Abstract
The moderately halophilic, chloride-dependent bacterium Halobacillus halophilus produces glutamate and glutamine as main compatible solutes at external salinities of 1.0 to 1.5 M NaCl. The routes for the biosynthesis of these solutes and their regulation were examined. The genome contains two genes potentially encoding glutamate dehydrogenases and two genes for the small subunit of a glutamate synthase, but only one gene for the large subunit. However, the expression of these genes was not salt dependent, nor were the corresponding enzymatic activities detectable in cell extracts of cells grown at different salinities. In contrast, glutamine synthetase activity was readily detectable in H. halophilus. Induction of glutamine synthetase activity was strictly salt dependent and reached a maximum at 3.0 M NaCl; chloride stimulated the production of active enzyme by about 300%. Two potential genes encoding a glutamine synthetase, glnA1 and glnA2, were identified. The expression of glnA2 but not of glnA1 was increased up to fourfold in cells adapted to high salt, indicating that GlnA2 is the glutamine synthetase involved in the synthesis of the solutes glutamate and glutamine. Furthermore, expression of glnA2 was stimulated twofold by the presence of chloride ions. Chloride exerted an even more pronounced effect on the enzymatic activity of preformed enzyme: in the absence of chloride in the assay buffer, glutamine synthetase activity was decreased by as much as 90%. These data demonstrate for the first time a regulatory role of a component of common salt, chloride, in the biosynthesis of compatible solutes.
Halobacillus halophilus is a moderately halophilic bacterium that requires salt for growth and grows optimally at NaCl concentrations ranging from 0.5 to 2.0 M NaCl. Interestingly, growth not only is salt dependent but also requires Na+ and Cl− ions (7, 24). Although Na+ dependence of growth is frequently observed in microorganisms from different physiological groups, H. halophilus was the first organism for which chloride dependence of growth was demonstrated (23). In addition to growth, the germination of endospores (9), flagellum synthesis, and thus motility were shown to be strictly chloride dependent (25). Moreover, production of the major component of the flagellum, FliC, was dependent on the chloride concentration. Interestingly, the cellular concentration of FliC was more affected by chloride than was fliC expression. In addition, two-dimensional gel electrophoresis revealed more chloride-induced proteins (23). Taken together, these studies indicated the presence of a chloride regulon in this moderate halophile (19). However, the processes identified so far to be chloride dependent are not essential for growth, and thus the essential nature of the chloride dependence of growth still remains to be elucidated.
H. halophilus is a moderate halophile, and like other halophiles, it prevents loss of water and adjusts turgor in a highly saline environment by accumulation of osmotically active compounds. In a systematic survey performed by the group of Trüper, H. halophilus was shown to accumulate a cocktail of different solutes when grown in the presence of 1.5 M NaCl in glucose mineral salt medium or rich medium (29). The solutes accumulated by H. halophilus include glycine betaine, Nɛ-acetyl lysine, Nδ-acetyl ornithine, alanine, citrulline, glutamate, glutamine, ectoine, and proline. A clue to the essentiality of chloride for growth may come from the observation that uptake of the compatible solute glycine betaine is strictly Cl− dependent (22). This observation might point to a global regulatory role of chloride in osmosensing and accumulation of compatible solutes, a feature clearly essential for life under high-salt conditions. Therefore, we have addressed the nature of compatible solutes synthesized by H. halophilus as well as their biosynthesis and regulation by salt and ions. Here we have concentrated on solutes synthesized at lower salt salinities, i.e., glutamate and glutamine.
MATERIALS AND METHODS
Organism and cultivation.
H. halophilus (DSMZ 2266T) was grown in glucose mineral salt medium (G10) containing 1% glucose, NH4Cl (2 g/liter), FeSO4 · 7H2O (10 mg/liter), Tris base (12 g/liter), K2HPO4 (0.49 g/liter), yeast extract (0.1 g/liter), DSM 141 vitamin solution (1 ml/liter), and DSM 79 artificial seawater (250 ml/liter). Sodium glutamate, NaNO3, or sodium gluconate was added to a final concentration of 1.0 M. The final concentration of NaCl varied depending on the assay conditions (values are given in the text). The pH was adjusted to 7.8 with H2SO4. H. halophilus was cultivated aerobically and shaken on a rotary shaker at 30°C.
Determination of compatible solutes.
