Abstract
In Salmonella enterica, the biosynthetic pathways for the generation of purines and the essential cofactor thiamine pyrophosphate branch after sharing five enzymatic steps. Phosphoribosyl amine (PRA) is the first intermediate in the common portion of the pathway and is generated from phosphoribosylpyrophosphate and glutamine by the PurF enzyme (phosphoribosylpyrophosphate amidotransferase). A null mutation in yjgF allows PurF-independent PRA formation by an unknown mechanism. The tryptophan biosynthetic enzyme complex anthranilate synthase-phosphoribosyltransferase, composed of the TrpD and TrpE proteins, was shown to be essential for PRA formation in strains lacking both yjgF and purF. The activity generating PRA in a yjgF mutant background has features that distinguish it from the TrpDE-mediated PRA formation shown previously for this enzyme in strains with an active copy of yjgF. The data presented here are consistent with a model in which the absence of YjgF uncovers a new catalytic activity of TrpDE.
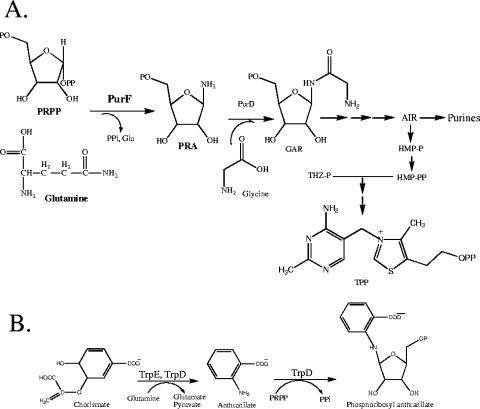
Thiamine pyrophosphate is an essential cofactor generated in bacteria via condensation and subsequent phosphorylation of 4-methyl-5-(β-hydroxymethyl) thiazole phosphate and 4-amino-5-hydroxymethyl-2-methylpyrimidine pyrophosphate (HMP-PP) (1). In most bacteria, the first five enzymes used to generate HMP-PP are shared with the purine biosynthetic pathway (25, 26). The formation of the first common intermediate, phosphoribosyl amine (PRA), is catalyzed by PurF (phosphoribosylpyrophosphate amidotransferase) from phosphoribosylpyrophosphate (PRPP) and glutamine (Fig. 1) (51). Under some conditions, Salmonella enterica mutants lacking the PurF enzyme generate enough PRA to satisfy the thiamine requirement for growth, although this synthesis is not sufficient to satisfy the purine requirement (10, 12, 32).
FIG. 1.
Thiamine pyrophosphate biosynthetic pathway in S. enterica. (A) Simplification of the biosynthetic pathway for thiamine production. Emphasis is placed on the two reactions relevant to this work. The reactions are catalyzed by the gene product indicated. The PurD reaction has been used in the coupled assay to monitor PRA formation in vitro (36). (B) Reaction catalyzed by the TrpDE enzyme complex that results in an intermediate in the biosynthesis of tryptophan.
A number of media that support PRA formation in a purF mutant have been described (10, 13). Of these media, only those which use ribose as the sole carbon source bypassed the need for a functional oxidative pentose phosphate pathway for growth in the absence of thiamine (13). This result led to the hypothesis that PRA could be generated from ribose-5-phosphate and an amino donor (35). In addition, two mutations that relieved the requirement for the oxidative pentose phosphate pathway in PRA synthesis on any carbon source were identified. First, an allele of trpD (trpD3611) enhanced a weak native activity of TrpDE (anthranilate synthase-phosphoribosyltransferase [AS-PRT]) to generate PRA from PRPP and NH4+ (36). Consistent with the characterized regulation of TrpDE (34), this PRA-forming activity was sensitive to the presence of tryptophan (36). Second, a null mutation in yjgF allowed thiamine-independent growth of purF mutants under nonpermissive conditions, as well as purF mutants defective in the oxidative pentose phosphate pathway (such as gnd mutants, defective in gluconate-6-phosphate dehydrogenase) (14).
YjgF is a small protein (128 amino acids) which is highly conserved throughout the three domains of life (14, 15, 21, 28, 29, 37, 39, 48). The biochemical function of the YjgF protein, and all proteins in the YjgF/YER057c/UK114 family, remains unknown (21, 41), although more than seven high-resolution structures of family members have been described (2, 7, 8, 22, 23, 31, 42, 46).
While the lack of yjgF enhanced PurF-independent thiamine synthesis, it also resulted in a defect in isoleucine biosynthesis in several organisms (14, 15, 21). Mutants of both Saccharomyces cerevisiae and S. enterica lacking yjgF have decreased transaminase B activity (encoded by ilvE). Since this enzyme catalyzes the final step of isoleucine biosynthesis, it was assumed that the effect of a yjgF lesion on isoleucine biosynthesis is via decreased IlvE activity (21, 41). Further studies in S. enterica led to a working model in which YjgF scavenges a hypothesized side product of threonine deaminase (encoded by ilvA) that catalyzes the first step in isoleucine biosynthesis (41). A more global model implicates YjgF in binding and/or degrading other metabolites, possibly generated in side reactions by central metabolic enzymes (11).
The biochemical mechanism(s) by which PRA is generated in strains lacking yjgF is not clear. This study was initiated to clarify the observed connection between tryptophan and PRA synthesis occurring in the absence of YjgF. Data herein demonstrate that the TrpDE enzyme complex is necessary and sufficient for PRA generation in a strain lacking both the purF and yjgF genes.
MATERIALS AND METHODS
Bacterial strains.
All strains used in this study are derivatives of Salmonella enterica serovar Typhimurium strain LT2 and are listed along with their respective genotypes in Table 1. Tn10d(Tc) refers to transposition-defective mini-Tn10 (Tn10Δ16Δ17) described previously (47). MudJ and MudK refer to derivatives of the Mud1734 transposon, each of which has been described previously (4, 20).
