Abstract
A novel tungstate and molybdate binding protein has been discovered from the hyperthermophilic archaeon Pyrococcus furiosus. This tungstate transport protein A (WtpA) is part of a new ABC transporter system selective for tungstate and molybdate. WtpA has very low sequence similarity with the earlier-characterized transport proteins ModA for molybdate and TupA for tungstate. Its structural gene is present in the genome of numerous archaea and some bacteria. The identification of this new tungstate and molybdate binding protein clarifies the mechanism of tungstate and molybdate transport in organisms that lack the known uptake systems associated with the ModA and TupA proteins, like many archaea. The periplasmic protein of this ABC transporter, WtpA (PF0080), was cloned and expressed in Escherichia coli. Using isothermal titration calorimetry, WtpA was observed to bind tungstate (dissociation constant [KD] of 17 ± 7 pM) and molybdate (KD of 11 ± 5 nM) with a stoichiometry of 1.0 mol oxoanion per mole of protein. These low KD values indicate that WtpA has a higher affinity for tungstate than do ModA and TupA and an affinity for molybdate similar to that of ModA. A displacement titration of molybdate-saturated WtpA with tungstate showed that the tungstate effectively replaced the molybdate in the binding site of the protein.
Molybdenum and tungsten have similar ionic radii and chemical properties. Tungsten is the heaviest atom and the only third-row transition element that exhibits biological activity in enzymes. Molybdenum is the only second-row transition metal that exhibits biological activity when it is present in a cofactor of a metalloenzyme. Both metals are present mainly in enzymes that catalyze oxygen atom transfer reactions. In these enzymes they are coordinated by the two dithiolene sulfur atoms of a pterin molecule, called a molybdopterin cofactor (25). In the case of tungsten, the metal is always coordinated by two pterin moieties, forming a so called bis-pterin cofactor (9, 13). Molybdenum is an essential trace metal for many forms of life whereas tungsten is found mostly in archaea and in some bacteria. The molybdenum cofactor-containing enzymes can be divided into three families depending on the coordination chemistry of the Mo ligand: the sulfite oxidases; the xanthine oxidoreductases, also including the aldehyde oxidases; and the dimethyl sulfoxide reductases, which are found only in prokaryotes (18, 35). The tungsten cofactor-containing enzymes consist only of the aldehyde oxidoreductases (AORs), formate dehydrogenases (FDHs), and an acetylene hydratase (13). Based on sequence comparison the FDHs and acetylene hydratase are part of the molybdenum-containing dimethyl sulfoxide reductase family.
The transport of molybdate has been well characterized in particular for Escherichia coli, which expresses a high-affinity ABC transporter for molybdate encoded by the modABC genes (27). The periplasmic molybdate binding protein ModA binds specifically molybdate and tungstate and not sulfate or other anions (27). Crystal structures of the E. coli and the Azotobacter vinelandii ModA indicate that the specificity for molybdate and tungstate is mostly determined by the size of the binding pocket. The Cambridge Structural Database (2) gives 1.75 ± 0.04 Å and 1.76 ± 0.02 Å for molybdate and tungstate, respectively, and 1.47 ± 0.02 Å for sulfate. The ModA proteins cannot discriminate between molybdate and tungstate. The first tungsten-specific ABC transporter was identified in Eubacterium acidaminophilum (17). The periplasmic tungsten uptake protein (TupA) was cloned and expressed in E. coli and was shown to bind only tungstate with a high affinity. A crystal structure of TupA is not yet available, and it is not clear what the structural basis is of the specificity for tungstate over molybdate. Recently, a high-affinity vanadate transporter, which was highly selective for vanadate compared to tungstate, was identified in the cyanobacterium Anabaena variabilis ATCC 29413 based on the sequence similarity with the TupA protein from E. acidaminophilum (58% sequence similarity) (24). A. variabilis ATCC 29413 expresses an alternative V-dependent nitrogenase for the fixation of nitrogen, and therefore it requires vanadate (24). The specificity of this transporter for vanadate indicates that high sequence similarities are not conclusive for the selectivity of the transporter.
Our goal was to study tungstate transport in an organism that is strictly dependent on tungstate, the hyperthermophilic archaeon Pyrococcus furiosus. This organism grows optimally at 100°C under strict anaerobic conditions (7). In the last decade five tungsten-containing aldehyde oxidoreductase enzymes were purified and characterized from P. furiosus. AOR (21), formaldehyde oxidoreductase (31), and tungsten-containing oxidoreductase 5 (WOR5) (5) all have a broad substrate specificity for aldehydes varying from shorter chains and C4 to C6 semialdehydes (FOR) to longer, aromatic, and aliphatic backbones (AOR and WOR5). These broad substrate specificities do not immediately imply a clear physiological function for these proteins; microarray experiments indicate that they might play a role in peptide fermentation or in stress response (34, 38). In contrast, glyceraldehyde-3-phosphate (GAP) oxidoreductase is known only to convert the substrate GAP (22). It is the only W-containing aldehyde oxidoreductase with an assigned function, namely, in the Embden-Meyerhof type of glycolysis, where it converts GAP to 3-phosphoglycerate, replacing glyceraldehyde-3-phosphate dehydrogenase and phosphoglycerate kinase. Tungsten oxidoreductase 4 (WOR4) was purified from P. furiosus grown in the presence of elemental sulfur (S0) (30). No substrate has been identified yet for WOR4. Besides these five tungsten-containing enzymes of the AOR family, the genome of P. furiosus also includes two genes for putative tungsten- or molybdenum-containing FDHs (29).
Cultivation experiments indicated that P. furiosus has a highly specific tungstate uptake mechanism. When molybdate was added to the growth medium in a 1,000-fold excess, the cells were able to selectively scavenge the traces of tungstate from the medium and use it for the incorporation in the cofactor of the AOR enzymes (23).
