Abstract
During infection of the cystic fibrosis (CF) lung, Pseudomonas aeruginosa microcolonies are embedded in the anaerobic CF mucus. This anaerobic environment seems to contribute to the formation of more robust P. aeruginosa biofilms and to an increased antibiotic tolerance and therefore promotes persistent infection. This study characterizes the P. aeruginosa protein PA4352, which is important for survival under anaerobic energy stress conditions. PA4352 belongs to the universal stress protein (Usp) superfamily and harbors two Usp domains in tandem. In Escherichia coli, Usp-type stress proteins are involved in survival during aerobic growth arrest and under various other stresses. A P. aeruginosa PA4352 knockout mutant was tested for survival under several stress conditions. We found a decrease in viability of this mutant compared to the P. aeruginosa wild type during anaerobic energy starvation caused by the missing electron acceptors oxygen and nitrate. Consistent with this phenotype under anaerobic conditions, the PA4352 knockout mutant was also highly sensitive to carbonyl cyanide m-chlorophenylhydrazone, the chemical uncoupler of the electron transport chain. Primer extension experiments identified two promoters upstream of the PA4352 gene. One promoter is activated in response to oxygen limitation by the oxygen-sensing regulatory protein Anr. The center of a putative Anr binding site was identified 41.5 bp upstream of the transcriptional start site. The second promoter is active only in the stationary phase, however, independently of RpoS, RelA, or quorum sensing. This is the second P. aeruginosa Usp-type stress protein that we have identified as important for survival under anaerobic conditions, which resembles the environment during persistent infection.
Pseudomonas aeruginosa is an adaptable opportunistic pathogen that causes a variety of nosocomial infections and burn-related sepsis and is also the dominant pathogen during infection of the cystic fibrosis (CF) lung (25, 27, 39). During infection, biofilm-like growth protects P. aeruginosa from the host immune response and the actions of antibiotics (11, 20, 37). Another factor important for persistent infection of the cystic fibrosis lung was identified by Worlitzsch et al. (45), who showed that the biofilm-like microcolonies of P. aeruginosa are embedded in oxygen-limited mucus. This oxygen-limited environment promotes the formation of more robust biofilms (45, 47). Particularly, the combination of oxygen restriction and biofilm growth seems to increase antibiotic tolerance and therefore promotes persistent infection by P. aeruginosa (9, 16, 20, 47).
Although anaerobic conditions are important for persistent infection, P. aeruginosa has only limited capabilities to grow and survive anaerobically. Anaerobic growth is sustained by denitrification (33, 49), which requires nitrate or nitrite. Both substrates were detected in small amounts (50 to 350 μM) in CF lung mucus and in airway surface liquid (19, 24, 45). In the absence of nitrate and nitrite, P. aeruginosa survives anaerobiosis by arginine fermentation, which allows moderate anaerobic growth (40). We recently identified a pyruvate fermentation pathway that allows anaerobic survival but does not sustain anaerobic growth (15). In the absence of pyruvate, arginine, or nitrate/nitrite, P. aeruginosa rapidly faces energy starvation in an anaerobic environment, and cell numbers decrease dramatically (7, 15). A proteome analysis of P. aeruginosa cells during pyruvate fermentation identified PA3309, a protein containing a universal stress protein (Usp) signature domain. This Usp-type protein was shown to be essential for survival during pyruvate fermentation (36) and was reported to be present in anaerobic biofilms (47). Moreover, we found the promoter of the corresponding gene to be induced in deeper layers of a biofilm. A proteome analysis of P. aeruginosa under pyruvate fermentation revealed 22-fold-increased production of a second Usp-type stress protein, PA4352 (36). In contrast to the PA3309 mutant, deletion of the PA4352 gene did not reduce survival during pyruvate fermentation. According to the Pfam database, the Usp signature is present in more than 1,000 proteins (4) from organisms of all domains of life (26). In Escherichia coli, Usp-type stress proteins are important for surviving various stress conditions and aerobic stationary phase (18, 26). However, their exact biological functions remain unclear, although more data have emerged from E. coli showing the physiological roles of the Usp proteins in iron metabolism, oxidative stress, adhesion, and motility (30).
In this study, we focused on the anaerobically induced Usp-type protein PA4352. This protein exhibits two Usp domains in tandem. We investigated the regulation of the PA4352 gene and the potential function of its product during starvation for energy, and thus its role in promoting survival in an anaerobic environment during persistent infection.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The bacterial strains and plasmids used in this study are shown in Table 1. For standard molecular biology protocols, E. coli and P. aeruginosa strains were grown in Luria-Bertani (LB) medium as described before (36).
TABLE 1.
Strains and plasmids used in this study
| Bacterial strain or plasmid | Genotype or phenotype | Reference |
|---|---|---|
| P. aeruginosa | ||
| PAO1 | Wild type | 13 |
| PAO6261 | PAO1 Δanr | 46 |
| PAO-MW20 | PAO1 rpoS::aacC1 Gmr | 44 |
| NB007 | PAO1 attB::(PPA4352-lacZ) | This study |
| NB015 | PAO1 ΔPA4352 | This study |
| NB023 | PAO6261 attB::(PPA4352-lacZ) | This study |
| NB071 | PAO1 attB::(PPA4352ΔANR-lacZ) | This study |
| NB072 | PAO6261 attB::(PPA4352ΔANR-lacZ) | This study |
| NB073 | PAO-MW20 attB::(PPA4352ΔANR-lacZ) | This study |
| NB074 | KS35 attB::(PPA4352ΔANR-lacZ) | This study |
| NB075 | NB071 ΔrhlR | This study |
| NB076 | NB071 ΔlasR | This study |
| NB058 | PAO1 attB::(mini-CTX2) | This study |
| NB059 | NB015 attB::(mini-CTX2) | This study |
| NB060 | NB015 attB::(pNB011) | This study |
| NB086 | BB71 attB::(PPA4352-lacZ) | This study |
| KS35 | PAO1 ΔrelA | 36 |
| BB71 | PAO1 Δdnr | This study |
| E. coli | ||
| DH10B | F−mcrA Δ(mrr-hsdRMS-mcrBC) φ80dlacZΔM15 ΔlacX74 deoR recA1 endA1 araD139 Δ(ara leu)7697 galU galK λ−rpsL nupG | GibcoBRL (Invitrogen) |
| S17 λ-pir | pro thi hsdR+ Tpr Smr chromosome::RP4-2 Tc::Mu-Km::Tn7/λpir | 12 |
| SM10 | thi-1 thr leu tonA lacY supE recA::RP4-2-Tc::Mu (Kmr) | 12 |
| Plasmids | ||
| pEX18Ap | AproriT+sacB+ gene replacement vector with multiple-cloning site from pUC18 | 22 |
| Mini-CTX-lacZ | Tcr; promoterless lacZ gene | 6 |
| Mini-CTX2 | Tcr; integration vector | 23 |
| pSB219.9A | Gmr; lasR::Gm suicide vector | 5 |
| pSB224.10A | Tcr; rhlR::Tc suicide vector | 5 |
| pPS858 | Apr Gmr; source of gentamicin cassette | 22 |
| pFLP2 | Apr; source of FLP recombinase | 22 |
| pNB003 | Tcr; mini-CTX-lacZ containing a 465-bp fragment of the putative promoter region of the PA4352 gene between EcoRI and BamHI | This study |
| pNB007 | Apr Gmr; pEX18Ap with 596-bp promoter PA4352, Gmr-gfp fragment from pPS858, and 617 bp downstream of the coding region of PA4352 between SacI and HindIII | This study |
| pNB010 | Apr; pUCP20T with a 1,670-bp PCR fragment covering the entire PA4352 gene, 659 bp of the putative promoter region, and 138 bp of the downstream region between EcoRI and SacI | This study |
| pNB011 | Tcr; mini-CTX2 with a 1,670-bp PCR fragment covering the entire PA4352 gene, 659 bp of the putative promoter region, and 138 bp of the downstream region between EcoRI and SacI | This study |
| pNB019 | Tcr; mini-CTX-lacZ containing a 465-bp fragment of the putative promoter region, with a mutation in the Anr box (TTGATGTGCATCAA→TTGATGTGCATACG) of the PA4352 gene between EcoRI and BamHI | This study |
| pBB25 | Apr Gmr; pEX18Ap with 589-bp promoter of the dnr gene, Gmr-gfp fragment from pPS858, and 553-bp fragment of the coding region of dnr between SacI and HindIII | This study |
Growth conditions to elucidate the phenotype of the ΔPA4352 mutant.
