Abstract
We report the presence of Mlc in a thermophilic bacterium. Mlc is known as a global regulator of sugar metabolism in gram-negative enteric bacteria that is controlled by sequestration to a glucose-transporting EIIGlc of the phosphotransferase system (PTS). Since thermophilic bacteria do not possess PTS, Mlc in Thermus thermophilus must be differently controlled. DNA sequence alignments between Mlc from T. thermophilus (MlcTth) and Mlc from E. coli (MlcEco) revealed that MlcTth conserved five residues of the glucose-binding motif of glucokinases. Here we show that MlcTth is not a glucokinase but is indeed able to bind glucose (KD = 20 μM), unlike MlcEco. We found that mlc of T. thermophilus is the first gene within an operon encoding an ABC transporter for glucose and mannose, including a glucose/mannose-binding protein and two permeases. malK1, encoding the cognate ATP-hydrolyzing subunit, is located elsewhere on the chromosome. The system transports glucose at 70°C with a Km of 0.15 μM and a Vmax of 4.22 nmol per min per ml at an optical density (OD) of 1. MlcTth negatively regulates itself and the entire glucose/mannose ABC transport system operon but not malK1, with glucose acting as an inducer. MalK1 is shared with the ABC transporter for trehalose, maltose, sucrose, and palatinose (TMSP). Mutants lacking malK1 do not transport either glucose or maltose. The TMSP transporter is also able to transport glucose with a Km of 1.4 μM and a Vmax of 7.6 nmol per min per ml at an OD of 1, but it does not transport mannose.
In Escherichia coli, glucose induction of several genes and operons involved in sugar transport and metabolism is mediated by the global repressor Mlc. ptsG, encoding enzyme IICB of the glucose-specific PEP-dependent phosphotransferase system (PTS), is the most prominent gene under the control of Mlc (22). Other genes regulated by Mlc include malT, encoding the activator of the maltose regulon (6); manXYZ, encoding three proteins of the mannose PTS (22); and the genes encoding the general components of the PTS (12, 23, 31).The expression of mlc is autoregulated (5) and partially under the control of the σH-mediated heat shock response (27).
The particularity of Mlc in E. coli is that, unlike normal prokaryotic transcriptional regulators, it is not a low-molecular-weight cytoplasmic molecule that inactivates the repressor by preventing its binding to DNA. Instead, the activity of Mlc as a repressor is regulated (i.e., inhibited) by its binding (sequestration) to the dephosphorylated state of the membrane-associated EIIBGlc domain of the PtsG protein occurring during glucose transport (14, 19, 27, 30).
The recent sequencing of the thermophilic bacterium Thermus thermophilus HB27 genome (10) revealed the presence of a gene (TTC0329) encoding a protein with similarity to the Mlc from E. coli. Its amino acid sequence contains the two consensus sequences that characterize the ROK family of transcriptional regulators (for repressors, open reading frames, and kinases), as well as the four residues (one histidine and three cysteines) corresponding to the zinc binding site necessary for the repressor function of Mlc in E. coli (25).
Strains of the species T. thermophilus are commonly isolated from marine hot springs and can grow at temperatures up to 70°C. Thus far, no PEP-dependent PTSs have been encountered in thermophilic bacteria or archaea (7), an observation in line with the concept of a hot origin of life (1, 11) and implying that the PTS only evolved later in mesophiles. It thus seemed intriguing that a homologue of Mlc would be found in such an organism, since the regulation of Mlc in E. coli is known to be dependent on the phosphorylated state of PtsG (13, 14, 19, 26, 30).
The present study began with the observation that the Mlc of T. thermophilus, when overexpressed in E. coli, had the ability to affect the expression of ptsG and malT of E. coli and also had residual ability to bind to the EIIBC domain of the E. coli PtsG. We became curious about the actual role of Mlc in T. thermophilus and about its mode of regulation, since it had to be different from PTS-dependent regulation in E. coli. DNA sequence alignments between the Mlc of T. thermophilus (MlcTth) and that of E. coli (MlcEco) revealed that MlcTth, unlike MlcEco, conserved five residues of the glucose-binding motif of glucokinases (15). However, we found that MlcTth was not a glucokinase but, unlike MlcEco, was able to bind glucose as well as mannose. We show here that the Mlc in T. thermophilus negatively regulates itself and an entire operon encoding a glucose/mannose ABC transport system in a glucose-dependent manner. The operon contains at least four genes encoding MlcTth, a glucose/mannose-binding protein, and two permeases. We show that the ATP-hydrolyzing subunit for this system is MalK1, which is also the ATP-hydrolyzing subunit of the ABC transporter for trehalose, maltose, sucrose, and palatinose (TMSP) described previously (28).
MATERIALS AND METHODS
Strains, plasmids, and chemicals.
T. thermophilus strain HB27 (DSM7039) was obtained from the Deutsche Sammlung von Mikroorganismen und Zellkulturen (DSMZ), Braunschweig, Germany. Other strains and plasmids used in the present study are listed in Table 1. All chemicals were reagent grade and were obtained from commercial sources.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Known genotypea | Source or reference |
|---|---|---|
| Strains | ||
| E. coli | ||
| SF120 | ptr32::cm degP4::kan ΔompT | 2 |
| JM-G2 | ptsG-lacZ mlc::Tn10 | 22 |
| T. thermophilus | ||
| DSM7029 | Wild-type strain (HB27) | DSMZ |
| CL3 | mlc::kan | This study |
| HB27 malK1::kan | malK1::kan | 28 |
| JN1 | ΔmalF::bleo | Jutta Nesper |
| CL4 | mlc::kan ΔmalF::bleo | This study |
| Plasmids | ||
| pFC4 | pGDR11 lacIq; N-terminally His6-tagged MlcTth (gene TTC0329); Ampr | This study |
| pEM1 | pCS19 lacIq; wild-type MlcTth (gene TTC0329); Ampr | This study |
| pCL2 | pQE30 (QIAGEN) lacIq; N-terminally His6-tagged Mlc916Tth::Kan; Ampr Kanr | This study |
| pTTC0328 | pQE30 (QIAGEN) lacIq; N-terminally His6-tagged glucose-binding protein (gene TTC0328) without its signal sequence | This study |
| pCL10 | pQE30(QIAGEN) lacIq; N-terminal His6-tagged TMSP transporter binding protein (gene TTC1627) without its signal sequence | This study |
| pCS19 | pQE60 (QIAGEN) lacIq; Ampr | 29 |
| pGDR11 | pQE31 (QIAGEN) lacIq; N-terminal His6 tag; Ampr | 20 |
| pMK18 | pUC18 derivative with a thermostable resistance to kanamycin | 6 |
| pREP4 | pACYC derivative containing the p15A replicon; Kanr | QIAGEN |
Standard DNA methods.
Chromosomal DNA from T. thermophilus HB27 was extracted by using the QIAampDNA blood minikit (QIAGEN). Plasmids were extracted from E. coli strains with the Mini-Plasmid kit (QIAGEN). Digestions by endonucleases (New England Biolabs), ligations (T4 DNA ligase; New England Biolabs), and PCR were performed by standard procedures (18, 24). Proofreading DNA polymerase (Pwo [Peqlab] or Phusion [Finnzymes]) was used for all PCR applications. Correct cloning was confirmed by sequencing analysis (GATC Biotech, Konstanz, Germany).
β-Galactosidase assays.
β-Galactosidase activity was determined according to the method of Miller (19) with alterations. We omitted β-mercaptoethanol from the Z buffer. Hydrolysis of ortho-nitrophenyl-β-galactoside (ONPGal) was done at a constant temperature of 28°C. After the reaction was stopped with sodium carbonate, we clarified the suspension by centrifugation before we measured the optical density at 405 nm (OD405). To calculate the specific activity, we used an extinction coefficient of 4,860/mol × cm for o-nitrophenol. The specific activity (U/mg protein) was given in μmol of ONPGal hydrolyzed per min per mg of protein at 28°C. A specific activity of 1 corresponds to about 1,000 Miller units.
Cloning of Mlc from T. thermophilus, overexpression, and purification of the recombinant proteins.
