Abstract
Pyrococcus furiosus (“rushing fireball”) was named for the ability of this archaeal coccus to rapidly swim at its optimal growth temperature, around 100°C. Early electron microscopic studies identified up to 50 cell surface appendages originating from one pole of the coccus, which have been called flagella. We have analyzed these putative motility organelles and found them to be composed primarily (>95%) of a glycoprotein that is homologous to flagellins from other archaea. Using various electron microscopic techniques, we found that these flagella can aggregate into cable-like structures, forming cell-cell connections between ca. 5% of all cells during stationary growth phase. P. furiosus cells could adhere via their flagella to carbon-coated gold grids used for electron microscopic analyses, to sand grains collected from the original habitat (Porto di Levante, Vulcano, Italy), and to various other surfaces. P. furiosus grew on surfaces in biofilm-like structures, forming microcolonies with cells interconnected by flagella and adhering to the solid supports. Therefore, we concluded that P. furiosus probably uses flagella for swimming but that the cell surface appendages also enable this archaeon to form cable-like cell-cell connections and to adhere to solid surfaces.
The best-characterized motility organelles of prokaryotes are the flagella; these organelles have been studied a great deal for bacterial models like, e.g., Escherichia coli and Salmonella enterica serovar Typhimurium. The mechanism of flagellar motion was identified for bacteria as rotation and can be explained in molecular detail (44), as can the mechanism of elongation of this motility organelle (15, 23, 54). Flagellar motion as a mode of force generation was originally visualized indirectly by using dark-field light microscopy (8). Another, more direct observation method uses fluorescence dyes covalently coupled to flagella and fluorescence microscopy (48). Flagellar motion can be observed easily with this technique for E. coli, S. enterica serovar Typhimurium, and Rhizobium lupini but not for all bacteria (43, 48). Our knowledge of the ultrastructure of bacterial flagella is based mainly on electron microscopy. Some bacterial flagella have been studied in great detail; in the case of S. enterica serovar Typhimurium they have been studied down to atomic resolution (55). This was achieved by step-by-step improvement of methods (34, 35, 36, 42). Meanwhile, models for the structure of other bacterial flagella (Rhodobacter sphaeroides, R. lupini, and Caulobacter crescentus) are available at a resolution of 1 to 2 nm.
In the case of archaea we have only limited data for the mode of motility and for structural components of the motility organelles themselves. To the best of our knowledge, rotation of archaeal flagella as a mode of force generation has been reported only for Halobacterium salinarum and the so-called “square bacterium,” recently described as Haloquadratum walsbyi or the SHOW (square haloarchaeum of Walsby) archaeon (1, 2, 10, 11, 33). Even for Methanococcus voltae, one of the few archaeal species which can be manipulated genetically (see reference 3 for a recent review) and which has been analyzed by K. Jarrell's group, rotation of flagella has not been proven yet (Jarrell, personal communication). In previous work with a focus on the ultrastructure of archaeal flagella workers reported distinct differences from bacterial flagella, especially with respect to diameter and helical structure (24, 49). In some cases the diameters of archaeal flagella were determined, and they ranged from 10 to 14 nm (Sulfolobus, 13 nm [49]; Metallosphaera, 13 nm [17]; Pyrodictium, 10 nm [38]; Ignicoccus, 14 nm [22; D. Müller, R. Rachel, and R. Wirth, unpublished]; Pyrococcus, 10 nm [49]; Methanococcus, 13 nm [27]; Archaeoglobus, 10 nm [Rachel, unpublished]; Halobacterium, 10 nm [13, 49]). There are no data on the ultrastructure in most cases; the first three-dimensional model with a resolution of ca. 2 nm was published recently for H. salinarum (13).
Archaeal and bacterial flagella differ in various respects (5, 6, 13, 27, 28, 46). Bacterial flagella form a helical tube with a diameter of ca. 20 nm and in most cases are composed of a single flagellin. In contrast, archaeal flagella are often composed of several flagellins, which in most cases are glycosylated. Bacterial flagella are synthesized via growth from the tip, while it is argued (but not proven) that polymerization of archaeal flagella occurs from the base. Anchoring of bacterial flagella is via a defined basal body containing “rings,” whereas anchoring of the flagellar bundle in a so-called polar cap has been described only for H. salinarum (28). In bacteria >50 highly conserved genes which are regulated in a hierarchical fashion are needed for formation of functional flagella. In archaea 12 fla genes are known to be needed for synthesis of flagella, and no genes needed for anchoring structures, switching, or Mot proteins are known at the moment. The structural proteins comprising the motility organelles show similarities to type IV pili of bacteria at the N terminus. Interestingly, only 15 proteins seem to be necessary for formation of type IV pili in the bacterium Neisseria meningitidis (12). The statements above refer to the rule, but, as usual, there are some exceptions.
Pyrococcus furiosus is a long-known euryarchaeon (16) with the potential to serve as a model organism for hyperthermophiles. Its main advantages are rapid growth at 100°C (doubling time, 37 min), high cell yield (3 × 108 cells/ml), known genome sequence (40), and good characterization with respect to physiology and molecular biology, including transcription (47). Using a high-temperature light microscope (21) especially equipped with electrically heated objectives and slide holder, swimming of P. furiosus at 95°C was observed previously (H. Huber, personal communication; unpublished results). Transmission electron microscopic (TEM) studies showed the existence of up to 50 monopolarly inserted cell surface appendages called flagella (16). Direct proof that these filaments are used for swimming motility is currently not available, but this function is very probable, because Ultraturrax treatment abolishes swimming. Also, rotation of P. furiosus flagella has not been demonstrated yet. We studied these organelles with respect to composition, and here we describe new functions for them, including establishing cell-cell connections and acting as an adhesin on various surfaces.
MATERIALS AND METHODS
Growth of cells and preparation of flagella.
P. furiosus Vc1 (= DSM 3638) was cultured anaerobically in modified SME medium (45) at 90°C in serum bottles. Cell masses were grown anaerobically at 95°C in a 50-liter fermentor (Bioengeneering, Wald, Switzerland) pressurized with 100 kPa of N2-CO2 (80:20) to early stationary phase and were collected by centrifugation for 30 min (3,000 × g, 4°C). Concentrated cells were sheared with an Ultraturrax T25 (IKA-Werke, Staufen, Germany) for 1 min at 13,000 rpm and for 10 s at 20,500 rpm; the suspension was centrifuged twice for 15 min (16,000 × g, 4°C) to remove cells. The supernatant containing flagella was centrifuged for 60 min at 75,000 × g, and the pellet was resuspended in a small volume of 0.1 M HEPES buffer (pH 7). Further purification on a CsCl gradient (0.45 g/ml) by centrifugation for 48 h (SW60-Ti rotor, 250,000 × g, 4°C, Beckman Optima LE-80K ultracentrifuge) resulted in three fractions that were isolated and dialyzed extensively against 5 mM HEPES buffer (pH 7) at 4°C. The isolated fractions were analyzed by TEM, and the lower one containing the purified flagella was used for further tests (the upper two bands contained protein aggregates whose identity was unknown).
