Abstract
Bacterial RNase P is composed of an RNA subunit and a single protein subunit (encoded by the rnpB and rnpA genes, respectively). We constructed Bacillus subtilis mutant strains that conditionally express the RNase P protein under control of the xylose promoter (Pxyl). In one strain (d7), rnpA expression was efficiently repressed in the absence of the inducer xylose, leading to cell growth arrest. Growth could be restored by a second functional rnpA allele. This is the first RNase P protein knockdown strain, providing the first direct proof that the rnpA gene is essential in B. subtilis and, by inference, in other bacteria. We further show (i) that, in the wild-type context, rnpA expression is attenuated by transcriptional polarity and (ii) that translation of rnpA mRNA in B. subtilis can be initiated at two alternative start codons. His-tagged RNase P protein variants are functional in vivo and permit purification of in vivo-assembled holoenzymes by affinity chromatography. Simultaneous expression of plasmid-encoded RNase P RNA and His-tagged protein increased RNase P holoenzyme yields. Massive overproduction of RNase P protein in strain d7 is compatible with cell viability.
Present knowledge about the in vivo function of the bacterial RNase P protein (termed P protein) originates from studies of the Escherichia coli system with a mutant strain (NHY322 [rnpA49]) encoding a P protein with a single amino acid mutation, which results in temperature-sensitive bacterial growth due to decreased affinity of the protein for its RNA subunit (1, 7, 11, 13). Although the rnpA49 mutant's phenotype has indicated that the P protein is essential in E. coli, one caveat is that complementation studies were performed under stress conditions at 43°C. The heat shock response has a global effect on gene expression (14, 19), and induced metabolic changes could exacerbate the mutant phenotype. Moreover, the mutant P protein is still expressed in the rnpA49 mutant strain under nonpermissive conditions and may interfere with the function of the P protein expressed from a plasmid in complementation studies.
Here we describe the construction and biological properties of Bacillus subtilis mutant strains with suppressible rnpA expression. In one type of mutant strain, termed d7, chromosomal rnpA expression could essentially be abolished, resulting in cell growth arrest. Expression of plasmid-borne rnpA genes encoding either the wild-type (WT) P protein or variants carrying an N- or C-terminal His tag was able to rescue the conditionally lethal phenotype. In contrast, an rnpA gene with internal stop codons was unable to restore growth under restrictive conditions. Our results demonstrate directly that the bacterial P protein is essential for cell viability. We further demonstrate the utility of the d7 strain for RNase P overproduction and affinity purification via His-tagged protein variants. Finally, we show that rnpA expression in B. subtilis is attenuated by transcriptional polarity and that translation of rnpA mRNA in B. subtilis can be initiated at two alternative start codons.
MATERIALS AND METHODS
Bacterial strains, media, and transformation.
E. coli strains DH5α (21) and XL-2 Blue (Stratagene) were used for plasmid preparations. B. subtilis YB886 (30), a prophage-cured derivative of B. subtilis 168, was used as the parent strain for the construction of mutant strains. B. subtilis and E. coli cells were grown in Luria-Bertani (LB) medium (21). For solid medium, 1.5% agar was added. LB broth and LB agar were supplemented with ampicillin (100 μg/ml), kanamycin (20 μg/ml), and/or chloramphenicol (5 μg/ml). For induction or repression of Pxyl-dependent rnpA expression in strains sb and d7, 2% (wt/vol) xylose or 2% (wt/vol) glucose was added to the medium. Plasmid-borne genes under control of the Pspac promoter were expressed by addition of 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG). E. coli cells were prepared for transformation with plasmid DNA according to Sambrook et al. (21). Plasmid DNA was introduced into B. subtilis by the method of Xue et al. (29).
Construction of mutant strains sb and d7 and expression plasmids.
Integration vectors pS2.1 and pD2b contained about 1.2 kb of genomic sequence on each side of rnpA for homologous recombination. Recombinant cell clones were analyzed and verified by standard Southern blot and genomic PCR techniques (for details, see the supplemental material).
Complementation analyses.
After transformation of rnpA mutant strains with complementation plasmids, cells were incubated at 37°C on LB agar plates containing 2% xylose, 20 μg/ml kanamycin, and 5 μg/ml chloramphenicol. Single colonies were picked and grown overnight in liquid LB medium supplemented with 2% xylose and then plated on LB agar containing 20 μg/ml kanamycin, 5 μg/ml chloramphenicol, and 2% xylose or 2% glucose.
RT-PCR.
Reverse transcription (RT)-PCR was performed with the Access RT-PCR System kit (Promega) as recommended by the manufacturer. Total RNA was extracted with the RNeasy Mini Kit (QIAGEN) (see Fig. 3); for quantitative RT-PCR (see Table 2), total RNA was isolated from exponentially growing B. subtilis cultures with the TRIzol reagent (Invitrogen). RNA preparations were subjected to an additional DNase I (Ambion) digestion step to remove any residual DNA (for further details, see the supplemental material).