Investigations of the ΔPA4352 mutant revealed sensitivity of the mutant to a breakdown of the proton motive force (PMF). To elucidate this phenotype, three different experiments were performed. First, the breakdown of the PMF was initiated by limiting nitrate after denitrification under anaerobic conditions. To achieve this, the P. aeruginosa wild type and the ΔPA4352 mutant were grown anaerobically in LB medium supplemented with 50 mM KNO3 into stationary phase (optical density at 578 nm [OD578], ∼2.0). Seven hours after the cells reached stationary phase, aliquots were taken periodically for the following 8 hours to determine the number of CFU by viable-cell counts (36) (see below). To rescue the phenotype of the ΔPA4352 mutant during anaerobic stationary phase, additional KNO3 (30 mM) was added to the anaerobic cultures 7 h after entry into stationary phase.
Secondly, the breakdown of the PMF was achieved by limiting oxygen in the aerobic electron transport chain after prolonged incubation in the aerobic stationary phase. Therefore, cultures of P. aeruginosa were first grown in LB medium under aerobic conditions to the stationary phase, which was reached at an OD578 of 7.0. After 15 h of incubation in the stationary phase, the cultures were transferred into hermetically sealed bottles to generate an anaerobic environment. Survival was determined by viable-cell counts during the following 33 h. To save the phenotype with the alternative electron acceptor nitrate, KNO3 (75 mM) was added to the prior aerobic cultures.
Thirdly, we showed that sensitivity of the mutant was caused by a depolarization of the membrane. PMF was destroyed with the protonophor carbonyl cyanide m-chlorophenylhydrazone (CCCP) in the anaerobic stationary phase. To prevent death in the anaerobic stationary phase caused by nitrate limitation, additional KNO3 (30 mM) was added after 7 h in the stationary phase, and 0.2 mM CCCP was added to cause depolarization of the membrane.
Viable-cell counts and control experiments.
Viable-cell counts were performed by plating aliquots of the anaerobic cultures on LB agar plates. After 24 h, aerobic-incubation colonies were counted and cell numbers were determined. In control experiments, we incubated LB agar plates containing 50 mM nitrate anaerobically. Under these conditions, we also observed small colonies and significantly reduced viability of the mutant, as with aerobic plating. However, anaerobic plating resulted in a delayed decrease in viability compared to aerobic plating but also confirmed clear premature death of the ΔPA4352 mutant strain (data not shown). We also verified a decrease in viability of the wild-type and mutant strains by fluorescence microscopic analysis of the samples after the cells were stained using the Live/Dead BacLight kit (Molecular Probes, Leiden, The Netherlands) following the instructions of the manufacturer, which confirmed the death of cells during the anaerobic stationary phase.
Construction and testing of the promoter-lacZ reporter gene fusions.
Chromosomal promoter-lacZ reporter gene fusions were constructed using the mini-CTX-lacZ vector (6). A 465-bp PCR product, covering the region between 454 bp and 6 bp upstream of the translational start site of the PA4352 gene, was generated using primers oKS18 (5′-GGAATTCACGGACTACGCCGACGAACC-3′) and oKS19 (5′-CGCGGATCCACCTCC ATTGGCAGAGC-3′). oKS18 contained an EcoRI restriction site at the 5′ end (underlined), and oKS19 contained a restriction site for BamHI, also at its 5′ end (underlined). The EcoRI- and BamHI-digested PCR product was cloned between the EcoRI and BamHI sites of mini-CTX-lacZ to generate pNB003. Transfer of pNB003 in P. aeruginosa was carried out by a diparental mating using E. coli S17λ-pir as the donor. The CTX integrase of pNB003 promoted integration of the vector into the attB site of the P. aeruginosa genome. The vector was transferred into the P. aeruginosa wild type, the anr mutant strain PAO6261, and the dnr mutant strain BB71 to generate the P. aeruginosa strains NB007, NB023, and NB086, respectively (Table 1). In these mutant strains, parts of the mini-CTX-lacZ vector containing the tetracycline resistance cassette were deleted using an FLP (flippase) recombinase encoded by the pFLP2 plasmid (22). β-Galactosidase assays were performed as outlined before in detail (15, 34, 36). The activities obtained were reported in Miller units (28).
To test the activation of PPA4352 under anaerobic conditions, cells were incubated aerobically in LB and were transferred to anaerobic flasks at an OD578 of 0.7. Activities were determined before and 4 h after the culture was shifted to anaerobic conditions.
The β-galactosidase activities of aerobically grown cultures were determined in the exponential growth phase (OD578, 0.7) and in the late stationary phase (16 h after entry into stationary phase) in LB.
Mutation of the putative Anr binding site in the PA4352 promoter.
The Anr binding site of the PA4352 promoter was mutated using the crossover-PCR technique (21) to generate the promoter PPA4352ΔANR. The putative Anr binding site TTGATGTGCATCAA (the target of the mutation is underlined) 75.5 bp upstream of the translational start site was mutated to TTGATGTGCATACG using the primers oNB044 (5′-GAAGCATCCGCCGTATGCACATCAAC-3′) and oNB045 (5′-CATACGGCGGATGCTT CGGACTGAAACA-3′) (mutated bases of the Anr binding site are underlined). The mutated promoter was amplified with oKS18 and oKS19 and cloned into the mini-CTX-lacZ vector as described above to generate the plasmid pNB019. This plasmid was introduced in the P. aeruginosa wild type and the mutants PAO6261 (Δanr), PAO-MW20 (rpoS::aacC1), and KS35 (ΔrelA) (Table 1) to generate the strains NB071, NB072, NB073, and NB074, respectively.
Construction of P. aeruginosa ΔPA4352, rhlR::Tcr, lasR::GmR, and Δdnr mutant strains.
To obtain unmarked gene deletion mutants, we used the well-established strategies based on sacB counterselection and FLP recombinase excision (22). To construct the suicide vector pNB007, the BamHI-digested gentamicin resistance cassette of pPS858 was cloned between two PCR fragments of the PA4352 gene in the multiple cloning site of pEX18Ap. The two PCR fragments contained DNA homologous to the upstream and downstream areas of the PA4352 gene. A 596-bp fragment containing the upstream promoter region of the PA4352 gene was amplified using primer oNB01 (5′-CGAGCTCTACGGCGACTTCGTCAAGG-3′), containing a SacI restriction site at the 5′ end, and oNB02 (5′-CGGGATCCAAGCGGATGCTTCGGACT-3′), containing a BamHI site (both underlined). The primers oNB03 (5′-CGCGGATCCCTTCCGCCGCGCGCTGA-3′), containing a BamHI site, and oNB04 (5′-CCCAAGCTTCCCTGGCGCCGCTGACC-3′), containing a HindIII site (both underlined), amplified 617 bp of the corresponding downstream region of PA4352. The suicide vector pNB007 was used to replace the PA4352 gene with a gentamicin cassette by sacB-based counterselection. The resulting mutant, NB011, was verified by Southern blot analysis. Finally, FLP recombinase encoded by the pFLP2 plasmid removed the FLP recombinase target-flanked gentamicin cassette to generate NB015. A dnr knockout mutant was constructed using the same strategy described above. The primer pairs for amplification of the upstream region were oBB50 (5′-CCCAAGCTTAGCGTCGCCTGCTGTTG-3′), containing a HindIII site, and oBB51 (5′CGGGATCCGCGCCGCCGCTTGATCG-3′), containing a BamHI site (both underlined). The primer pairs for amplification of the downstream region were oBB53 (5′-CGGGATCCAAGGCTCGCGATGATGA-3′), containing a BamHI site, and oBB55 (5′-CGAGCTCTGCAGGCGGAACTGAAC-3′), containing a SacI site (both underlined). The resulting suicide plasmid was named pBB25, and the dnr knockout mutant was named BB71.
The two quorum-sensing mutants rhlR::Tcr (NB075) and lasR::Gmr (NB076) were constructed as described previously (5), using NB071 as the parental strain.
Complementation of the PA4352 knockout mutant.