Cells were grown to an optical density at 578 nm (OD578) of about 0.8, harvested, and freeze-dried. Ten milligrams of lyophilized cells was then extracted with 570 μl extraction mixture (methanol-chloroform-water, 10:5:4 [vol/vol/vol]) by being vigorously shaken for 5 min. Next, 170 μl chloroform and 170 μl water were added, and the solution was shaken again for 10 min. The aqueous supernatant was recovered and dried. The pellet was then resuspended in 500 μl water. In order to remove residual proteins and other high-molecular-weight compounds, the solution was then filtered through a Microcon YM-3 filter. The sample was then derivatized with 9-fluorenylmethoxy carbonyl (FMOC) according to the method of Kunte et al. (14). One hundred fifty microliters of FMOC reagent (1.5 mM in acetone) was added to 150 μl sodium borate buffer (0.5 M, pH 7.7) and 150 μl sample or standard solution and then mixed by vortexing. Thereafter, 200 μl amantadine hydrochloride reagent (12 mM in borate buffer) was added to remove excess FMOC reagent. In order to make ectoine accessible to FMOC, it has to be hydrolyzed to Nγ-acetyldiaminobutyric acid and Nα-acetyldiaminobutyric acid. This was accomplished by incubating the sample solution with NaOH (final concentration, 0.1 M) at 50°C for 20 h. The sample was neutralized with perchloric acid before FMOC derivatization. FMOC-derivatized amino acids were separated by reversed-phase chromatography (4-μm Supersphere 60 RP-8 endcapped column; Merck, Germany). The solvent system used comprised solvent A (20% acetonitrile and 0.5% tetrahydrofuran in 50 mM sodium acetate buffer [pH 4.2]) and solvent B (80% acetonitrile in 50 mM sodium acetate buffer [pH 4.2]). The gradient used was as follows: 0 to 2 min, 100% solvent A; 2 to 8 min, 80% solvent A; 8 to 16 min, 73% solvent A; 16 to 29 min, 46% solvent A; and 29 to 31 min, 0% solvent A. Chromatography was carried out at a flow rate of 1.25 ml/min at 45°C. FMOC-labeled derivatives were monitored with an RF 2000 fluorescence detector (Dionex, Idstein, Germany) with an excitation wavelength of 254 nm and an emission wavelength of 315 nm.
Real-time PCR analysis.
For real-time PCR analysis, H. halophilus cells were harvested in the early exponential growth phase (OD578, 0.15 to 0.3). Total RNA was prepared by using a NucleoSpin RNA II kit (Macherey-Nagel, Düren, Germany). To break the cells, they were incubated with 40 mg/ml (final concentration) lysozyme for 5 min at room temperature. The cell suspension was then transferred to a 2-ml reaction tube that already contained 350 μl lysis buffer RA1 (Macherey-Nagel, Düren, Germany), 3.5 μl β-mercaptoethanol, and about 30 zirconoxide balls (2 mm in diameter, yttrium stabilized; Retsch, Germany). The tubes were applied to an MM 301 mixer mill (Retsch, Germany) at 30 Hz for 5 min. The subsequent steps were done according to the protocol provided by Macherey-Nagel. To remove residual DNA, the samples were treated with RQ1 RNase-free DNase (Promega) according to the manufacturer's protocol. cDNA synthesis was accomplished using Moloney murine leukemia virus reverse transcriptase RNase H Minus, point mutant (Promega, Mannheim, Germany) according to the manufacturer's protocol. To prevent RNA degradation during this step, 48 U of recombinant RNasin RNase inhibitor (Promega, Mannheim, Germany) was added. Quantitative PCR was run in a Rotor-Gene RG-3000 qPCR cycler (Corbett Research, Cambridge, United Kingdom), using SYBR green as a fluorescent dye. For amplification of glnA1 and glnA2 fragments, the following oligonucleotides were used as primers: RT_glnA1_for, GATGTCTGTGTTACTAGCC; RT_glnA1_rev, AGCGCATTGAACAGCGTG; RT_glnA2_for, ACGTAGAACTCCCTATCGATG; and RT_glnA2_rev, TTGATCTTCGTCTACCAGG. The fragments amplified comprised 150 bp and 164 bp, respectively.
Data analysis was accomplished using the 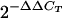 method (15). Real-time PCR analysis was done with three independent physiological parallels to ensure statistical relevance. Two open reading frames, encoding a malate dehydrogenase and a glycerate dehydrogenase, respectively, served as internal normalizers. The expression of these two genes did not change with the salinity of the medium.
method (15). Real-time PCR analysis was done with three independent physiological parallels to ensure statistical relevance. Two open reading frames, encoding a malate dehydrogenase and a glycerate dehydrogenase, respectively, served as internal normalizers. The expression of these two genes did not change with the salinity of the medium.
Enzyme assays.