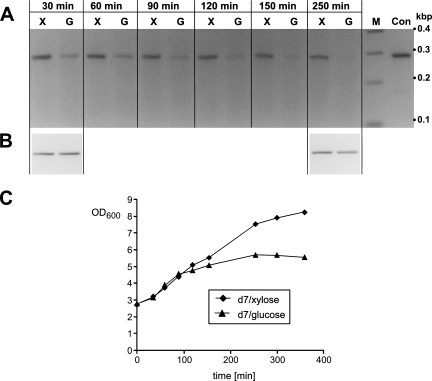
FIG. 3.
RT-PCR analysis of strain d7. PCR products were analyzed on 2.5% agarose gels. (A) Time course of rnpA expression after transfer into medium supplemented with 2% xylose (X) or 2% glucose (G). Total RNA was isolated at the indicated time points from the respective d7 culture. Con, PCR control with genomic DNA as the template; M, DNA marker with fragment sizes indicated at the right. (B) The rpsR transcript encoding ribosomal protein S18 was used to control for fluctuations in total RNA amounts. (C) Growth curves of the cultures used for RT-PCR in panels A and B. OD600, optical density at 600 nm.
TABLE 2.
Quantitative RT-PCR of various B. subtilis strains
| B. subtilis strain | rpmH/rnpA mRNA levelc | rnpA mRNA levelc (relative to WT) | No. of expt |
|---|---|---|---|
| WTa | 92.4 ± 28 | 1 | 13 |
| sba | 8.5 ± 2 | 20.4 ± 8 | 9 |
| d7b | 248 ± 82 | 1.2 ± 0.4 | 10 |
| d7(pDG148)a | 268 ± 97 | 1.2 ± 0.7 | 5 |
| d7(pB.s.[rpmH(Oc)-rnpA-CH])b | 16 ± 11 | 506 ± 182 | 6 |
| d7(pB.s.rnpA-NH)b | 0.09 ± 0.08 | 21,275 ± 9,028 | 7 |
Grown in the presence of 2% xylose.
Induced for 1.5 h with 1 mM IPTG.
Values are averages and standard deviations.
Protein analysis.
Preparation of total cellular protein, sodium dodecyl sulfate-polyacrylamide gel electrophoresis (PAGE), and immunoblotting were performed according to standard techniques (for details, see the supplemental material).
Isolation of RNase P holoenzyme by batch purification and activity assay.
Plasmid-bearing B. subtilis strains were grown in 50 ml LB broth to mid-log phase, induced for 1.5 h with 1 mM IPTG, and harvested by centrifugation (7 min at 5,000 × g and 4°C). About 0.3 g of cell pellet was washed once and resuspended in 4 to 5 ml of buffer R+ (100 mM KCl, 8 mM MgCl2, 10 mM HEPES, 9 mM imidazole, pH 6.2). After preparation of cell lysates by sonication (10 min, 50% pulses on ice), cell debris was removed by centrifugation for 12 min at 18,000 × g at 4°C. A 1.3-ml volume of supernatant was then incubated with 0.6 ml of Ni-nitrilotriacetic acid (NTA) agarose slurry (preequilibrated in buffer R+; binding capacity, 5 to 10 mg six-His-tagged protein per ml of matrix; QIAGEN, Chatsworth, CA) for 45 min on an end-to-end shaker at 4°C in order to allow the RNase P holoenzyme to adsorb to the matrix. The slurry was washed five times with buffer R+ and then four times with buffer R (100 mM KCl, 8 mM MgCl2, 10 mM HEPES, pH 6.2). The activity of RNase P coupled to Ni-NTA agarose (20 μl in a final reaction volume of 50 μl) was assayed at 37°C in buffer R with 5′-end-labeled precursor tRNAGly (100 nM) from Thermus thermophilus as the substrate. The substrate was preincubated for 5 min at 55°C and subsequently for 25 min at 37°C in buffer R. Aliquots were withdrawn at different time points, and cleavage products were analyzed by denaturing 20% PAGE and visualized and quantified as previously described (27).
RESULTS
Construction and phenotype of B. subtilis strains with the chromosomal rnpA gene under control of the Pxyl promoter.
In the majority of bacteria, the rnpA gene encoding the P protein is located immediately downstream of rpmH, coding for ribosomal protein L34 (10). In E. coli, rpmH and rnpA have been demonstrated to be part of the same operon with two major promoters preceding the rpmH gene (8, 9). E. coli cells contain a 60- to 80-fold molar excess of ribosomes over RNase P RNA (5), and expression of ribosomal protein L34 is expected to exceed that of RNase P protein by a similar factor. Regulation seems to occur at the (post)transcriptional level, as three mRNA species derived from the rpmH-rnpA operon were detected, two shorter ones lacking the rnpA cistron and a longer and much less abundant one including it (17). Also, the codon usage of rnpA was reported to deviate from that of highly expressed E. coli genes such as rpmH (9). In B. subtilis, cotranscription of rpmH and rnpA is also likely since two promoters have been identified upstream of the rpmH-rnpA tandem genes, but none is evident between rpmH and rnpA (15, 16).