TABLE 1.
Bacterial strains
| Strain | Genotype |
|---|---|
| DM728 | purF2085 gnd-181 |
| DM1936 | purF2085 |
| DM6417 | purF2085 gnd-181 zdd-9147::Tn10d(Tc)atrpD3611 |
| DM7379 | purF2085 gnd-181 zdd-9147::Tn10d trpD3611 trpE3613 |
| DM7435 | purF2085 gnd-181 yjgF3::MudJbtrpE3613 |
| DM7436 | purF2085 gnd-181 yjgF3::MudJ |
| DM8229 | purF2085 gnd-181 yjgF3::MudJ trpE3613 ΔtrpR3614::Cm |
| DM8230 | purF2085 gnd-181 yjgF3::MudJ ΔtrpR3614::Cm |
| DM8239 | purF2085 gnd-181 zdd-9147::Tn10d ΔtrpR3614::Cm |
| DM8724 | purF2085 gnd-181 yjgF3::MudJ ΔtrpCBA3616::Cm |
| DM8725 | purF2085 gnd-181 yjgF3::MudJ ΔtrpDCBA3617::Cm |
| DM8726 | purF2085 gnd-181 yjgF3::MudJ ΔtrpEDCBA3618::Cm |
| DM8820 | trp3615::MudJ yjgF::Tn10d |
| DM8821 | trp3615::MudJ |
| DM8885 | trp3615::MudJ ΔtrpR3614::Cm yjgF::Tn10d |
| DM8886 | trp3615::MudJ ΔtrpR3614::Cm |
| DM9154 | purF2085 trpE3613 |
| DM9227 | purF2085 gnd-181 (pBAD24) |
| DM9228 | purF2085 gnd-181 (pBAD-TrpED) |
| DM9229 | purF2085 gnd-181 (pBAD-TrpE(P289T)D) |
| DM9230 | purF2085 gnd-181 yjgF3::MudJ (pBAD24) |
| DM9231 | purF2085 gnd-181 yjgF3::MudJ (pBAD-TrpED) |
| DM9232 | purF2085 gnd-181 yjgF3::MudJ (pBAD-TrpE(P289T)D) |
Culture media and chemicals.
The no-carbon E (NCE) medium of Vogel and Bonner (6, 45) was utilized as a minimal medium and supplemented with MgSO4 (1 mM) and either glucose or gluconate as a carbon source (11 mM). When necessary, the following compounds were provided at the indicated final concentrations: adenine, 0.4 mM; thiamine, 100 nM; tryptophan, 0.1 mM; and arabinose, 1 mM. Difco nutrient broth (8 g/liter) with NaCl (5 g/liter) and Luria-Bertani broth were used as rich media. Difco BiTek agar was added (15 g/liter) for solid medium. Antibiotics were added when needed at the following concentrations to rich and minimal media, respectively: ampicillin, 30 and 15 μg/ml; chloramphenicol, 20 and 4 μg/ml; kanamycin, 50 and 125 μg/ml; and tetracycline, 20 and 10 μg/ml. Antibiotics and chemicals were purchased from Sigma-Aldrich Chemical Co., St. Louis, MO.
Genetic methods. (i) Transduction method.
The high-frequency general transducing mutant of bacteriophage P22 (HT105/1 int-201) (38, 40) was used to perform all transductions, as described elsewhere (9, 32).
(ii) Isolation of a feedback-resistant allele of trpE.
The basis for the mutant screen was that trpD3611, in combination with a feedback-insensitive allele of trpE, resulted in a strain in which PRA formation was not affected by tryptophan (36). Soft agar (0.7% agar) seeded with strain DM6417 (purF gnd trpD3611) was overlaid on agar plates containing minimal glucose medium supplemented with adenine and tryptophan. Spontaneously arising colonies were saved as mutants of interest and screened by assaying glutamine-dependent anthranilate synthase activity in crude cell extracts (16, 44, 50). Strains in which the anthranilate synthase activity was not reduced in the presence of tryptophan were considered candidates for carrying the feedback-resistant allele of trpE. After reconstruction, the trpE gene from candidate mutants was amplified by PCR and sequenced at the University of Wisconsin Biotechnology Center—Nucleic Acid and Protein Facility.
(iii) Generation of a trp::MudJ transcriptional fusion.
Lysate from phage P22 grown on strain JE1652 containing hisD10381::MudK (Kanr) was used to transduce a strain containing trp::MudA (Ampr) (JE1234) to Kanr. The Kanr transductants were screened for sensitivity to ampicillin and presumed to contain MudJ at the site of the original MudA, as described elsewhere (4, 20).
(iv) Phenotypic analysis.
Nutritional requirements were assessed on solid and/or liquid medium.
(a) Liquid growth.
Strains to be analyzed were grown to full density in nutrient medium at 37°C. Cells were pelleted and resuspended in an equal volume of saline (85 mM). Two microliters of the cell suspension was used to inoculate 198 μl of the medium in each well of a 96-well microtiter plate. Growth at 37°C was monitored using a microplate spectrophotometer (Spectro-Max Plus; Molecular Devices, Sunnyvale, CA). Specific growth rate (μ) was determined as ln(X/X0)/T, where X is the absorbance at 650 nm during the linear portion of the growth curve and T is time. Growth lag was considered to be the time required for the cells to start growing at the rate that continued until stationary phase.
(b) Solid media.
Nutritional requirements were measured on solid agar medium by replica printing. Growth was scored after incubation at 37°C for 24 and 48 h.
Molecular biology techniques. (i) Plasmid construction.