All enzyme assays were performed at 37°C. One unit (U) corresponds to 1 μmol substrate produced per minute.
(i) Glutamine synthetase.
Glutamine synthetase activity was measured by the methods of Woolfolk et al. (30) and Bender et al. (2), which follow the production of γ-glutamyl hydroxamate from glutamine and hydroxylamine, respectively. For the preparation of cell suspensions, the growth of cell cultures was stopped in mid-exponential growth phase by adding 0.1% (wt/vol) cetyltrimethylammonium bromide (CTAB) and incubating the cells for 10 min on a shaker at 30°C. Cells were then harvested and washed in a 0.2 volume of KCl solution. The KCl concentration corresponded to the NaCl concentration used to grow the cells. The pellet was then resuspended in 1.5 M KCl solution to an OD578 of 70. The cell suspension was stored on ice. The standard reaction mixture (4 ml) contained 126 mM imidazole hydrochloride, 17 mM hydroxylamine hydrochloride, 0.26 mM MnCl2, 24 mM potassium arsenate, 84 μg/ml CTAB, and 0.37 mM Na-ADP. The pH was adjusted to 7.8. The cell suspension (0.5 ml) was preincubated with this mixture for 2 min at 37°C on a shaker, and the reaction was started by the addition of l-glutamine to a final concentration of 25 mM. Samples (0.5 ml) were withdrawn for 5 min in 1-min intervals, the reaction was stopped by the addition of 1 ml of stop mix (0.2 M FeCl3, 0.15 M trichloroacetic acid, 0.25 M HCl), and the samples were incubated on ice for 30 min. Cells were removed by centrifugation (2 min, 15,000 × g). The formation of γ-glutamyl hydroxamate, which forms a brownish complex together with FeCl3, was measured at 540 nm.
(ii) Glutamate dehydrogenase.
A spectrophotometric assay described by Bonete et al. (4) was used to monitor the glutamate dehydrogenase-dependent depletion of NAD(P)H during the formation of glutamate from ammonium and 2-oxoglutarate (reductive amination reaction) or the NAD(P)H formation in the reverse reaction (oxidative deamination reaction). The standard reaction mixture (1.125 ml) contained 0.3 mM NAD(P)H, 64 mM ammonium acetate, 1 M NaCl, and protein (amination reaction) or 3 mM NAD(P)+, 19 mM Tris-HCl, 1 M NaCl, and protein (deamination reaction). The reactions were started with 20 mM 2-oxoglutarate and 50 mM l-glutamate, respectively. NAD(P)H depletion or formation was monitored at 340 nm.
Variations of the assays included different concentrations of NAD(P)+/NAD(P)H (0.3 to 0.6 mM), different protein concentrations, different pH values in the assay buffer (pH 7.0 to 8.0), the presence or absence of MgCl2 (20 mM), replacement of Na+ ions with K+ ions, and different salt concentrations (250 mM to 1 M). Additionally, different methods for cell rupture (use of a French press versus sonication or the addition of lysozyme) and different conditions of the cell resuspension buffer, such as the addition or depletion of glycerol (20% [vol/vol]), the presence or absence of β-mercaptoethanol (3 mM) or EDTA (1 mM), or temperature (4°C versus room temperature), were tested. In order to identify a putative catabolic glutamate dehydrogenase, H. halophilus was cultivated with alternative nitrogen sources, such as glutamate, arginine, or ornithine, instead of ammonium.
(iii) Glutamate synthase.
To measure the glutamate synthase-dependent formation of glutamate, the method described by Bohannon et al. (3) was used. Again, the depletion of NAD(P)H was monitored spectrophotometrically. A standard reaction mixture (1 ml) contained 30 mM Tris-HCl (pH 8.0), 1.6 M KCl, 5 mM β-mercaptoethanol, 35 mM 2-oxoglutarate, 0.1 mM NAD(P)H, and protein. The reaction was started by the addition of l-glutamine to a final concentration of 20 mM.
Protein content.
Forty microliters of perchloric acid (3 M) was added to 100 μl of cell suspension. This mixture was incubated for 10 min at 100°C and then cooled on ice. After the addition of 560 μl of H2O and 233 μl of trichloroacetic acid (25% [wt/vol]), precipitated protein was pelleted by centrifugation (15,000 × g for 15 min). The pellet was then resuspended in 200 μl Na2HPO4 buffer (20 mM) and 200 μl NaOH (0.1 M). The protein concentrations of these solutions as well as of cell extracts were determined by the method of Bradford (5) or Lowry et al. (16), using bovine serum albumin as the standard. The protein content of CTAB-permeabilized cells was determined before the addition of CTAB.