Two versions of MlcTth were produced: an N-terminal His6-tagged version and a wild-type version. Primers were designed based on the retrieved sequence of gene TTC0329 in T. thermophilus. The N-terminal His-tagged protein was constructed by producing a PCR product using the genomic DNA of T. thermophilus (strain HB27) and the primers 5′-CGC GGA TCC GCG TAA GGG CGA CGT CCA AAC-3′ (forward) and 5′-AAA AAG CTT CTA AGC CCC AAG ACC ATA CCG ATC-3′ (reverse). After gel purification of the PCR fragment (QIAGEN gel purification kit) and digestion with the restriction enzymes BamHI and HindIII (restriction sites underlined), the fragment was ligated into plasmid pGDR11 (a pQE31 derivative harboring the lacIq gene (20), yielding plasmid pFC4 (N-terminally His6-tagged MlcTth).
Plasmid pFC4 was subsequently used as a template for the construction of the wild-type Mlc using the primers 5′-CAT GCCATGG TGC GTA AGG GCG ACG TCC-3′ (forward) and 5′-GGA AGATCTA TTA AGC CCC AAG ACC ATA CCG-3′ (reverse). These primers were designed so that a methionine start codon was introduced at the N terminus of the construct, while an additional stop codon (TAA) was introduced at the C terminus. After purification of the PCR product and digestion with the NcoI and BglII restriction enzymes (underlined), the PCR fragment was ligated into plasmid pCS19 (a pQE60 derivative harboring the lacIq gene (29), yielding plasmid pEM1 (wild-type MlcTth). This plasmid was transformed into E. coli SF120 (2)-competent cells, which were grown in 1 liter of Luria-Bertani (LB) broth containing 100 μg of ampicillin/ml at 37°C. When the OD578 reached 0.6, the cells were induced with 0.2 mM IPTG (isopropyl-β-d-thiogalactopyranoside) and grown for an additional 5 h. The cells were centrifuged for 20 min at 5,000 rpm in a Sorvall SS34 rotor (as in all of the following centrifugation steps) and washed once with 0.9% NaCl. The resulting pellets were stored at −80°C until further use. The frozen cells were resuspended in 15 ml of lysis buffer (50 mM sodium phosphate, 300 mM NaCl, 10 mM imidazole [pH 8]) and French pressed four times at 16,000 lb/in2. The crude cell extract was centrifuged at 12,000 rpm for 20 min, the supernatant was incubated at 70°C for 15 min, and the denaturated proteins were removed by centrifugation (15,000 rpm for 20 min). The His6-tagged construct of Mlc was further purified by using a Ni-NTA Superflow column. The column was first washed with 2 volumes (40 ml) of lysis buffer containing 20 mM imidazole, and the His tag protein was then eluted by using a linear gradient from 20 to 500 mM imidazole. The protein was dialyzed in equilibration buffer (25 mM Tris, 150 mM NaCl). Wild-type Mlc was purified as described above, except that the lysis buffer was 50 mM HEPES-20 mM NaCl-10 mM β-mercaptoethanol (pH 7.5). After heat treatment at 70°C, the soluble protein was loaded onto a cationic-exchange column (Hi-Load SP HP; Amersham Bioscience), washed with 10 volumes (10 ml) of lysis buffer, and eluted by using a linear gradient of 20 mM to 1 M NaCl.
Cloning of the putative glucose-binding protein from T. thermophilus, overexpression, and purification of the recombinant protein.
Gene TTC0328 (downstream of MlcTth) encoding a putative glucose-binding protein was amplified by PCR without its signal leader peptide encoding sequence, using the genomic DNA of T. thermophilus (strain HB27) as a template and the primers 5′-GCC GGATCC CAA GGA GGC AAG CTG GAG ATC TT-3′ (forward) and 5′-GGC CC AAGCTTC GCG GCC TCT TAC TGG-3′ (reverse). The PCR product was digested with BamHI and Hind III (underlined) and ligated into vector pQE30 (QIAGEN). Competent E. coli M15 (QIAGEN) harboring the pREP4 (QIAGEN) lacIq repressor-carrying plasmid was transformed with the ligation mixture and plated on 1.5% LB agar plates supplemented with 100 μg of ampicillin/ml and 25 μg of kanamycin/ml. The resulting plasmid, pTTC0328, carries gene TTC0328 under the control of the T5 promoter lacIq operator system. The N-terminal leader peptide sequence of the gene is exchanged by a His6 tag-encoding sequence.
E. coli M15 harboring plasmids pREP4 and pTTC0328 was grown in 2 liters of NZA medium (0.5% yeast extract, 0.75% NaCl, 1% N-Z-Amine A [Sigma Aldrich, Munich, Germany]) containing 100 μg of ampicillin/ml and 25 μg of kanamycin/ml at 37°C. When the OD587 reached 0.8, IPTG was added to a final concentration of 100 μM. Cells were grown for an additional 3 h and then harvested by centrifugation. The pellet was resuspended in 6 ml of lysis buffer (20 mM sodium phosphate buffer [pH 7.1], containing 300 mM NaCl). Cells were disrupted by passing them four times at 12,000 lb/in2 through a French pressure cell, followed by centrifugation at 17,000 × g for 90 min at 4°C. The supernatant was loaded onto a Ni affinity column (HiTrap chelating HP 1-ml column) equilibrated with lysis buffer. Bound protein was eluted with a linear gradient of 0 to 500 mM imidazole within 20 column volumes. In order to remove imidazole, the protein was extensively dialyzed against 50 mM Tris-300 mM NaCl (pH 7.5).
Cloning and purification of the binding protein of the TMSP transporter.
Gene TTC1627, encoding the TMSP binding protein, was PCR amplified without its signal leader peptide-encoding sequence, using the genomic DNA of T. thermophilus (strain HB27) as a template and the primers 5′-CCGGATCCCAGTCCGGCCCCGTGATC-3′ (MBPTth forward) and 5′-CCCGGGAAGCTTGGGGCTAGCGCAGGA-3′ (MBPTth reverse). The PCR product was digested with BamHI and HindIII (sites are underlined) and ligated into vector pQE30 (QIAGEN). Competent E. coli M15 (QIAGEN) harboring the pREP4 (QIAGEN) the lacIq repressor-carrying plasmids were transformed with the ligation mixture and plated on 1.5% LB agar plates supplemented with 100 μg of ampicillin/ml and 25 μg of kanamycin/ml. The resulting plasmid, pCL10, carries TTC1627 under the control of the T5 promoter lacIq operator system. The N-terminal leader peptide sequence (27 amino acids) of the gene is exchanged by a His6 tag- encoding sequence.
E. coli M15 harboring plasmids pREP4 and pCL10 was grown in 2 liters of NZA medium containing 100 μg of ampicillin/ml and 25 μg of kanamycin/ml at 37°C. When the OD600 reached 0.6, IPTG was added to a final concentration of 100 μM. Cells were grown for an additional 3 h and then harvested by centrifugation. The pellet was resuspended in 6 ml of lysis buffer (50 mM Tris [pH 7.5] containing 300 mM NaCl and 6 M guanidinium hydrochloride). Cells were disrupted by passing them four times at 12,000 lb/in2 through a French pressure cell. Afterward, the suspension was incubated at 70°C for 30 min and centrifuged at 17,000 × g for 90 min at 4°C. The supernatant was loaded onto a Ni-affinity column (HiTrap chelating HP 1-ml column) equilibrated with lysis buffer. Bound protein was eluted with a linear gradient of 0 to 500 mM imidazole within 20 column volumes in a refolding buffer (50 mM Tris [pH 7.5] containing 300 mM NaCl). In order to remove imidazole, the protein was extensively dialyzed against 50 mM Tris-300 mM NaCl (pH 7.5).
Construction of the mlc::kan mutant in T. thermophilus.
The thermoresistant kanamycin resistance cassette from plasmid pMK18 (6) was amplified by PCR using the primers 5′-ATT TGC GGC CGC AGT ATA ACA GAA ACC TTA AGG CCC GAC-3′ (forward) and 5′-ATT TGC GGC CGC CAT CTG TGC GGT ATT TCA CAC C-3′ (reverse). The kanamycin fragment was gel purified (QIAGEN kit), digested with restriction enzyme NotI (underlined), and inserted into the unique NotI site of Mlc (at position 916 bp) by ligation into the previously NotI-digested and dephosphorylated plasmid pFC4 (pGDR11 with N-terminally His6-tagged Mlc between the BamHI and HindIII sites). Competent E. coli DH5α was transformed with the ligation mixture and plated on 1.5% agar LB plates containing 30 μg of kanamycin/ml. The resulting plasmid was called pCL2 (N-terminally His6-tagged MlcTth::kan). The orientation of the thermo-kanamycin cassette in mlc proved to be in the opposite direction of mlc transcription, as tested by digestion of pCL2 plasmid with restriction enzymes BglII and PstI.