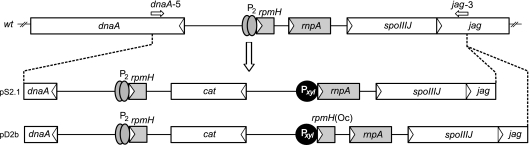
Since little information is known about the cellular levels of rnpA mRNA in B. subtilis, we pursued two strategies for mutant strain construction. In the pS2.1 construct (Fig. 1; for more details, see Fig. S1 in the supplemental material), we put rnpA under direct control of the Pxyl promoter including an optimized Shine-Dalgarno (SD) sequence and an AUG start codon (see Fig. S2 in the supplemental material). Here we expected high-level expression of rnpA under derepressed conditions. In the pD2b construct (Fig. 1), we inserted the entire rpmH-rnpA region (for details, see Fig. S1 in the supplemental material) downstream of the Pxyl promoter, which preserved the native 5′ region of rnpA including its potential GUG and UUG start codons (Fig. 2). This configuration was expected to result in weaker rnpA expression. We then noticed that the Pxyl-regulated rpmH gene in pD2b had a 5-bp deletion in its 5′ coding region which introduced two ochre stop codons at the seventh and ninth codon positions of rpmH [rpmH(Oc), Fig. 2]. As a consequence, translation of this second rpmH cistron copy terminated after synthesis of the hexapeptide MNIPTE, and production of a functional L34 protein was solely directed from the native rpmH copy downstream of cat (Fig. 1). This fortuitous deletion, leading to inactivation of the second rpmH copy, was seen as an advantage, since it preserved the native situation where only one chromosomal copy of rpmH directs expression of L34.
FIG. 1.
Schematic of the WT rpmH-rnpA region in B. subtilis YB886 (wt, top) in comparison with those of integration vectors pS2.1 and pD2b (for construction details, see Fig. S1 in the supplemental material). In construct pS2.1, the Pxyl promoter was directly fused to the rnpA gene, whereas Pxyl was fused to the upstream rpmH gene in construct pD2b; here, rpmH has a 5-bp deletion in its 5′ region [rpmH(Oc)], preventing translation of a functional rpmH gene product. The chloramphenicol resistance gene (cat) was used as a selection marker. Primer pair dnaA-5 and jag-3 (horizontal arrows at the top) was used to amplify this chromosomal region for insertion into plasmid pSP64 to yield integration vectors pS2.1 and pD2b. The direction of transcription is indicated by the open arrowheads within boxes representing the coding regions of cat, dnaA, jag, rnpA, rpmH, and spoIIIJ. P2 indicates the natural tandem promoters preceding rpmH; the rpmH and rnpA genes are shaded in gray.
FIG. 2.
Sequence of the genomic rpmH-rnpA region in B. subtilis 168 derivative strain YB886. Tandem promoters (16) and the SD sequence preceding rpmH are indicated by horizontal dashed or solid lines above the sequence. Coding regions for rpmH and rnpA are depicted by gray boxes. The highlighted nucleotides at the beginning of rpmH indicate a 5-bp deletion (del.) in construct pD2b (Fig. 1). The rnpA cistron contains two potential start codons (boxed); a possible SD sequence is absent with respect to the GUG start codon; a potential SD sequence for the putative UUG start codon of rnpA is indicated by the dotted line; the third position of each potential start codon was mutated singly or simultaneously to C in plasmids d7(pB.s.[rpmH(Oc)-rnpA-GTc]), d7(pB.s.[rpmH(Oc)-rnpA-TTc]), and d7(pB.s.[rpmH(Oc)-rnpA-GTcTTc]) (Table 1). G (stop) and T (stop) above the rnpA sequence depict two point mutations, resulting in stop codons, which were introduced simultaneously into rnpA in plasmid pB.s.[rpmH(Oc)-rnpA(Am,Op)]. Arrowheads below the intergenic sequence depict regions of complementarity, the first one fulfilling the requirements of a transcription terminator.
Representative cell clones derived from pS2.1 (named sb) and pD2b (named d7), respectively, were verified by Southern blotting and genomic PCR (see Fig. S3 of the supplemental material).
Growth of strain d7 was dependent on xylose (Table 1), as expected for tight regulation of the Pxyl promoter and if rnpA expression is essential for cell viability. The xylose-independent growth of mutant strain sb suggested that basal rnpA expression from the Pxyl promoter occurred in this strain because of nonsaturating intracellular concentrations of the XylR repressor. Indeed, overexpression of XylR [strain sb(pB.s.xylR)] led to xylose-dependent growth (Table 1). These observations are in line with ca. 20-fold higher levels of rnpA mRNA in strain sb compared with strain d7 (Table 2).