Cloning and DNA sequence determination.
DNA sequences were retrieved from the genome sequence of H. halophilus that will be described elsewhere.
Nucleotide sequence accession numbers.
DNA sequences were deposited in the GenBank database with accession numbers AM286406 (gdh-1), AM286407 (gdh-2), AM286408 (glnA1), AM286409 (glnA2), AM286410 (glnR), AM286411 (gltA), AM286412 (gltB1), and AM286413 (gltB2).
RESULTS
Glutamate and glutamine are major compatible solutes in H. halophilus grown at moderate salt concentrations.
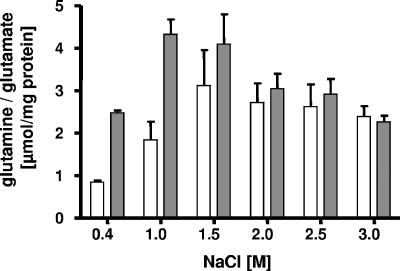
Glutamate and glutamine were shown before to be accumulated by H. halophilus grown at 1.5 M NaCl (29). To establish their cellular concentrations at different salinities, H. halophilus was grown at NaCl concentrations ranging from 0.4 to 3.0 M in G10 mineral medium up to an OD578 of 0.5 to 0.8 (late exponential growth phase) and harvested by centrifugation, and the cellular content of glutamine and glutamate was analyzed by high-performance liquid chromatography. As shown in Fig. 1, the intracellular concentrations of glutamate and glutamine were clearly salt dependent. The maximal glutamate accumulation of 4.3 μmol/mg protein was observed at 1.0 M NaCl, whereas the maximum glutamine accumulation was found at 1.5 M NaCl (3.1 μmol/mg protein). Thereafter, the glutamate and glutamine concentrations decreased slightly, by 25%, at salinities from 2.0 to 3.0 M NaCl. At 1.0 M NaCl, glutamate was the predominant solute, but the Glu/Gln ratio decreased with the increasing salinity of the medium, from 3.1 (at 0.4 M NaCl) to 1.0 (at 3.0 M NaCl).
FIG. 1.
Cellular concentrations of glutamate and glutamine in H. halophilus at different salinities. Cells were grown in glucose mineral salt medium. Compatible solutes were extracted and analyzed by high-performance liquid chromatography. Glutamate concentrations are shown by gray bars, and glutamine concentrations are shown by white bars.
Routes for biosynthesis of glutamate.
Glutamate can be produced from 2-oxoglutarate by the action of glutamate dehydrogenase or by the combined action of glutamine synthetase and glutamate synthase. We addressed the possible routes for the biosynthesis of glutamate and glutamine in H. halophilus by enzymatic assays and molecular studies.
(i) Glutamate dehydrogenase.
Glutamate dehydrogenase catalyzes the following reaction: 2-oxoglutarate + NH4+ + 2 [H] ⇄ glutamate + H2O. Its activity can be measured by amination of 2-oxoglutarate, with NADH as the electron donor, or by determining the deamination of glutamate, with NAD+ as the electron acceptor. Neither assay revealed measurable glutamate dehydrogenase activity in cells of H. halophilus grown in G10 mineral medium at various salinities. Using the same assay conditions, we were able to detect glutamate dehydrogenase activity in cell extracts of Escherichia coli. Even after variation of the assay conditions, we were not able to detect any glutamate dehydrogenase activity in H. halophilus.