The genome of P. furiosus does not carry a tupA homologue; however, a putative sulfate/thiosulfate/molybdate transporter is present that has 30% sequence similarity with the ModA protein from E. coli (PF0080/PF0081/PF0082) (18% sequence identity). The other components of this putative transporter, WtpB and WtpC, have a high sequence similarity to ModB/TupB (53% and 50% similarity) and ModC/TupC (51% and 56% similarity), respectively. Besides this putative sulfate/thiosulfate/molybdate transporter, the only ABC transporter encoded in the genome with some similarity (28%) to ModA is annotated as a putative phosphate transporter (PF1003/PF1006/PF1007/PF1008). However, the sequence identity is much lower, only 11%.
Since no molybdenum enzymes have been identified yet from P. furiosus, we hypothesized that the operon that contains the PF0080, PF0081, and PF0082 genes codes for a tungstate-selective ABC transporter. An mRNA fragment carrying the PF0080 gene has previously been detected in microarray experiments (38), indicating that the protein is expressed in vivo.
In this paper we describe the cloning, expression, and binding characteristics of this new tungstate transport protein (WtpA).
MATERIALS AND METHODS
Materials.
All chemicals used were of the highest quality available. Prepacked Strep-tag columns were used as recommended by the supplier (IBA).
Cloning of the WtpA gene.
The WtpA gene (PF0080) was amplified by PCR using Pfx polymerase (Invitrogen); sense (WtpA_F_Bsa1) and antisense (WtpA_R_Bsa1) primers (Thermohybaid) 5′-ATGGTACGTCTCAAATGCGAGAGGG-3′ and 5′-ATGGTACGTCTCAGCGCTCTTTTCAAT-3′, respectively; and chromosomal DNA from P. furiosus as a template. Extraction of the chromosomal DNA was performed with phenol-chloroform-isoamyl alcohol (32). The PCR product was treated with Taq polymerase (Amersham Bioscience) for 10 min at 72°C to obtain single 3′-adenine overhangs for subcloning into the pCR2.1-TOPO vector (Invitrogen). This TOPO construct was transformed into competent E. coli TOP10 cells (Invitrogen), and plasmid was isolated from an overnight culture. Both primers contain a BsaI restriction site, and they were used to clone the wtpA gene into the BsaI site of the pASK-IBA2 expression vector (IBA), resulting in a WtpA fusion protein with an N-terminal OmpA E. coli signal peptide and a C-terminal Strep-tag. This construct was transformed into competent E. coli BL21-CodonPlus-(DE3)-RIL cells (Stratagene) and sequenced for confirmation.
Protein expression and purification.
E. coli BL21(DE3) cells that contained the plasmid encoding the WtpA fusion protein were grown on LB medium containing 100 μg/ml ampicillin. Protein synthesis was induced with 200 μl of an anhydrotetracycline solution (2 mg/ml in dimethyl sulfoxide) per liter of culture, when the absorbance of the culture reached 0.5 at 600 nm. Cells were induced for 4 h at 30°C and harvested by centrifugation. The cells were washed with 100 mM Tris-HCl, pH 8.0, and were broken in the same buffer (1 g of cells per 5 ml buffer) with a cell disrupter system (Constant Systems). Cell extract was obtained by centrifugation for 20 min at 15,000 × g at 4°C. As a first purification step the supernatant was heated for 30 min at 60°C. Precipitated protein was removed by centrifugation, and the remaining cell extract was applied to a 1-ml Strep-Tactin column (IBA) equilibrated with buffer W (100 mM Tris-HCl, pH 8.0, 150 mM NaCl, 1 mM EDTA). The column was washed with 5 ml buffer W, and the protein was eluted in 3 ml buffer W containing 2.5 mM desthiobiotin.
Gel shift assay.
The gel shift assay developed by Rech et al. (28) was used to qualitatively observe the binding of different oxoanions to the protein. Samples of 10 μl containing 25 μM of purified WtpA in 10 mM Tris-HCl, pH 8.0, were incubated in 50 mM potassium acetate (pH 5.0) and 10 mM of one of the oxoanions (sulfate, phosphate, chlorate, molybdate, and tungstate) for 30 min on ice. Samples were analyzed on a high-density (20%) native polyacrylamide gel on a Phast System (GE Healthcare). The electrophoresis was performed at a voltage of 150 V, at 4°C for approximately 4 h.
Size-exclusion chromatography.
Size-exclusion chromatography was performed using an analytical HR10/30 Superdex 200 column (GE Healthcare) equilibrated with 20 mM Tris-HCl, pH 8.0, 150 mM NaCl. WtpA (35 μM) was incubated with different concentrations of tungstate and/or molybdate in a total volume of 100 μl for 30 min at room temperature. The protein was separated from unbound oxoanion with a flow rate of 0.5 ml/min. Fractions were collected, and the molybdenum and tungsten content was determined by catalytic adsorptive stripping voltammetry (8).
Radioactively labeled tungstate experiments.
187W (half-life, 23.8 h) was produced by irradiation of 10 mg Na2WO4 in a thermal neutron flux of 4 × 1016 m−2 s−1 for 10 h. The specific activity was 8 × 1012 Bq 187W per mole W. The target material was dissolved in 1.0 ml 20 mM Tris-HCl, pH 8.0.
WtpA (0.5 μM) was incubated with various concentrations (25 nM to 20 μM) of radioactively labeled sodium tungstate for 5 min in 20 mM Tris-HCl, pH 8.0, at room temperature in a total volume of 250 μl. Dowex AG-1X8 (100/200 mesh, Cl− form; Fluka), 50 mg, was added as a slurry (1 volume water/volume resin) to remove unbound tungstate from the protein solution. The Dowex was allowed to settle for 5 min, and the 187W in 200 μl of the supernatant was determined in a Wallac (Turku, Finland) 1480 Automatic 3" gamma counter. The amount of 187W in the Dowex and the residual supernatant was also determined in this gamma counter.
ITC.