Biochemical characterization of flagella.
Protein samples were resolved by sodium dodecyl sulfate-polyacrylamide (12.5%) gel electrophoresis (SDS-PAGE), as described previously (30); proteins were stained with Coomassie brilliant blue G250 or were silver stained as described previously (9). Periodic acid-Schiff staining (56) was used for detection of protein glycosylation. N-terminal sequencing by Edman degradation was performed by the central protein analytic facility of the Biology Department of the University of Regensburg.
Adherence studies: growth on carbon-coated gold grids for TEM.
Methods to study growth of microorganisms directly on carbon-coated gold grids used for TEM have been developed in our labs (39). Gold grids were placed in small Teflon holders in serum bottles containing anaerobic medium. For TEM cells were fixed with 2.5% (final concentration) glutaraldehyde for 30 min at room temperature. In the case of cell or flagellum suspensions, a drop was placed on a carbon-coated 200-mesh copper grid (Plano, Wetzlar, Germany). The samples were either unidirectionally shadowed with Pt/C at 15° (CFE 50; Cressington Ltd., Watford, United Kingdom) or negatively stained for 1 min with 2% uranyl acetate. All TEM micrographs were recorded using a slow-scan charge-coupled device camera (TEM 1000; TVIPS-Tietz, Gauting, Germany) attached to a CM 12 transmission electron microscope (FEI, Eindhoven, The Netherlands) operated at 120 keV.
Analyzing adherence to various surfaces using light microscopy.
To analyze adherence to various surfaces using light microscopy, various particulate materials (maximum size, 10 by 10 by 1 mm) were added to serum bottles used for growth of P. furiosus. The following materials were tested: gold, nickel, and copper grids for TEM; stainless steel (V4A quality used for fermentors); household aluminum foil; Plexiglas, polycarbonate, polyvinyl chloride, and nylon (various labware consumables); enamel (coating of fermentors used for growth of P. furiosus); various types of glass; mica (glass substitute for light microscopy); and Si wafers (Infineon). Another solid material tested for adhesion was a sintered quartz material used for aquarium filters (ca. 1-mm-diameter particles of Substrat pro from Eheim, Deizisau, Germany, were obtained using a mortar and pestle). After overnight growth of P. furiosus the particles were removed using tweezers, and adhering cells were stained by use of the double-stranded DNA-specific fluorescence dye 4′,6′-diamidino-2-phenylindole (DAPI). Detection was performed by using an Olympus BX50 fluorescence microscope; this approach allowed detection with nontransparent materials since the UV light used for detection was provided through the objective. The particulate materials used in adhesion studies could also be analyzed by scanning electron microscopy (SEM), as outlined below.
Analyzing adherence to solids using scanning electron microscopy.
To analyze adherence to solids using scanning electron microscopy, sand grains from the beach of Porto di Levante, Italy, or other particulate materials were added to serum bottles instead of the gold grids used for TEM studies. After incubation, the solids with adhering cells were collected by sedimentation and fixed for at least 30 min in 0.1 M HEPES-buffered (pH 7) SME medium with 2.5% (final concentration) glutaraldehyde. After washing in double-distilled water and dehydration with a graded series of acetone solutions, cells were critical point dried from liquid CO2, mounted on stubs with conductive tabs (Plano, Wetzlar, Germany), and sputter coated (3 to 5 nm platinum) with a magnetron sputter coater (model SCD 050; BAL-TEC, Walluf, Germany). Specimens were examined with a Hitachi S-4100 field emission scanning electron microscope. SEM images were recorded with Digiscan hardware and were processed with the Digital micrograph 3.4.4 software (Gatan Digital Micrograph, Inc., Pleasanton, CA).
Characterization of cell-cell connections using freeze-etching.
The methods used for freeze-etching experiments have been described previously (37). Cells from supernatants were harvested by centrifugation, loaded onto a gold carrier, and plunged into liquid nitrogen. The samples were cut with a cold knife (temperature, less than −185°C) in a freeze-etch unit (CFE-50; Cressington, Watford, United Kingdom) and then shadowed (1 nm Pt/C at 45°, 10 nm C at 90°), and the replicas were cleaned with 70% H2SO4.
Immunological methods.
Flagella prepared by CsCl gradient centrifugation as described above were used to raise antibodies in rabbits, which were purified from serum by protein G affinity chromatography. The specificity of this antibody preparation was proven by reaction with crude cell lysates before and after shearing and with purified flagella (see Fig. S1 in the supplemental material) and by reaction with whole cells (see Fig. S2 in the supplemental material). Detection in the latter case was by use of a secondary gold-labeled antibody and TEM using established methods (52). For studies aimed at determining whether flagella were responsible for the establishment and maintenance of microcolonies growing on various surfaces, the particulate materials (maximum size, 10 by 10 by 1 mm) with adhering cells were incubated (at room temperature) with 2 ml of a 1:250 dilution (in sterile medium) of the purified antibodies. After 90 min of treatment the particles were removed with tweezers, placed into 2 ml of sterile medium, and incubated for 5 min; cells were detected by DAPI staining as described above.
RESULTS
P. furiosus flagella are composed mainly of one glycoprotein.
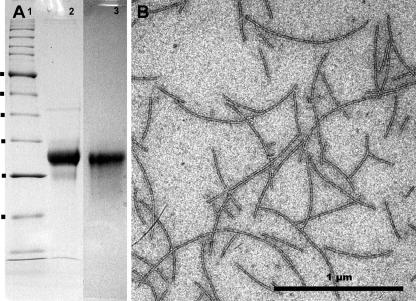
Using a slightly modified protocol (25) for preparation of archaeal flagella, we were able to obtain a pure preparation, which allowed us to analyze these cell surface organelles. Our preparation, obtained after flagella were sheared from cells and purified via CsCl gradient centrifugation, consisted of long filaments with diameters of 9 to 10 nm (Fig. 1B). SDS-PAGE revealed that these flagella were composed of >95% of one protein with an apparent molecular mass of ca. 30 kDa (Fig. 1A). The protein was identified as an archaeal flagellin by N-terminal sequencing; the sequence obtained, AVGIGTLIVFIA, was found to perfectly match the consensus sequence for archaeal flagellins defined previously (46). As Fig. 1A shows, use of periodic acid-Schiff staining revealed that the P. furiosus flagellin is a glycoprotein.