Plasmid pCL2 was then double digested with restriction enzymes BamHI and HindIII, and the linear fragment was gel purified and transformed into T. thermophilus cells prepared as follows. An overnight culture of T. thermophilus HB27 (strain DSM7029) grown at 70°C in the medium developed by Brouns et al. (3) and composed of 0.8% Trypton, 0.4% yeast extract, 0.3% NaCl, 3.9 mM CaCl2, and 1.9 mM MgCl2 in Evian mineral water (referred to as Trafo medium here) was diluted 1:25 in Trafo medium and grown for 8 h at 70°C. Linearized pCL2 (1 μg) was added directly into 0.5 ml of the growing culture, followed by incubation for 1 h at 70°C. The mlc::kan recombined mutants were selected on Trafo medium plates (containing 2.8% agar and 30 μg of kanamycin/ml) incubated at 70°C for 2 days. Positive mutants were confirmed by PCR using the primers MlcTthdown (5′-GAG CTT GCC GTT CCA GAG GTT GTT CCA GTC-3′) and MlcTthup (5′-CTC CCT TCC CTG CGG GCT TCC CAG TAT AC-3′). Strain CL3 contains a kanamycin insertion in the chromosome of T. thermophilus at 916 bp from the start codon of mlc (which has a total length of 1,197 bp).
Sugar-binding assays.
The following sugars were tested for their ability to be bound by MlcTth (N-terminally His6-tagged Mlc, purified from a strain harboring pFC4): glucose, glucose-6-phosphate, α-methyl glycopyranoside, mannose, maltose, maltotriose, sucrose, fructose, lactose, galactose, and trehalose. Binding assays were performed at 70°C using a 2.5 or 5 μM concentration of the purified protein incubated with 0.8 μM 14C-labeled sugar in a final reaction volume of 100 μl in binding buffer (50 mM Tris, 200 mM NaCl, 20 mM MgSO4 [pH 7.6]). The reaction was stopped after 5 min by the addition of 2 ml of ice-cold saturated ammonium sulfate (25 mM Tris, 150 mM NaCl [pH 7.5]) and kept on ice for a further 10 min. After filtration of the protein-sugar complex through a nitrocellulose membrane (0.45-μm pore size), unbound sugar was washed out with 2 ml of saturated ammonium sulfate solution, and the radioactivity of the membrane-bound 14C-labeled sugar was measured by using a scintillation counter.
Glucose, mannose, maltose, sucrose, α-methyl glycopyranoside, glucose-6-phosphate, ribose, and fructose were also tested for their ability to be bound by glucose-binding protein (N-terminal His tag purified from pTTC0328) using the protocol described above, except that the quantities were 5 μM glucose-binding protein and 0.8 μM substrate ([14C]glucose). To determine the KD values of glucose-binding protein, a 5 μM concentration of protein (in 100 μl of buffer) was incubated with 50 nM to 1 μM [14C]glucose using undiluted [14C]glucose (311 μCi/μmol; Amersham). In the range of 1 to 50 μM glucose, the sugar concentration was adjusted by the addition of unlabeled glucose but maintaining 1 μM [14C]glucose.
The same procedure was used to determine the glucose-binding kinetics for the TMSP-binding protein. In order to ensure that the binding protein did not contain any unlabeled ligand, the protein was treated with 6 M guanidinium-HCl in binding buffer and dialyzed twice against 200 ml of the same solution. To remove the chaotropic reagent, the sample was dialyzed overnight against binding buffer. The solution was freed from precipitate and used in the assay described above.
TLC for the measurement of glucokinase activity.
The reaction was performed with 10 μg of protein (N-terminally His6-tagged MlcTth purified from pFC4) in a total volume of 500 μl containing 50 mM Tris-HCl (pH 7.5), 50 mM glucose, 50 mM ATP (50 mM ADP, 10 mM GTP, 10 mM CTP), and 10 mM MgCl2. Portions (5 μl) of the reaction mixture were spotted onto a thin-layer chromatography (TLC) plate, and the plate was developed with butanol-ethanol-water (5:3:2). The TLC plate was dipped into methanol containing 5% H2SO4, and after the plate was dried, the sugar-containing spots were visualized by heating at 170°C for 5 min.
Electrophoretic mobility shift assay (EMSA).
Promoter regions of mlc (TTC0329), malE1 (TTC1627), and malK1 (TTC0211) were amplified by hot start PCRs using genomic DNA of T. thermophilus and the respective primers as follows: TthMlcPro_for (5′-TCC AAG AGG GCG TCC AGG ACC TTG GCG TA-3′) and TthMlcPro_rev (5′-CTG AGC TGG TTC AGG ATG GCC CTG CGG TTG-3′) for the mlc promoter amplification, TthE1Pro_for (5′-GTG TAC GAA CAC GTC GGG ACC TTC CT-3′) and TthE1Pro_rev (5′-GCT TGC CGC CAC GCC TAC GCC GA-3′) for the malE1 promoter amplification, and TthK1Pro_for (5′-TCG CCC TCC TCG CCC TGA GGC AG-3′) and TthK1Pro_rev (5′-TTG ACC GCC ACC ACC TTG CCG AA-3′) for the malK1 promoter amplification. The DNA promoter regions were then purified by using a PCR purification kit (QIAGEN), and the amplification step was repeated as described above using the purified promoter regions as a template this time.
Labeled DNA of each promoter region was obtained from the appropriate PCR product by end labeling with T4 polynucleotide kinase (MBI Fermentas) according to the instructions of the manufacturer. The labeled DNA was purified by using Mini-Quick-Spin columns (Roche). The binding buffer was composed of 50 mM HEPES, 200 mM NaCl, and 20 mM MgSO4 (pH 7.5). All samples contained about 7 nCi of labeled DNA (ca. 44 fmol) and 250 ng of poly(dI-dC)-poly(dI-dC) competitor DNA (Roche, Germany) per 10 μl. Mlc (wild type, purified from pEM1) and glucose were added at the concentrations indicated in the figure legends. The reaction mixtures were incubated at 70°C for 10 min, mixed with 5 μl of loading buffer (100 mM HEPES, 400 mM NaCl, 50% glycerol), loaded directly onto 6% native polyacrylamide electrophoresis gels, and run at room temperature under a constant voltage of 200 V.
Transport assays in T. thermophilus.
Precultures were grown at 70°C in minimal medium A (19) with additions as described by Silva et al. (28). The precultures were diluted 1:100 in fresh medium supplemented with 0.4% glucose, maltose, or both. Cells were then grown at 70°C in minimum medium A with Casamino Acids (1%), glucose (0.4%), maltose (0.4%), or a combination of Casamino Acids with glucose or maltose (same proportions as described above). After 6 h at 70°C the cultures were harvested by centrifugation (5,000 × g, 20°C, 5 min), washed three times with minimum medium without carbon source, and resuspended in minimum medium. To measure the transport of glucose or maltose, a cell suspension with an OD600 of 0.03 (wild type) or 0.1 (mlc mutant) was used. To 3 ml of the cell suspension, prewarmed for 2 min at 70°C, 14C-labeled sugars were added to a final concentration for glucose of 112 nM (311 μCi/μmol) and for maltose of 48 nM (680 μCi/μmol). Cells were further incubated at 70°C. At each time point (15, 30, 45, and 60 s), 0.5 ml of the cell suspension was filtered through Millipore filters (pore size, 0.45 μm) with a rapid filtration apparatus and washed once with 5 ml of minimal medium at room temperature. The filters were counted in a toluene-based scintillation fluid by using a scintillation counter (LS 1801). Linear correlations of the number of counts versus time were obtained. The rate of transport in T. thermophilus is expressed as nanomoles per minute per milliliter of cell culture at an OD600 of 1. To determine the Km and Vmax of glucose transport in the wild type and mlc::kan strains, as well as in the ΔmalF::bleo mutant, cells were grown in MMA with CAA as carbon source and 0.4% glucose (wild type and ΔmalF::bleo mutant) and 0.4% maltose (mlc::kan).
RESULTS
Mlc of E. coli has a homologue in the thermophilic bacterium T. thermophilus.