Prior to all isothermal titration calorimetry (ITC) experiments WtpA was extensively dialyzed against large volumes of ITC buffer (10 mM Tris-HCl, 50 mM NaCl, pH 8.0) at 4°C. Tungstate and molybdate stock solutions (1 M) were prepared in H2O and diluted to an 0.1 to 0.4 mM final concentration using ITC buffer. Tungstate and molybdate were titrated as ligand into the sample cell (1.42 ml) containing 7 to 15 μM WtpA. Tungstate and molybdate were injected in 2- to 4-μl injections to reach a final molar ratio of ligand to WtpA ranging from 2:1 to 4:1 at the end of the experiment. Blank injections of titrant into the buffer were performed to estimate the heat of injection, mixing, and dilution, which was similar to the heat release that was seen at the end of each titration after saturation was reached. At least three experiments were performed with tungstate and molybdate, and two displacement titrations with tungstate were done using molybdate-saturated WtpA (containing a 1.5-fold molar excess of molybdate). All experiments were performed at 25°C using a VP-ITC Microcalorimeter (MicroCal, Northampton, Mass.). For every injection the binding enthalpy was calculated by integration of the peak area using ORIGIN software. The heat change after each injection is related to the calorimetric enthalpy of binding (ΔHcal) and is also dependent on the stoichiometry of the WtpA/anion complex. The association constant (Ka) and additional binding parameters (binding stoichiometry, enthalpy, and entropy) were obtained through curve fitting with ORIGIN. Baseline subtraction was performed manually by averaging the last 5 to 10 injections after reaching saturation and subtracting from the ΔHcal.
Protein assays.
Protein concentration was determined using the bicinchoninic acid assay method with bovine serum albumin as the standard. Molecular weight and degree of purity were determined with sodium dodecyl sulfate-polyacrylamide gel electrophoresis on a Phast System (GE Healthcare) in 8 to 25% gradient sodium dodecyl sulfate-polyacrylamide gels.
RESULTS
Cloning and purification of WtpA.
The N-terminal amino acid sequence of WtpA (PF0080) starts with a putative lipobox of a lipoprotein signal peptide. The consensus sequence for a lipobox in bacteria is [I/L/G/A]-[A/G/S]-C (1), and in the case of WtpA it is AGC (Fig. 1). Furthermore, significant homology was found with leader peptides from other periplasmic components of anion ABC transporters in archaea with the putative motif KK-X14-GC (GC is part of the lipobox sequence [Fig. 1]) (1). The cysteine is always conserved in this motif. In bacteria it has been shown that this residue is lipid modified prior to cleavage by type II signal peptidases (1). These lipoproteins have not yet been identified in archaea. Some archaeal solute binding proteins have been characterized that also contain a typical lipobox motif (SGC), e.g., the maltotriose binding protein of P. furiosus (14). However, these proteins were N-terminally blocked, and therefore the presence of the lipid moiety could not be confirmed. The leader peptides are split at the N-terminal side of the cysteine in the case of bacteria; the cleavage site in archaea is unknown. In bacteria the mature lipoprotein remains anchored in the cytoplasmic membrane after cleavage.
FIG. 1.
Amino acid sequence alignment of periplasmic binding proteins: E. coli ModA, E. acidaminophilum TupA, P. furiosus WtpA, and the 11 WtpA homologues. The sequence alignment was performed with all the protein sequences that were also used to create the phylogenetic tree (Fig. 4). The boldface residues (shaded gray) in the ModA protein are involved in the binding of the molybdate, based on the resolved crystal structures (10, 16). The boldface residues in the WtpA protein indicate the lipobox consensus sequence, and the ones postulated to play a role in binding of the oxoanion are printed in white on gray. The residues printed in white on black in the TupA and WtpA sequences are conserved among their homologues.
For the TupA gene of E. acidaminophilum a similar leader sequence was found (17). When the TupA protein was expressed in E. coli containing its native leader peptide, 90% of the recombinant protein was found associated with the cytoplasmic membrane of E. coli and only a small fraction of soluble protein was obtained. Therefore, the WtpA (PF0080) protein from P. furiosus was cloned without its native leader sequence into the pASK-IBA2 vector. This vector contains an N-terminal E. coli leader sequence, coding for the OmpA leader peptide that causes the protein to be exported across the cytoplasmic membrane. The WtpA gene was cloned into the pASK-IBA2 vector and expressed as a C-terminal Strep-tag fusion protein. Purification resulted in a yield of approximately 5 mg WtpA per liter of induced E. coli culture. The protein remained stable and soluble during the purification step in which the E. coli cell extract was heated for 30 min at 60°C. The heat stability of WtpA was tested more thoroughly on the purified protein; the 280-nm absorption remained constant for at least 6 h after incubation of the protein at 80°C, and there was no precipitation observed (data not shown).
Purified WtpA showed multiple bands with apparent molecular masses between 35 and 45 kDa (data not shown) on native polyacrylamide gel electrophoresis, which was also observed for the TupA protein from E. acidaminophilum (17). These different forms of WtpA are obtained because they are processed differently by the signal peptidases of E. coli. The major band was observed around 40 kDa, which is in agreement with the gene PF0080 coding for a 37-kDa protein (without the leader sequence), suggesting that the WtpA protein occurs as a monomer in its native form.
Qualitative binding experiments.
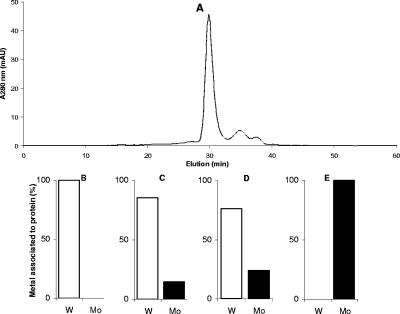
WtpA (35 μM) was incubated with 50 μM tungstate or molybdate, and the protein was subsequently separated from the unbound salt with size-exclusion chromatography. The presence of the oxoanions did not change the elution profile of the monomeric protein. The metal content of the protein fraction and the low-molecular-weight fraction (unbound oxoanion) was determined by catalytic adsorptive stripping voltammetry (8). It was found that molybdate and tungstate coeluted with the protein (Fig. 2) and the excess of oxoanion eluted in the low-molecular-weight fraction. The molar ratio of tungstate and/or molybdate and protein was determined to be less than 1 in all experiments. This can be explained by a loss of bound oxoanion during the time course of the experiment of approximately 30 min. Still 20% of the tungstate or molybdate was bound to the protein at the end of the chromatography run, which indicates an off rate on the order of 10−3 per second.