To circumvent the use of antibiotics during the complementation experiments, the PA4352 gene was integrated chromosomally into the attB site using the vector mini-CTX2. We amplified a 1,674-bp PCR product covering 659 bp of the PA4352 promoter region, the PA4352 gene, and 138 bp downstream of PA4352 using primers oNB01 (5′-CGAGCTCTACGGCGACTTCGTCAAGG-3′), containing a SacI restriction site at the 5′ end, and oNB22 (5′-CCCAAGCTTCGGCAGCGTGCATATCC-3′), containing a HindIII site (both underlined). The product was digested with SacI and HindIII and ligated into mini-CTX2 to generate pNB011.
This vector was transferred into P. aeruginosa NB015 to generate NB060 by diparental mating using E. coli S17λ-pir as the donor and integrated into the attB site of the genome as described above. As a control, the empty mini-CTX2 vector was integrated into the genome of wild-type PAO1 and NB015 to generate NB058 and NB059, respectively.
Proteomic analysis.
Cultures for proteome analysis were incubated aerobically in LB medium at 37°C. After 15 h of incubation in the stationary phase, cells were harvested by mixing the cell culture with a double volume of ice-cold potassium phosphate buffer (0.1 M; pH 7.4) and centrifugation at 8,000 × g for 30 min at 4°C. The cells were washed twice with potassium phosphate buffer and were resuspended in 1 ml potassium phosphate buffer. Proteins were isolated form the whole-cell suspension by extraction with an equal volume of phenol and subsequent acetone precipitation (36). The precipitated proteins were solubilized in sample buffer consisting of 7 M urea, 2 M thiourea, 4% 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate (CHAPS), 50 mM dithiothreitol, and 2% ampholytes (Bio-Lyte; Bio-Rad, Munich, Germany). The protein concentration was determined in the sample buffer using the PlusOne 2D Quant kit (Amersham Biosciences, Freiburg, Germany). Two-dimensional (2D) gel electrophoresis was performed using 17-cm immobilized pH gradient (IPG) strips covering a pH range from 5 to 8 (IPG Ready Strips; Bio-Rad, Munich, Germany). The IPG strips were loaded with 500 μg of protein, and isoelectric focusing was conducted for a total of 110,000 V · h. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis was performed at a constant temperature of 20°C with 1 W per gel for approximately 20 h using 10% polyacrylamide gels (25.5 by 20.5 cm). All gels were stained with ruthenium(II)-Tris-bathophenanthroline disulfonate as described before (36). The gels were documented with an FX-Scanner (Bio-Rad, Munich, Germany). Identification of protein spots was performed at the Gesellschaft für Biotechnologische Forschung, using a Bruker Ultraflex matrix-assisted laser desorption ionization-time of flight mass spectrometer.
Preparation of RNA for primer extension.
Protocols for RNA preparation were based on a modified hot-phenol method (1, 41). For preparations of RNA, appropriate aliquots of cultures (25 ml of a culture with an OD578 of 0.7, 5 ml of a culture with an OD578 of 2, and 2.5 ml of a culture with an OD578 of 5.0) were added to 25 ml of an ice-cold solution containing 20 mM Tris-HCl (pH 7.5), 5 mM MgCl2, and 20 mM NaN3. The cells were harvested by centrifugation (10 min at 4,400 × g and 4°C). The cell pellets were immediately frozen in liquid N2.
The pellets were resuspended in cell lysis solutions consisting of 125 μl 0.3 M sucrose and 0.01 M sodium acetate (pH 5.2) and 125 μl 2% (wt/vol) sodium dodecylsulfate and 0.01 M sodium acetate (pH 5.2). For complete cell lysis, the resuspended cells were incubated at 65°C for 1 min before the addition of prewarmed (65°C) acidic phenol. After incubation at 65°C for 3 min, samples were dropped into liquid N2 and centrifuged (12,110 × g) for 10 min at room temperature. This procedure was repeated with the upper phase three times following an extraction with acidic phenol-chloroform-isoamyl alcohol (25:24:1 [vol/vol/vol]) and once with chloroform-isoamyl alcohol (24:1 [vol/vol]). After ethanol precipitation, the RNA pellet was resuspended in 180 μl of 20 mM phosphate buffer (pH 6.5), 1 mM EDTA and 20 μl of a solution containing 200 mM sodium acetate (pH 4.5), 100 mM MgCl2, 100 mM NaCl, and 15 U of DNase I and was then incubated for 30 min at room temperature. Finally, the RNA was purified by phenol-chloroform extraction and ethanol precipitation. The resulting RNA pellet was dissolved in 30 μl of H2O.
Identification of the PA4352 transcriptional start site.
The transcriptional initiation site of PA4352 was determined in the aerobic and anaerobic stationary phases (3 h after entering stationary phase) and in the exponential growth phase of an anaerobic denitrifying culture (OD578 = 0.7). Thirty micrograms of RNA was used for primer extension analysis of the PA4352 transcript. Reverse transcription was initiated from the γ-32P end-labeled primer oNB21 (5′-AGGACAGGTCGGTCGCCACCAGGATTC-3′) by a standard procedure (3). The sequencing reaction was performed with the same primer and plasmid pNB010 containing the PA4352 gene. The primer extension products and the sequencing reactions were analyzed in a 6% denaturing polyacrylamide gel in Tris-borate buffer. The dried gel was analyzed by using a K-Screen (Kodak) and a phosphorimager (FX-Scanner; Bio-Rad).
RESULTS
Phenotype of the ΔPA4352 mutant.
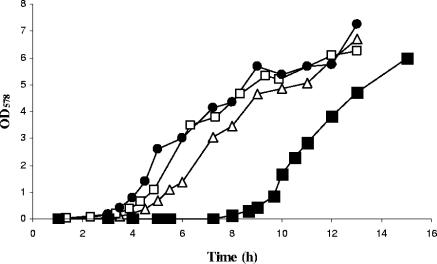
Recently, we found increased production of the hypothetical protein PA4352 in a proteome analysis of Pseudomonas aeruginosa during pyruvate fermentation (36). However, a P. aeruginosa PA4352 knockout mutant showed no reduced survival during pyruvate fermentation (36). According to the Pfam database, the PA4352 protein belongs to the Usp-type stress proteins and contains two Usp domains in tandem (4). Escherichia coli usp mutants show decreased viability in response to various stress conditions (18). To determine if the P. aeruginosa ΔPA4352 mutant shared a similar phenotype, we tested its sensitivity to H2O2, heat shock at 53°C, UV light, and osmotic shock with 3 M NaCl and 10% ethanol. No increased sensitivity was detected for the ΔPA4352 mutant (data not shown), in contrast to published data for the E. coli uspA, uspC, uspD, and uspE mutants, which showed decreased survival under the tested growth conditions (18). Investigations of the P. aeruginosa ΔPA4352 mutant under anaerobic growth conditions revealed no growth phenotype compared to wild-type P. aeruginosa during exponential growth in LB supplemented with 50 mM KNO3. However, when the ΔPA4352 mutant reached the anaerobic stationary phase, we observed the following phenotypes after 9 and 15 h of incubation in the stationary phase. First, after 9 h of incubation in the anaerobic stationary phase, we noticed a remarkably smaller colony size of the ΔPA4352 mutant than of the wild-type colonies, when cells were plated on LB agar plates and incubated aerobically for viable-cell counts (see Materials and Methods) (Fig. 1). However, the numbers of CFU of the wild type and mutant did not differ at this time. To test whether smaller colony size corresponded to reduced growth rates, we determined the growth curve of the mutants. Aliquots of the 9-h anaerobic stationary-phase cultures were taken to inoculate fresh LB cultures and to monitor aerobic growth. The growth curve of the ΔPA4352 mutant showed an extended lag phase compared to that of the wild type, while the growth rate after recovery was comparable to that of wild-type cells (doubling times: PAO1, 34 min; ΔPA4352, 36 min) (Fig. 2).
FIG. 1.
Phenotype of the CFU obtained from viable plating of P. aeruginosa wild-type cells and the ΔPA4352 mutant on LB agar plates after 9 hours of anaerobic stationary phase. Cultures of P. aeruginosa were incubated anaerobically in LB supplemented with 50 mM KNO3. After 9 hours of incubation in the stationary phase, aliquots from (A) wild-type P. aeruginosa containing the empty mini-CTX2 vector (NB058), (B) the ΔPA4352 mutant containing the empty mini-CTX2 vector (NB059), and (C) the complemented ΔPA4352 mutant containing pNB011 with the PA4352 gene (NB060) were taken, and 50 μl of a 10−5 dilution was plated on LB agar plates. The plates were incubated aerobically for 20 h at 37°C.