TABLE 1.
Growth of colonies of the strains used in this study under various conditions
| Strain | Colony growtha in:
|
|||
|---|---|---|---|---|
| 2% xylose-1 mM IPTG | 2% xylose | 2% glucose | 2% glucose-1 mM IPTG | |
| sb | + | + | + | + |
| sb(pDG148) | + | + | + | + |
| sb(pB.s.xylR) | + | + | − | − |
| d7 | + | + | − | − |
| d7(pDG148) | + | + | − | − |
| d7[pB.s.rnpA(Am,Op)] | + | + | − | − |
| d7(pB.s.rnpA) | + | + | + | + |
| d7(pB.s.rnpA-NH) | + | + | + | + |
| d7(pB.s.[rpmH(Oc)-rnpA(Am,Op)]) | + | + | − | − |
| d7(pB.s.[rpmH(Oc)-rnpA]) | + | + | + | + |
| d7(pB.s.[rpmH(Oc)-rnpA-CH]) | + | + | + | + |
| d7(pB.s.[rpmH(Oc)-rnpA-GTc])c | NAb | + | + | NA |
| d7(pB.s.[rpmH(Oc)-rnpA-TTc])c | NA | + | + | NA |
| d7(pB.s.[rpmH(Oc)-rnpA-GTcTTc])c | NA | + | − | NA |
+, colony growth after overnight incubation at 37°C on LB agar plates; −, no colony growth overnight.
NA, not analyzed.
rnpA gene with potential GUG and UUG start codons mutated to GUC or UUC, respectively, either singly or simultaneously (for more details, see Fig. 2).
RT-PCR analysis of strain d7.
We next analyzed transcription of the chromosomal rnpA gene in strain d7 in the presence and absence of xylose by RT-PCR. The mRNA encoding ribosomal protein S18 (rpsR) was used as an internal standard to control for variation in the total RNA concentration. Levels of rpsR mRNA were assumed to be unaffected by the presence or absence of xylose. A culture of d7 cells was grown in the presence of xylose to late exponential phase. After two washing steps, cells were used to inoculate two fresh cultures, one supplemented with 2% xylose and the other supplemented with 2% glucose. At different time points after inoculation, total RNA was prepared and used as the template for RT-PCR analysis with primers specific for rnpA and rpsR, respectively. PCRs were terminated in the log-linear range of amplification to obtain semiquantitative RT-PCR results (for details, see the supplemental material). After transfer of d7 cells into the medium without xylose (Fig. 3A, lanes G), the rnpA-specific amplification product decreased over time, with complete disappearance after 250 min. In contrast, product levels remained constant when cells were grown in the presence of xylose (lanes X). The rpsR-specific RT-PCR product was not affected by the presence or absence of xylose or by the time point of RNA extraction (Fig. 3B). These results demonstrated efficient repression of Pxyl-directed rnpA transcription in the absence of the inducer xylose.
In addition, we monitored the growth of the bacterial cultures used for RT-PCR. Consistent with the RT-PCR results, growth of strain d7 stagnated after 100 to 150 min in the absence of xylose (Fig. 3C), indicating that cellular levels of P protein became rate limiting for growth. The relatively slow manifestation of the mutant phenotype can probably be explained by a delay in the reduction of intracellular xylose levels after transfer of cells to xylose-free medium.
rnpA complementation in strain d7.
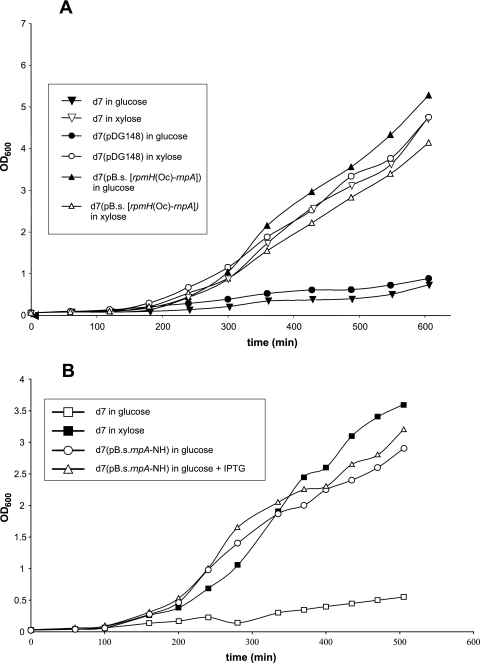
Complementation studies were performed to examine whether the conditionally lethal phenotype of strain d7 in the absence of xylose could be rescued by expression of a plasmid-borne rnpA gene. We constructed two vectors with different rnpA expression strengths (for details, see Fig. S2 in the supplemental material). In vector pB.s.rnpA, we placed rnpA under direct control of the Pspac promoter, including an optimized SD sequence and an AUG start codon, whereas the inactivated rpmH sequence [rpmH(Oc)] plus the intergenic region was inserted between the Pspac promoter and rnpA in pB.s.[rpmH(Oc)-rnpA]. As a control, we employed vector pB.s.[rpmH(Oc)-rnpA(Am,Op)], containing an rnpA gene with two consecutive (opal and amber) stop codons in the 5′-proximal part of rnpA (for details, see Fig. 2). Plasmids pB.s.rnpA and pB.s.[rpmH(Oc)-rnpA], but not pB.s.[rpmH(Oc)-rnpA(Am,Op)], restored the growth of strain d7 in the absence of xylose (Table 1). Complementation with pB.s.[rpmH(Oc)-rnpA] even occurred in the absence of IPTG (Table 1 and Fig. 4 A), without any changes in growth behavior, indicating that basal levels of rnpA transcription from the Pspac promoter in the absence of an inducer are sufficient for normal cell growth, in line with previous observations (3, 25). Our finding that suppression of the lethal phenotype strictly depended on the expression of an intact plasmid-encoded rnpA gene demonstrated that the growth defect of strain d7 in the absence of xylose is due to depletion of the rnpA gene product.