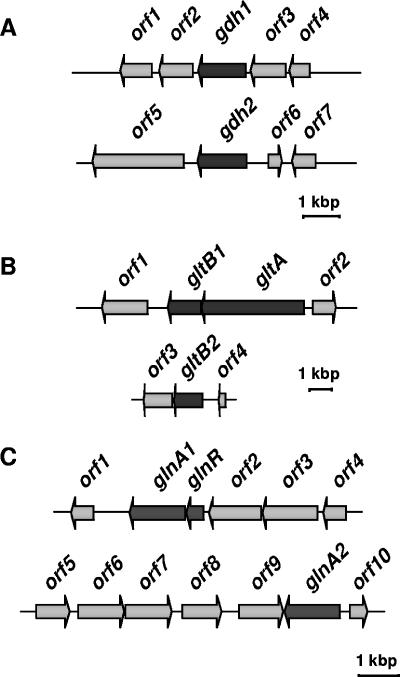
The genome of H. halophilus was searched for genes potentially encoding glutamate dehydrogenases. Interestingly, two genes (gdh-1 and gdh-2) were identified (Fig. 2A). gdh-1 is 1,281 bp long, has a putative σL promoter 120 to 135 bp upstream, and has a terminator sequence downstream. gdh-1 is preceded by a gene potentially encoding a d-alanyl-d-alanine ligase and followed by a putative thioredoxin reductase gene. The deduced protein is very similar to glutamate dehydrogenases from Bacillus halodurans (421 amino acids [aa]; 85% identity to a putative glutamate dehydrogenase), Bacillus cereus (428 aa; 83% identity to the product of gdhA), Bacillus subtilis (426 aa; 82% identity to the product of gudB), and Oceanobacillus iheyensis (426 aa; 80% identity to the product of gudB). gdh-2 (1,377 bp) is preceded by genes encoding hypothetical proteins and a gene annotated as an enoyl-(acyl carrier protein) reductase and followed by a gene encoding a hypothetical protein. Upstream (374 to 393 bp) of gdh-2 is a putative σL promoter, and downstream is a terminator sequence. The deduced protein is less conserved than Gdh1 (46% identity to BH0824 [464 aa] from B. halodurans, 38% identity to alr4255 [437 aa] from a Nostoc sp., and 37% identity to the product of gdhA [413 aa] from Treponema denticola).
FIG. 2.
Genetic organization of genes potentially involved in the biosynthesis of glutamine and glutamate in H. halophilus. (A) Glutamate dehydrogenase (gdh). Two ORFs encoding a putative glutamate dehydrogenase (gdh-1 and gdh-2) could be identified. The other ORFs shown encode the following proteins: orf1, l-asparaginase; orf2, pyridine nucleotide-disulfide oxidoreductase family protein (thioredoxin reductase); orf3, d-alanyl-d-alanine ligase A; orf4, negative regulator of genetic competence; orf5, unknown; orf6, enoyl-(acyl carrier protein) reductase; and orf7, unknown. (B) Glutamate synthase (glt). The glutamate synthase is a heterodimer encoded by gltA (large subunit) and gltB (small subunit). In the genome of H. halophilus, only one ORF could be identified encoding GltA, whereas two ORFs were found encoding GltB (gltB1 and gltB2). gltB1 and gltA are organized in an operon (data not shown). The other ORFs shown encode the following proteins: orf1, catalase; orf2, l-aminopeptidase/d-esterase; orf3, dihydropyrimidine dehydrogenase; and orf4, beta-alanine synthase. (C) Glutamine synthetase (glnA). The ORFs shown encode the following proteins: orf1, hypothetical conserved protein; glnR, transcriptional regulator; orf2, aluminum resistance protein; orf3, GTP-binding protein; orf4, spore formation protein; orf5, ABC transporter protein; orf6, oxidoreductase; orf7, putative dehydrogenase; orf8, conserved protein; orf9, conserved hypothetical protein/GTPase of unknown function; and orf10, hypothetical protein.
Expression of the gdh genes was analyzed by quantitative real-time PCR. For this assay, cells were grown at different salinities and harvested, RNAs were isolated and transcribed into cDNAs, and the gdh genes were amplified by gene-specific primers. These experiments clearly demonstrated that gdh-1 expression was not influenced by the salinity of the medium. In contrast, gdh-2 expression was very low at every salt concentration tested (at least 2 orders of magnitude less than that of gdh-1), so the statistical error increased dramatically, making it impossible to make statements about the regulation of gene transcription.
(ii) Glutamate synthase.
Glutamate synthase catalyzes the following reaction: glutamine + 2-oxoglutarate + 2 [H] ⇆ 2 glutamate. The buffer used to determine glutamate synthase activity contained 2-oxoglutarate and glutamine as substrates and cell extracts of H. halophilus grown at various salinities to early exponential growth phase. Glutamate synthase activity was detectable but low (0.023 mU/mg protein). Variation of the buffer system, growth conditions, and methods for cell disruption did not increase the glutamate synthase activity.
Glutamate synthases are known to be heterodimers comprising a large (GltA) and a small (GltB) subunit (13, 17, 18, 20, 27). Despite the inability to detect glutamate synthase activity in cell extracts of H. halophilus, the genome contains two tandemly arranged genes that potentially encode the large and small subunits of glutamate synthase, i.e., gltA and gltB1 (Fig. 2B). gltA is 4,581 bp long, and gltB1 has a length of 1,488 bp. The intergenic region comprises 20 bp. The genes are organized in an operon structure and are translated from a common dicistronic transcript (data not shown).