FIG. 1.
Characterization of purified flagella. (A) Flagella prepared by shearing and purified by CsCl gradient centrifugation were analyzed by SDS-PAGE. Coomassie brilliant blue G250 staining indicated that the filament consists of >95% of one protein (lane 2); periodic acid-Schiff staining revealed that this flagellin is glycosylated (lane 3). Lane 1 contained a protein size standard; molecular masses of 66, 56, 43, 34, 27, and 20 kDa are indicated on the left. (B) TEM analysis proved the identity of the flagellum preparation.
Various heat treatments indicated the very high thermal stability of P. furiosus flagella. After incubation in 5 mM HEPES buffer for various times (15 min, 30 min, 60 min, 120 min) at different temperatures (from room temperature to 121°C), TEM analysis indicated that disintegration began after 60 min of treatment at 121°C because some shorter filaments were detected (data not shown). Even after 2 h of treatment at 121°C 30 to 50% of the flagella still were detected as long filaments. For denaturation of flagella into monomers a 60-min treatment at 25°C with one of the following detergents was found to be sufficient: 0.1% (final concentration) SDS, 0.05% (final concentration) Triton X-100, or 0.05% (final concentration) cetyltrimethylammonium bromide. Solubilization of flagella into monomers also could be achieved by a 60-min treatment at 80°C with 1.5 M (final concentration) guanidine hydrochloride.
Flagella of P. furiosus enable the cells to adhere to carbon-coated gold grids and to form cell-cell connections.
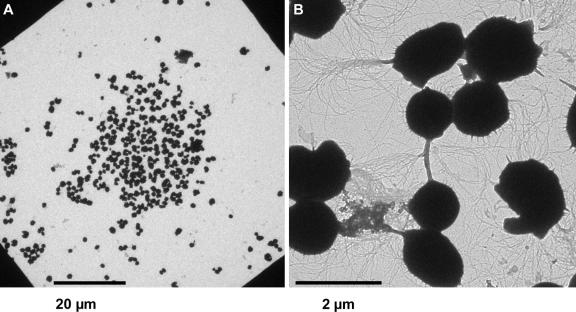
During our attempts to develop techniques to study the three-dimensional structure of P. furiosus flagella via tomography, we realized that P. furiosus cells adhered to carbon films supported by gold grids, like those used for TEM. Following incubation in liquid growth medium, microscopic studies of such grids indicated that cells grew in the form of small colonies (Fig. 2A). Nearly all cells of these preparations had tufts of flagella (Fig. 2B), compared to cells freely floating in the medium and later prepared for TEM, where only about 50% of the cells had flagella. SEM studies indicated that the cells adhered to the solid support by means of their flagella (see below).
FIG. 2.
Growth of P. furiosus on carbon-coated gold grids for TEM. (A) Low-magnification TEM micrograph of a single P. furiosus colony. (B) Approximately 5% of the cells were connected by aggregated flagella.
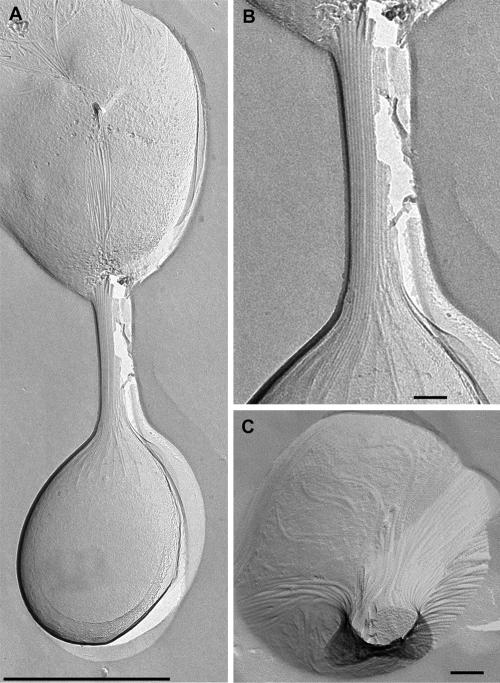
Another observation was stunning: up to 5% of all cells were observed to form pairs, in which the two cells were interconnected via cable-like structures, with a total diameter of 100 to 200 nm. These pairs of cells were also observed after freeze-etching. The micrographs obtained clearly demonstrate that the cell-cell connections are made from a multitude of flagella aggregating into a highly ordered parallel structure resembling a cable (Fig. 3A and B). In a few cases we obtained preparations in which the cables were freeze fractured, which provided a view of the cross axis; each single filament in the cables had the diameter of a single flagellum (Fig. 3C).
FIG. 3.
Transmission electron micrographs of freeze-etched P. furiosus cell-cell connections. (A) Two cells connected by cable-like structures composed of parallel flagella. Bar = 1 μm. (B) “Cable” connecting two cells at higher magnification. Bar = 100 nm. (C) Freeze fracturing, resulting in breakage of the cables across the long axis. The highly ordered structure of the cables is evident. Bar = 100 nm.
Flagella of P. furiosus allow adherence to and growth in a biofilm-like manner on natural substrates.
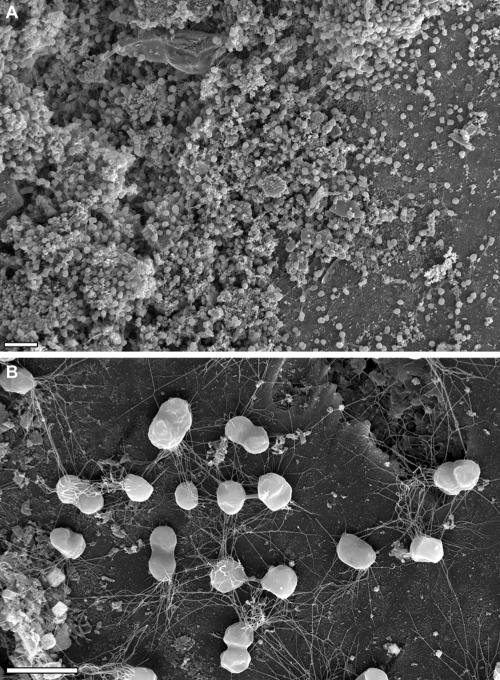
It could be argued that growth of P. furiosus on carbon-coated gold grids might not reflect physiological conditions; therefore, we tested growth on other surfaces identical to or mimicking the natural habitat. For this we chose sand grains from the beach of Porto di Levante (island of Vulcano, Italy), the location from which P. furiosus was originally isolated (16). Different experimental settings clearly proved not only that the cells could attach to these surfaces via their flagella, but also that flagella enabled them to grow in a three-dimensional manner (Fig. 4B), forming (micro)colonies in a biofilm-like structure. As an example, Fig. 4A shows part of a microcolony grown on medium-washed and autoclaved but otherwise untreated sand grains. The microcolonies could develop into cell masses containing a few thousand cells. To exclude the possibility that some kind of surface preconditioning of the sand grains is needed for adherence, any potentially present coating was removed from the sand grains by treatment with chromosulfuric acid, followed by extensive washing in medium. Again, P. furiosus exhibited growth on the sand grains in a biofilm-like manner (data not shown). A second solid material tested for adhesion was a sintered quartz material used for aquarium filters. This material, according to the supplier's information, is processed from lava and has a very high pore content. Use of chromosulfuric acid-treated quartz or untreated quartz allowed P. furiosus to grow in the (micro)colony-like manner observed for sand grains (Fig. 5). It should be noted that in all these cases the cells were connected by a multitude of flagella which aggregated along part of their lengths, forming cables. The thinnest filaments had an estimated diameter of ca. 10 nm, which perfectly matches the value obtained for flagella using TEM. In addition, the filaments were fastened to the sand grains and quartz not only at their tips but also over a distance of up to 1 μm (Fig. 4B). Cells in such microcolonies again exhibited the cell-cell connections that we identified during growth on carbon-coated gold grids (Fig. 4B and 5B).