FIG. 2.
Effect of the PA4352 knockout in P. aeruginosa on recovery from nitrate starvation in the anaerobic stationary phase. Cultures of P. aeruginosa were incubated anaerobically in LB supplemented with 50 mM KNO3 and allowed to pass 9 h of stationary phase before being diluted in fresh LB medium. The inoculum contained 4.2 × 108 to 6.1 × 108 viable cells per ml as determined by viable-cell counts and Live/Dead staining (see Materials and Methods). The diluted cultures were incubated aerobically at 37°C to generate the growth curves shown of the wild-type strain containing the empty mini-CTX2 (NB058) (open triangles), the ΔPA4352 mutant containing the empty mini-CTX2 vector (NB059) (filled squares), and the complemented ΔPA4352 mutant (NB060) containing pNB011 with the PA4352 gene (open squares). To rescue the phenotype of the ΔPA4352 mutant, one culture of the ΔPA4352 mutant was supplemented with additional KNO3 (30 mM) 5 hours prior to dilution (filled circles).
The absence of PA4352 during the anaerobic stationary phase impairs P. aeruginosa adaptation to energy upshift conditions. In additional experiments, we confirmed that the upshift conditions were independent of the electron acceptors oxygen and nitrate (data not shown). This phenotype was completely restored by complementation of the ΔPA4352 mutant with pNB011 harboring the PA4352 gene (Fig. 1 and 2).
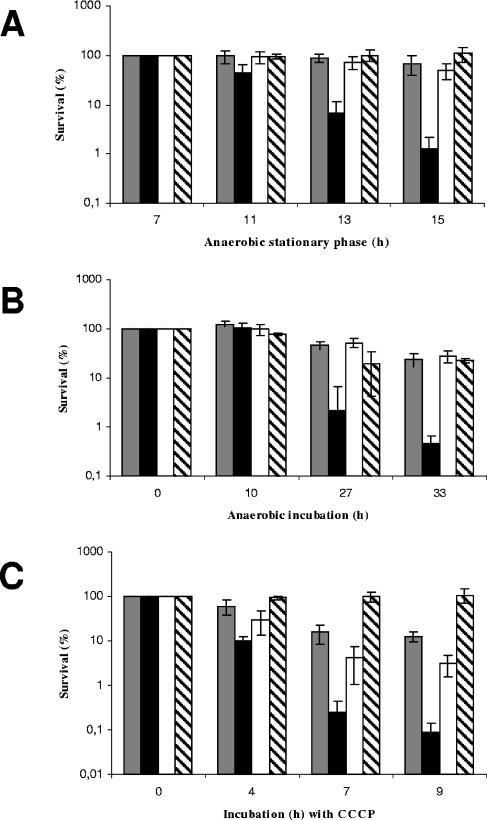
Secondly, extended incubation in the anaerobic stationary phase for up to 15 h led to premature death of the ΔPA4352 mutant. Again, viable-cell numbers were determined after serial dilutions of the anaerobic stationary-phase cultures were plated on LB agar, and CFU were monitored after aerobic incubation. We observed a 77-fold decrease in viability of the ΔPA4352 mutant, while wild-type cells and complemented mutant cells showed only a 1.5- to 2-fold loss of viability. Figure 3A shows the time-dependent reduction of viability in the stationary phase after 7, 11, 13, and 15 h.
FIG. 3.
Premature death of the ΔPA4352 mutant caused by restriction of the terminal electron acceptor nitrate (A), by restriction of oxygen (B), and by addition of CCCP in the anaerobic stationary phase (C). (A) Cultures of P. aeruginosa were incubated anaerobically in LB supplemented with 50 mM KNO3. The survival (%) of wild-type cells containing the empty mini-CTX2 vector (NB058) (gray bars), of the ΔPA4352 mutant containing the empty mini-CTX2 vector (NB059) (black bars), and of the complemented ΔPA4352 mutant (NB060) (white bars) was determined by viable-cell counts during the stationary phase. One culture of the ΔPA4352 mutant was supplemented with additional KNO3 (30 mM) 7 hours after entry into the stationary phase (striped bars). (B) Cultures of P. aeruginosa were incubated aerobically in LB, and as a control, an additional culture of the ΔPA4352 mutant was incubated in LB supplemented with KNO3 (50 mM). After reaching the stationary phase, the cultures were incubated for an additional 15 h under aerobic conditions. To initiate anaerobiosis, the cultures were transferred to hermetically sealed bottles. The control culture of the ΔPA4352 mutant grown in LB with 50 mM KNO3 was supplemented with additional KNO3 (30 mM) immediately after the shift to anaerobiosis to prevent nitrate limitation (striped bars). The survival (%) of wild-type cells containing the empty mini-CTX2 vector (NB058) (gray bars), the ΔPA4352 mutant containing the empty mini-CTX2 vector (NB059) (black bars), and the complemented ΔPA4352 mutant (NB060) (white bars) was determined by viable-cell counts during the stationary phase. (C) CCCP sensitivity was examined in the anaerobic stationary phase. After 7 h of stationary phase, cultures of P. aeruginosa wild-type cells containing the empty mini-CTX2 vector (NB058) (gray bars), the ΔPA4352 mutant containing the empty mini-CTX2 vector (NB059) (black bars), and the complemented ΔPA4352 mutant (NB060) (white bars) were supplemented with an additional 30 mM KNO3 and challenged with CCCP (0.2 mM). One culture of the ΔPA4352 mutant was supplemented with KNO3 without CCCP (striped bars). All experiments were repeated three times, and standard deviations are indicated.
Nitrate limitation causes premature death of the ΔPA4352 mutant.
A systematic analysis of the stationary-phase culture led to the identification of the factor that caused the observed phenotypes. We noticed that nitrate is limiting during stationary phase (data not shown) and that addition of extra KNO3 to cultures in the anaerobic stationary phase abolished both phenotypes completely (Fig. 2 and 3A). Therefore, we conclude that PA4352 is involved in surviving prolonged anaerobic energy stress conditions caused by nitrate limitation. In the absence of nitrate, the ΔPA4352 mutant died under these conditions, while addition of KNO3 rescued the phenotype.
Restriction of oxygen initiates premature death of the ΔPA4352 mutant.
P. aeruginosa also faces energy stress when aerobically grown cells enter an anaerobic environment. To analyze the effect of oxygen limitation, we performed a shift experiment. Wild-type cells (NB058), the ΔPA4352 mutant (NB059) containing the empty mini-CTX2 vector, and the complemented ΔPA4352 mutant (NB060) harboring the PA4352 gene cloned into mini-CTX2 (pNB011) were grown aerobically in LB to stationary phase. After 15 h of incubation in the aerobic stationary phase, the cultures were shifted to anaerobic conditions by transfer into hermetically sealed bottles. The viability of the ΔPA4352 mutant declined 220-fold within 33 h of anaerobiosis, while the wild type and complemented mutant showed only a 4- and a 3.5-fold loss of viability, respectively (Fig. 3B). This experiment confirmed the role of PA4352 in surviving energy stress even after a shift from aerobic to anaerobic conditions.
Sensitivity of the ΔPA4352 mutant to CCCP in the anaerobic stationary phase.
To initiate energy starvation by depolarization of the membrane, we added CCCP to different strains in the anaerobic stationary phase. Wild-type cells (NB058), the ΔPA4352 mutant (NB059) containing the empty mini-CTX2 vector, and the complemented ΔPA4352 mutant (NB060) harboring the PA4352 gene cloned into mini-CTX2 (pNB011) were incubated anaerobically in LB supplemented with 50 mM KNO3. After 7 h of incubation in the anaerobic stationary phase, the cultures were supplemented with additional KNO3 (30 mM) to prevent premature death caused by nitrate limitation. At the same time, we initiated depolarization of the membrane by adding CCCP (0.2 mM). The numbers of CFU were determined at different time points during the incubation with CCCP, and survival was calculated as a percentage (Fig. 3C). After 9 h of incubation with CCCP, we observed a dramatic 1,110-fold decrease in viability for the ΔPA4352 mutant, whereas only an 8-fold loss of viability was observed for the wild type. Complementation with pNB011 harboring PA4352 did not fully restore the phenotype but still reduced the 1,110-fold decline to a 32-fold decrease in viability.