FIG. 2.
(A) Size-exclusion chromatography of WtpA (35 μM, 100-μl sample volume, 0.5 ml/min). (B to E) Percentage of total metal, tungstate (white bars) or molybdate (black bars), detected in the protein fraction after incubation for 30 min at room temperature with 50 μM sodium tungstate (B), a mixture of 46 μM sodium tungstate and 92 μM sodium molybdate (twofold excess of molybdate) (C), a mixture of 44 μM sodium tungstate and 222 μM sodium molybdate (fivefold excess of molybdate) (D), and 50 μM sodium molybdate (E).
To qualitatively examine the preference for either tungstate or molybdate, the protein was incubated with a mixture of the two oxoanions. In all experiments both metals were detected in the protein fraction. However, the amount of bound tungstate was significantly higher, even in the presence of an excess of molybdate during the incubation (Fig. 2). These data demonstrate that WtpA is able to bind tungstate and molybdate, with a preference for tungstate, without changing the oligomeric state of the protein.
To test the affinity for other oxoanions, a ligand-dependent protein gel shift assay was performed with reference to the migration rate of the uncomplexed protein in native polyacrylamide gels (28). The protein was incubated with a 400-fold molar excess of sulfate, phosphate, chlorate, molybdate, and tungstate. Only the tungstate-incubated samples showed a slight but significant mobility shift compared to the nonincubated protein and to the samples incubated with the other anions (data not shown). A similar gel shift assay has been used before to estimate the dissociation constant (KD) for tungstate to be 0.5 μM for the E. acidaminophilum TupA (17) (Table 1). In the present case, the sensitivity of this gel shift mobility assay was not sufficient to determine dissociation constants of P. furiosus WtpA for tungstate or molybdate.
TABLE 1.
KD values for the binding of tungstate and molybdate to the periplasmic binding proteins
Ligand-dependent spectral changes have also been used to estimate values for the apparent KD for the binding of molybdate or tungstate to periplasmic binding proteins. In the case of the ModA protein, binding of tungstate or molybdate has been shown to cause a slight change in the spectrum in the far-UV wavelength range; also the intrinsic fluorescence spectrum of ModA changes upon molybdate binding (12, 28). However, for P. furiosus WtpA these spectral differences were not significant enough to make a valid estimation for the KD (data not shown).
An isotopic binding method provided the most accurate KD value of 20 ± 8 nM for molybdate binding of ModA (Table 1) (12). Therefore, a similar experiment was carried out for determining the KD of P. furiosus WtpA for tungstate. However, the determined ligand-to-protein stoichiometry was much lower than unity; this indicates that this experimental setup is not reliable, as it is likely to result in a serious overestimation of the magnitude of KD. Most likely, the Dowex resin was able to strip bound tungstate from the protein.
In summary, the above-mentioned assays, which were all used in previous reports to determine the KD for periplasmic binding proteins, were not sensitive and/or accurate enough to determine the KD in the case of WtpA. However, they qualitatively confirm the ability of the WtpA protein to bind tungstate.
Isothermal titration calorimetry.
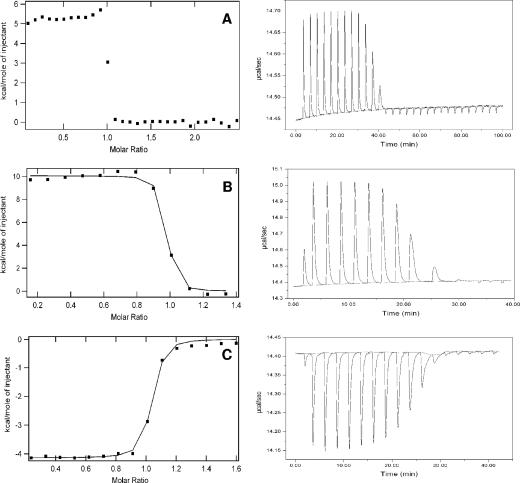
ITC of WtpA showed that the protein endothermically binds tungstate and molybdate with a stoichiometry of 1 mole oxoanion per mole of protein, as deduced from the heat consumption upon addition of tungstate or molybdate to the protein solution (Fig. 3). The obtained binding curve for molybdate was used to determine the KD value to be 11 ± 5 nM. The extremely high affinity of the protein for tungstate resulted in a very steep binding curve, and this precluded an accurate fit to determine the KD value of the protein for tungstate. It was not possible to significantly decrease both the protein concentration and the amount of titrated tungstate to obtain more data points in the steep region in view of the signal-to-noise ratio. However, an upper limit for the KD of 1 nM was estimated from the data. Binding of molybdate resulted in a greater heat consumption (ΔHcal = 9.9 ± 0.5 kcal/mole of injectant) compared to the heat consumption upon the binding of tungstate (ΔHcal = 5.3 ± 0.2 kcal/mole of injectant). These numbers were calculated by taking the average heat consumption of the first data points of the curve where all the ligand was directly bound to the protein.
FIG. 3.
(A and B) ITC of 10 μM WtpA titrated with injections of 0.8 μM tungstate (A) and 1 μM molybdate (B). (C) Displacement titration of 10 μM WtpA incubated with 15 μM molybdate, with injections of 1 μM tungstate. Data were fitted (continuous line in panels B and C) with ORIGIN software. The raw ITC data are shown in the right graphs.
A displacement titration of the molybdate-saturated protein with tungstate showed a heat release which corresponds to the difference in heat release between the two oxoanions (ΔHcal = −4.1 ± 0.4 kcal/mole of injectant) (Fig. 3C). This shows clearly that the protein favors the binding of tungstate, even when the binding site is occupied by a molybdate molecule. The apparent KD for tungstate when the protein is saturated with molybdate was determined to be 15 ± 4 nM (Fig. 3C). The KD value of a displacement titration in combination with the KD value for the inhibiting ligand in the absence of strong binding ligand can be used to calculate the actual KD for the strong binding ligand with the following competition equation: Kapp = KA/(1 + KB[B]) (37) where KA is the binding constant for the strong binding ligand (tungstate) and KB is that for the competitively inhibiting ligand (molybdate). The apparent binding constant depends on the concentration of free molybdate [B], which changes during the experiment from 5 μM to 15 μM. An average value of 10 μM was used in the equation to calculate a KD of 17 ± 7 pM of WtpA for tungstate (KA) (37), which is the lowest KD value determined for any tungstate or molybdate periplasmic binding protein. The displacement titration and the extremely low KD value for tungstate indicate the latter to be the physiological substrate for WtpA.