FIG. 4.
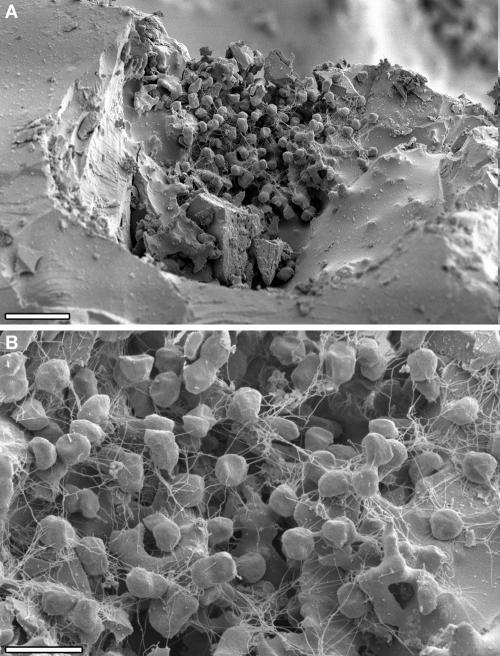
Growth of P. furiosus on the surface of sand grains from its natural habitat, visualized by scanning electron microscopy. (A) Low-magnification scanning electron micrograph. Bar = 5 μm. (B) Higher magnification. Flagella attach the cells of the microcolony to the sand grain and to each other. Bar = 2 μm.
FIG. 5.
Growth of P. furiosus on the surface of sintered quartz, visualized by scanning electron microscopy. (A) Low-magnification scanning electron micrograph. Bar = 5 μm. (B) Higher magnification, demonstrating that flagella are used to attach the cells of the microcolony to the quartz surface and to each other. Bar = 2 μm.
Flagella of P. furiosus allow adherence to and growth in a biofilm-like manner on various surfaces.
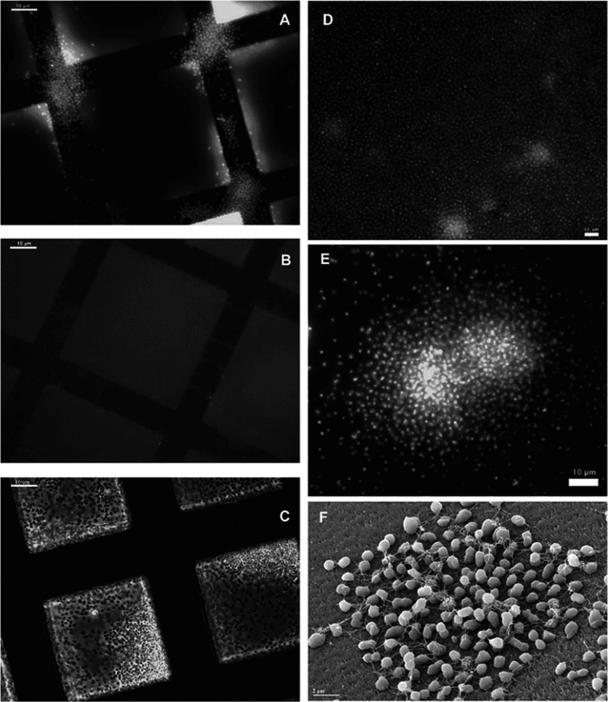
The data presented above raised the question whether P. furiosus can adhere to surfaces other than sand grains, sintered quartz, and TEM gold grids. Therefore, various materials were tested for adherence, and the first step in detection of cells was DAPI staining. As Fig. 6 shows, adherence to various surfaces was observed; in addition, it is evident that two different types of growth patterns were observed. In the case of gold, nickel, Si wafers, nylon, Plexiglas, and quartz, cells formed more or less dense aggregates resembling colonies, while on copper and polycarbonate growth was more dispersed, with single cells separated by distances of ca. 2 to 5 μm. For both growth patterns SEM data clearly showed that flagella connected the cells (Fig. 6F). Final proof that it was indeed the flagella that enabled adherence to the various surfaces and establishment of biofilms on them came from experiments using antibodies against flagella. Since the antibodies would have been denatured under growth conditions for P. furiosus (95°C), they were added after growth to established biofilms on surfaces at room temperature. Figure 6B very clearly shows that addition of antibodies against P. furiosus flagella did dissolve the aggregates. As a control, antibodies against a cytoplasmic multienzyme complex of P. furiosus (namely, DNA-dependent RNA polymerase) were used, which very clearly did not influence the aggregates (Fig. 6C).
FIG. 6.
P. furiosus adheres to various surfaces via flagella. (A) P. furiosus cells adhere to gold grids (detection via DAPI staining). Bar = 10 μm. (B) After addition of antibodies against P. furiosus flagella, no adhering cells are observed on gold grids (detection via DAPI staining). Bar = 10 μm. (C) Addition of antibodies against P. furiosus RNA polymerase does not remove adhering cells from gold grids (detection via phase-contrast microscopy). Bar = 10 μm. (D) P. furiosus adheres very well to polycarbonate (detection via DAPI staining). Bar = 10 μm. (E) P. furiosus adheres to Si wafers and grows on these wafers in the form of microcolonies (detection via DAPI staining). Bar = 10 μm. (F) Microcolony of P. furiosus growing on Si wafer (detection via SEM). Bar = 2 μm.
DISCUSSION
We showed that the long filaments inserted at one pole of the coccoid P. furiosus cells enable this archaeon to form cell-cell connections and to adhere to various solid surfaces. We therefore concluded that flagella of P. furiosus can act as adhesins.