Two promoters control expression of the PA4352 gene.
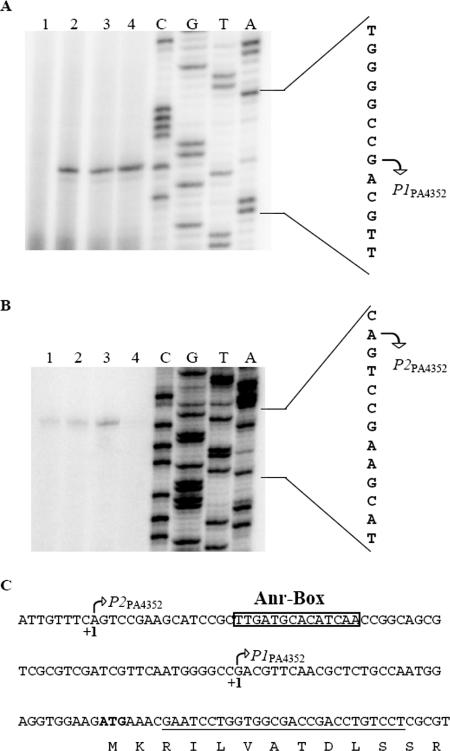
The phenotypic characterization indicated induction of the PA4352 gene in response to oxygen limitation and in the stationary phase. To identify the transcriptional start point of PPA4352, we performed primer extension analysis. Total cellular RNA was extracted from anaerobically grown P. aeruginosa wild-type cells in the exponential and early stationary phases. Additionally, we extracted the RNAs of wild-type cells and the anr mutant grown aerobically to early stationary phase (Fig. 4). The Anr protein is the global oxygen-sensing regulator of anaerobic metabolism in P. aeruginosa (35, 48) and was selected because of the presence of a putative Anr binding site in the upstream region of PA4352 (see below). We identified two transcriptional start sites in front of the PA4352 coding region. The first transcriptional start site was localized 33 bp upstream of the predicted start codon and was designated P1PA4352. The second transcriptional start site was designated P2PA4352 and was located 64 bp upstream of P1PA4352.
FIG. 4.
Primer extension analysis of PA4352 transcription, which identified the positions of P1PA4352 (A) and P2PA4352 (B). Total cellular RNA was extracted from aerobically grown cultures of the P. aeruginosa anr mutant (lanes 1) and wild-type cells (lanes 2) harvested in the early stationary phase and also wild-type cells cultivated under anaerobic conditions in the early stationary phase (lanes 3) and during exponential growth (OD578, 0.7) (lanes 4) in LB supplemented with 50 mM KNO3. Thirty micrograms of each prepared RNA was applied to primer extension analysis, using the primer oNB021, shown in panel C. The primer extension products were separated on a 6% urea-acrylamide gel, together with a sequencing ladder generated with oNB021. The complementary sequence of the transcription initiation start site is represented, and the 5′ ends of the PA4352 transcripts are indicated by arrows. (C) Nucleotide sequence of the upstream and downstream regions flanking the transcriptional start sites of PA4352. The Anr binding site is boxed. The first few N-terminal amino acids of the PA4352 protein are shown in one-letter code. The position of the oligonucleotide (oNB021) used for primer extension is underlined.
The P1PA4352 promoter is Anr dependent.
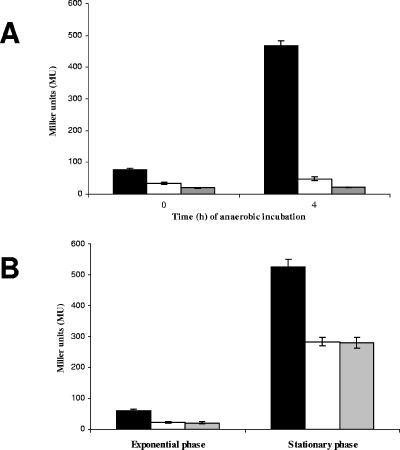
We analyzed the P1PA4352 promoter region of PA4352 using the Virtual Footprint tool of the PRODORIC database (29). This software tool identifies patterns of regulator binding sites using the position weight matrix model (29). The center of a putative Anr binding site was identified 41.5 bp upstream of the P1PA4352 transcriptional start site, which is the characteristic distance of an Anr-dependent promoter (Fig. 4C). The Fnr-type regulator Anr is activated in response to oxygen limitation. Anr controls the transcription of the dnr gene, which encodes a second Fnr-type regulator in P. aeruginosa. Dnr presumably detects nitric oxide and binds to a recognition sequence, which seems to be similar to the Anr binding site (2). To test if the PA4352 promoter was Anr or Dnr dependent, we transferred a transcriptional PPA4352-lacZ fusion into the P. aeruginosa wild-type, the Δanr mutant, and the Δdnr mutant strains, generating the strains NB007, NB023, and NB086, respectively (see Materials and Methods). Furthermore, we constructed a P. aeruginosa wild-type strain with a chromosomal PPA4352ΔANR-lacZ fusion (NB071) harboring a mutated Anr binding site of PPA4352. The binding site TTGATGTGCATCAA was changed to TTGATGTGCATACG (the boldface letters indicate the Anr consensus sequence, and the exchanged nucleotides are underlined). Since the anr and dnr mutant strains are incapable of anaerobic growth, we had to perform a shift experiment to monitor the β-galactosidase activities of the strains during anaerobiosis. In this experiment, the strains were grown aerobically in LB medium to an OD578 of 0.7 and were immediately shifted to anaerobic conditions by transfer into hermetically sealed bottles. β-Galactosidase activities were determined after 4 h of anaerobic incubation and increased up to 470 ± 15 Miller units in the wild-type strain (NB007) and up to 430 ± 80 Miller units in the dnr mutant strain, both harboring the PPA4352-lacZ fusion, while the anr mutant containing PPA4352-lacZ (NB023) failed to induce PPA4352 under anaerobic conditions (Fig. 5A). This clearly shows regulation of the PPA4352 promoter by Anr and no influence of the Dnr regulator. Moreover, the mutated Anr binding site abolished the activity of PPA4352ΔANR-lacZ in the wild-type P. aeruginosa (NB071) during anaerobiosis (Fig. 5A).
FIG. 5.
(A) Induction of the PPA4352-lacZ fusion during anaerobiosis in P. aeruginosa wild type (black bars), the anr mutant (white bars), and the mutated promoter PPA4352ΔANR-lacZ in wild-type cells (gray bars). The strains were grown at 37°C in LB to an OD578 of 0.7 (time zero) and transferred to hermetically sealed bottles for a 4-hour incubation (time 4). β-Galactosidase activities were determined at the indicated time points. (B) Expression patterns of PPA4352-lacZ in P. aeruginosa wild-type cells (black bars) and the anr mutant (white bars) and the PPA4352ΔANR-lacZ fusion containing a mutated Anr recognition site in wild-type cells (gray bars). The cells were grown aerobically in LB at 37°C. β-Galactosidase activities were determined in exponential and late stationary phases (see Materials and Methods). All experiments were repeated three times, and standard deviations are indicated.
Primer extension experiments also confirmed the Anr-dependent induction of the P1PA4352 promoter in wild-type cells in the anaerobic exponential and anaerobic stationary growth phases (Fig. 4A). We also observed promoter activity in the aerobic stationary phase in wild-type cells, but not in the anr mutant (Fig. 4A), which confirms Anr-dependent induction during anaerobiosis. The Anr-dependent induction of this promoter during the aerobic stationary phase indicates oxygen-limiting conditions, which have been observed by others (10).
The P2PA4352 promoter is active in the stationary phase.