BLAST analysis of the sequence of Mlc of E. coli (i.e., MlcEco) against the full genome of the thermophilic bacterium T. thermophilus HB27 (10) revealed the presence of a homologue of MlcEco in T. thermophilus. Gene TTC0329 encodes a protein with 17% amino acid identities with MlcEco over its entire length. It contains the two consensus sequences that characterize the ROK family of transcriptional regulators (for repressors, open reading frames and kinases) (9), as well as the four residues (one histidine and three cysteines) corresponding to the zinc-binding site involved in the Mlc repressor function in E. coli (25). The sequence of TTC0329 also showed that the protein from T. thermophilus conserved five residues of the glucose-binding motif of glucokinases, unlike MlcEco that lost one of them (His for Asn; Fig. 1). With 17% amino acid identity, the sequence homology between MlcEco and MlcTth is rather low. The reverse analysis, comparing the sequence of MlcTth with all E. coli proteins, revealed again MlcEco but also NagC, a close homologue of MlcEco. However, MlcTth, in contrast to NagC (as discussed below), does bind to PtsG of E. coli, suggesting a close relationship of MlcTth to MlcEco rather than to a number of regulators of the ROK family known to act as repressors. Comparison of MlcTth to all bacterial proteins showed the close relationship of MlcTth with the widely distributed class of the ROK family, notably, the XylR regulator of B. subtilis. However, the genome of T. thermophilus does contain a sequence that has been annotated as XylR and is distinctly different from MlcTth. Therefore, we conclude that MlcTth is evolutionarily related to MlcEco. In this respect it is interesting that the glucose-binding motif appears to be quite common among other Mlc homologues and that Mlc (VC2007) from Vibrio cholerae, a mesophilic bacterium with PTS transporters, actually conserved all five binding sites of the glucose-binding motif of glucokinase (Swiss-Prot accession no. Q9KQJ1).
FIG. 1.
Sequence alignment of MlcTth (TtMlc) and MlcEco (EcMlc). The two consensus sequences that characterize the ROK family of transcriptional regulators (gray shading) are shown: the amino acids involved in the zinc-binding site according to Schiefner et al. (25) are in boldface, the amino acids involved in the glucose binding site of E. coli glucokinase (15) are white on a black background, and the HTH binding motif is indicated by a bar above the sequence.
MlcTth, when overexpressed in E. coli, affects the expression of ptsG.
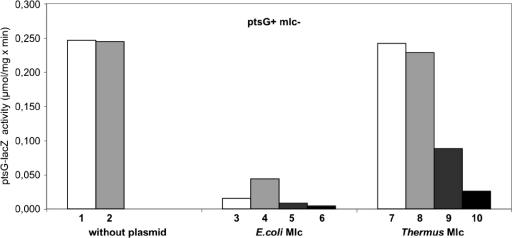
The DNA sequence of MlcTth (TTC0329) was cloned in an IPTG-inducible E. coli vector, yielding pFC4, and transformed into strains of E. coli containing a ptsG-lacZ translational fusion (22). To our surprise, MlcTth influenced the expression of this major known Mlc target gene. As shown in Fig. 2, MlcTth reduced the activity of ptsG, but the effect was much less than that observed for MlcEco (noticeable only when MlcTth is overexpressed). These assays were conducted with strain JM-G2, which contains the ptsG-lacZ translational fusion but lacks mlc and is ptsG+. Although the expression of ptsG was lifted by glucose in the case of MlcEco (only when not overexpressed), glucose noticeably increased the repression of ptsG by MlcTth when overexpressed (Fig. 2). In contrast to the data reported previously (22), we did not observe a significant repression of ptsG-lacZ by glucose in a mutant lacking Mlc, even though ptsG is known to be weakly dependent on cyclic AMP and CAP.
FIG. 2.
Effect of plasmid-encoded MlcTth on ptsG-lacZ (translational fusion) in E. coli after growth in MMA with CAA as a carbon source. These assays were conducted with strain JM-G2, which lacks Mlc but is PtsG+. The data are specific activities of the ptsG-lacZ fusion (μmol of ONPG hydrolyzed per minute per milligram of protein). Bars: 1, without plasmid; 2, without plasmid but with glucose in the growth medium; 3, harboring pSA1 (encoding MlcEco); 4, harboring pSA1 with glucose in the growth medium; 5, harboring pSA1 with IPTG; 6, harboring pSA1 with IPTG and glucose in the growth medium; 7, harboring pFC4 (encoding MlcTth); 8, harboring pFC4 and glucose in the growth medium; 9, harboring pFC4 with IPTG; 10, harboring pFC4 with IPTG and glucose in the growth medium.
As a control, we also tested the effect of MlcTth on a tsx-lacZ fusion that in E. coli is not controlled by Mlc. No effect was observed (data not shown).
We also found that MlcTth had residual ability to bind to the EIIBC domain of E. coli PtsG, but this binding was not released by glucose transport as is the case with MlcEco (data not shown). However, since T. thermophilus does not possess PtsG, the role of MlcTth in its real host had to be different from that of MlcEco in E. coli. That is the next question we sought to address here.
MlcTth, unlike MlcEco, binds glucose and mannose and shows no glucokinase activity.
Since the sequence of gene TTC0329 retained the five residues necessary for glucose binding in glucokinases and given the high structural similarity between Mlc and kinases (25), it seemed likely that the corresponding protein (MlcTth) was in fact a glucokinase. We therefore investigated its glucose-binding and glucokinase activities.
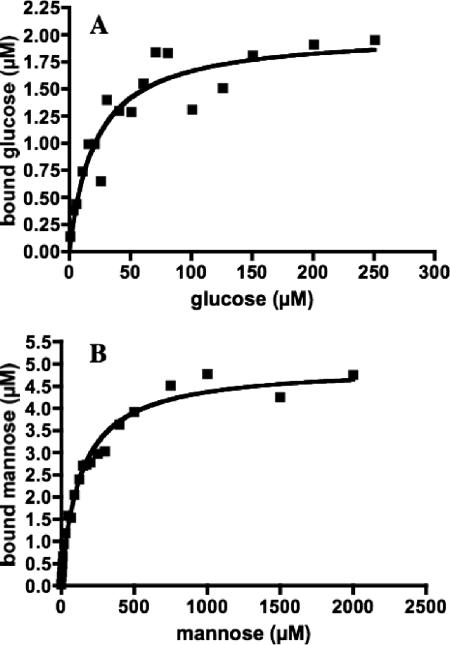
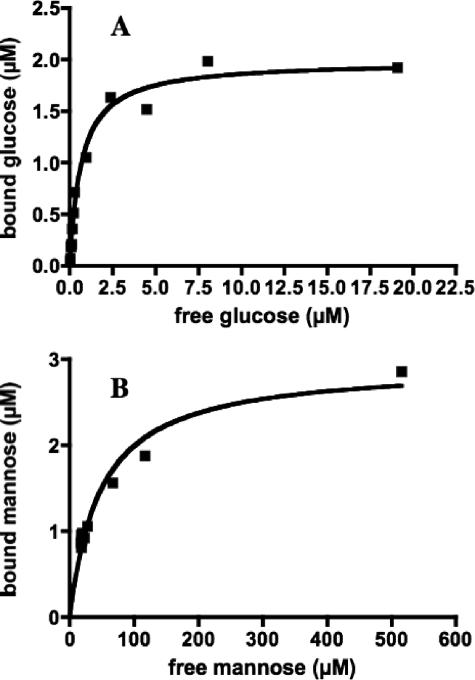
MlcTth was purified to homogeneity (as judged by sodium dodecyl sulfate-polyacrylamide gel electrophoresis) both as an N-terminally His6-tagged version and in its native form. The His6-tagged version was used for the binding and enzymatic assays. MlcTth indeed showed glucose-binding activity. At a 0.8 μM initial substrate concentration and a 2.5 μM protein concentration, it showed its highest binding activity at 70°C (294 nM bound glucose), whereas its activity at 37°C was 30% lower (208 nM bound glucose). The protein worked best in the presence of MgSO4 in the binding buffer (294 nM bound glucose versus 207 nM bound glucose in a binding buffer without MgSO4). In contrast, ZnCl2 reduced its activity (145 nM bound glucose). We therefore kept MgSO4 in the buffer for our standard assays. The apparent KD value for glucose binding was determined to be 20 μM, and the 2.5 μM protein solution bound maximally a 2.1 μM concentration of substrate (Fig. 3A). It is surprising that MlcTth would bind glucose with a much higher affinity than the E. coli glucokinase, which shows a Km for glucose of 0.78 mM (17).
FIG. 3.