The deduced proteins are very similar to the corresponding proteins from O. iheyensis (73% identity for GltA [1,530 aa] and 74% for GltB1 [494 aa]). In the neighborhood of this cluster, open reading frames (ORFs) were found coding for a putative catalase, aminopeptidase, or σL protein. However, these genes do not seem to form a transcriptional unit together with gltAB1 or to be functionally related to glutamate/glutamine biosynthesis. Real-time PCR analysis did not reveal a salt dependence of gltAB1 expression.
Interestingly, a second gltB homologue (gltB2; 1,275 bp), which is not clustered with a gltA gene, was identified elsewhere on the chromosome. The deduced protein showed highest similarity to the glutamate synthase of Geobacillus kaustophilus (449 aa; 73% identity). ORFs in proximity to gltB2 do not seem to be functionally related to the gene encoding GltB2, and most of them likely play a role in nucleotide metabolism.
(iii) Glutamine synthetase.
To determine glutamine synthetase activity, we took advantage of its glutamyltransferase activity. In the glutamyltransferase activity assay, glutamine is converted to γ-glutamylhydroxamate in the presence of hydroxylamine, which can be measured photometrically (30). In the assay, whole cells were used that had been permeabilized with CTAB. In a first set of experiments, glutamine synthetase activity was readily detectable using this assay system. Its characterization is described below.
Glutamine synthetase is salt induced.
H. halophilus was grown routinely at 37 mM NH4Cl. Since it is known that glutamine synthetase is induced at low ammonium concentrations (10), we determined the effects of various NH4Cl concentrations (0 to 370 mM) on growth. Growth rates were only marginally affected by the NH4Cl concentration. In contrast, final optical densities were significantly reduced at 0, 278, or 370 mM NH4Cl but maximal at 4 to 185 mM NH4Cl. This experiment ruled out the possibility that growth at 37 mM NH4Cl is limited by ammonium. Next, the effect of the growth phase on glutamine synthetase activity was examined. The activity increased during growth but stayed at a high level for about 80 h, from the last third of the exponential growth phase until stationary phase. To exclude regulation of glutamine synthetase by the salt concentration in the assay buffer, cells were grown at different salinities, and glutamine synthetase activity was measured for each culture at three different KCl concentrations in the assay buffer. Independent of the salinity used to grow the cells, glutamine synthetase activity was maximal in the presence of 1.5 M KCl in the assay buffer. Therefore, the following experiments were performed with cells grown at 37 mM NH4Cl and various salt concentrations up to late exponential growth phase, and the assay was performed in the presence of 1.5 M KCl.
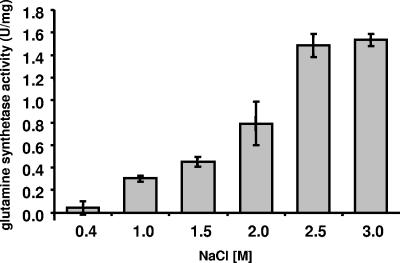
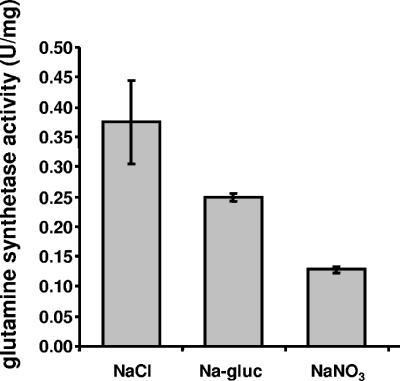
In cells grown at 0.4 M NaCl, glutamine synthetase activity was negligible, but it increased steadily with increasing salinities. Maximal activities of 1.49 and 1.53 U/mg protein were observed at 2.5 and 3.0 M NaCl, respectively (Fig. 3). These data demonstrate that glutamine synthetase activity is clearly salt induced. Next, the effect of the anion on induction of glutamine synthetase activity was determined. Therefore, cells grown at 1 M NaCl were transferred to medium containing 1 M NaNO3 or sodium gluconate. Although this concentration is suboptimal for induction of glutamine synthetase activity, higher salt concentrations could not be used due to the fact that cells do not grow at higher salt concentrations in the absence of chloride. As shown in Fig. 4, the specific activity of glutamine synthetase was reduced 34% during growth in the presence of sodium gluconate and 66% in the presence of NaNO3. At the same time, growth rates and final densities were reduced. For cells grown in the presence of NaCl, sodium gluconate, and NaNO3, growth rates of 0.12 h−1, 0.08 h−1, and 0.06 h−1, respectively, were determined. These experiments revealed, for the first time, a stimulatory effect of chloride on the induction of glutamine synthetase.
FIG. 3.