FIG. 4.
Genetic complementation of strain d7. (A) Growth curves of B. subtilis d7, d7(pDG148), and d7(pB.s.[rpmH(Oc)-rnpA]) in the presence of either 2% xylose or 2% glucose. Initially, cultures were grown in medium supplemented with 2% xylose. At time point zero, duplicate cultures were inoculated to an optical density at 600 nm (OD600) of ca. 0.2 into fresh LB medium supplemented with either xylose or glucose. (B) Corresponding growth curves for strain d7(pB.s.rnpA-NH) grown in the presence of 2% glucose, with or without induction by 1 mM IPTG.
Quantitation of relative rpmH and rnpA mRNA levels.
The relative levels of the rpmH and rnpA mRNAs in the different B. subtilis strains were determined by quantitative RT-PCR (for details, see Fig. S4 in the supplemental material). The level of rpmH mRNA exceeded that of rnpA by a factor of ∼90 in exponentially growing B. subtilis YB886 WT cells (Table 2). Furthermore, the rnpA levels in the WT and d7 strains were comparable. For strain d7 harboring the pB.s.[rpmH(Oc)-rnpA-CH] or pB.s.rnpA-NH expression vector, rnpA mRNA levels increased by factors of ∼500 and ∼21,000, respectively, relative to the WT strain. The rpmH/rnpA ratio of ∼250 in strain d7 is a result of the additional chromosomal rpmH(Oc) copy (Fig. 1).
Western blot analysis to examine P protein levels in vivo.
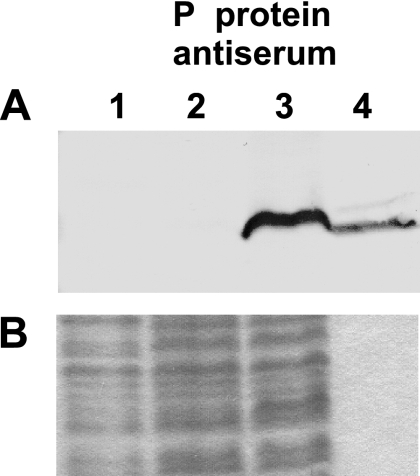
C- or N-terminally His-tagged P protein variants were able to restore growth of the d7 mutant strain (Table 1). By use of a polyclonal antiserum raised against the B. subtilis P protein, we could detect the P protein in preparations from strain d7(pB.s.rnpA-NH) but not in those from d7(pB.s.[rpmH(Oc)-rnpA-CH]) (Fig. 5, lanes 2 and 3) or in those from strain d7 grown in the presence of xylose (lane 1). This result shows that P protein levels are largely increased in strain d7(pB.s.rnpA-NH), consistent with increased rnpA mRNA levels in these bacteria (Table 2). The failure to detect the P protein in lanes 1 and 2 can be attributed to the limited sensitivity of the antiserum used.
FIG. 5.
Western blot analysis to determine levels of P protein expression. Total protein was prepared from strain d7(pDG148) (lane 1), d7(pB.s.[rpmH(Oc)-rnpA-CH]) (lanes 2), or d7(pB.s.rnpA-NH) (lane 3) 1.5 h after IPTG induction by treatment of cell suspensions with trichloroacetic acid. Recombinant N-terminally His-tagged B. subtilis P protein (60 ng) was loaded as a control in lane 4. Samples were subjected to 13% sodium dodecyl sulfate-PAGE and either blotted (panel A) or stained with Coomassie blue (panel B) to control for differences in the amount of loaded total protein.
rnpA start codon utilization in B. subtilis.