Binding of glucose and mannose by MlcTth. (A) MlcTth at 2.5 μM was incubated at 70°C with 14C-labeled glucose, and the amount of bound glucose was determined by ammonium sulfate precipitation. The points obtained can be fitted with a Michaelis-Menten curve exhibiting a KD of 20 μM and binding saturation at 2.1 μM, indicating a stoichiometry of 1:1 (polypeptide-substrate). (B) MlcTth at 5 μM was incubated at 70°C with 14C-labeled mannose. The points obtained can be fitted with a Michaelis-Menten curve exhibiting a KD of 134 μM mannose and a binding saturation of 4.76 μM, again indicating a 1:1 stoichiometry.
Other sugars were also tested for their possible binding to MlcTth. Among them, mannose, the 2-epimer of glucose, was bound by MlcTth with a KD value of 134 μM and in equimolar proportion (ca. 5 μM Mlc bound maximally 4.76 μM mannose; Fig. 3B). In contrast, we did not observe any binding between MlcTth and the following sugars: maltose, maltotriose, sucrose, fructose, lactose, galactose, trehalose, α-methyl-glucopyranoside, or glucose-6-phosphate.
Given its high affinity for glucose and its structural homology to E. coli glucokinase, the glucokinase activity of MlcTth was investigated by using TLC for product identification and compared to the activity of E. coli glucokinase. No glucokinase activity was found for MlcTth (data not shown). Neither divalent ions such as Mg2+ or Zn2+ nor different phosphoryl donors (ATP, ADP, GTP, and CTP) would initiate glucose phosphorylation.
MlcTth is a repressor of its own gene.
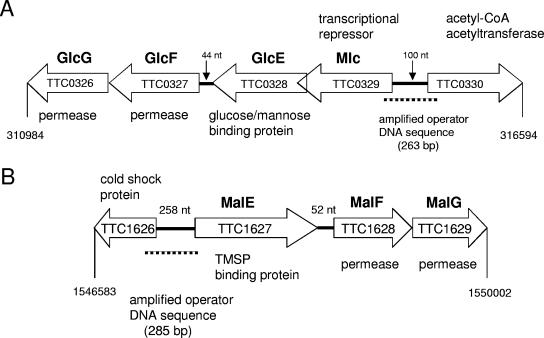
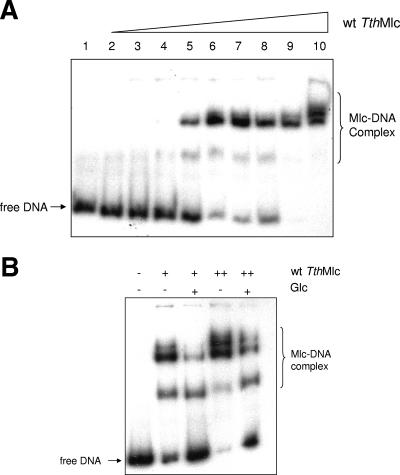
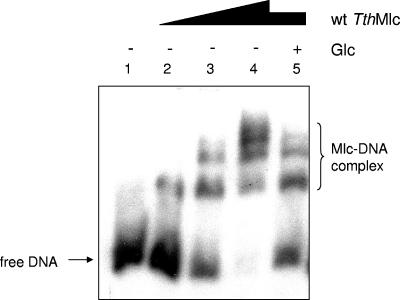
A closer examination of the genomic surroundings of mlc in T. thermophilus revealed that, unlike the gene encoding MlcEco, which is on its own in E. coli, the gene encoding MlcTth belonged to an operon encoding at least four proteins: MlcTth, a putative (annotated) glucose-binding protein, and two putative (annotated) permeases (Fig. 4). Given this information, we tested whether MlcTth would act as a transcriptional regulator on this operon and whether it acted as a repressor inactivated by glucose or as an activator activated by glucose. We used EMSAs to test this proposal. Figure 5A shows that MlcTth was able to shift DNA upstream of the mlc gene, and Fig. 5B shows that glucose was able to counteract the shift. Thus, Mlc acts as a transcriptional repressor on its own gene and, in consequence, on the distal genes in the operon. We also tested mannose for its ability to counteract MlcTth band shifting, since mannose is also bound, albeit weakly, by MlcTth. However, mannose did not counteract DNA shifting by MlcTth. It is not clear why mannose was bound by MlcTth without inactivating the protein. One possibility is that, by competing with glucose binding, mannose would interfere with glucose-dependent induction.
FIG. 4.
Mlc glucose/mannose transport operon and TMSP ABC transport operon organization in T. thermophilus. The TTC numbers refer to the gene numbering in the genome sequencing of T. thermophilus HB27 (10). We propose the names GlcE, GlcF, and GlcG for the glucose/mannose-binding protein and the two membrane components of the glucose/mannose ABC transporter.
FIG. 5.
EMSA between MlcTth and the T. thermophilus mlc promoter. (A) Lanes 1 to 10 represent reactions using 8 ng of labeled DNA amplified from the promoter region of mlc (TTC0329) with the following concentrations of wild-type MlcTth: 0, 0.21, 0.28, 0.56, 0.67, 0.84, 1.11, 1.26, 1.67, and 3.32 μM. (B) Each reaction was done with the same quantity of labeled DNA (8 ng). + and ++, 1.11 and 1.67 μM Mlc, respectively. Glucose was added to a concentration of 200 μM.
Identification of the mlc operon encoding a glucose/mannose-specific ABC transporter.
To demonstrate that the genes distal to mlc encode an ABC transporter, we cloned the gene encoding the binding protein downstream of mlc, purified the protein to homogeneity, and tested its binding of glucose, mannose, maltose, sucrose, α-methyl glycopyranoside, glucose-6-phosphate, ribose, and fructose. The purified binding protein (N terminally His tagged and purified from pTTC0328) bound glucose and mannose with respective apparent KD values of 0.67 and 47 μM and an apparent stoichiometry of 1:2 (substrate-polypeptide), whereas no binding activity was detected for any of the other sugars tested (Fig. 6). At present it is unclear why the glucose/mannose-binding protein only showed an approximate stoichiometry of 1:2. It was not possible to test whether all protein molecules were active.
FIG. 6.
Binding of glucose and mannose by the glucose/mannose-binding protein (GlcE). (A) GlcE at 5 μM was incubated at 70°C with 14C-labeled glucose, and the amount of bound glucose was determined by ammonium sulfate precipitation. The points obtained can be fitted with a Michaelis-Menten curve exhibiting a KD of 0.67 μM and binding saturation at 2.1 μM, indicating a stoichiometry of 2:1 (polypeptide-substrate). (B) MlcTth at 5 μM was incubated at 70°C with 14C-labeled mannose. The points obtained can be fitted with a Michaelis-Menten curve exhibiting a KD of 47 μM and binding saturation of 2.9 μM, again indicating a 2:1 stoichiometry. The low stoichiometry may indicate that not all protein molecules are active.
Recently, we became aware of the work H. W. Hellinga and coworkers at Duke University Medical Center, who crystallized a glucose/galactose-binding protein from Thermus thermophilus (4). Therefore, we tested whether the glucose/mannose-binding protein described here was identical to the protein crystallized by Cuneo et al. (4). Indeed, we found that the glucose/mannose-binding protein bound also galactose with the same affinity as glucose, indicating identity of the two binding proteins.
BLAST analysis of the adjacent two open reading frames, i.e., the annotated permeases, revealed homology to membrane components of the ABC transporters. Thus, the operon seemed to encode a standard ABC transporter for the uptake of glucose and mannose in T. thermophilus.
Transport and regulation of glucose and maltose.