The trpED genes were amplified from strains DM7436 and DM7435 using the primers TrpE EcoR1 (5′ GGCGCGAATTCATGCAAACACCAAAACCCACGCTCG 3′) and TrpD Pst1 Rev (5′ GGGCCCTGCAGTTACCCTCTTGCCGCCAGTGCGGTG 3′), generating plasmids pBAD-TrpED and pBAD-TrpE(P289T)D, respectively. The primers were designed with a 5′ EcoRI restriction site and a 3′ PstI restriction site to facilitate cloning of the purified and digested amplification product into the double-digested pBAD24 vector (17). Plasmids were electroporated into competent cells of DM728 and DM7436.
(ii) Mutation generation by linear transformation.
Deletion/insertion mutations of trpR, trpCBA, trpDCBA, and trpEDCBA were generated using the λ-red recombination method (5). The following forward primers were used for the deletions of various portions of the trp operon: TrpE-FWan (5′ GAGAATAACCATGCAAACACCAAAACCCACGCTCGAACTGGTGTAGGCTGGAGCTGCTTC 3′), TrpD-FWan (5′ TGGCTGATATTCTGCTGCTCGATAACATCGACTCGTTTACGTGTAGGCTGGAGCTGCTTC 3′), and TrpC-FWan (5′ATGCAAACCGTTTTAGCGAAAATCGTCGCAGACAAGGCGAGTGTAGGCTGGAGCTGCTTC 3′). The reverse primer used for all three was TrpA-RWan (5′ TTATGCGCGGCTGGCGGCTTTCATGGCTGAGACAAAGGACCATATGAATATCCTCCTTAG 3′). The primers used for trpR were TrpR-FWan (5′ ATGACCCAGCATTCCCCTTATTCATCGGCTATCGCCGAACGTGTAGGCTGGAGCTGCTTCG 3′) and TrpR-RWan (5′ TCAGGCGTTTTTCAGCAGTACGTTCTCAAGCCAATGACGCCATATGAATATCCTCCTTAG 3′).
Preparation of cell extracts.
Cell extracts were prepared from 25-ml cultures grown for 24 h at 37°C in NCE minimal medium supplemented with 11 mM glucose, 1 mM MgSO4, 0.4 mM adenine, 100 nM thiamine, and 0.2% Casamino Acids (autoclave sterilized). The cells were harvested as previously described (36), resuspended in 1 ml of PED buffer (36), and disrupted by sonication on ice using a 550 Sonic Dismembrator (Fisher Scientific, Pittsburgh, PA). Cell debris was removed by centrifugation (30 min at 16,060 × g at 4°C). The supernatant was used as the cell extract.
Enzymatic assays. (i) AS assays.
Glutamine-dependent and ammonium-dependent AS activity was determined as described previously (16, 36, 44, 50).
(ii) PRT assays.
PRT activity of component II of the AS-PRT complex was determined as described previously (16, 18, 36).
(iii) β-Galactosidase assays.
β-Galactosidase assays were performed using the Miller method (49).
RESULTS
TrpDE is necessary for PRA formation in a purF gnd yjgF background.
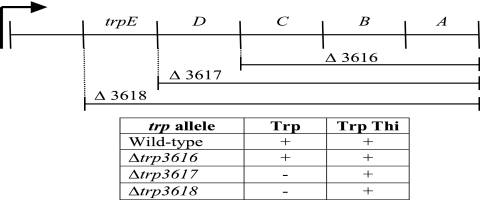
Previous observations have shown that exogenous tryptophan impacted thiamine-independent growth of purF yjgF mutants (36). Based on these reports, the possibility that the trp operon was involved in PRA formation in the absence of purF and yjgF was addressed. Three mutations deleting the trp operon to different extents were constructed and introduced into strain DM7436 (purF gnd yjgF). The three resulting strains and the parental control were analyzed for growth in the presence of tryptophan with and without added thiamine, in an experiment schematically represented in Fig. 2. Only the parental (Trp+) strain and the one containing trpDE but lacking trpCBA (DM8724) grew in the absence of thiamine. It was concluded that TrpD and/or TrpE was required for the PRA formation allowed by the yjgF mutant background. It is important to note that thiamine-independent growth of a mutant lacking only purF on gluconate is independent of the trp operon (data not shown).
FIG. 2.
The tryptophan operon in S. enterica, illustrating the three constructed deletions. A series of three deletions (designated by allele numbers) of various portions of the tryptophan operon were introduced into a purF gnd yjgF mutant background (strain DM7436), and the resulting growth phenotypes on media containing gluconate, adenine, and tryptophan in the presence and absence of exogenous thiamine are presented.
Expression of the trp operon is not significantly altered in yjgF mutants.
It was previously demonstrated that overproduction of TrpDE provided sufficient PRA for thiamine-independent growth of a purF gnd mutant (36). This result suggested a simple scenario in which a yjgF lesion increased expression of the trp operon, allowing growth of a purF gnd yjgF strain under conditions in which the purF gnd parent was unable to grow (Table 2). The β-galactosidase activity of a transcriptional fusion in the tryptophan operon was assayed in wild-type (DM8821) and yjgF mutant (DM8820) strains. The β-galactosidase activity in strain DM8820 (46 ± 4.2 Miller units) was less than twice that in DM8821 (26 ± 2.7 Miller units) in glucose medium supplemented with 0.1 mM tryptophan. In contrast, a null mutation in trpR (encoding the tryptophan repressor) resulted in a 13- to 23-fold induction in activity (DM8885 [trp::MudJ trpR yjgF], 627 ± 37 Miller units; DM8886 [trp::MudJ trpR], 595 ± 25 Miller units) relative to DM8820 (trp::MudJ yjgF) and DM8821 (trp::MudJ), respectively. However, a purF gnd yjgF strain showed significantly better thiamine independent growth in vivo than a purF gnd trpR strain (Table 2), eliminating transcription derepression as a mechanism of the yjgF mutant effect.