Glutamine synthetase activity in H. halophilus cells increases with increasing salinity of the medium. Cells were harvested in the late exponential growth phase, washed in an isosmolar KCl solution, and resuspended in a 1.5 M KCl solution.
FIG. 4.
Induction of glutamine synthetase activity in H. halophilus is stimulated by chloride. Cells were grown with 1 M NaCl, sodium gluconate (Na-gluc), or NaNO3, harvested in the late exponential growth phase, washed in an iso-osmolar KCl solution, and resuspended in 1.5 M KCl.
Glutamine synthetase activity is chloride dependent.
To determine a possible dependence of glutamine synthetase activity on the nature of the anion, cells were grown in the presence of 2.5 M NaCl, and the assay was performed with different salts at a concentration of 1.5 M. As shown in Fig. 5, glutamine synthetase activity was reduced 80 to 90% in the absence of chloride. The lowest activities were observed in the presence of NaNO3, followed by sodium gluconate, NaBr, KNO3, and potassium gluconate. Chloride salts stimulated glutamine synthetase activity, and the highest activity was observed in the presence of choline chloride. Because it was shown before that nitrate is not inhibitory and since the effect was observed with different anions, the experiments demonstrate a Cl− dependence of glutamine synthetase activity.
FIG. 5.
Glutamine synthetase activity in H. halophilus is dependent on chloride. Cells were cultivated in the presence of 2.5 M NaCl, harvested in the late exponential growth phase, washed in isosmolar KCl solution, and resuspended in 1.5 M KCl. Different salts were used in the assay buffer at a final concentration of 1.5 M, whereas the final concentration of KCl was 188 mM, originating from the cell suspension.
Molecular characterization of glutamine synthetase.
Two ORFs encoding putative glutamine synthetases were identified in the genome of H. halophilus (Fig. 2C). The first one (glnA1; 1,332 bp) is preceded by a gene with similarity to glnR, which codes for a putative negative transcriptional regulator of glnA. Both genes are part of one message (data not shown), indicating that they form an operon. Downstream of the glnRA operon is a gene encoding a putative lipoprotein, and upstream is a gene encoding an aluminum resistance protein, both of which are obviously not related to nitrogen metabolism. The derived amino acid sequence of glnA1 is 85% identical to that of the corresponding protein of O. iheyensis (445 aa; accession number BAC13607) and 81% identical to that of the corresponding protein from B. subtilis (444 aa; accession number BAA00730). The glnR product is 57% and 62% identical, respectively, to the corresponding proteins in these organisms.
Inspection of the genomic sequence revealed another protein with similarity to glutamine synthetase, namely, the glnA2 product. The derived amino acid sequence of GlnA2 is 52% identical to those of O. iheyensis (445 aa; accession number BAC13607) and B. halodurans (449 aa; accession number BAB06079) and 53% identical to that of B. subtilis (444 aa; accession number BAA00730). In contrast to glnA1, glnA2 (1,311 bp) is transcribed as a monocistronic mRNA. It is not clustered together with a glnR gene, and its promoter does not have sequence similarity to σA-dependent promoters. Instead, a sequence with 92% similarity to σB-dependent promoters in B. subtilis was found 131 to 157 bp upstream of the start codon. σB-dependent promoters are found in front of genes that are involved in the general stress response, including the response to salt stress (12). In the same neighborhood, we could not detect ORFs that seem to be in functional correlation to glnA2.
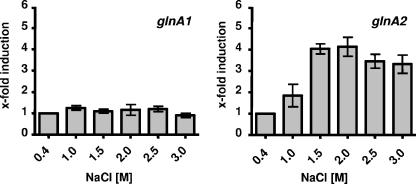
Transcription of glnA2, but not that of glnA1, is salt dependent.
To determine the effects of salinity on the expression of glnA1 and glnA2, we determined cellular mRNA levels by quantitative reverse transcription-PCR with gene-specific primers. Cells were grown in the presence of different NaCl concentrations, ranging from 0.4 to 3.0 M NaCl, and total RNA was isolated in the early exponential growth phase (OD578, ca. 0.2). The real-time PCR analyses revealed no effect of salinity on glnA1 expression. In contrast, glnA2 expression was clearly salt dependent (Fig. 6). The induction increased up to about fourfold from 0.4 M NaCl to 1.5 to 2.0 M NaCl and then decreased only slightly at higher salt concentrations. This finding strongly corroborates the finding of salt-induced glutamine synthetase activity in H. halophilus and identifies GlnA2 as being involved in osmoprotection. To prove the relevance of this finding, we compared the relative transcription levels of glnA1 and glnA2, since an increase of glnA2 transcription is only of importance when the transcription levels of glnA2 and glnA1 are in the same range. These analyses demonstrated that the levels of glnA1 and glnA2 were comparable at 0.4 and 1 M NaCl but that, at higher salt concentrations, the glnA2/glnA1 ratio increased due to increased expression of glnA2.