Biochemical analyses of flagella.
Using slight modifications of established procedures for isolating archaeal flagella (25), we obtained a preparation which according to TEM and SDS-PAGE data contained pure flagella. We have shown that this cell surface appendage consists of >95% of one flagellin, which is rather unusual for archaea (46). The apparent molecular mass, ca. 30 kDa, does agree well with a previous report (50) on the existence of two flagellins (32 and 32.5 kDa) in the P. furiosus cell surface appendages. The discrepancy with respect to the number of flagellins reported here and previously (one flagellin versus two flagellins) cannot be resolved easily; the genome data (40) indicate that there are two flagellin genes encoding proteins with molecular masses of ca. 21 kDa (PF0338) and 29 kDa (PF0337). Unfortunately, both of these genes originally were referred to as flab. To differentiate between them, we named PF0338 (genome nucleotides 352341 to 351751) flaB1 and PF0337 (genome nucleotides 351738 to 350947) flaB2. The N terminus of the ca. 30-kDa protein identified here was determined to be AVGIGTLIVFIA, demonstrating that FlaB2 constituted the long filaments in our cultures. The genome data indeed predict that the second amino acid in mature FlaB2 is isoleucine instead of valine; therefore, we resequenced this part of the genome twice and confirmed the occurrence of valine at this position. It is not known if the original genome sequence in this region is not correct or if the strain of P. furiosus that we are used differs in this respect from the strain originally sequenced (40) by 1 bp; in either case this is only a minor difference because of the conservative nature of this amino acid substitution (isoleucine to valine). We have no evidence that significant amounts of the 21-kDa protein FlaB1 are present in the filaments. It is not known if FlaB1 is a minor flagellin located at the base of the organelle, as has been reported for flagellin FlaB3 of M. voltae (5). Our data (see Fig. S1 in the supplemental material) showing that an antiserum raised against sheared flagella reacted weakly with a ca. 27-kDa protein on whole unsheared cells (but not with purified flagella) might be considered support for this possibility. In addition, it should be noted that very probably the published genome sequence for the region coding for the potential N terminus of FlaB1 is not correct. An AVGIGTLIVFIA amino acid motif is directly upstream of the predicted N terminus (MLVAAVAA). The AVGIGTLIVFIA motif is present in all other Pyrococcus flagellins, which could be taken as evidence that the original annotation is not correct. The genome sequence does not contain a start codon in front of the AVGIGTLIVFIA motif but rather contains multiple stop codons in all three reading frames. Furthermore, our preliminary data indicate that there are rather large differences between the published genome sequence and the genome sequence of our strain, which will be clarified in further experiments.
Our finding that P. furiosus FlaB2 is glycosylated is in line with other reports, because such a modification seems to be more the rule than the exception for archaeal flagellins (46), and it is corroborated by our data showing that the apparent molecular mass of the protein is somewhat larger than the predicted molecular mass. At present, we have no data concerning the chemical nature of the glycosylation; we plan to determine this in a separate project because “structural characterization of glycoproteins is a time-consuming and challenging process especially in cases where only limited amounts of protein can be recovered form biological samples” (51).
Our biochemical data for the heat stability, structural integrity, and detergent sensitivity of the flagella are in good agreement with the natural habitat of P. furiosus, marine water (without detergents) at temperatures around 100°C.
Formation of cell-cell connections.
During our experiments to determine under which growth conditions maximum flagellation is observed, we realized that some cells in stationary phase had very unusual structures, namely cell-cell connections. These cell-cell connections could be visualized with a fluorescence light microscope by staining cells with fluorescence dyes (data not shown); the use of electron microscope techniques allowed detailed analyses. These experiments indicated that up to 5% of all cells in stationary phase were connected via cable-like structures. The structures were composed of flagella, as indicated by their occasional dissociation into single filaments. Formation of “cables” rarely was observed in exponential growth phase. Our antiserum raised against purified flagella reacted with dissociated single flagella but not with the cables. This can be explained by our finding that the cables are covered by some material whose chemical composition is not known.
An obvious question arises: why does P. furiosus form these cell-cell connections? At the moment we do not have a definite answer; a possible explanation is that the connections are a prerequisite for allowing gene transfer between P. furiosus cells. It can be argued that gene transfer takes place between P. furiosus cells and also with other species, although this has not been proven so far. Such potential gene transfer is supported by the fact that a 16-kb DNA region differing by only 151 point mutations has been identified in P. furiosus and Thermococcus litoralis (14). In P. furiosus this DNA region is flanked by identical IS elements with direct and inverted repeats, implying that it is a mobile DNA element. For P. furiosus no plasmids which might be naturally transferred between different strains have been described so far; this is in contrast to other species of Pyrococcus for which plasmids have been reported (e.g., pGT5 in Pyrococcus abyssi [32], pGE27 in P. abyssi [7], and pRT1 in Pyrococcus sp. strain JT1 [53]). On the other hand, it has been shown that at least two shuttle vectors are maintained stably in P. furiosus (4). Very recently, a comparative analysis of the genomes of P. furiosus and Pyrococcus woesei provided evidence that P. furiosus acquired more than 100 open reading frames from other organisms by lateral gene transfer (20).
Taken together, these data suggest that P. furiosus should have the capacity for gene transfer; the formation of cell-cell connections might be the structural prerequisite for such a transfer. It should be noted in this context that we did observe various lengths (>2 μm to <0.2 μm) of cable-like connections. An attractive hypothesis is that the first cell-cell contact is established by formation of the cables, which is followed by shortening of the cables that results in pairs of cells close enough to allow direct gene transfer.
The cell-cell connections that we observed here for P. furiosus do not resemble those reported previously for Haloferax (previously Halobacterium) volcanii (41). In the latter organism cytoplasmic bridges (up to 2 μm long and 0.1 μm in diameter) have been reported to support exchange of chromosomal DNA but not of immobile plasmids. It was argued that these bridges were made of the regular H. volcanii membrane and cellular envelope and that they maintain cytoplasmic continuity between the two connected cells. It was concluded that the cytoplasm of H. volcanii did not mix and that the cytoplasmic continuity should be restricted to the movement of certain molecules. In the case of P. furiosus it is very clear that no cytoplasmic bridges connect cells; rather, cables consisting of aggregated flagella connect cells. Clearly, the formation of cell fusions in the newly described species Thermococcus coalescens (29) also does not resemble the interactions that we observed here for P. furiosus.
Adhesion to solid surfaces.