The rnpA gene in B. subtilis is preceded by one of two potential start codons (GUG or UUG, Fig. 2). To analyze start codon utilization, we inactivated one or both of the two potential rnpA start codons in the context of strain d7(pB.s.[rpmH(Oc)-rnpA]). Surprisingly, single inactivation of the GUG or UUG codon did not abrogate cell vitality. Only simultaneous inactivation of the two codons prevented complementation (Table 1). This suggests that either start codon can be used for rnpA translation.
Isolation of RNase P holoenzyme.
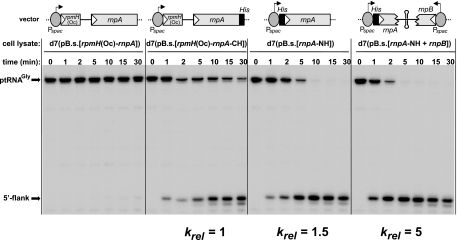
We considered Ni-NTA affinity chromatography as an effective strategy to enrich the in vivo-assembled RNase P holoenzyme via the His tag of the plasmid-encoded P protein. For this purpose, cell lysates were allowed to adsorb to the Ni-NTA resin and the retained RNase P activity was tested in a precursor tRNA processing assay (Fig. 6). Within the experimental time window, the Ni-NTA resin lacked RNase P activity after incubation with the cell lysate from the control strain expressing a P protein without the His tag (leftmost panel of Fig. 6). RNase P activity could be purified from cells expressing the P protein with the C- or N-terminal His tag (central panels of Fig. 6). The greatest enrichment of activity was seen for lysates from cells carrying a plasmid that directed expression of both RNase P subunits in B. subtilis d7 in order to increase the limiting intracellular concentration of P RNA (rightmost panel of Fig. 6). Thus, affinity purification seems very useful for the rapid isolation of in vivo-assembled homologous or chimeric RNase P holoenzymes from cell lysates for biochemical and structural studies. The d7 strain serves this purpose particularly well, as it allows repression of expression of the chromosomally encoded P protein lacking a His tag, which would otherwise compete with the plasmid-borne His-tagged protein for P RNA binding.
FIG. 6.
Endonucleolytic processing of 5′-32P-labeled ptRNAGly by RNase P holoenzyme bound to Ni-NTA agarose via His-tagged B. subtilis P protein. The enrichment of RNase P activity was determined after incubation of Ni-NTA resin with cell lysate from strain d7(pB.s.[rpmH(Oc)-rnpA]), d7(pB.s.[rpmH(Oc)-rnpA-CH]), d7(pB.s.rnpA-NH), or d7(pB.s.[rnpA-NH+rnpB]). Relative processing rates (krel), given at the bottom, are normalized to activity with extract from strain d7(pB.s.[rpmH(Oc)-rnpA-CH]). Vector pB.s.[rnpA-NH+rnpB] additionally encoded the B. subtilis rnpB gene under control of the Pspac promoter, including the natural Rho-independent transcription terminator downstream of rnpB. The terminator is of the type that can function in both transcription directions (4). This was assumed to prevent potential antisense interference due to the opposite orientation of rnpA and rnpB in pB.s.[rnpA-NH+rnpB].
DISCUSSION
Introduction of a plasmid carrying a homologous rnpA gene rescued growth of strain d7 in the absence of xylose. This is the first rnpA knockdown strain and the first direct proof that expression of rnpA is essential in B. subtilis and, by inference, in other bacteria, although we are aware that the essential character of RNase P components has been suggested by the previously identified E. coli rnpA and rnpB mutant alleles that caused a temperature-sensitive phenotype (22, 24). The highest levels of rnpA expression were achieved with strains d7(pB.s.rnpA) and d7(pB.s.rnpA-NH). For strain d7(pB.s.rnpA-NH), we determined a ca. 21,000-fold overexpression of rnpA mRNA (Table 2) and observed a corresponding overproduction of the P protein (Fig. 5). Thus, in contrast to E. coli (7, 11), overexpression of rnpA is not toxic to B. subtilis (Fig. 4B).
On the basis of the estimate of 200 copies of RNase P per 6,000 ribosomes in rapidly growing B. subtilis cells (2), the ca. 90-fold lower level of rnpA versus rpmH mRNA determined for the WT context (Table 2) indicates that differences in L34 and P protein expression are fully explainable by (post)transcriptional regulation. The canonical transcription terminator (4) immediately downstream of rpmH (Fig. 2) may be the main cause or should at least contribute substantially to this transcription polarity. If we assume, however, a number of 20 to 50 RNase P molecules and 6,000 ribosomes per cell (18), then additional attenuation of rnpA expression, relative to rpmH expression, may occur posttranscriptionally, either at the level of translation or by cleavage of rmpH-rnpA primary transcripts and differential degradation rates of the two RNA fragments.