TABLE 2.
The presence of tryptophan impacts thiamine synthesis in a yjgF mutant
| Strain | Relevant genotype | Specific growth rate (μ)a
|
||
|---|---|---|---|---|
| Ade | Ade Trp | Ade Trp Thi | ||
| DM728 | purF gnd | NG | NG | 0.472 |
| DM7436 | purF gnd yjgF | 0.433 | 0.408 | 0.446 |
| DM8239 | purF gnd trpR | 0.188 | 0.158 | 0.479 |
| DM7436 | purF gnd yjgF | 0.445 | 0.389 | 0.463 |
| DM8230 | purF gnd yjgF trpR | 0.456 | 0.458 | 0.454 |
| DM7435 | purF gnd yjgF trpE3613 | 0.405 | 0.100 | 0.464 |
| DM8229 | purF gnd yjgF trpE3613 trpR | 0.472 | 0.456 | 0.446 |
Growth studies were conducted as described in Materials and Methods. Medium contained glucose and the indicated additions. The specific growth rate was calculated by the equation ln(X/X0)/T, where X is A650, X0 is A650 at time zero, and T is time (in hours). NG, no growth (μ ≤ 0.018).
TrpDE-mediated PRA formation in a yjgF mutant is insensitive to tryptophan.
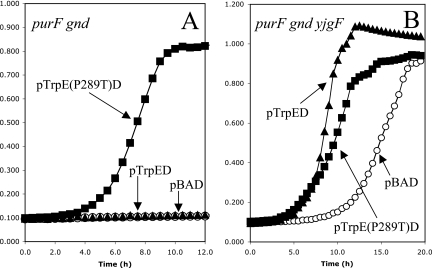
To further probe PRA synthesis by TrpDE in a yjgF background, a number of strains were constructed. Three plasmids were introduced into both DM728 (purF gnd) and DM7436 (purF gnd yjgF), and the resulting strains were analyzed for thiamine-independent growth. A plasmid containing no insert and two that had the trpDE genes under the control of the araBAD promoter (17) were used. In one case, the trpDE genes were wild type, and in the other, the trpE3613 allele that resulted in a feedback resistant enzyme (TrpEP289TD) was present in the plasmid. The data are shown in Fig. 3. All six strains grew at similar rates on glucose medium containing adenine/tryptophan/thiamine (data not shown).
FIG. 3.
TrpDE-dependent PRA formation in a yjgF mutant is not sensitive to tryptophan. Growth at 37°C in glucose adenine tryptophan medium with arabinose (1 mM) added was monitored (A650 versus time). Two parental strains and three plasmids are involved. (A) Data for strains with a wild-type allele of yjgF, including strains DM9227 [purF gnd (pBAD)] ([circo]), DM9228 [purF gnd (pBAD-TrpED)] (▴), and DM9229 [purF gnd (pBAD-TrpE(P289T)D)] (▪). (B) Data for strains lacking yjgF, including strains DM9230 [purF gnd yjgF (pBAD)] (○), DM9231 [purF gnd yjgF (pBAD-TrpED)] (▴), and DM9232 [purF gnd yjgF (pBAD-TrpE(P289T)D)] (▪).
As shown in Fig. 3, strains carrying the empty vector (pBAD) had the growth pattern previously described for the relevant parental strain (14). Significantly, the two strains carrying the plasmid with the wild-type trpDE alleles (DM9228 and DM9231) had distinct phenotypes. In the strain containing wild-type yjgF, with a plasmid carrying trpDE, tryptophan prevented growth in the absence of thiamine (Fig. 3A) as reported previously (36). However, in the yjgF mutant, the same plasmid actually enhanced the background (vector alone) growth (Fig. 3B). These results suggested that the function of the plasmid was probably different in the two backgrounds and indicated that in a yjgF mutant background, tryptophan did not allosterically inhibit the catalytic activity responsible for PRA formation. Introduction of a plasmid expressing a feedback-resistant variant, TrpEP289TD, into the purF gnd strain (DM9229) restored thiamine-independent growth in the presence of tryptophan (Fig. 3A) (36). However, when a yjgF mutation was in the strain (DM9232), the same plasmid allowed less growth than the plasmid containing the wild-type genes.
An allele of trpE impairs thiamine synthesis in a yjgF mutant.
The data in Fig. 3B suggested that the variant complex (TrpEP289TD) encoded by the trpE3613 allele was less proficient at PRA formation than the wild-type protein when present in a yjgF mutant. The trpE3613 allele was a C-to-A transversion at nucleotide 865, resulting in a P289T amino acid change in the TrpE protein. Residue 289 is in close proximity to residues of the tryptophan allosteric binding site of TrpE in S. enterica and is the site of previously characterized feedback-insensitive alleles (3, 24). A pair of strains isogenic at the trpE locus was assayed in crude cell extracts for three reactions inherent to TrpDE (glutamine-dependent anthranilate synthase, ammonia-dependent anthranilate synthase, and phosphoribosyltransferase activity). The results (Table 3) were consistent with those reported previously (3) and show that the mutant protein retained catalytic activity but was no longer sensitive to inhibition by 0.5 mM tryptophan. Further, the catalytic activities of the variant enzyme were not affected by the status of yjgF in the strain (Table 3).
TABLE 3.