Sequence alignments. (i) WtpA (PF0080).
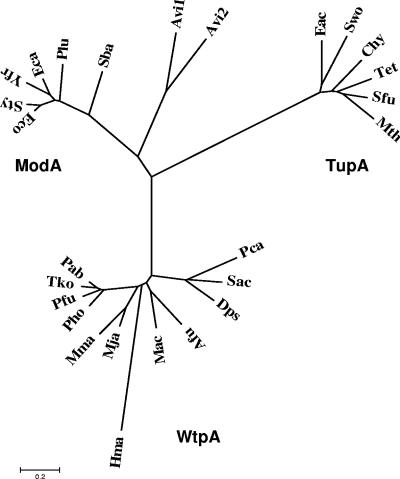
The highest similarities with the PF0080 gene were found with hypothetical proteins from Thermococcus kodakarensis KOD1 (YP_182428) (87%), Pyrococcus abyssi GE5 (NP_125843) (87%), and Pyrococcus horikoshii OT3 (NP_142154) (90%). They are all included in operons encoding putative ABC transporters and represent the periplasmic binding protein. WtpA has only weak similarity with the ModA protein of E. coli (17.6% identity, 30% similarity) and the TupA protein of E. acidaminophilum (15.6% identity, 30.7% similarity) (Fig. 1 and 4), and therefore it forms a new class of tungstate and molybdate binding proteins (Fig. 4). A BLAST search of the WtpA sequence against the nonredundant database (NCBI) resulted in the identification of 11 homologues (P < 10−20) (Fig. 1 and 4). These homologues of the P. furiosus WtpA protein, the five closest homologues of the E. coli ModA protein, the five closest homologues of the E. acidaminophilum TupA protein, and the A. vinelandii ModA1 and ModA2 proteins were used to make the alignment in Fig. 1.
FIG. 4.
Unrooted phylogenetic tree of molybdate and tungstate periplasmic binding proteins. The alignment was made with the 11 homologues (P < 10−20) of the P. furiosus WtpA protein and the five closest homologues, each of the E. coli ModA protein, the E. acidaminophilum TupA protein, and the two A. vinelandii ModA1 and ModA2 proteins. ModA homologues (accession number, identity, similarity): Salmonella enterica serovar Typhimurium LT2 (AE008732.1, 86%, 91%), Erwinia carotovora subsp. atroseptica SCRI1043 (BX950851.1, 69%, 80%), Yersinia frederiksenii ATCC 33641 (NZ_AALE01000012.1, 70%, 80%), Photorhabdus luminescens subsp. laumondii TTO1 (BX571864.1, 61%, 75%), Shewanella baltica OS155 (NZ_AAIO01000008.1, 50%, 71%); TupA homologues (accession number, identity, similarity): Syntrophomonas wolfei strain Goettingen (NZ_AAJG01000003.1, 51%, 67%), Thermoanaerobacter ethanolicus ATCC 33223 (NZ_AAKQ01000001.1, 50%, 70%), Carboxydothermus hydrogenoformans Z-2901 (CP000141.1, 50%, 65%), Syntrophobacter fumaroxidans MPOB (NZ_AAJF01000122.1, 48%, 66%), Moorella thermoacetica ATCC 39073 (NC_007644.1, 46%, 64%); WtpA homologues (accession number, identity, similarity): Thermococcus kodakarensis KOD1 (AP006878.1, 75%, 87%), Pyrococcus abyssi GE5 (AJ248283.1, 74%, 87%), Pyrococcus horikoshii shinkaj OT3 (BA000001.2, 73%, 90%), Methanococcus jannaschii DSM2661 (L77117.1, 49%, 69%), Archaeoglobus fulgidus DSM4304 (NC_000917.1, 46%, 67%), Methanococcus maripaludis S2 (BX957223.1, 46%, 63%), Methanosarcina acetivorans C2A (AE010299.1, 43%, 63%), Syntrophus aciditrophicus SB (NC_007759.1, 39%, 60%), Desulfotalea psychrophila LSv54 (CR522870.1, 40%, 56%), Pelobacter carbinolicus DSM 2380 (CP000142.1, 36%, 55%), Haloarcula marismortui ATCC 43049 (AY596297.1, 29%, 47%).
The crystal structures of the ModA protein of E. coli and the ModA2 protein of A. vinelandii have been solved to high resolution (10, 16). The residues that bind molybdate through hydrogen bonds in the ModA proteins are S12, S39, A125, V152, and Y170 in E. coli ModA (Fig. 1) and T9, N10, S37, Y118, and V147 in A. vinelandii ModA2. Unfortunately, there is no crystal structure of TupA available yet, and therefore it is not known which residues play a role in the binding of tungstate in this protein. A structural homology model was made for WtpA based on the amino acid sequence and a homologue of a known structure (4). The protein 1AMF (E. coli ModA) was selected by the program from the Protein Data Bank database as parental structure to which the WtpA structure could be modeled with an e-value of 10−139. This indicates a significant structural similarity, and therefore we use the obtained structure to hypothesize which residues might play a role in the binding of tungstate or molybdate. Three of the five amino acids that bind molybdate in E. coli ModA have identical residues at identical positions in the modeled structure of WtpA. The S42, S75, and Y164 residues in the WtpA protein are positioned homologously to the S12, S39, and Y170 residues in ModA, respectively. Furthermore, these residues are completely conserved among all WtpA homologues, and this is an additional indication that they might play a role in the binding of the molybdate or tungstate (Fig. 1). The ModA residues A125 and V152 that bind the molybdate through their backbone NH group show no obvious similar residues in the WtpA model. A crystal structure of the WtpA protein is required for the confirmation of these residues being involved in binding of the oxoanion. Crystallization studies with WtpA are currently in progress in our laboratory.