In addition to cell-cell connections which are formed by aggregation of many flagella into cable-like structures, P. furiosus cells can adhere to solid surfaces by means of single flagella. We are not aware of any report that a motility organelle of an archaeon can also function as an adhesin. In bacteria, flagella originally were not suspected to have a function other than generating force for motion of the cells; this picture has to been modified. Recently, adhesion to surfaces via flagella has been reported for a variety of bacteria, especially pathogenic bacteria. For example, it was not until 2002 that it was realized that certain strains of the model bacterium E. coli can adhere to epithelial cells via their flagella (18). Further examples of adhesion of bacteria to surfaces via flagella were reviewed by Jonson et al. (26).
The adhesion of P. furiosus to surfaces is specific, because the cells do not interact in similar ways with all surfaces. Initial experiments in which adhesion to different materials was tested revealed that P. furiosus does not form colonies on glass surfaces; only a few single cells were observed very rarely on glass surfaces (data not shown). We did test if P. furiosus could adhere to a variety of other surfaces which are not related to the natural habitat of the archaeon (e.g., sand grains) or to growth under laboratory conditions (e.g., glass for serum bottles and enamel used as a coating of our fermentors for mass cultivation). Such surfaces, including metals and plastics, were also selected based on potential biotechnological applications; the results of these studies are summarized in Table 1. A few examples are shown in Fig. 6, which also provides the final proof that adhesion was via flagella: addition of antibodies against purified flagella “dissolved” the preformed aggregates (compare Fig. 6B with Fig. 6A), while antibodies against a cytoplasmic component (RNA polymerase) of P. furiosus had no effect (Fig. 6C). From this we concluded that adhesion is not an “all or nothing” effect but rather is “dynamic”; flagella coming free from surfaces are coated by the antibodies, finally resulting in dissolution of aggregates.
TABLE 1.
Adherence of P. furiosus to various surfaces
| Material | Adherence strengtha | Growth pattern |
|---|---|---|
| Gold | +++ | Very dense growth in microcolonies |
| Nickel | +++ | Very dense growth in microcolonies |
| Copper | +++ | Very dense growth, evenly distributed |
| Steel | (+) | Few cells |
| Aluminum | + | Few cells in very small microcolonies |
| Enamel | + | Few cells |
| Glass | − | Single cells |
| Si wafer | ++ | Dense growth in microcolonies |
| Mica | (+) | Few cells at edges of single mica layers |
| Polyvinyl chloride | + | Single cells in loose growth |
| Polycarbonate | +++ | Very dense growth, evenly distributed |
| Nylon | +++ | Very dense growth, plus microcolonies |
| Plexiglas | +++ | Very dense growth in colonies |
| Wood | + | Very small microcolonies |
| Quartz | +++ | Very dense growth in microcolonies |
−, only rare single cells; (+), less than 50 cells per 15 mm2; +, at least 100 cells per 15 mm2; ++, 100 to 1,000 cells per 15 mm2; +++, dense growth with ≫1,000 cells per 15 mm2.
Adhesion to sand grains collected from the natural habitat of P. furiosus seems reasonable, because this would allow the archaeon to stay in an environment that supports its growth. P. furiosus was isolated from a marine vent emerging a few meters below sea level (16). Due to their adhesion to solids, cells would not be washed away from locations which provide optimal growth conditions. P. furiosus does not need any special “surface conditioning” of the sand grains; cell adherence was neither improved nor impaired by treating the sand with chromosulfuric acid. Given the fact that flagella of P. furiosus allow the cells to adhere to solid surfaces and to each other, it was not surprising (in retrospect) to observe growth of the archaeon as (micro)colonies on such surfaces, indicating the initial formation of a biofilm. While biofilm formation by archaea can often be observed in the natural environment (19), to the best of our knowledge growth of cultivated archaea in biofilms has been reported only for Archaeoglobus fulgidus (31) (according to the unpublished results of LaPaglia and Hartzell, biofilm formation was also observed for Archaeoglobus profundus, Methanobacterium thermoautotrophicum, and Methanococcus jannaschii). In the case of A. fulgidus various stress conditions, like nonphysiological extremes of pH and temperature and also high concentrations of metals and other compounds, induced the formation of biofilms consisting of variable structures containing proteins, polysaccharide, and metals; induction of flagellum-like cell appendages has not been noted for A. fulgidus. The (micro)colonies which we describe here, therefore, represent new structures for cultivated archaea.
From the data presented above we concluded that flagella of P. furiosus enable this archaeon to form cell-cell connections and to adhere to solid surfaces. This raises the question whether the filaments (which have diameters of 9 to 10 nm and are up to 7 μm long) are correctly named flagella, which indicates that they are used for swimming motility. Our biochemical analyses have clearly shown that these flagella are composed mainly of one protein which is homologous to other archaeal flagellins, and therefore designation of the cell surface appendages as flagella seems reasonable. On the other hand, as discussed above, a direct correlation of archaeal flagella with motility has been obtained for very few species. Archaeal flagellins show some homology to type IV pili of bacteria in the N-terminal region (6), and the latter bacterial cell surface organelles combine two functions, namely adhesion and motility. Type IV pili bind at the tip to surfaces and are retracted rapidly, resulting in the so-called twitching motility of bacteria. In the case of P. furiosus we show here that the flagella are used for adhesion; a challenging task for future investigations will be to understand the motility mechanism of this archaeon.
Supplementary Material
Acknowledgments
This work was supported by a grant from the University of Regensburg (for extension of our high-temperature light microscope).
We thank S. Naji and M. Thomm for the gift of anti-RNA polymerase antibodies and S. Dobner for expert technical help. The helpful comments of the anonymous referees are gratefully acknowledged.