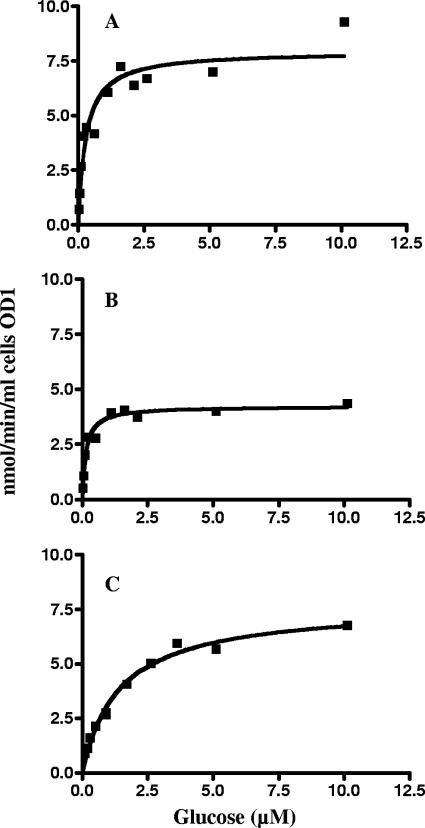
Glucose transport assays with wild-type T. thermophilus (HB27) demonstrated glucose- and maltose-inducible transport of glucose and maltose (Table 2) when measured at fixed substrate concentrations of 320 nM glucose and 48 nM maltose, respectively. The kinetic analysis of glucose uptake in glucose-grown wild-type cells is shown in Fig. 7A. A kan insertion in mlc of T. thermophilus was isolated (strain CL3) that most likely is polar on the distal genes encoding the glucose/mannose ABC transporter. Transport activity of glucose at a 0.32 μM substrate concentration in this mutant was lower than in the wild type. It was weakly induced by glucose but induced threefold by maltose (Table 2). Considering the polar effect of the kan insertion on the glucose transport genes, we suspected that the remaining glucose transport in the mutant was mediated by another system, most likely the TMSP transporter, as shown below. Kinetic analysis of glucose transport in the mlc::kan mutant grown in the presence of maltose gave a Km of 1.4 μM and a Vmax of 7.6 nmol/min per ml of cells at an OD of 1 (Fig. 7C). Also, as shown in Table 3, mannose no longer inhibited glucose uptake.
TABLE 2.
Transport of glucose and maltose in T. thermophilus
| Strain | Growth conditionsa | Initial rate of glucose transportb at 0.32 μM [14C]glucose | Initial rate of maltose transportb at 0.048 μM [14C]maltose |
|---|---|---|---|
| HB27 (wild type) | — | 2.70 | 0.65 |
| Glucose | 10.23 | 0.91 | |
| Maltose | 7.01 | 1.73 | |
| Glucose plus maltose | 6.26 | 0.99 | |
| CL3 (mlc::Kan) | — | 0.58 | 0.41 |
| Glucose | 0.81 | 0.74 | |
| Maltose | 1.84 | 0.91 |
Growth conditions were MMA with Casamino Acids (1%) with no addition (—) or with glucose (0.2%) or maltose (0.2%) or both added in the logarithmic phase 6 h prior to harvest.
The rate of transport is given in nanomoles of substrate taken up per minute per milliliter of culture at an OD600 of 1.
FIG. 7.
Glucose transport kinetics of T. thermophilus. (A) Wild type (HB27); (B) ΔmalF::bleo mutant (JN1); (C) mlc::kan mutant (CL3). Transport by JN1 represents the activity of the glucose/mannose ABC transporter, and transport by CL3 represents the activity of the TMSP ABC transporter. The curves can be fitted according to the Michaelis-Menten equation and yield a Km of 0.15 μM for glucose uptake via the glucose/mannose transporter and a Km of 1.4 μM for the TMSP transporter. A double mutant harboring both ΔmalF::bleo and mlc::kan exhibits neither glucose nor maltose transport activity.
TABLE 3.
Inhibition of glucose transport by mannose and maltose
| Strain | Growth condition | Inhibitor concn (μM) | % Remaining transporta |
|---|---|---|---|
| HB27 (wild type) | Glucose | 10 (mannose) | 24 |
| CL3 (mlc::Kan) | Maltose | 10 (mannose) | 90 |
| CL3 (mlc::Kan) | Maltose | 10 (maltose) | 3.2 |
| JN1 (ΔmalF::Bleo) | Glucose | 10 (maltose) | 89 |
| Glucose | 50 (maltose) | 82 | |
| Glucose | 100 (maltose) | <0.1 |
That is, compared to glucose transport in the wild-type strain HB27 without inhibitor.
We then also measured glucose uptake kinetics in a mutant lacking the TMSP transporter (ΔmalF::bleo). As displayed in Fig. 7B, glucose transport in this mutant showed a Km of 0.15 μM and a Vmax of 4.22 nmol/min per ml cell at an OD of 1 corresponding to a fourfold-higher affinity than that of the cognate glucose/mannose-binding protein for glucose (0.67 μM). The transport kinetics were in accord with Michaelis-Menten kinetics. When [14C]glucose transport at 0.1 μM was measured in the presence of 100 μM unlabeled mannose, glucose transport was abolished (data not shown).
We also transferred the malF::bleo mutation from strain JN1 into the T. thermophilus strain CL3 harboring the mlc::kan insertion, yielding strain CL4 harboring both mutations. As expected, we could no longer detect transport of maltose in this double mutant. The transport of glucose in the double mutant was still measurable but amounted to only 3.7% of that in the wild type.
We conclude that uptake of glucose in the malF::bleo mutant represents the activity of the glucose/mannose ABC transporter only. Thus, transport of glucose, as shown in Fig. 7, is mediated by two transporters systems (Fig. 7A), exclusively by the glucose/mannose system (Fig. 7B), and exclusively by the TMSP system (Fig. 7C).
Surprisingly, the presence of glucose in the growth medium also induced the uptake of maltose. This indicated that MlcTth may also be involved in the regulation of the TMSP ABC transporter. However, the mlc::kan mutant, even though reduced in maltose transport, was still maltose inducible. Thus, MlcTth may stimulate the expression of the TMSP system, but it cannot be its major regulator. MlcTth did shift DNA containing the promoter region of malE, the first gene of the TMSP ABC transport gene cluster, a finding consistent with its stimulatory action, but glucose did not interfere (Fig. 8). When both glucose and maltose were present in the medium, transport of either sugar was reproducibly reduced (Table 2). We interpret this finding by MalK1 becoming limiting for transport through either system when both transporters were induced. As shown below, MalK1 serves the glucose and the TMSP transporters as an ATP-hydrolyzing subunit.
FIG. 8.
EMSA between MlcTth and the malE1 promoter region. Lanes 1 to 5 represent reaction mixtures with 12 ng of labeled DNA amplified from the promoter region of malE1 and increasing concentrations of wild-type MlcTth. Lanes 1 to 4, 0, 0.84, 1.67, and 3.32 μM, respectively. Lane 5 contained 1.67 μM MlcTth (identical to lane 3) but was done in the presence of 200 μM glucose.
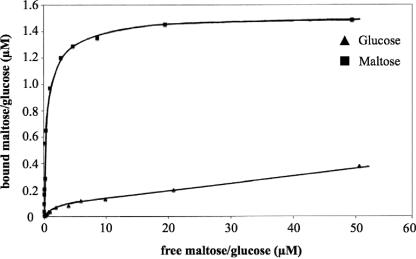
In parallel, we cloned and purified the binding protein from the TMSP transporter to homogeneity (as judged by sodium dodecyl sulfate-polyacrylamide gel electrophoresis) and assessed its binding affinity for glucose and maltose. Although the protein bound maltose very well with a standard Michaelis-Menten pattern and a KD of 0.1 μM (Fig. 9, upper curve) as reported previously (30), the affinity for glucose did not show Michaelis-Menten characteristics but appeared negatively cooperative (Fig. 9, lower curve). We could not reach saturation at a reasonably high concentration of glucose (ca. 100 μM). In order to remove any possible hidden unlabeled ligand, both binding tests were done with the same protein preparation (and the same protein concentration of 5 μM) that had been denatured with 6 M guanidinium chloride, dialyzed, and renaturated in dilute buffer. Although this treatment resulted in substantial loss of binding activity (at saturation only 1.6 μM maltose was bound), it did not affect the maltose-binding characteristics (Fig. 9, upper curve). However, it did not alter the peculiar binding pattern for glucose.
FIG. 9.
Binding of maltose and glucose by the TMSP-binding protein (MalE). A 5 μM concentration of the TMSP-binding protein was incubated at 70°C with 14C-labeled maltose (▪) or 14C-labeled glucose (▴), and the bound sugar was determined by ammonium sulfate precipitation in each case. The points obtained for maltose can be fitted with a Michaelis-Menten curve exhibiting a KD of 0.1 μM (upper curve), whereas the points for glucose could not be fitted by applying the Michaelis-Menten equation (lower curve). The protein used was identical for the maltose- and glucose-binding assays. It had been denatured by 6 M guanidinium-HCl and renatured in order to remove any potential hidden ligand. The treatment reduced the binding activity but did not alter the binding characteristics.
Thus, a hidden unlabeled ligand cannot be the reason for this peculiar binding behavior toward glucose. Inhibition studies of glucose uptake showed that mannose was not transported via the TMSP ABC transporter. As seen in Table 3, mannose inhibited ca. 80% of the glucose transport in the wild-type strain possessing both transporters. In the mlc::kan mutant, mannose only inhibited glucose transport by 10%. In contrast, 100 μM mannose completely inhibited transport of 0.32 μM [14C]glucose in the ΔmalF::bleo mutant. Thus, in contrast to glucose, mannose is only transported by the glucose/mannose transporter but not by the TMSP transporter.