The sequence similarities of the other components of the novel tungstate ABC transporter, between WtpB and ModB/TupB (53% and 50% similarity, respectively) and between WtpC and ModC/TupC (51% and 56% similarity, respectively), are much higher than the similarities between the A components. WtpB and WtpC both exhibited highest similarity with the permease and ATPase components from the putative ABC transporters in T. kodakarensis KOD1 (87% and 90%, respectively), P. abyssi GE5 (87% and 86%, respectively), and P. horikoshii OT3 (89% and 90%, respectively), whose binding protein component also exhibited the highest similarity with the periplasmic WtpA component.
(ii) WtpB (PF0081).
Analysis of the WtpB sequence by using the Goldman, Engelman, and Steitz hydrophobicity scale (6) identified the presence of five transmembrane helices. This indicates that WtpB is located in the membrane. The conserved C-terminal region EAA-X2-G-X9-I-X-LP, which is generally present in permease components of ABC transporters, was identified to be VARTLG-X9-I/V-X-LP in the case of the WtpB proteins. This conserved sequence is thought to be the recognition site for the C component of the transporter (19).
(iii) WtpC (PF0082).
Sequence analysis of the WtpC component of the tungstate transporter identifies the presence of characteristic motifs that are responsible for binding nucleotides (33). These so-called Walker A (GPSGAGKT) and Walker B (LDEPF) motifs and a Q loop (LSGGEQQ) are conserved in WtpC as in ModC and TupC. Hung et al. have postulated a role in nucleotide binding for a conserved histidine residue, which is also present in ModC, TupC, and WtpC (11). Sequence analysis of the WtpC protein in the InterPro database for domains and functional sites (39) identifies an ATPase domain (IPR003593/SM00382). Therefore, WtpC is proposed to be the ATPase part of the ABC transporter. Besides the ATPase domain, a transport-associated oligonucleotide/oligosaccharide binding domain, the TOBE domain (IPR005116/PF03459) (15), and a molybdate/tungstate binding domain, the MOP domain (IPR008995/SSF50331), are also recognized. These two domains are also found in E. coli ModC immediately after the ATPase domain. Probably the TOBE and MOP domains are involved in the recognition of molybdate and tungstate.
Additional BLAST searches confirm that this new class of tungstate transporters clarifies the uptake mechanism of many organisms that express tungsten-containing enzymes or encode putative tungsten-containing enzymes on the genome. To corroborate this proposal, a group of organisms that most likely use tungstate in their metabolism was obtained by performing a BLAST search (3) of the sequence of the P. furiosus AOR against the nonredundant database (NCBI). AOR is the only enzyme known thus far that can use only tungsten and not molybdenum. This search resulted in 33 organisms that have a gene coding for a putative tungsten-containing aldehyde oxidoreductase (P < 10−30) and therefore most likely require a tungstate uptake mechanism. Subsequently, BLAST searches of the sequences of E. acidaminophilum TupA, E. coli ModA, and P. furiosus WtpA were performed against these genomes (P < 10−20) (Table 2). The discovery of WtpA as a new class of tungstate transporters identifies the tungstate uptake system of a significant number of archaea, and some bacteria (see below), that do not express homologues of the E. acidaminophilum TupA or the E. coli ModA. Some of the archaea and bacteria have homologue genes for more than one transporter system. There are no WtpA, TupA, or ModA homologues found in eukaryotic organisms even though they do express molybdenum enzymes.
TABLE 2.
Organisms containing a putative member of the tungsten-containing aldehyde:ferredoxin oxidoreductase family encoded in the genome (P < 10−30)
| Organism | Anaerobic | Homologue
|
||
|---|---|---|---|---|
| TupA | ModA | WtpA | ||
| Archaea | ||||
| Pyrococcus furiosus | Yes | − | − | + |
| Pyrococcus abyssi | Yes | − | − | + |
| Pyrococcus horikoshii | Yes | − | − | + |
| Thermococcus kodakarensis | Yes | − | − | + |
| Thermoplasma volcanium | Facultative | − | − | +a |
| Thermoplasma acidophilum | Facultative | − | − | +a |
| Archaeoglobus fulgidus | Yes | − | − | + |
| Methanosarcina acetivorans | Yes | − | + | + |
| Methanosarcina mazei | Yes | + | + | − |
| Methanocaldococcus jannaschii | Yes | − | − | + |
| Methanococcus maripaludis | Yes | − | + | + |
| Haloarcula marismortui | No | + | − | + |
| Pyrobaculum aerophilum | Facultative | + | − | − |
| Aeropyrum pernix | No | − | +a | − |
| Thermoproteus tenaxb | Yes | |||
| Thermococcus litoralisb | Yes | |||
| Bacteria | ||||
| Eubacterium acidaminophilumb | Yes | + | ||
| Clostridium acetobutylicum | Yes | − | +a | − |
| Geobacter sulfurreducens | Yes | + | + | − |
| Geobacter metallireducens | Yes | + | + | − |
| Desulfuromonas acetoxidans | Yes | + | + | − |
| Desulfovibrio vulgaris | Yes | + | + | − |
| Desulfovibrio desulfuricans | Yes | + | − | − |
| Desulfotalea psychrophila | Yes | + | +a | + |
| Wolinella succinogenes | No | + | +a | − |
| Magnetospirillum magnetotacticum | Facultative | + | + | − |
| Rhodospirillum rubrum | Facultative | − | − | − |
| Azoarcus sp. strain EbN1 | Facultative | + | +a | − |
| Rubrivivax gelatinosus | Facultative | + | + | − |
| Shigella flexneri | Facultative | − | + | − |
| Escherichia coli | Facultative | − | + | − |
| Thermus thermophilus HB8 | Facultative | + | − | − |
| Thermus thermophilus HB27 | No | + | − | − |
| Symbiobacterium thermophilum | No | + | + | − |
These homologues have P values higher than the threshold of 10−20; however, they still exhibit significant homology with the protein in the corresponding column (between 10−8 and 10−16).