Neither the trpE3613 allele nor a yjgF null mutation affects the catalytic activities of the AS-PRT complex
| Strain | Relevant genotype | Activitya
|
|||||
|---|---|---|---|---|---|---|---|
| AS (NH4)b
|
AS (Gln)c
|
PRTd
|
|||||
| No Trp | Trp | No Trp | Trp | No Trp | Trp | ||
| DM7436 | purF gnd yjgF | 0.29 ± 0.04 | 0.03 ± 0.01 | 0.32 ± 0.03 | 0.10 ± 0.01 | 0.30 ± 0.06 | 0.20 ± 0.05 |
| DM7435 | purF gnd yjgF trpE3613 | 0.27 ± 0.02 | 0.25 ± 0.01 | 0.31 ± 0.04 | 0.28 ± 0.01 | 0.33 ± 0.05 | 0.20 ± 0.03 |
| DM8126 | purF gnd trpE3613 | 0.28 ± 0.04 | 0.24 ± 0.00 | 0.38 ± 0.09 | 0.34 ± 0.05 | 0.20 ± 0.03 | 0.14 ± 0.01 |
| DM7435 | purF gnd yjgF trpE3613 | 0.28 ± 0.09 | 0.24 ± 0.07 | 0.33 ± 0.10 | 0.31 ± 0.10 | 0.27 ± 0.07 | 0.12 ± 0.06 |
Strains were grown in minimal glucose medium supplemented with adenine, thiamine, and acid-hydrolyzed casein, as described in the Materials and Methods. Assays were performed with cell extracts in the presence and absence of tryptophan.
AS (NH4), NH4-dependent anthranilate synthase activity. One unit of activity is defined as the appearance of 1 nmol of anthranilate in 1 min per mg of protein.
AS (Gln), glutamine-dependent anthranilate synthase activity. One unit of activity is defined as the appearance of 1 nmol of anthranilate in 1 min per mg of protein.
PRT, phosphoribosyl transferase. One unit of activity is defined as the disappearance of 1 nmol of anthranilate in 1 min per mg of protein.
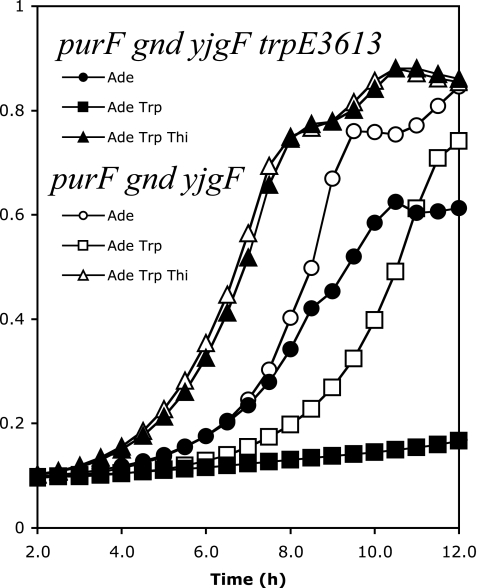
To address the implications of the data in Fig. 3 without the complications generated by a multicopy plasmid system, strains carrying a wild-type or mutant allele of trpE in the chromosome were constructed. When isogenic strains were compared, the purF gnd yjgF strain containing trpE3613 grew more poorly in the absence of thiamine than when the wild-type trp allele was present (Fig. 4). Exogenous tryptophan virtually eliminated thiamine-independent growth of the strain carrying trpE3613 while only slightly affecting (2-h increase in lag) growth of the strain carrying wild-type trp genes. This result contrasted with the plasmid studies (Fig. 3), in which tryptophan had no deleterious effect on the strain with the TrpEP289TD variant. A significant difference between these two studies was the presence of the regulatory region for the trp operon in the chromosome, which was absent in the plasmid constructs. It was considered that the inhibitory effect of tryptophan was due to transcription repression, a possibility in the chromosome but not with the plasmid constructs. A deletion/insertion (Cmr) mutation of trpR (encoding the tryptophan repressor) was generated and transduced into strains DM7436 (purF gnd yjgF) and DM7435 (purF gnd yjgF trpE3613), generating strains DM8230 and DM8229, respectively. In contrast to the parental strains, the trpR derivatives grew equally well, in the absence of thiamine with or without tryptophan (Table 2). These results were consistent with a scenario in which decreased growth was due to transcriptional repression by tryptophan reducing the levels of a compromised enzyme (TrpEP289TD).
FIG. 4.
The trpE3613 allele is detrimental to thiamine synthesis in a yjgF mutant. Growth of DM7436 (purF gnd yjgF) at 37°C in glucose adenine, glucose adenine tryptophan, and glucose adenine tryptophan thiamine was compared to that of DM7435 (purF gnd yjgF trpE3613) under similar growth conditions (A650 versus time).
The trpE3613 allele affects PRA formation specifically in a yjgF background.
Taken together, all the results above were consistent with a model in which the mechanism of PRA formation by TrpDE was distinct in a strain lacking yjgF. This hypothesis predicted that in a situation, other than a yjgF mutant, where PRA formation was due to TrpDE, the trpE3613 allele would not decrease thiamine-independent growth. This prediction was tested with four strains: DM6417 (purF gnd trpD3611), DM7379 (purF gnd trpD3611 trpE3613), DM7436 (purF gnd yjgF), and DM7435 (purF gnd yjgF trpE3613). Strains DM6417 and DM7436 both require TrpDE to grow in the absence of thiamine. In the former strain, growth is allowed by the trpD3611 mutation (36), and in the latter, growth is due to the yjgF mutation (14). As described above, PRA formation in the former strain is sensitive to tryptophan and in the latter it is not. Introduction of trpE3613 into these strains reversed this behavior, making one strain sensitive to tryptophan and the other insensitive. When PurF-independent PRA synthesis depended on the trpD3611 allele, tryptophan eliminated growth (μ ≤ 0.001). The trpE3613 allele (or another feedback-insensitive trpE allele) restored growth (μ = 0.338) (36). However, when the yjgF mutation was responsible for the thiamine-independent growth, tryptophan had little effect on growth (μ = 0.368) unless the trpE3613 allele was present, in which case growth was essentially eliminated (μ = 0.06). All strains grew equally well in the presence of adenine, tryptophan, and thiamine (data not shown).