MalK1 is the shared ATP-hydrolyzing subunit for both the glucose and the TMSP transporters.
We had previously identified MalK1 as the ATP-hydrolyzing subunit of the TMSP ABC transporter. A kan insertion mutant in malK1 had lost the ability to grow on maltose or trehalose (28). We now tested the transport of glucose and maltose in the malK1::kan mutant and found that both activities had been lost completely. Thus, MalK1 is shared by both the glucose and the TMSP transporters.
Band shift analysis showed that MlcTth did not shift the malK1 operator (not shown), meaning that the regulation of malK1 is MlcTth independent.
Quaternary structure of MlcTth.
MlcTth cloned as an N-terminally His-tagged version was purified, and its molecular weight was determined in the presence or absence of glucose by molecular sieve chromatography. In both cases, an identical molecular weight of 145,000 at ambient temperature was estimated, indicating a tetrameric quaternary structure (not shown). However, native MlcTth purified by ion-exchange chromatography behaved differently. It showed a strong tendency to multimerize and to precipitate from solutions, preventing a meaningful characterization of its quaternary structure.
DISCUSSION
We report here for the first time the presence and function of Mlc in a thermophilic bacterium. Mlc has been widely studied in E. coli, and it is well known that its activity as a global repressor for sugar uptake is inhibited by binding to the dephosphorylated state of the membrane-associated EIIBGlc domain of the PtsG protein occurring during the transport of glucose (13, 14, 19, 26, 30). What makes the presence of Mlc in a thermophilic bacterium so special is that thermophilic bacteria do not possess PTS transport systems. Therefore, the inactivation of Mlc in T. thermophilus cannot occur via sequestration to a PTS transporter. Nevertheless, MlcTth did affect the expression of ptsG in E. coli, revealing its relatedness to MlcEco. The effect on ptsG expression was weaker and in the same direction as that of MlcEco, even though glucose increased the repression instead of releasing it.
We found that MlcTth is a transcriptional regulator for the glucose/mannose ABC transporter in T. thermophilus and that it is controlled by glucose binding, which affected (reduced) its operator binding. The best evidence for the function as a glucose-specific transcriptional regulator was its ability to shift a DNA fragment containing the upstream regulatory region of the operon harboring the glucose ABC transporter. The presence of glucose counteracts the shift, identifying it as an inducer.
An insertion mutation in mlc did not lead to constitutivity of glucose transport, as expected for a repressor, but resulted in strongly reduced transport activity. This can be explained by the polar effect that the kan insertion in mlc exerts on the downstream glucose transport genes.
Glucose transport remaining in the mlc mutant was due to the action of the TMSP ABC transporter. This could be demonstrated by near-complete inhibition of glucose transport by maltose in the mlc mutant and by the fact that the isolated TMSP-binding protein could also recognize glucose.
The TMSP ABC transporter has been characterized as a constitutive system of high activity (28). Here, we demonstrate that, with Casamino Acids as the major carbon source and maltose or glucose as an additive in the medium, the transport activity of maltose was slightly induced by glucose and threefold induced by maltose. The expression of maltose transport activity is clearly lower in the mlc mutant than in the wild type, indicating that MlcTth is involved in the regulation of the TMSP ABC transporter genes as well. MlcTth shifted a DNA fragment containing the promoter/operator sequence of the TMSP ABC transporter genes. However, in contrast to its action on the mlc promoter/operator sequence, glucose does not prevent shifting. Thus, if anything, MlcTth has to act as an auxiliary activator of the TMSP system rather than as a repressor, as observed with the glucose/mannose ABC transporter genes.
In contrast, glucose was seen to weakly induce the TMSP ABC transporter, meaning that either the uptake of glucose in the cytoplasm leads to inducer formation for the TMSP ABC transporter genes or the glucose itself is acting as an inducer. Since all substrates of the TMSP ABC transporter contain glucose, the metabolism of these sugars will form free glucose that may act as an inducer.
The characterization of the MlcTth-controlled ABC glucose transporter in a mutant lacking the TMSP system revealed a high affinity (Km = 0.15 μM) and a Vmax of 4.22 nmol/min per 1 ml of cells at an OD of 1, which is sufficient for the maintenance of growth on glucose as the sole source of carbon. The corresponding glucose-binding protein exhibited a KD for glucose binding of 0.67 μM, which is somewhat higher than the transport Km. This may well be due to an in vivo stoichiometric excess of binding protein over the membrane components and the exclusive interaction of only the substrate-loaded binding protein with the latter, which is in contrast to the situation of the maltose transporter in E. coli (16). The glucose-binding protein also recognizes d-mannose, the 2-epimer of glucose, with a clearly reduced affinity (KD = 47 μM). This and the inhibition of glucose transport in the wild type (but not in the mlc mutant) by mannose showed that the glucose transporter of T. thermophilus also accommodates d-mannose. A similar overlap of specificities is also seen in the PTS-dependent glucose and mannose transporter of E. coli (8). The overlap between the glucose and the mannose specificity of the cognate-binding protein is reflected in the specificity of Mlc. The KD for glucose binding was 20 μM and for mannose binding was 134 μM, both with a stoichiometry of 1:1 (polypeptide-substrate). However, whereas glucose interfered with the binding of MlcTth to its operator, mannose did not. Thus, the inducer of the system is only glucose. This is reminiscent of the situation wherein MlcEco regulates both the glucose-specific PtsG (22) and the mannose/glucose-specific PtsM (ManXYZ) (21), but only transport of glucose via PtsG controls the activity of MlcEco (13, 14, 19, 26, 30).
We have demonstrated that glucose can also be taken up by the TMSP ABC transporter. This became clear when we analyzed the remaining glucose transport in the mlc::kan mutant: it was no longer inhibited by mannose but was completely inhibited by maltose. In addition, a strain carrying a mlc::kan and a malF::bleo mutation could no longer transport glucose (3.7% remaining transport). Glucose transport via the TMSP ABC transporter (as determined in the mlc::kan mutant) is somewhat peculiar. Its transport Km of 1.4 μM reflects a reasonably high affinity, but high concentrations of glucose do not completely inhibit maltose or trehalose transport in the wild type (28). The recognition site of the system, the TMSP-binding protein which binds maltose with high affinity (Fig. 9, upper curve) and Michaelis-Menten characteristics, does bind glucose, but its binding isotherm is not of the Michaelis-Menten type. Rather, it indicated that binding of glucose is negatively cooperative.
We observed that mutants lacking MalK1, which had previously been shown to be devoid of the TMSP ABC transport activity (28), had also lost glucose transport via the glucose/mannose ABC transporter. Thus, this ABC subunit must be shared by both systems. One observation is relevant in this respect. When wild-type cells were grown in the presence of both glucose and maltose, the transport activity for either glucose or maltose was significantly less than when the cells were grown in the presence of either sugar. Possibly, MalK1 (which is not controlled by ThMlc) is constitutively expressed and becomes limiting for transport when both systems are induced. Are there other ABC transporters that would make use of MalK1? Sequence analysis of sugar ABC transporters in T. thermophilus showed seven systems (including the glucose/mannose and TMSP systems) without an ATP-hydrolyzing enzyme encoded within their gene clusters. Moreover, there are only two “isolated” genes encoding “sugar ATPases” including MalK1. Therefore, it is not unlikely that MalK1 may serve yet another ABC transporter as an energizing subunit.
Upon examination of the structure of MlcEco and glucokinase of E. coli, their close structural relatedness becomes apparent (25). It seems reasonable to conclude that MlcEco has evolved from a glucokinase by the acquisition of the DNA-binding domain. MlcTth has lost the kinase activity but kept glucose binding to control gene expression. Interestingly, MlcTth does have residual binding affinity for the PtsG of E. coli even though T. thermophilus has not yet acquired the PTS-type transporters. One might speculate that it was only after the appearance of PTS in mesophilic bacteria that PtsG was optimized for the binding of Mlc, which in turn lost its ability to recognize glucose as repressor-controlling principle, replacing it by sequestration to PtsG.
It will be interesting to compare the structure of MlcTth to that of MlcEco with respect to the evolutionary alteration toward the sequestration mode of repressor regulation.