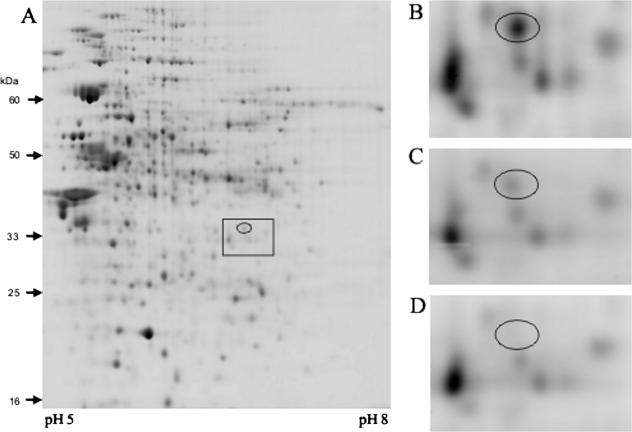
Primer extension experiments clearly showed that P2PA4352 is active in wild-type cells in the stationary phase under aerobic and anaerobic conditions but not during anaerobic exponential growth (Fig. 4B). The P2PA4352 transcript was also found in the anr mutant in the aerobic stationary phase (Fig. 4B). However, since only a faint P2PA4352 transcript was detected, we used 2D gel electrophoresis to investigate if P2PA4352 contributes to protein biosynthesis in the stationary phase. Wild-type cells, the anr mutant, and the PA4352 knockout mutant were grown aerobically in LB. Cells were harvested in the late stationary phase, and proteins were extracted for 2D gel electrophoresis. A protein spot corresponding to the PA4352 protein was identified by matrix-assisted laser desorption ionization-time of flight mass spectrometry in wild-type cells and the anr mutant strain (Fig. 6A to C). Since no Anr-dependent P1PA4352 transcript was detected by primer extension experiments in the anr mutant strain, this protein is translated from the P2PA4352 promoter alone. As expected, no PA4352 protein could be detected in the ΔPA4352 mutant (Fig. 6D). Therefore, P2PA4352 enables synthesis of the PA4352 protein in the stationary phase, independently of Anr.
FIG. 6.
(A) Image of a 2D polyacrylamide gel showing the protein pattern of the P. aeruginosa wild type. Cells were grown to the stationary phase aerobically in LB and harvested for protein extraction after 15 h in the stationary phase. 2D gel electrophoresis was performed by using IPG strips covering a pH range from 5 to 8 (IPG Ready Strips; Bio-Rad, Munich, Germany). The ellipse marks the position of the PA4352 protein spot, and the boxed area indicates the enlarged zone shown in Fig. 6B to D. (B to D) Enlarged areas of 2D gel images indicating the positions of PA4352 in the P. aeruginosa wild type (B), the anr mutant (C), and the ΔPA4352 mutant (D). The positions of the PA4352 protein spots are marked by ellipses.
The induction of the P2PA4352 promoter in the aerobic stationary phase was also confirmed using lacZ fusions. We found a clear 8- to 14-fold induction in the aerobic stationary phase when we measured β-galactosidase activities from the wild-type strain harboring the PPA4352ΔANR-lacZ fusion with the mutated Anr binding site (NB071) (Table 2) and the anr mutant strain with the PPA4352-lacZ fusion (NB023). In both strains, β-galactosidase activities result only from the P2PA4352 promoter, since the Anr-dependent P1PA4352 promoter is inactive because of either the missing Anr protein in the anr mutant strain (NB023) or the mutated Anr binding site of the lacZ fusion (NB071). As a control, we monitored β-galactosidase activity from the complete promoter-lacZ fusion containing P1PA4352 and P2PA4352 integrated into the wild-type strain (strain NB007) and detected a ninefold induction upon entry into the aerobic stationary phase (Fig. 5B).
TABLE 2.
Induction of PPA4352ΔANR-lacZ in wild-type P. aeruginosa and various mutant strainsa
| P. aeruginosa strain or relevant genotype | β-Galactosidase activity (Miller units)
|
Fold inductiond | |
|---|---|---|---|
| Aerobic exponential phaseb | Aerobic stationary phasec | ||
| Wild type | 20 ± 5 | 280 ± 20 | 14 |
| rpoS::aacC1 | 40 ± 10 | 325 ± 20 | 8.1 |
| ΔrelA | 25 ± 10 | 185 ± 30 | 7.4 |
| ΔrhlR | 20 ± 10 | 290 ± 30 | 14.5 |
| ΔlasR | 20 ± 5 | 210 ± 20 | 10.5 |
| Δanr | 45 ± 10 | 370 ± 30 | 8.2 |
Rounded β-galactosidase activities of the Anr-independent promoter P2PA4352 measured from the PPA4352ΔANR-lacZ fusion in wild-type PAO1 and various mutants under aerobic conditions as described in Materials and Methods.
Exponential growth phase; OD578, 0.7.
Stationary phase, 16 h after entry into the stationary phase.
Fold induction from aerobic exponential to aerobic stationary phase.
Regulation of the P2PA4352 promoter.
To determine the regulator responsible for stationary-phase activation of P2PA4352, we used β-galactosidase assays. The PPA4352ΔANR-lacZ fusion contains both promoters P1PA4352 and P2PA4352. However, we had already shown that, due to the mutated Anr box, P1PA4352 is inactive in PPA4352ΔANR-lacZ (Fig. 5A). Therefore, induction of β-galactosidase in the stationary phase depends solely on P2PA4352 and is not affected by Anr (see the wild-type and the anr mutant in Table 2). This promoter-lacZ fusion harboring only the active Anr-independent promoter was transferred into the wild type and the mutant strains deficient in rpoS, relA, rhlR, or lasR. The resulting strains were grown aerobically in LB, and β-galactosidase activities were determined in the exponential and stationary phases (Table 2). The activity of PPA4352ΔANR-lacZ in the wild type increased 14-fold in the stationary phase compared to aerobic-exponential growth. The same pattern of induction (between 7- and 14-fold induction) was observed for the mutant strains lacking rpoS, relA, rhlR, or lasR. None of the tested regulators and sigma factors was responsible for stationary-phase activation of P2PA4352.
DISCUSSION
During our investigation of Pseudomonas aeruginosa anaerobic long-term survival, we identified increased production of two proteins containing Usp domains: PA3309 and PA4352 (36). Recently, we reported the regulation of the PA3309 gene and its gene product during long-term survival via pyruvate fermentation (36). While PA3309 is essential for survival during pyruvate fermentation, PA4352 is not. In order to elucidate the biological function of PA4352, we screened for mutant phenotypes of a ΔPA4352 mutant strain and studied the regulation of the PA4352 promoter.
Primer extension analysis revealed two transcriptional start sites of PA4352, P1PA4352 and P2PA4352. We showed that P1PA4352 is a typical Anr-dependent promoter. Anaerobic induction was shown to be independent of Dnr. Anr of P. aeruginosa is a homolog of the E. coli redox regulator Fnr and detects oxygen (35, 48). In the absence of oxygen, the Anr regulator activates transcription from Anr-dependent promoters by binding to a specific binding site termed the Anr box. The center of an Anr binding site of P1PA4352 was located at position −41.5 bp with respect to the transcriptional start point, which is the reported optimal distance (17). Moreover, the Anr box is highly conserved and displays a perfect palindrome, TTGAT-XXXX-ATCAA. Mutation of this Anr box abolished promoter activity under anaerobic conditions, as shown by the promoter-lacZ fusions (Fig. 5A). Primer extension experiments confirmed that P1PA4352 was active under anaerobic conditions but also showed promoter activity in the aerobic stationary phase. Stationary-phase induction of P1PA4352 depends on Anr, since it is abolished in an anr mutant strain. Oxygen limitation in the stationary phase of aerobically shaken cultures was already shown by Cooper et al. (10), using an O2 microelectrode, and could be explained by the high respiration level of high cell densities (1010 CFU/ml) in the stationary phase. The second promoter of PA4352, P2PA4352, was induced only in the stationary phase under anaerobic and aerobic conditions and was also active in the anr mutant, showing an Anr-independent induction. Primer extension did not detect a transcript of P2PA4352 in the exponential growth phase. Genes encoding Usp-type stress proteins in E. coli are induced in the stationary phase by guanosine 3′-diphosphate 5′-diphosphate, and this induction is abolished in a relA/spoT double mutant (26). In P. aeruginosa, stringent response was shown to depend strictly on the RelA protein (14, 38). Our lacZ reporter gene experiments showed that stationary-phase induction of P2PA4352 does not depend on stringent response (RelA), the stationary-phase sigma factor RpoS, or quorum sensing (LasR and RhlR). Therefore, the regulatory system inducing P2PA4352 in the stationary phase remains unknown. Stationary-phase induction was also observed for PA3309, the first Usp-type stress protein we identified in P. aeruginosa, but the regulation has not been elucidated (36). The identification of the regulator activating transcription in the stationary phase is currently our research focus.