Footnotes
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Alam, M., M. Claviez, D. Oesterhelt, and M. Kessel. 1984. Flagella and motility behaviour of square bacteria. EMBO J. 3:2899-2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alam, M., and D. Oesterhelt. 1984. Morphology, function and isolation of halobacterial flagella. J. Mol. Biol. 176:459-475. [DOI] [PubMed] [Google Scholar]
- 3.Allers, T., and M. Mevarech. 2005. Archaeal genetics—the third way. Nat. Rev. Genet. 6:58-73. [DOI] [PubMed] [Google Scholar]
- 4.Aravalli, R. N., and R. A. Garrett. 1997. Shuttle vectors for hyperthermophilic archaea. Extremophiles 1:183-191. [DOI] [PubMed] [Google Scholar]
- 5.Bardy, S. L., S. Y. M. Ng, and K. F. Jarrell. 2003. Prokaryotic motility structures. Microbiology 140:295-304. [DOI] [PubMed] [Google Scholar]
- 6.Bardy, S. L., T. Mori, K. Komoriya, S.-I. Aizawa, and K. F. Jarrell. 2002. Identification and localization of flagellins FlaA and FlaB3 within flagella of Methanococcus voltae. J. Bacteriol. 184:5223-5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benbouzid-Rollet, N., P. Lopez-Garcia, L. Watrin, G. Erauso, D. Prieur, and P. Forterre. 1997. Isolation of new plasmids from hyperthermophilic archaea of the order Thermococcales. Res. Microbiol. 148:767-775. [DOI] [PubMed] [Google Scholar]
- 8.Berg, H., and R. A. Anderson. 1973. Bacteria swim by rotating their flagellar filaments. Nature 245:380-382. [DOI] [PubMed] [Google Scholar]
- 9.Blum, H., H. Beier, and H. J. Gross. 1987. Improved silver staining of plant proteins, RNA and DNA in polyacrylamide gels. Electrophoresis 8:93-99. [Google Scholar]
- 10.Bolhuis, H., E. M. te Poele, and F. Rodriguez-Valera. 2004. Isolation and cultivation of Walsby's square archaeon. Environ. Microbiol. 6:1287-1291. [DOI] [PubMed] [Google Scholar]
- 11.Burns, D. G., H. M. Camarkis, P. H. Janssen, and M. L. Dyall-Smith. 2004. Cultivation of Walsby's square haloarchaeon. FEMS Microbiol. Lett. 238:469-473. [DOI] [PubMed] [Google Scholar]
- 12.Carbonelle, E., S. Helaine, L. Prouvensier, X. Nassif, and V. Pelicic. 2005. Type IV pilus biogenesis in Neisseria meningitidis: PilW is involved in a step occurring after pilus assembly, essential for fibre stability and function. Mol. Microbiol. 55:54-64. [DOI] [PubMed] [Google Scholar]
- 13.Cohen-Krausz, S., and S. Trachtenberg. 2002. The structure of the archaebacterial flagellar filament of the extreme halophile Halobacterium salinarum R1M1 and its relation to eubacterial flagellar filaments and type IV pili. J. Mol. Biol. 321:383-395. [DOI] [PubMed] [Google Scholar]
- 14.DiRuggiero, J., D. Dunn, D. L. Maeder, R. Holley-Shanks, J. Chatard, R. Horlacher, F. T. Robb, W. Boos, and R. B. Weiss. 2000. Evidence of recent lateral gene transfer among hyperthermophilic archaea. Mol. Microbiol. 38:684-693. [DOI] [PubMed] [Google Scholar]
- 15.Emerson, S. U., K. Tokuyasu, and M. I. Simon. 1970. Bacterial flagella: polarity of elongation. Science 169:190-192. [DOI] [PubMed] [Google Scholar]
- 16.Fiala, G., and K. O. Stetter. 1986. Pyrococcus furiosus sp. nov. represents a novel genus of marine heterotrophic archaebacteria growing optimally at 100°C. Arch. Microbiol. 146:56-61. [Google Scholar]
- 17.Fuchs, T., H. Huber, K. Teiner, S. Burggraf, and K. O. Stetter. 1995. Metallosphaera prunae, sp. nov., a novel metal-mobilizing, thermoacidophilic archaeum, isolated from a uranium mine in Germany. Syst. Appl. Microbiol. 18:560-566. [Google Scholar]
- 18.Giron, J. A., A. G. Torres, E. Freer, and J. B. Kaper. 2002. The flagella of enteropathogenic Escherichia coli mediate adherence to epithelial cells. Mol. Microbiol. 44:361-379. [DOI] [PubMed] [Google Scholar]
- 19.Hall-Stoodley, L., J. W. Costerton, and P. Stoodley. 2004. Bacterial biofilms: from the natural environment to infectious diseases. Nat. Rev. Microbiol. 2:95-107. [DOI] [PubMed] [Google Scholar]
- 20.Hamilton-Brehm, S. D., G. J. Schut, and M. W. W. Adams. 2005. Metabolic and evolutionary relationships among Pyrococcus species: genetic exchange within a hydrothermal vent environment. J. Bacteriol. 187:7492-7499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Horn, C., B. Paulmann, G. Kerlen, N. Junker, and H. Huber. 1999. In vivo observation of cell division of anaerobic hyperthermophiles by using a high-intensity dark-field microscope. J. Bacteriol. 181:5114-5118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huber, H., S. Burggraf, T. Mayer, I. Wyschkony, R. Rachel, and K. O. Stetter. 2000. Ignicoccus gen. nov., a novel genus of hyperthermophilic, chemolithoautotrophic archaea, represented by two new species, Ignicoccus islandicus sp. nov. and Ignicoccus pacificus sp. nov. Int. J. Syst. Evol. Microbiol. 50:2093-2100. [DOI] [PubMed] [Google Scholar]
- 23.Iino, T. 1969. Polarity of flagellar growth in Salmonella. J. Gen. Microbiol. 56:227-239. [DOI] [PubMed] [Google Scholar]
- 24.Jarrell, K. F., D. P. Bayley, and A. S. Kostyukova. 1996. The archaeal flagellum: a unique motility structure. J. Bacteriol. 178:5057-5064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jarrell, K. F., M. L. Kalmakoff, S. F. Koval, D. M. Faguy, T. M. Karnauchow, and D. P. Bayley. 1995. Purification of the flagellins from methanogenic archaea, p. 307-314. In F. T. Robb (ed.), Archaea: a laboratory manual. Cold Spring Harbor Press, Cold Spring Harbor, N.Y.