DISCUSSION
Certain genetic backgrounds and growth conditions permit synthesis of thiamine in Salmonella enterica strains lacking PurF. This observation resulted in efforts to identify the cellular mechanism(s) used to generate PRA in the absence of the primary catalytic enzyme PurF. Relevant to this study is the fact that the need for both PurF and the oxidative pentose phosphate pathway enzymes (e.g., Gnd) for PRA synthesis can be overcome by inactivating yjgF (14). Though YjgF is conserved throughout the three domains of life and has been the subject of numerous structural studies (2, 7, 8, 22, 23, 31, 42, 46), its specific role in the cell is not known. This study was initiated to understand the cellular role of the YjgF protein in the context of thiamine synthesis by probing the mechanism used to generate PRA in a purF yjgF gnd mutant background.
Taken together, results herein support the conclusion that in a purF gnd yjgF mutant the TrpDE enzyme complex is essential and sufficient for the PRA synthesis (Fig. 2). Previously two other conditions where PRA synthesis depends on TrpDE (but yjgF is wild type) have been reported; either trpD3611 is present or wild-type trpDE is overexpressed (36). The behavior of the PRA-forming activity under either of these two conditions is similar to that described for the AS and PRT activities of TrpDE in tryptophan biosynthesis (33). Specifically, the PRA-forming and AS-PRT activities are inhibited by tryptophan; this inhibition is overcome by mutations altering the allosteric binding site for tryptophan, and the allosteric-insensitive mutants remain catalytically proficient (3, 19, 30, 36, 43, 44, 50). In contrast, the TrpDE-dependent PRA-forming activity of a yjgF mutant is not sensitive to tryptophan, and mutations altering the allosteric site significantly compromise the PRA-forming activity.
Significant to this study was the fact that the TrpEP289TD variant complex has different effects on PRA formation in the two distinct genetic backgrounds. The relevant mutation (trpE3613) is located near the allosteric binding site (24). One scenario to explain the behavior of this mutant protein is that the tryptophan-binding site overlaps with a site critical for the PRA-forming activity of TrpDE functioning in a yjgF mutant. Consistent with this possibility was the finding that additional mutations, producing the feedback-resistant protein complexes TrpEM293TD and TrpES40FD (3), also compromised thiamine-independent growth in a purF gnd yjgF background (data not shown). Both alleles allow PRA formation in a trpD3611 background in the presence of tryptophan (data not shown). Additional mutagenesis of both trpE and trpD will help identify the residues important for the PRA-formation unique to a yjgF mutant.
While the lack of YjgF affects the characteristics of TrpDE-dependent PRA formation, how the YjgF protein (or lack thereof) mediates this effect is not clear. Previous work implicating YjgF in binding metabolites (31, 41) and showing the promiscuity of enzymes to perform side reactions (27) as well as the results of this study continue to shape our working model for the role of the YjgF protein in the cell (11, 41). Our working model, displayed in Fig. 5, is an extrapolation of the model previously proposed for YjgF in the context of branched chain amino acid biosynthesis (41). We suggest that the lack of YjgF allows detection of an activity of TrpDE that is made possible by an altered metabolic environment in the cell. Further work is required to identify the hypothesized metabolite that serves as a substrate for the PRA-forming activity of TrpDE that is only detected in the absence of YjgF.
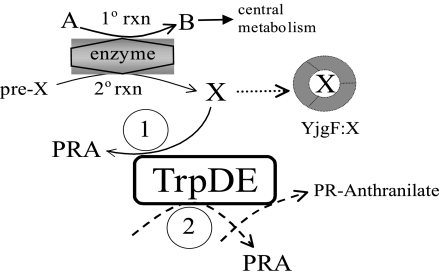
FIG. 5.
Working model for the influence of a yjgF mutation on purF-independent PRA synthesis. The features of this model are described in the text (see Discussion). Depicted is a generic enzyme in central metabolism that has a 2o reaction generating metabolite X. The two distinct TrpDE-dependent PRA-forming reactions supported by the data herein are depicted and labeled 1 and 2. Activity 1 is shown to generate PRA from substrate X that is normally sequestered and/or eliminated by YjgF. Activity 2 has been described previously (36) and is considered to be a weak activity that can be enhanced by overexpression or mutation.
Acknowledgments
This work was supported by NIH competitive grant GM47296 to D.M.D. Funds were also provided from a 21st Century Scientists Scholars Award from the J.M. McDonnell fund to D.M.D.
We acknowledge the assistance of Inna Larsen in the preparation of the manuscript.