DISCUSSION
The discovery of the tungstate-specific ABC transporter from P. furiosus, WtpABC, uncovers a new class of tungstate and molybdate transporters, given the low sequence similarity between WtpA and the earlier-characterized periplasmic binding proteins ModA and TupA, which are part of the ABC transporters ModABC (28) and TupABC (17). BLAST searches of WtpA against the nonredundant database (NCBI) indicate that WtpA is an archaeal tungstate transporter, whereas TupA (and homologue VupA) and ModA occur predominantly in bacteria. Homologues of the wtpA gene are found in the genomes of only three bacteria: Syntrophus aciditrophicus, Desulfotalea psychrophila, and Pelobacter carbinolicus. D. psychrophila encodes a tungsten-containing AOR homologue on its genome (Table 2), but for the other two bacteria it is not known if they use tungsten in their metabolism. They might also use the WtpABC transporter for the uptake of molybdate. The bacterium Rhodospirillum rubrum is identified by BLAST to contain a tungsten-containing AOR homologue; however, there is no putative ModA, TupA, or WtpA protein present in its genome. This might indicate that there are still other tungstate and/or molybdate uptake systems that have not been identified yet. However, with the discovery of the WtpABC transporter, together with the earlier-characterized ModABC and TupABC, the tungstate and molybdate uptake systems of most bacteria and archaea can be identified.
The discovery of the very high affinity WtpABC transporter explains the earlier observed ability of P. furiosus cells to scavenge traces of tungstate from growth medium (23). However, it does not explain the highly selective incorporation of tungstate in the cofactor of the AOR enzymes in the presence of a 1,000-fold excess of molybdate in the growth medium (23) because the WtpABC transporter also has a high affinity for molybdate. As a consequence, this indicates the existence of an additional intracellular mechanism that determines the selective incorporation of tungstate in the pterin cofactor of these AOR enzymes.
Acknowledgments
This research was supported by a grant from the Council for Chemical Sciences of The Netherlands Organization for Scientific Research (700.51.301).
REFERENCES
- 1.Albers, S.-V., and A. J. M. Driessen. 2002. Signal peptides of secreted proteins of the archaeon Sulfolobus solfataricus: a genomic survey. Arch. Microbiol. 177:209-216. [DOI] [PubMed] [Google Scholar]
- 2.Allen, F. H., J. E. Davies, J. J. Galloy, O. Johnson, O. Kennard, C. F. Macrae, E. M. Mitchell, G. F. Mitchell, J. M. Smith, and D. G. Watson. 1991. The development of versions 3 and 4 of the Cambridge Structural Database System. J. Chem. Inf. Comput. Sci. 31:187-204. [Google Scholar]
- 3.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bates, P. A., L. A. Kelley, R. M. MacCallum, and M. J. Sternberg. 2001. Enhancement of protein modeling by human intervention in applying the automatic programs 3D-JIGSAW and 3D-PSSM. Proteins Suppl. 5:39-46. [DOI] [PubMed] [Google Scholar]
- 5.Bevers, L. E., E. Bol, P.-L. Hagedoorn, and W. R. Hagen. 2005. WOR5, a novel tungsten-containing aldehyde oxidoreductase from Pyrococcus furiosus with a broad substrate specificity. J. Bacteriol. 187:7056-7061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Engelman, D. M., T. A. Steitz, and A. Goldman. 1986. Identifying nonpolar transbilayer helixes in amino acid sequences of membrane proteins. Annu. Rev. Biophys. Biophys. Chem. 15:321-353. [DOI] [PubMed] [Google Scholar]
- 7.Fiala, G., and K. O. Stetter. 1986. Pyrococcus furiosus sp. nov. represents a novel genus of marine heterotrophic archaebacteria growing optimally at 100°C. Arch. Microbiol. 145:56-61. [Google Scholar]
- 8.Hagedoorn, P. L., P. van't Slot, H. P. van Leeuwen, and W. R. Hagen. 2001. Electroanalytical determination of tungsten and molybdenum in proteins. Anal. Biochem. 297:71-78. [DOI] [PubMed] [Google Scholar]
- 9.Hille, R. 2002. Molybdenum and tungsten in biology. Trends Biochem. Sci. 27:360-367. [DOI] [PubMed] [Google Scholar]
- 10.Hu, Y., S. Rech, R. P. Gunsalus, and D. C. Rees. 1997. Crystal structure of the molybdate binding protein ModA. Nat. Struct. Biol. 4:703-707. [DOI] [PubMed] [Google Scholar]
- 11.Hung, L. W., I. X. Wang, K. Nikaido, P. Q. Liu, G. F. Ames, and S. H. Kim. 1998. Crystal structure of the ATP-binding subunit of an ABC transporter. Nature 396:703-707. [DOI] [PubMed] [Google Scholar]
- 12.Imperial, J., M. Hadi, and N. K. Amy. 1998. Molybdate binding by ModA, the periplasmic component of the Escherichia coli mod molybdate transport system. Biochim. Biophys. Acta Biomembranes 1370:337-346. [DOI] [PubMed] [Google Scholar]
- 13.Kletzin, A., and M. W. W. Adams. 1996. Tungsten in biological systems. FEMS Microbiol. Rev. 18:5-63. [DOI] [PubMed] [Google Scholar]
- 14.Koning, S. M., M. G. L. Elferink, W. N. Konings, and A. J. M. Driessen. 2001. Cellobiose uptake in the hyperthermophilic archaeon Pyrococcus furiosus is mediated by an inducible, high-affinity ABC transporter. J. Bacteriol. 183:4979-4984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koonin, E. V., Y. I. Wolf, and L. Aravind. 2000. Protein fold recognition using sequence profiles and its application in structural genomics. Adv. Protein Chem. 54:245-275. [DOI] [PubMed] [Google Scholar]
- 16.Lawson, D. M., C. E. M. Williams, L. A. Mitchenall, and R. N. Pau. 1998. Ligand size is a major determinant of specificity in periplasmic oxyanion-binding proteins: the 1.2 Å resolution crystal structure of Azotobacter vinelandii ModA. Structure (London) 6:1529-1539. [DOI] [PubMed] [Google Scholar]