Efficient translation in B. subtilis requires a strong SD sequence preceding the start codon with an optimal spacing of seven to nine nucleotides (20, 26). In B. subtilis rnpA, the potential UUG start codon is preceded by a weak SD sequence (GGAG) with a spacing of eight nucleotides, whereas an SD element is completely missing in front of the potential GUG three codons upstream (Fig. 2). Surprisingly, our genetic data indicate that both the GUG and UUG codons can be used to initiate translation (Table 1). This is unexpected on the basis of the absence of an SD sequence upstream of the GUG codon but points to an elaborate mechanism of translational initiation at the rnpA cistron. Complementation analyses with heterologous or mutant rnpA alleles will be a major application of the d7 strain. However, expression levels are expected to affect complementation efficiency (12). The strength of plasmid-encoded rnpA expression can be modulated in several ways in strain d7. With plasmids pB.s.[rpmH(Oc)-rnpA-CH] and pB.s.rnpA-NH, the rnpA mRNA was overexpressed by a factor of 500 to 21,000, respectively (Table 2). This correlated with massive overproduction of the P protein in the case of plasmid pB.s.rnpA-NH (Fig. 5). Also, expression of plasmid-borne rnpA genes under control of the Pspac promoter is expected to be adjustable by variation of the IPTG concentration, providing another means to regulate rnpA expression in complementation analyses. In conclusion, the d7 strain is well suited to testing the in vivo function of heterologous, chimeric, or truncated RNase P proteins at various expression levels. For example, the cyanobacterial P protein from Synechocystis can restore growth of strain d7 in the presence of glucose as the carbon source (unpublished results). On the basis of the structure of the RNase P protein of B. subtilis, the effect of mutations at specific positions can be investigated in vivo. Despite the low sequence similarity among bacterial RNase P proteins, it seems that they have the same fold (6). However, some bacterial P proteins vary substantially in length. The functional role of such deletions or insertions, at present unclear, can be analyzed in the B. subtilis d7 strain. Other interesting candidates to test are P protein subunit Pop5 from Archaea, which displays structural similarity to the bacterial P protein (28), or the human RNase P protein Rpp29, which was reported to be able to activate bacterial P RNA (23).
Supplementary Material
Acknowledgments
We thank S. Niranjanakumari and Carol A. Fierke for providing the antiserum specific for the B. subtilis RNase P protein, Simona Cuzic for support with kinetic experiments, Barbara Wegscheid for the construction of plasmid pB.s.(rnpA-NH+rnpB), and Ciarán Condon for critical reading of the manuscript.
This work was supported by the Deutsche Forschungsgemeinschaft (HA 1672/4-2/4-3/13-1) and the Fonds der Chemischen Industrie.
Footnotes
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Baer, M. F., D. Wesolowski, and S. Altman. 1989. Characterization in vitro of the defect in a temperature-sensitive mutant of the protein subunit of RNase P from Escherichia coli. J. Bacteriol. 171:6862-6866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barrera, A., and T. Pan. 2004. Interaction of the Bacillus subtilis RNase P with the 30S ribosomal subunit. RNA 10:482-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhavsar, A. P., X. Zhao, and E. D. Brown. 2001. Development and characterization of a xylose-dependent system for expression of cloned genes in Bacillus subtilis: conditional complementation of a teichoic acid mutant. Appl. Environ. Microbiol. 67:403-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Hoon, M. J. L., Y. Makita, K. Nakai, and S. Miyano. 2005. Prediction of transcriptional terminators in Bacillus subtilis and related species. PLoS Comput. Biol. 1:e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dong, H., L. A. Kirsebom, and L. Nilsson. 1996. Growth rate regulation of 4.5 S RNA and M1 RNA the catalytic subunit of Escherichia coli RNase P. J. Mol. Biol. 261:303-308. [DOI] [PubMed] [Google Scholar]
- 6.Evans, D., S. M. Marquez, and N. R. Pace. 2006. RNase P: interface of the RNA and protein worlds. Trends Biochem. Sci. 31:333-341. [DOI] [PubMed] [Google Scholar]
- 7.Gopalan, V., A. D. Baxevanis, D. Landsman, and S. Altman. 1997. Analysis of the functional role of conserved residues in the protein subunit of ribonuclease P from Escherichia coli. J. Mol. Biol. 267:818-829. [DOI] [PubMed] [Google Scholar]
- 8.Hansen, F. G., E. B. Hansen, and T. Atlung. 1982. The nucleotide sequence of the dnaA gene promotor and of the adjacent rpmH gene, coding for the ribosomal protein L34, of Escherichia coli. EMBO J. 1:1043-1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hansen, F. G., E. B. Hansen, and T. Atlung. 1985. Physical mapping and nucleotide sequence of the rnpA gene that encodes the protein component of ribonuclease P in Escherichia coli. Gene 38:85-93. [DOI] [PubMed] [Google Scholar]