Acknowledgments
We thank Jutta Nesper for strain JN1, Stefan Schönert for technical support and advice, and Erika Oberer-Bley for the lectorship.
This study was supported by several grants from the Deutsche Forschungsgemeinschaft (esp. TR-SFB 11).
REFERENCES
- 1.Achenbach-Richter, L., R. Gupta, K. O. Stetter, and C. R. Woese. 1987. Were the original eubacteria thermophiles? Syst. Appl. Microbiol. 9:34-39. [DOI] [PubMed] [Google Scholar]
- 2.Baneyx, F., and G. Georgiou. 1991. Construction and characterization of Escherichia coli strains deficient in multiple secreted proteases: protease III degrades high-molecular-weight substrates in vivo. J. Bacteriol. 173:2696-2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brouns, S. J., H. Wu, J. Akerboom, A. P. Turnbull, W. M. de Vos, and J. van der Oost. 2005. Engineering a selectable marker for hyperthermophiles. J. Biol. Chem. 280:11422-11431. [DOI] [PubMed] [Google Scholar]
- 4.Cuneo, M. J., A. Changela, J. J. Warren, L. Beese, and H. W. Hellinga. Submitted for publication.
- 5.Decker, K., J. Plumbridge, and W. Boos. 1998. Negative transcriptional regulation of a positive regulator: the expression of malT, encoding the transcriptional activator of the maltose regulon of Escherichia coli, is negatively controlled by Mlc. Mol. Microbiol. 27:381-390. [DOI] [PubMed] [Google Scholar]
- 6.de Grado, M., P. Castan, and J. Berenguer. 1999. A high-transformation-efficiency cloning vector for Thermus thermophilus. Plasmid 42:241-245. [DOI] [PubMed] [Google Scholar]
- 7.Galperin, M. Y., K. M. Noll, and A. H. Romano. 1996. The glucose transport system of the hyperthermophilic anaerobic bacterium Thermotoga neapolitana. Appl. Environ. Microbiol. 62:2915-2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garcia-Alles, L. F., A. Zahn, and B. Erni. 2002. Sugar recognition by the glucose and mannose permeases of Escherichia coli. Steady-state kinetics and inhibition studies. Biochemistry 41:10077-10086. [DOI] [PubMed] [Google Scholar]
- 9.Hansen, T., B. Reichstein, R. Schmid, and P. Schonheit. 2002. The first archaeal ATP-dependent glucokinase, from the hyperthermophilic crenarchaeon Aeropyrum pernix, represents a monomeric, extremely thermophilic ROK glucokinase with broad hexose specificity. J. Bacteriol. 184:5955-5965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henne, A., H. Bruggemann, C. Raasch, A. Wiezer, T. Hartsch, H. Liesegang, A. Johann, T. Lienard, O. Gohl, R. Martinez-Arias, C. Jacobi, V. Starkuviene, S. Schlenczeck, S. Dencker, R. Huber, H. P. Klenk, W. Kramer, R. Merkl, G. Gottschalk, and H. J. Fritz. 2004. The genome sequence of the extreme thermophile Thermus thermophilus. Nat. Biotechnol. 22:547-553. [DOI] [PubMed] [Google Scholar]
- 11.Howland, J. L. 2001. Microbial survivors: thermophiles, halophiles, and other prodigies. Biologist 48:278-282. [PubMed] [Google Scholar]
- 12.Kim, S. Y., T. W. Nam, D. Shin, B. M. Koo, Y. J. Seok, and S. Ryu. 1999. Purification of Mlc and analysis of its effects on the pts expression in Escherichia coli. J. Biol. Chem. 274:25398-25402. [DOI] [PubMed] [Google Scholar]
- 13.Lee, S. J., W. Boos, J. P. Bouche, and J. Plumbridge. 2000. Signal transduction between a membrane-bound transporter, PtsG, and a soluble transcription factor, Mlc, of Escherichia coli. EMBO J. 19:5353-5361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee, S. J., C. Moulakakis, S. M. Koning, W. Hausner, M. Thomm, and W. Boos. 2005. TrmB, a sugar sensing regulator of ABC transporter genes in Pyrococcus furiosus exhibits dual promoter specificity and is controlled by different inducers. Mol. Microbiol. 57:1797-1807. [DOI] [PubMed] [Google Scholar]
- 15.Lunin, V. V., Y. Li, J. D. Schrag, P. Iannuzzi, M. Cygler, and A. Matte. 2004. Crystal structures of Escherichia coli ATP-dependent glucokinase and its complex with glucose. J. Bacteriol. 186:6915-6927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Merino, G., W. Boos, H. A. Shuman, and E. Bohl. 1995. The inhibition of maltose transport by the unliganded form of the maltose-binding protein of Escherichia coli: experimental findings and mathematical treatment. J. Theor. Biol. 177:171-179. [DOI] [PubMed] [Google Scholar]
- 17.Meyer, D., C. Schneider-Fresenius, R. Horlacher, R. Peist, and W. Boos. 1997. Molecular characterization of glucokinase from Escherichia coli K-12. J. Bacteriol. 179:1298-1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller, J. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 19.Nam, T. W., S. H. Cho, D. Shin, J. H. Kim, J. Y. Jeong, J. H. Lee, J. H. Roe, A. Peterkofsky, S. O. Kang, S. Ryu, and Y. J. Seok. 2001. The Escherichia coli glucose transporter enzyme IICB(Glc) recruits the global repressor Mlc. EMBO J. 20:491-498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peist, R., A. Koch, P. Bolek, S. Sewitz, T. Kolbus, and W. Boos. 1997. Characterization of the aes gene of Escherichia coli encoding an enzyme with esterase activity. J. Bacteriol. 179:7679-7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Plumbridge, J. 1998. Control of the expression of the manXYZ operon in Escherichia coli: Mlc is a negative regulator of the mannose PTS. Mol. Microbiol. 27:369-380. [DOI] [PubMed] [Google Scholar]
- 22.Plumbridge, J. 1998. Expression of ptsG, the gene for the major glucose PTS transporter in Escherichia coli, is repressed by Mlc and induced by growth on glucose. Mol. Microbiol. 29:1053-1063. [DOI] [PubMed] [Google Scholar]
- 23.Plumbridge, J. 1999. Expression of the phosphotransferase system both mediates and is mediated by Mlc regulation in Escherichia coli. Mol. Microbiol. 33:260-273. [DOI] [PubMed] [Google Scholar]
- 24.Sambrook, J. F., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 25.Schiefner, A., K. Gerber, S. Seitz, W. Welte, K. Diederichs, and W. Boos. 2005. The crystal structure of Mlc, a global regulator of sugar metabolism in Escherichia coli. J. Biol. Chem. 280:29073-29079. [DOI] [PubMed] [Google Scholar]
- 26.Seitz, S., S. J. Lee, C. Pennetier, W. Boos, and J. Plumbridge. 2003. Analysis of the interaction between the global regulator Mlc and EIIBGlc of the glucose-specific phosphotransferase system in Escherichia coli. J. Biol. Chem. 278:10744-10751. [DOI] [PubMed] [Google Scholar]
- 27.Shin, D., S. Lim, Y. J. Seok, and S. Ryu. 2001. Heat shock RNA polymerase (E sigma(32)) is involved in the transcription of mlc and crucial for induction of the Mlc regulon by glucose in Escherichia coli. J. Biol. Chem. 276:25871-25875. [DOI] [PubMed] [Google Scholar]
- 28.Silva, Z., M. M. Sampaio, A. Henne, A. Bohm, R. Gutzat, W. Boos, M. S. da Costa, and H. Santos. 2005. The high-affinity maltose/trehalose ABC transporter in the extremely thermophilic bacterium Thermus thermophilus HB27 also recognizes sucrose and palatinose. J. Bacteriol. 187:1210-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spiess, C., A. Beil, and M. Ehrmann. 1999. A temperature-dependent switch from chaperone to protease in a widely conserved heat shock protein. Cell 97:339-347. [DOI] [PubMed] [Google Scholar]
- 30.Tanaka, Y., K. Kimata, and H. Aiba. 2000. A novel regulatory role of glucose transporter of Escherichia coli: membrane sequestration of a global repressor Mlc. EMBO J. 19:5344-5352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tanaka, Y., K. Kimata, T. Inada, H. Tagami, and H. Aiba. 1999. Negative regulation of the pts operon by Mlc: mechanism underlying glucose induction in Escherichia coli. Genes Cells 4:391-399. [DOI] [PubMed] [Google Scholar]