- 17.Makdessi, K., J. R. Andreesen, and A. Pich. 2001. Tungstate uptake by a highly specific ABC transporter in Eubacterium acidaminophilum. J. Biol. Chem. 276:24557-24564. [DOI] [PubMed] [Google Scholar]
- 18.Mendel, R. R. 2005. Molybdenum: biological activity and metabolism. Dalton Trans. 2005:3404-3409. [DOI] [PubMed] [Google Scholar]
- 19.Mourez, M., M. Hofnung, and E. Dassa. 1997. Subunit interactions in ABC transporters: a conserved sequence in hydrophobic membrane proteins of periplasmic permeases defines an important site of interaction with the ATPase subunits. EMBO J. 16:3066-3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mukund, S., and M. W. Adams. 1993. Characterization of a novel tungsten-containing formaldehyde ferredoxin oxidoreductase from the hyperthermophilic archaeon, Thermococcus litoralis. A role for tungsten in peptide catabolism. J. Biol. Chem. 268:13592-13600. [PubMed] [Google Scholar]
- 21.Mukund, S., and M. W. Adams. 1991. The novel tungsten-iron-sulfur protein of the hyperthermophilic archaebacterium, Pyrococcus furiosus, is an aldehyde ferredoxin oxidoreductase. Evidence for its participation in a unique glycolytic pathway. J. Biol. Chem. 266:14208-14216. [PubMed] [Google Scholar]
- 22.Mukund, S., and M. W. W. Adams. 1995. Glyceraldehyde-3-phosphate ferredoxin oxidoreductase, a novel tungsten-containing enzyme with a potential glycolytic role in the hyperthermophilic archaeon Pyrococcus furiosus. J. Biol. Chem. 270:8389-8392. [DOI] [PubMed] [Google Scholar]
- 23.Mukund, S., and M. W. W. Adams. 1996. Molybdenum and vanadium do not replace tungsten in the catalytically active forms of the three tungstoenzymes in the hyperthermophilic archaeon Pyrococcus furiosus. J. Bacteriol. 178:163-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pratte, B. S., and T. Thiel. 2006. High-affinity vanadate transport system in the cyanobacterium Anabaena variabilis ATCC 29413. J. Bacteriol. 188:464-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rajagopalan, K. V., and J. L. Johnson. 1992. The pterin molybdenum cofactors. J. Biol. Chem. 267:10199-10202. [PubMed] [Google Scholar]
- 26.Rauh, D., A. Graentzdoerffer, K. Granderath, J. R. Andreesen, and A. Pich. 2004. Tungsten-containing aldehyde oxidoreductase of Eubacterium acidaminophilum. Isolation, characterization and molecular analysis. Eur. J. Biochem. 271:212-219. [DOI] [PubMed] [Google Scholar]
- 27.Rech, S., U. Deppenmeier, and R. P. Gunsalus. 1995. Regulation of the molybdate transport operon, modABCD, of Escherichia coli in response to molybdate availability. J. Bacteriol. 177:1023-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rech, S., C. Wolin, and R. P. Gunsalus. 1996. Properties of the periplasmic ModA molybdate-binding protein of Escherichia coli. J. Biol. Chem. 271:2557-2562. [DOI] [PubMed] [Google Scholar]
- 29.Robb, F. T., D. L. Maeder, J. R. Brown, J. DiRuggiero, M. D. Stump, R. K. Yeh, R. B. Weiss, and D. M. Dunn. 2001. Genomic sequence of hyperthermophile, Pyrococcus furiosus: implications for physiology and enzymology. Methods Enzymol. 330:134-157. [DOI] [PubMed] [Google Scholar]
- 30.Roy, R., and M. W. W. Adams. 2002. Characterization of a fourth tungsten-containing enzyme from the hyperthermophilic archaeon Pyrococcus furiosus. J. Bacteriol. 184:6952-6956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roy, R., S. Mukund, G. J. Schut, D. M. Dunn, R. Weiss, and M. W. W. Adams. 1999. Purification and molecular characterization of the tungsten-containing formaldehyde ferredoxin oxidoreductase from the hyperthermophilic archaeon Pyrococcus furiosus: the third of a putative five-member tungstoenzyme family. J. Bacteriol. 181:1171-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 33.Schmitt, L., and R. Tampe. 2002. Structure and mechanism of ABC transporters. Curr. Opin. Struct. Biol. 12:754-760. [DOI] [PubMed] [Google Scholar]
- 34.Schut, G. J., S. D. Brehm, S. Datta, and M. W. W. Adams. 2003. Whole-genome DNA microarray analysis of a hyperthermophile and an archaeon: Pyrococcus furiosus grown on carbohydrates or peptides. J. Bacteriol. 185:3935-3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schwarz, G. 2005. Molybdenum cofactor biosynthesis and deficiency. Cell. Mol. Life Sci. 62:2792-2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Siebers, B., B. Tjaden, K. Michalke, C. Doerr, H. Ahmed, M. Zaparty, P. Gordon, C. W. Sensen, A. Zibat, H.-P. Klenk, S. C. Schuster, and R. Hensel. 2004. Reconstruction of the central carbohydrate metabolism of Thermoproteus tenax by use of genomic and biochemical data. J. Bacteriol. 186:2179-2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sigurskjold, B. W. 2000. Exact analysis of competition ligand binding by displacement isothermal titration calorimetry. Anal. Biochem. 277:260-266. [DOI] [PubMed] [Google Scholar]
- 38.Weinberg, M. V., G. J. Schut, S. Brehm, S. Datta, and M. W. W. Adams. 2005. Cold shock of a hyperthermophilic archaeon: Pyrococcus furiosus exhibits multiple responses to a suboptimal growth temperature with a key role for membrane-bound glycoproteins. J. Bacteriol. 187:336-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zdobnov, E. M., and R. Apweiler. 2001. InterProScan—an integration platform for the signature-recognition methods in InterPro. Bioinformatics (Oxford) 17:847-848. [DOI] [PubMed] [Google Scholar]