REFERENCES
- 1.Begley, T. P., D. M. Downs, S. E. Ealick, F. W. McLafferty, A. P. Van Loon, S. Taylor, N. Campobasso, H. J. Chiu, C. Kinsland, J. J. Reddick, and J. Xi. 1999. Thiamin biosynthesis in prokaryotes. Arch. Microbiol. 171:293-300. [DOI] [PubMed] [Google Scholar]
- 2.Burman, J. D., C. E. M. Stevenson, K. A. Hauton, G. Sawers, and D. M. Lawson. 2003. Crystallization and preliminary X-ray analysis of the E. coli hypothetical protein TdcF. Acta Crystallogr. Sect. D 59:1076-1078. [DOI] [PubMed] [Google Scholar]
- 3.Caligiuri, M. G., and R. Bauerle. 1991. Identification of amino acid residues involved in feedback regulation of the anthranilate synthase complex from Salmonella typhimurium. Evidence for an amino-terminal regulatory site. J. Biol. Chem. 266:8328-8335. [PubMed] [Google Scholar]
- 4.Castilho, B. A., P. Olfson, and M. J. Casadaban. 1984. Plasmid insertion mutagenesis and lac gene fusion with mini-mu bacteriophage transposons. J. Bacteriol. 158:488-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis, R. W., D. Botstein, and J. R. Roth. 1980. Advanced bacterial genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 7.Deaconescu, A. M., A. Roll-Mecak, J. B. Bonanno, S. E. Gerchman, H. Kycia, F. W. Studier, and S. K. Burley. 2002. X-ray structure of Saccharomyces cerevisiae homologous mitochondrial matrix factor 1 (Hmf1). Proteins 48:431-436. [DOI] [PubMed] [Google Scholar]
- 8.Deriu, D., C. Briand, E. Mistiniene, V. Naktinis, and M. G. Grutter. 2003. Structure and oligomeric state of the mammalian tumour-associated antigen UK114. Acta Crystallogr. Sect. D 59:1676-1678. [DOI] [PubMed] [Google Scholar]
- 9.Downs, D. M., and L. Petersen. 1994. apbA, a new genetic locus involved in thiamine biosynthesis in Salmonella typhimurium. J. Bacteriol. 176:4858-4864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Downs, D. M., and J. R. Roth. 1991. Synthesis of thiamine in Salmonella typhimurium independent of the purF function. J. Bacteriol. 173:6597-6604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Downs, D. M., G. E. Schmitz, and E. Skovran. 2005. Probing the complex system of metabolic integration. Prog. Nucleic Acid Res. Mol. Biol. 80: 43-94. [DOI] [PubMed] [Google Scholar]
- 12.Enos-Berlage, J. L., and D. M. Downs. 1999. Biosynthesis of the pyrimidine moiety of thiamine independent of the PurF enzyme (phosphoribosylpyrophosphate amidotransferase) in Salmonella typhimurium: incorporation of stable isotope-labeled glycine and formate. J. Bacteriol. 181:841-848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Enos-Berlage, J. L., and D. M. Downs. 1997. Mutations in sdh (succinate dehydrogenase genes) alter the thiamine requirement of Salmonella typhimurium. J. Bacteriol. 179:3989-3996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Enos-Berlage, J. L., M. J. Langendorf, and D. M. Downs. 1998. Complex metabolic phenotypes caused by a mutation in yjgF, encoding a member of the highly conserved YER057c/YjgF family of proteins. J. Bacteriol. 180:6519-6528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goupil-Feuillerat, N., M. Cocaign-Bousquet, J.-J. Gocon, S. D. Ehrlich, and P. Renault. 1997. Dual role of α-acetolactate decarboxylase in Lactococcus lactis subsp. lactis. J. Bacteriol. 179:6285-6293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grieshaber, M., and R. Bauerle. 1974. Monomeric and dimeric forms of component II of the anthranilate synthetase-anthranilate 5-phosphoribosylpyrophosphate phosphoribosyltransferase complex of Salmonella typhimurium. Implications concerning the mode of assembly of the complex. Biochemistry 13:373-383. [DOI] [PubMed] [Google Scholar]
- 17.Guzman, L. M., D. Belin, M. J. Carson, and J. Beckwith. 1995. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 177:4121-4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henderson, E. J., H. Nagano, H. Zalkin, and L. H. Hwang. 1970. The anthranilate synthetase-anthranilate 5-phosphoribosylpyrophosphate phosphoribosyltransferase aggregate. Purification of the aggregate and regulatory properties of anthranilate synthetase. J. Biol. Chem. 245:1416-1423. [PubMed] [Google Scholar]
- 19.Henderson, E. J., and H. Zalkin. 1971. On the composition of anthranilate synthetase-anthranilate 5-phosphoribosylpyrophosphate phosphoribosyltransferase from Salmonella typhimurium. J. Biol. Chem. 246: 6891-6898. [PubMed] [Google Scholar]
- 20.Hughes, K., and J. Roth. 1984. Conditionally transposition-defective derivative of MudI (Amp, Lac). J. Bacteriol. 159:130-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim, J. M., H. Yoshikawa, and K. Shirahige. 2001. A member of the YER057c/yjgf/Uk114 family links isoleucine biosynthesis and intact mitochondria maintenance in Saccharomyces cerevisiae. Genes Cells 6:507-517. [DOI] [PubMed] [Google Scholar]
- 22.Manjasetty, B. A., H. Delbruck, D. Pham, U. Mueller, M. Fieber-Erdmann, C. Scheich, V. Sievert, K. Bussow, F. H. Neisen, W. Weihofen, B. Loll, and W. H. Saenger. 2004. Crystal structure of Homo sapiens protein hp14.5. Proteins Struct. Function Bioinformatics 54:797-800. [DOI] [PubMed] [Google Scholar]
- 23.Mistiniene, E. 2003. Oligomeric assembly and ligand binding of the members of the protein family YER057C/YIL051c/YJGF. Bioconjugate Chem. 14:1243-1252. [DOI] [PubMed] [Google Scholar]
- 24.Morollo, A. A., and M. J. Eck. 2001. Structure of the cooperative allosteric anthranilate synthase from Salmonella typhimurium. Science 8:243-247. [DOI] [PubMed] [Google Scholar]
