, and M. V. Martinez-Toledo. 1996. Enhancement of nodulation, N2-fixation and growth of faba-bean (Vicia faba L.) by combined inoculation with Rhizobium leguminosarum bv viciae and Azosprillum brasilense. Symbiosis 21:175-186. [Google Scholar]
- 43.Rodionov, D. A., A. G. Vitreschak, A. A. Mironov, and M. S. Gelfand. 2002. Comparative genomics of thiamine biosynthesis in procaryotes. J. Biol. Chem. 277:48949-48959. [DOI] [PubMed] [Google Scholar]
- 44.Ruiz-Argueso, T., J. Hanus, and H. J. Evans. 1978. Hydrogen production and uptake by pea nodules as affected by strains of Rhizobium leguminosarum. Arch. Microbiol. 116:113-118. [Google Scholar]
- 45.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 46.Schwinghamer, E. A. 1970. Requirement of riboflavin for effective symbiosis of clover by an auxotrophic mutant strain of Rhizobium trifolii. Aust. J. Biol. Sci. 26:1118-1196. [Google Scholar]
- 47.Stanley, J., D. N. Dowling, and W. J. Broughton. 1988. Cloning of hemA from Rhizobium sp. NGR234 and symbiotic phenotype of a gene-directed mutant in diverse legume genera. Mol. Gen. Genet. 215:32-37. [Google Scholar]
- 48.Streit, W. R., C. M. Joseph, and D. A. Phillips. 1996. Biotin and other water-soluble vitamins are key growth-factors for alfalfa root colonization by Rhizobium meliloti 1021. Mol. Plant-Microbe Interact. 9:330-338. [DOI] [PubMed] [Google Scholar]
- 49.Streit, W. R., and D. A. Phillips. 1996. Recombinant Rhizobium meliloti strains with extra biotin synthesis capability. Appl. Environ. Microbiol. 62:3333-3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Strzelczyk, E., and U. Leniarska. 1985. Production of B-group vitamins by mycorrhizal fungi and actinomycetes isolated from the root zone of pine (Pinus sylvestris L.). Plant Soil 86:387-394. [Google Scholar]
- 51.Sullivan, J. T., H. N. Patrick, W. L. Lowther, D. B. Scott, and C. W. Ronson. 1995. Nodulating strains of Rhizobium loti arise through chromosomal symbiotic gene-transfer in the environment. Proc. Natl. Acad. Sci. USA 92:8985-8989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Webb, E., F. Febres, and D. M. Downs. 1996. Thiamine pyrophosphate (TPP) negatively regulates transcription of some thi genes of Salmonella typhimurium. J. Bacteriol. 178:2533-2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wijffelman, C. A., E. Pees, A. A. N. Vanbrussel, and P. J. J. Hooykaas. 1983. Repression of small bacteriocin excretion in Rhizobium leguminosarum and Rhizobium trifolii by transmissible plasmids. Mol. Gen. Genet. 192:171-176. [Google Scholar]
- 54.Winkler, W., A. Nahvi, and R. Breaker. 2002. Thiamine derivatives bind messenger RNAs directly to regulate bacterial gene expression. Nature 419:952-956. [DOI] [PubMed] [Google Scholar]
- 55.Young, J. P., L. Crossman, A. Johnston, N. Thomson, Z. Ghazoui, K. Hull, M. Wexler, A. Curson, J. Todd, P. Poole, T. Mauchline, A. East, M. Quail, C. Churcher, C. Arrowsmith, I. Cherevach, T. Chillingworth, K. Clarke, A. Cronin, P. Davis, A. Fraser, Z. Hance, H. Hauser, K. Jagels, S. Moule, K. Mungall, H. Norbertczak, E. Rabbinowitsch, M. Sanders, M. Simmonds, S. Whitehead, and J. Parkhill. 2006. The genome of Rhizobium leguminosarum has recognizable core and accessory components. Genome Biol. 7:R34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zilles, J. L., L. R. Croal, and D. M. Downs. 2000. Action of the thiamine antagonist bacimethrin on thiamine biosynthesis. J. Bacteriol. 182:5606-5610. [DOI] [PMC free article] [PubMed] [Google Scholar]