- 10.Hartmann, E., and R. K. Hartmann. 2003. The enigma of ribonuclease P evolution. Trends Genet. 19:561-569. [DOI] [PubMed] [Google Scholar]
- 11.Jovanovic, M., R. Sanchez, S. Altman, and V. Gopalan. 2002. Elucidation of structure-function relationships in the protein subunit of bacterial RNase P using a genetic complementation approach. Nucleic Acids Res. 30:5065-5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim, M., B. Hyun Park, and Y. Lee. 2000. Effects of terminal deletions in C5 protein on promoting RNase P catalysis. Biochem. Biophys. Res. Commun. 268:118-123. [DOI] [PubMed] [Google Scholar]
- 13.Kirsebom, L. A., M. F. Baer, and S. Altman. 1988. Differential effects of mutations in the protein and RNA moieties of RNase P on the efficiency of suppression by various tRNA suppressors. J. Mol. Biol. 204:879-888. [DOI] [PubMed] [Google Scholar]
- 14.Li, Y., K. Cole, and S. Altman. 2003. The effect of a single, temperature-sensitive mutation on global gene expression in Escherichia coli. RNA 9:518-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moriya, S., N. Ogasawara, and H. Yoshikawa. 1985. Structure and function of the region of the replication origin of the Bacillus subtilis chromosome. III. Nucleotide sequence of some 10,000 base pairs in the origin region. Nucleic Acids Res. 13:2251-2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ogasawara, N., S. Moriya, K. von Meyenburg, F. G. Hansen, and H. Yoshikawa. 1985. Conservation of genes and their organization in the chromosomal replication origin region of Bacillus subtilis and Escherichia coli. EMBO J. 4:3345-3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Panagiotidis, C. A., D. Drainas, and S. C. Huang. 1992. Modulation of ribonuclease P expression in Escherichia coli by polyamines. Int. J. Biochem. 24:1625-1631. [DOI] [PubMed] [Google Scholar]
- 18.Reich, C., K. J. Gardiner, G. J. Olsen, B. Pace, T. L. Marsh, and N. R. Pace. 1986. The RNA component of Bacillus subtilis RNase P. Sequence, activity, and partial secondary structure. J. Biol. Chem. 261:7888-7893. [PubMed] [Google Scholar]
- 19.Richmond, C. S., J. D. Glasner, R. Mau, H. Jin, and F. R. Blattner. 1999. Genome-wide expression profiling in Escherichia coli K-12. Nucleic Acids Res. 27:3821-3835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rocha, E. P., A. Danchin, and A. Viari. 1999. Translation in Bacillus subtilis: roles and trends of initiation and termination, insights from a genome analysis. Nucleic Acids Res. 27:3567-3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sambrook, J., E. F. Fritsch, and T. E. Maniatis. 1989. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 22.Schedl, P., P. Primakoff, and J. Roberts. 1974. Processing of E. coli tRNA precursors. Brookhaven Symp. Biol. 26:53-76. [PubMed] [Google Scholar]
- 23.Sharin, E., A. Schein, H. Mann, Y. Ben-Asouli, and N. Jarrous. 2005. RNase P: role of distinct protein cofactors in tRNA substrate recognition and RNA-based catalysis. Nucleic Acids Res. 33:5120-5132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shiraishi, H., and Y. Shimura. 1988. Functional domains of the RNA component of ribonuclease P revealed by chemical probing of mutant RNAs. EMBO J. 7:3817-3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vagner, V., E. Dervyn, and S. D. Ehrlich. 1998. A vector for systematic gene inactivation in Bacillus subtilis. Microbiology 144:3097-3104. [DOI] [PubMed] [Google Scholar]
- 26.Vellanoweth, R. L., and J. C. Rabinowitz. 1992. The influence of ribosome-binding-site elements on translational efficiency in Bacillus subtilis and Escherichia coli in vivo. Mol. Microbiol. 6:1105-1114. [DOI] [PubMed] [Google Scholar]
- 27.Warnecke, J. M., R. Held, S. Busch, and R. K. Hartmann. 1999. Role of metal ions in the hydrolysis reaction catalyzed by RNase P RNA from Bacillus subtilis. J. Mol. Biol. 290:433-445. [DOI] [PubMed] [Google Scholar]
- 28.Wilson, R. C., C. J. Bohlen, M. P. Foster, and C. E. Bell. 2006. Structure of Pfu Pop5, an archaeal RNase P protein. Proc. Natl. Acad. Sci. USA 103:873-878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xue, G.-P., J. S. Johnson, and B. P. Dalrymple. 1999. High osmolarity improves the electro-transformation efficiency of the gram-positive bacteria Bacillus subtilis and Bacillus licheniformes. J. Microbiol. Methods 34:183-191. [Google Scholar]
- 30.Yasbin, R. E., P. I. Fields, and B. J. Andersen. 1980. Properties of Bacillus subtilis 168 derivatives freed of their natural prophages. Gene 12:155-159. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.