- 25.Newell, P. C., and R. G. Tucker. 1968. Biosynthesis of the pyrimidine moiety of thiamine. A new route of pyrimidine biosynthesis involving purine intermediates. Biochem. J. 106:279-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Newell, P. C., and R. G. Tucker. 1966. The derepression of thiamine biosynthesis by adenosine. A tool for investigating this biosynthetic pathway. Biochem. J. 100:512-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O'Brien, P. J., and D. Herschlag. 1999. Catalytic promiscuity and the evolution of new enzymatic activities. Chem. Biol. 6:R91-R105. [DOI] [PubMed] [Google Scholar]
- 28.Oka, T., H. Tsuji, C. Noda, K. Sakai, Y.-M. Hong, I. Suzuki, S. Munoz, and Y. Natori. 1995. Isolation and characterization of a novel perchloric-acid soluble protein inhibiting cell-free protein synthesis. J. Biol. Chem. 270:30060-30067. [DOI] [PubMed] [Google Scholar]
- 29.Oxelmark, E., A. Marchini, I. Malanchi, F. Magherini, L. Jaquet, M. A. Hajibagheri, K. J. Blight, J. C. Jauniaux, and M. Tommasino. 2000. Mmf1p, a novel yeast mitochondrial protein conserved throughout evolution and involved in maintenance of the mitochondrial genome. Mol. Cell. Biol. 20:7784-7797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pabst, M. J., J. C. Kuhn, and R. L. Somerville. 1973. Feedback regulation in the anthranilate aggregate from wild type and mutant strains of Escherichia coli. J. Biol. Chem. 248:901-914. [PubMed] [Google Scholar]
- 31.Parsons, L., N. Bonander, E. Eisenstein, M. Gilson, V. Kairys, and J. Orban. 2003. Solution structure and functional ligand screening of HI0719, a highly conserved protein from bacteria to humans in the YjgF/YER057c/UK114 family. Biochemistry 42:80-89. [DOI] [PubMed] [Google Scholar]
- 32.Petersen, L., and D. M. Downs. 1996. Mutations in apbC (mrp) prevent function of the alternative pyrimidine biosynthetic pathway in Salmonella typhimurium. J. Bacteriol. 178:5676-5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pittard, A. J. 1996. Biosynthesis of the aromatic amino acids, p. 458-484. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed., vol. 1. ASM Press, Washington, D.C. [Google Scholar]
- 34.Pittard, J. 1996. The various strategies within the TyrR regulation of Escherichia coli to modulate gene expression. Genes Cells 1:717-725. [DOI] [PubMed] [Google Scholar]
- 35.Ramos, I. 2006. Metabolic robustness of phosphoribosylamine biosynthesis in Salmonella enterica. Ph.D. thesis. University of Wisconsin, Madison.
- 36.Ramos, I., and D. M. Downs. 2003. Anthranilate synthase can generate sufficient phosphoribosyl amine for thiamine synthesis in Salmonella enterica. J. Bacteriol. 185:5125-5132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robello, C., B. Dallagiovanna, J. C. Engel, F. Gamarro, and S. Castanys. 1998. A new member of YER057c family in Trypanosoma cruzi is adjacent to an ABC-transporter. Gene 220:1-12. [DOI] [PubMed] [Google Scholar]
- 38.Roberts, G. P. 1978. Isolation and characterization of informational suppressors in Salmonella typhimurium. Ph.D. thesis. University of California, Berkeley.
- 39.Schmiedeknecht, G., C. Kerkhoff, E. Orso, J. Stohr, C. Aslanidis, G. M. Nagy, R. Knuechel, and G. Schmitz. 1996. Isolation and characterization of a 14.5-kDa trichloroacetic-acid-soluble translational inhibitor protein from human monocytes that is upregulated upon cellular differentiation. Eur. J. Biochem. 242:339-351. [DOI] [PubMed] [Google Scholar]
- 40.Schmieger, H. 1972. Phage P22-mutants with increased or decreased transduction abilities. Mol. Gen. Genet. 119:75-88. [DOI] [PubMed] [Google Scholar]
- 41.Schmitz, G., and D. M. Downs. 2004. Reduced transaminase B (IlvE) activity caused by the lack of yjgF is dependent on the status of threonine deaminase (IlvA) in Salmonella enterica serovar Typhimurium. J. Bacteriol. 186:803-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sinha, S., P. Rappu, S. C. Lange, P. Mantsala, H. Zalkin, and J. L. Smith. 1999. Crystal structure of Bacillus subtilis YabJ, a purine regulatory protein and member of the highly conserved YjgF family. Proc. Natl. Acad. Sci. USA 96:13074-13079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Somerville, R. I., and C. Yanofsky. 1965. Studies on the regulation of tryptophan biosynthesis in Escherichia coli. J. Mol. Biol. 11:747-759. [DOI] [PubMed] [Google Scholar]
- 44.Tamir, H., and P. R. Srinivasan. 1969. Purification and properties of anthranilate synthase from Salmonella typhimurium. J. Biol. Chem. 244:6507-6513. [PubMed] [Google Scholar]
- 45.Vogel, H. J., and D. M. Bonner. 1956. Acetylornithase of Escherichia coli: partial purification and some properties. J. Biol. Chem. 218:97-106. [PubMed] [Google Scholar]
- 46.Volz, K. 1999. A test case for structure-based functional assignment: the 1.2 A crystal structure of the yjgF gene product from Escherichia coli. Protein Sci. 8:2428-2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Way, J. C., M. A. Davis, D. Morisato, D. E. Roberts, and N. Kleckner. 1984. New Tn10 derivatives for transposon mutagenesis and for construction of lacZ operon fusions by transposition. Gene 32:369-379. [DOI] [PubMed] [Google Scholar]
- 48.Weng, M., and H. Zalkin. 2000. Mutations in the Bacillus subtilis purine repressor that perturb PRPP effector function in vitro and in vivo. Curr. Microbiol. 41:56-59. [DOI] [PubMed] [Google Scholar]
- 49.Winston, F., D. Botstein, and J. H. Miller. 1979. Characterization of amber and ochre suppressors in Salmonella typhimurium. J. Bacteriol. 137:433-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zalkin, H., and D. Kling. 1968. Anthranilate synthetase. Purification and properties of component I from Salmonella typhimurium. Biochemistry 7:3566-3573. [DOI] [PubMed] [Google Scholar]
- 51.Zalkin, H., and P. Nygaard. 1996. Biosynthesis of purine nucleotides, p. 561-579. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed., vol. 1. ASM Press, Washington, D.C. [Google Scholar]