A systematic screening for the phenotype of the ΔPA4352 mutant revealed reduced survival in the anaerobic stationary phase, which was triggered by nitrate limitation. Addition of extra KNO3 to ΔPA4352 mutant cells in the stationary phase rescued the observed phenotype. After 9 h of anaerobic incubation, the restriction of nitrate as a terminal electron acceptor resulted first in delayed growth of the mutant after transfer to upshift conditions and, second, in premature death of the ΔPA4352 mutant during extended (15-h) anaerobic incubation. We observed a 77-fold decrease in viability of the ΔPA4352 mutant, whereas wild-type cells and the complemented mutant showed only a 1.5- and 2.0-fold decline, respectively. A similar phenotype was observed when cells were shifted from the aerobic stationary phase to anaerobic conditions. We observed a 220-fold loss in viability of the ΔPA4352 mutant, while wild-type cells and the complemented mutant showed only a slight 4-fold decrease of viability after 33 h of anaerobiosis. Since we complemented the mutant with the PA4352 gene, we showed that the observed phenotype is restricted to the absence of the PA4352 gene. We confirmed the observation that PA4352 is involved in surviving energy stress in an independent experiment. The uncoupler of the electron transport chain, CCCP, was used to depolarize the membrane and to induce energy starvation under anaerobic conditions. Again, we observed a significant loss of viability of the ΔPA4352 mutant compared to the wild type. This confirms that PA4352 is important for surviving anaerobic energy stress. Therefore, PA4352 might play an important role during persistent infection of the CF lung and could contribute to survival of P. aeruginosa cells inside microcolonies in anaerobic mucus plaques.
A comparison of the properties of P. aeruginosa PA4352 to those of the E. coli Usp-type stress proteins indicates different functions. None of the typical uspA mutant phenotypes, such as UV sensitivity or reduced survival in the aerobic stationary phase, were observed for the P. aeruginosa ΔPA4352 mutant (data not shown). CCCP sensitivity was reported for UspG (named UP12 in reference 8), however, under aerobic conditions. No anaerobic phenotype has been described for the E. coli Usp-type stress proteins. Interestingly, Usp-type stress proteins are found to be induced in response to oxygen starvation in mycobacteria (31, 32). Mycobacteria face oxygen limitation during infection when incorporated into fibrous granulomas, which leads to a nonreplicative persistent state (42, 43). It will be interesting to see if Usp-type stress proteins contribute to anaerobic survival of bacteria and if Usp-type stress proteins are important for persistent infection in P. aeruginosa and other pathogens.
. . . .
Acknowledgments
We thank Dieter Jahn for continuous support and for critically reading the manuscript and Beatrice Benkert from our laboratory for the construction of the unmarked dnr mutant strain. We are indebted to H. P. Schweizer (University of Colorado) for providing the pEX18Ap, mini-CTX-lacZ, pFLP2, and mini-CTX2 plasmids; Dieter Haas (Université de Lausanne, Lausanne, Switzerland) for providing the P. aeruginosa anr mutant; and E. P. Greenberg (University of Iowa) for supplying the P. aeruginosa rpoS mutant. We thank Miguel Camara (University of Nottingham, United Kingdom) for providing the plasmids pSB219.9A and pSB224.10A for construction of the lasR and rhlR knockout mutants in P. aeruginosa.
The investigation was supported by funds from the Deutsche Forschungsgemeinschaft, the German Research Centre for Biotechnology, and the Fonds der Chemischen Industrie. K.S. was supported by the DFG-European Graduate College 653.
REFERENCES
- 1.Aiba, H., S. Adhya, and B. de Crombrugghe. 1981. Evidence for two gal promoters in intact Escherichia coli. J. Biol. Chem. 256:11905-11910. [PubMed] [Google Scholar]
- 2.Arai, H., T. Kodama, and Y. Igarashi. 1997. Cascade regulation of the two CRP/FNR-related transcriptional regulators (ANR and DNR) and the denitrification enzymes in Pseudomonas aeruginosa. Mol. Microbiol. 25:1141-1148. [DOI] [PubMed] [Google Scholar]
- 3.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1995. Current protocols in molecular biology. John Wiley & Sons, New York, N.Y.
- 4.Bateman, A., L. Coin, R. Durbin, R. D. Finn, V. Hollich, S. Griffiths-Jones, A. Khanna, M. Marshall, S. Moxon, E. L. Sonnhammer, D. J. Studholme, C. Yeats, and S. R. Eddy. 2004. The Pfam protein families database. Nucleic Acids Res. 32:D138-D141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beatson, S. A., C. B. Whitchurch, A. B. Semmler, and J. S. Mattick. 2002. Quorum sensing is not required for twitching motility in Pseudomonas aeruginosa. J. Bacteriol. 184:3598-3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Becher, A., and H. P. Schweizer. 2000. Integration-proficient Pseudomonas aeruginosa vectors for isolation of single-copy chromosomal lacZ and lux gene fusions. BioTechniques 29:948-950, 952. [DOI] [PubMed] [Google Scholar]
- 7.Binnerup, S. J., and J. Sorensen. 1993. Long-term oxidant deficiency in Pseudomonas aeruginosa PAO303 results in cells which are non-culturable under aerobic conditions. FEMS Microbiol. Ecol. 13:79-84. [Google Scholar]
- 8.Bochkareva, E. S., A. S. Girshovich, and E. Bibi. 2002. Identification and characterization of the Escherichia coli stress protein UP12, a putative in vivo substrate of GroEL. Eur. J. Biochem. 269:3032-3040. [DOI] [PubMed] [Google Scholar]
- 9.Borriello, G., E. Werner, F. Roe, A. M. Kim, G. D. Ehrlich, and P. S. Stewart. 2004. Oxygen limitation contributes to antibiotic tolerance of Pseudomonas aeruginosa in biofilms. Antimicrob. Agents Chemother. 48:2659-2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cooper, M., G. R. Tavankar, and H. D. Williams. 2003. Regulation of expression of the cyanide-insensitive terminal oxidase in Pseudomonas aeruginosa. Microbiology 149:1275-1284. [DOI] [PubMed] [Google Scholar]
- 11.Costerton, J. W., P. S. Stewart, and E. P. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318-1322. [DOI] [PubMed] [Google Scholar]
- 12.de Lorenzo, V., and K. N. Timmis. 1994. Analysis and construction of stable phenotypes in gram-negative bacteria with Tn5- and Tn10-derived minitransposons. Methods Enzymol. 235:386-405. [DOI] [PubMed] [Google Scholar]
- 13.Dunn, N. W., and B. W. Holloway. 1971. Pleiotrophy of p-fluorophenylalanine-resistant and antibiotic hypersensitive mutants of Pseudomonas aeruginosa. Genet. Res. 18:185-197. [DOI] [PubMed] [Google Scholar]
- 14.Erickson, D. L., J. L. Lines, E. C. Pesci, V. Venturi, and D. G. Storey. 2004. Pseudomonas aeruginosa relA contributes to virulence in Drosophila melanogaster. Infect. Immun. 72:5638-5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eschbach, M., K. Schreiber, K. Trunk, J. Buer, D. Jahn, and M. Schobert. 2004. Long-term anaerobic survival of the opportunistic pathogen Pseudomonas aeruginosa via pyruvate fermentation. J. Bacteriol. 186:4596-4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fux, C. A., J. W. Costerton, P. S. Stewart, and P. Stoodley. 2005. Survival strategies of infectious biofilms. Trends Microbiol. 13:34-40. [DOI] [PubMed] [Google Scholar]
- 17.Gamper, M., A. Zimmermann, and D. Haas. 1991. Anaerobic regulation of transcription initiation in the arcDABC operon of Pseudomonas aeruginosa. J. Bacteriol. 173:4742-4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gustavsson, N., A. Diez, and T. Nystrom. 2002. The universal stress protein paralogues of Escherichia coli are co-ordinately regulated and co-operate in the defence against DNA damage. Mol. Microbiol. 43:107-117. [DOI] [PubMed] [Google Scholar]