- 26.Jonson, A.-B., S. Normark, and M. Rhen. 2005. Fimbriae, pili, flagella and bacterial virulence, p. 67-89. In A. Schmidt and H. Herwald (ed.), Concepts in bacterial virulence. Karger, Basel, Switzerland. [DOI] [PubMed]
- 27.Kalmokoff, M. L., K. F. Jarrell, and S. F. Koval. 1988. Isolation of flagella from the archaebacterium Methanococcus voltae by phase separation with Triton X-114. J. Bacteriol. 170:1752-1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kupper, J., W. Marwan, D. Typke, H. Grunberg, U. Uwer, M. Gluch, and D. Oesterhelt. 1994. The flagellar bundle of Halobacterium salinarium is inserted into a distinct polar cap structure. J. Bacteriol. 176:5184-5187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuwabara, T., M. Minaba, Y. Iwayama, I. Inouye, M. Nakashima, K. Marumo, A. Maruyama, A. Sugai, T. Itoh, J.-I. Ishibashi, T. Urabe, and M. Kamekura. 2005. Thermococcus coalescens sp. nov., a cell-fusing hyperthermophilic archaeon from Suiyo seamount. Int. J. Syst. Evol. Microbiol. 55:2507-2514. [DOI] [PubMed] [Google Scholar]
- 30.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 31.LaPaglia, C., and P. L. Hartzell. 1997. Stress-induced production of biofilm in the hyperthermophile Archaeoglobus fulgidus. Appl. Environ. Microbiol. 63:3158-3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marsin, S., and P. Forterre. 1998. A rolling circle replication initiator protein with a nucleotidyl-transferase activity encoded by the plasmid pGT5 from the hyperthermophilic archaeon Pyrococcus abyssi. Mol. Microbiol. 27:1183-1192. [DOI] [PubMed] [Google Scholar]
- 33.Marwan, W., M. Alam, and D. Oesterhelt. 1991. Rotation and switching of the flagellar motor assembly in Halobacterium halobium. J. Bacteriol. 173:1971-1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mimori, Y., I. Yamashita, K. Murata, Y. Fujiyoshi, K. Yonekura, C. Toyoshima, and K. Namba. 1995. The structure of the R-type flagellar filament of Salmonella at 9 Å resolution by electron cryomicroscopy. J. Mol. Biol. 249:69-87. [DOI] [PubMed] [Google Scholar]
- 35.Mimori-Kiyosue, Y., I. Yamashita, Y. Fujiyoshi, S. Yamaguchi, and K. Namba. 1998. Role of the outermost subdomain of Salmonella flagellin in the filament structure revealed by electron cryomicroscopy. J. Mol. Biol. 284:521-530. [DOI] [PubMed] [Google Scholar]
- 36.Morgan, D. G., C. Owen, L. A. Melanson, and D. J. DeRosier. 1995. Structure of bacterial flagellar filaments at 11 Å resolution: packing of the α-helices. J. Mol. Biol. 249:88-110. [DOI] [PubMed] [Google Scholar]
- 37.Rachel, R., I. Wyschkony, S. Riehl, and H. Huber. 2002. The ultrastructure of Ignicoccus: evidence for a novel outer membrane and for intracellular vesicle budding in an archaeon. Archaea 1:9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rieger, G. 1998. Elektronenmikroskopische und biochemische Untersuchungen zum Aufbau des Netzwerks bei Pyrodictium. Ph.D. Dissertation. University of Regensburg, Regensburg, Germany.
- 39.Rieger, G., R. Rachel, R. Herrmann, and K. O. Stetter. 1995. Ultrastructure of the hyperthermophilic archaeon Pyrodictium abyssi. J. Struct. Biol. 115:78-87. [Google Scholar]
- 40.Robb, F. T., D. L. Maeder, J. R. Brown, J. DiRuggiero, M. D. Stump, R. K. Yeh, R. B. Weiss, and D. M. Dunn. 2001. Genomic sequence of hyperthermophile, Pyrococcus furiosus: implications for physiology and enzymology. Methods Enzymol. 330:134-157. [DOI] [PubMed] [Google Scholar]
- 41.Rosenshine, I., R. Tchelet, and M. Mevarech. 1989. The mechanism of DNA transfer in the mating system of an archaebacterium. Science 245:1387-1389. [DOI] [PubMed] [Google Scholar]
- 42.Samatey, F. A., K. Imada, S. Nagashima, F. Vonderviszt, T. Kumasaka, M. Yamamoto, and K. Namba. 2001. Structure of the bacterial flagellar protofilament and implications for a switch for supercoiling. Nature 410:331-337. [DOI] [PubMed] [Google Scholar]
- 43.Scharf, B. 2002. Real-time imaging of fluorescent flagellar filaments of Rhizobium lupini H13-3: flagellar rotation and pH-induced polymorphic transitions. J. Bacteriol. 184:5979-5986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schmitt, R. 2003. Helix rotation model of the flagellar rotary motor. Biophys. J. 85:843-852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stetter, K. O., R. Huber, E. Blöchl, M. Kurr, R. D. Eden, M. Fiedler, H. Cash, and I. Vance. 1983. Hyperthermophilic archaea are thriving in deep North Sea and Alaskan oil reservoirs. Nature 365:743-745. [Google Scholar]
- 46.Thomas, N. A., S. L. Bardy, and K. F. Jarrell. 2001. The archaeal flagellum: a different kind of prokaryotic motility structure. FEMS Microbiol. Rev. 25:147-174. [DOI] [PubMed] [Google Scholar]
- 47.Thomm, M. 1996. Archaeal transcription factors and their role in transcription initiation. FEMS Microbiol. Rev. 18:159-171. [DOI] [PubMed] [Google Scholar]
- 48.Turner, L., W. S. Ryu, and H. C. Berg. 2000. Real-time imaging of fluorescent flagellar filaments. J. Bacteriol. 182:2793-2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Typke, D., M. Nitsch, A. Möhrle, R. Hegerl, M. Alam, D. Grogan, and J. Trent. 1998. Structural studies of the flagellar filaments of some archaebacteria. Inst. Phys. Conf. Ser. 93:379-380. [Google Scholar]
- 50.Vassilenko, K. S., R. Rachel, and A. S. Kostyukova. 1994. Structural studies of Pyrococcus furiosus flagella, p. 289. Abstr. Int. Symp. Biol. Motility.
- 51.Voisin, S., R. S. Houliston, J. Kelly, J.-R. Brisson, D. Watson, S. L. Bardy, K. F. Jarrell, and S. M. Logan. 2005. Identification and characterization of the unique N-linked glycan common to the flagellins and S-layer glycoprotein of Methanococcus voltae. J. Biol. Chem. 280:16586-16593. [DOI] [PubMed] [Google Scholar]
- 52.Wanner, G., H. Formanek, D. Galli, and R. Wirth. 1989. Localization of aggregation substances of Enterococcus faecalis after induction by sex pheromones. Arch. Microbiol. 151:491-497. [DOI] [PubMed] [Google Scholar]
- 53.Ward, D. E., I. M. Revet, R. Nandakumar, J. H. Tuttle, W. M. deVos, J. van der Oost, and J. DiRuggiero. 2002. Characterization of plasmid pRT1 from Pyrococcus sp. strain JT1. J. Bacteriol. 184:2561-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yonekura, K., S. Maki, D. G. Morgan, D. J. DeRosier, F. Vonderviszt, K. Imada, and K. Namba. 2000. The bacterial flagellar cap as the rotary promoter of flagellin self-assembly. Science 290:2148-2152. [DOI] [PubMed] [Google Scholar]
- 55.Yonekura, K., S. Maki-Yonekura, and K. Namba. 2003. Complete atomic model of the bacterial flagellar filament by electron cryomicroscopy. Nature 424:643-650. [DOI] [PubMed] [Google Scholar]
- 56.Zacharius, R. M., T. Zell, J. H. Morrison, and J. J. Woodlock. 1969. Glycoprotein staining following electrophoresis on acrylamide gels. Anal. Biochem. 172:320-329. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.