- 19.Hassett, D. J. 1996. Anaerobic production of alginate by Pseudomonas aeruginosa: alginate restricts diffusion of oxygen. J. Bacteriol. 178:7322-7325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hassett, D. J., J. Cuppoletti, B. Trapnell, S. V. Lymar, J. J. Rowe, S. Sun Yoon, G. M. Hilliard, K. Parvatiyar, M. C. Kamani, D. J. Wozniak, S. H. Hwang, T. R. McDermott, and U. A. Ochsner. 2002. Anaerobic metabolism and quorum sensing by Pseudomonas aeruginosa biofilms in chronically infected cystic fibrosis airways: rethinking antibiotic treatment strategies and drug targets. Adv. Drug. Deliv. Rev. 54:1425-1443. [DOI] [PubMed] [Google Scholar]
- 21.Ho, S. N., H. D. Hunt, R. M. Horton, J. K. Pullen, and L. R. Pease. 1989. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 77:51-59. [DOI] [PubMed] [Google Scholar]
- 22.Hoang, T. T., R. R. Karkhoff-Schweizer, A. J. Kutchma, and H. P. Schweizer. 1998. A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally-located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene 212:77-86. [DOI] [PubMed] [Google Scholar]
- 23.Hoang, T. T., A. J. Kutchma, A. Becher, and H. P. Schweizer. 2000. Integration-proficient plasmids for Pseudomonas aeruginosa: site-specific integration and use for engineering of reporter and expression strains. Plasmid 43:59-72. [DOI] [PubMed] [Google Scholar]
- 24.Jones, K. L., A. H. Hegab, B. C. Hillman, K. L. Simpson, P. A. Jinkins, M. B. Grisham, M. W. Owens, E. Sato, and R. A. Robbins. 2000. Elevation of nitrotyrosine and nitrate concentrations in cystic fibrosis sputum. Pediatr. Pulmonol. 30:79-85. [DOI] [PubMed] [Google Scholar]
- 25.Koch, C., and N. Hoiby. 2000. Diagnosis and treatment of cystic fibrosis. Respiration 67:239-247. [DOI] [PubMed] [Google Scholar]
- 26.Kvint, K., L. Nachin, A. Diez, and T. Nystrom. 2003. The bacterial universal stress protein: function and regulation. Curr. Opin. Microbiol. 6:140-145. [DOI] [PubMed] [Google Scholar]
- 27.Lyczak, J. B., C. L. Cannon, and G. B. Pier. 2002. Lung infections associated with cystic fibrosis. Clin. Microbiol. Rev. 15:194-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller, J. M. 1992. A short course in bacterial genetics. A laboratory manual and handbook for Escherichia coli and related bacteria. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 29.Münch, R., K. Hiller, A. Grote, M. Scheer, J. Klein, M. Schobert, and D. Jahn. 2005. Virtual Footprint and PRODORIC: an integrative framework for regulon prediction in prokaryotes. Bioinformatics 21:4187-4189. [DOI] [PubMed] [Google Scholar]
- 30.Nachin, L., U. Nannmark, and T. Nystrom. 2005. Differential roles of the universal stress proteins of Escherichia coli in oxidative stress resistance, adhesion, and motility. J. Bacteriol. 187:6265-6272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O'Toole, R., M. J. Smeulders, M. C. Blokpoel, E. J. Kay, K. Lougheed, and H. D. Williams. 2003. A two-component regulator of universal stress protein expression and adaptation to oxygen starvation in Mycobacterium smegmatis. J. Bacteriol. 185:1543-1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O'Toole, R., and H. D. Williams. 2003. Universal stress proteins and Mycobacterium tuberculosis. Res. Microbiol. 154:387-392. [DOI] [PubMed] [Google Scholar]
- 33.Palleroni, N. J. 1984. Family I. Pseudomonaceae, p. 141-219. In J. G. Hold (ed.), Bergey's manual of systematic bacteriology. The Williams and Wilkins Co., Baltimore, Md.
- 34.Rompf, A., C. Hungerer, T. Hoffmann, M. Lindenmeyer, U. Romling, U. Gross, M. O. Doss, H. Arai, Y. Igarashi, and D. Jahn. 1998. Regulation of Pseudomonas aeruginosa hemF and hemN by the dual action of the redox response regulators Anr and Dnr. Mol. Microbiol. 29:985-997. [DOI] [PubMed] [Google Scholar]
- 35.Sawers, R. G. 1991. Identification and molecular characterization of a transcriptional regulator from Pseudomonas aeruginosa PAO1 exhibiting structural and functional similarity to the FNR protein of Escherichia coli. Mol. Microbiol. 5:1469-1481. [DOI] [PubMed] [Google Scholar]
- 36.Schreiber, K., N. Boes, M. Eschbach, L. Jaensch, J. Wehland, M. Givskov, T. Bjarnsholt, M. Hentzer, and M. Schobert. 2006. Anaerobic survival of Pseudomonas aeruginosa by pyruvate fermentation requires an Usp-type stress protein. J. Bacteriol. 188:659-668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singh, P. K., A. L. Schaefer, M. R. Parsek, T. O. Moninger, M. J. Welsh, and E. P. Greenberg. 2000. Quorum-sensing signals indicate that cystic fibrosis lungs are infected with bacterial biofilms. Nature 407:762-764. [DOI] [PubMed] [Google Scholar]
- 38.van Delden, C., R. Comte, and A. M. Bally. 2001. Stringent response activates quorum sensing and modulates cell density-dependent gene expression in Pseudomonas aeruginosa. J. Bacteriol. 183:5376-5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Van Delden, C., and B. H. Iglewski. 1998. Cell-to-cell signaling and Pseudomonas aeruginosa infections. Emerg. Infect. Dis. 4:551-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vander Wauven, C., A. Pierard, M. Kley-Raymann, and D. Haas. 1984. Pseudomonas aeruginosa mutants affected in anaerobic growth on arginine: evidence for a four-gene cluster encoding the arginine deiminase pathway. J. Bacteriol. 160:928-934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.von Gabain, A., J. G. Belasco, J. L. Schottel, A. C. Chang, and S. N. Cohen. 1983. Decay of mRNA in Escherichia coli: investigation of the fate of specific segments of transcripts. Proc. Natl. Acad. Sci. USA 80:653-657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wayne, L. G., and L. G. Hayes. 1996. An in vitro model for sequential study of shiftdown of Mycobacterium tuberculosis through two stages of nonreplicating persistence. Infect. Immun. 64:2062-2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wayne, L. G., and C. D. Sohaskey. 2001. Nonreplicating persistence of Mycobacterium tuberculosis. Annu. Rev. Microbiol. 55:139-163. [DOI] [PubMed] [Google Scholar]
- 44.Whiteley, M., M. R. Parsek, and E. P. Greenberg. 2000. Regulation of quorum sensing by RpoS in Pseudomonas aeruginosa. J. Bacteriol. 182:4356-4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Worlitzsch, D., R. Tarran, M. Ulrich, U. Schwab, A. Cekici, K. C. Meyer, P. Birrer, G. Bellon, J. Berger, T. Weiss, K. Botzenhart, J. R. Yankaskas, S. Randell, R. C. Boucher, and G. Doring. 2002. Effects of reduced mucus oxygen concentration in airway Pseudomonas infections of cystic fibrosis patients. J. Clin. Investig. 109:317-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ye, R. W., D. Haas, J. O. Ka, V. Krishnapillai, A. Zimmermann, C. Baird, and J. M. Tiedje. 1995. Anaerobic activation of the entire denitrification pathway in Pseudomonas aeruginosa requires Anr, an analog of Fnr. J. Bacteriol. 177:3606-3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yoon, S. S., R. F. Hennigan, G. M. Hilliard, U. A. Ochsner, K. Parvatiyar, M. C. Kamani, H. L. Allen, T. R. DeKievit, P. R. Gardner, U. Schwab, J. J. Rowe, B. H. Iglewski, T. R. McDermott, R. P. Mason, D. J. Wozniak, R. E. Hancock, M. R. Parsek, T. L. Noah, R. C. Boucher, and D. J. Hassett. 2002. Pseudomonas aeruginosa anaerobic respiration in biofilms: relationships to cystic fibrosis pathogenesis. Dev. Cell. 3:593-603. [DOI] [PubMed] [Google Scholar]
- 48.Zimmermann, A., C. Reimmann, M. Galimand, and D. Haas. 1991. Anaerobic growth and cyanide synthesis of Pseudomonas aeruginosa depend on anr, a regulatory gene homologous with fnr of Escherichia coli. Mol. Microbiol. 5:1483-1490. [DOI] [PubMed] [Google Scholar]
- 49.Zumft, W. G. 1997. Cell biology and molecular basis of denitrification. Microbiol. Mol. Biol. Rev. 61:533-616. [DOI] [PMC free article] [PubMed] [Google Scholar]