FIG. 6.
Expression of glnA2 but not of glnA1 is salt dependent. H. halophilus cells were cultivated at the indicated salinities. Total RNA was isolated and transcribed into cDNA, and relative transcription levels were measured using real-time PCR analysis. In the diagram, relative quantitation of transcript levels is given. All values were compared to the value at 0.4 M, which was defined as 1. The experiment was repeated in three independent parallels to ensure statistical relevance.
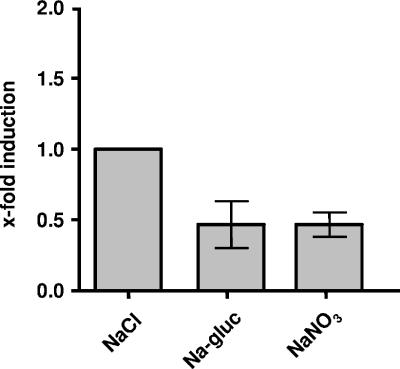
To elucidate the role of chloride in the transcription of glnA2, cells were cultivated in the presence of different anions, such as nitrate or gluconate, supplied as sodium salts. Again, these experiments could not be done with cells grown at salt concentrations above 1.0 M due to the lack of cell growth in the absence of chloride. Real-time quantitative PCR analyses with RNAs isolated from these cells revealed a decrease of about 50% of glnA2 transcripts in cells grown in the presence of NaNO3 or sodium gluconate compared to those in NaCl-grown cells (Fig. 7), demonstrating a stimulation of gene expression by chloride.
FIG. 7.
Expression of glnA2 is stimulated by chloride. H. halophilus was cultivated in glucose mineral salt medium in the presence of NaCl, sodium gluconate (Na-gluc), or NaNO3 at a final concentration of 1.0 M. Total RNA was isolated and transcribed into cDNA, and relative transcription levels were measured using real-time PCR analysis. In the diagram, relative quantitation of transcript levels is given. All values were compared to the value for NaCl, which was defined as 1. The experiment was repeated in three independent parallels to ensure statistical relevance.
DISCUSSION
Glutamine and glutamate are common compatible solutes found in members of all three domains of life (11, 21). Some halophiles and moderate halophiles accumulate glutamine and glutamate as a final response to saline environments and maintain them at rather high concentrations throughout the cell cycle. In contrast, the halotolerant organism E. coli accumulates glutamate along with potassium only transiently in a first, fast response to salt stress, but during long-term adaptation, glutamate is replaced by trehalose (8). Despite the ubiquitous distribution of glutamate and glutamine as compatible solutes, little is known about the genes and enzymes involved in biosynthesis and their regulation. Here we have demonstrated that the production of glutamate and glutamine is a salt-induced process in the moderate halophile H. halophilus. In principle, glutamate (and glutamine) can be produced by the action of glutamate dehydrogenase or the combined action of glutamine synthetase and glutamate synthase.
Two glutamate dehydrogenase genes were identified in H. halophilus. gdh-1 is expressed, but its expression is apparently not affected by the salt concentration. Upstream (120 to 135 bp) of gdh-1 is a DNA sequence with 67% identity to the nitrogen-regulated σL promoter of RocG, the glutamate dehydrogenase of B. subtilis. This would argue for a role of Gdh1 in nitrogen metabolism. Taken together, the molecular data and the enzyme assays make a role for the glutamate dehydrogenase in the production of glutamate/glutamine as compatible solutes unlikely. It should be mentioned that H. halophilus can use glutamate as a carbon and energy source. An additional function of Gdh1/Gdh2 might therefore be involved in catabolism.
In contrast to glutamate dehydrogenase activity, glutamine synthetase activity was readily detectable. Interestingly, expression of glnA2 and the specific activity of glutamine synthetase were strictly dependent on the salinity of the growth medium, indicating that this enzyme is involved in biosynthesis of the compatible solute glutamine and that its synthesis is regulated by the salinity of the medium. Maximal glutamine synthetase activities were found in cells grown at 2.5 to 3.0 M NaCl, but maximal glutamine concentrations were already observed at 1.5 M NaCl. At higher salinities, the glutamine concentration remained fairly constant. This seemingly contradictory finding may reflect a function of glutamine synthetase activity in additional biosynthetic pathways that lead to different compatible solutes at higher salinities, such as ectoine and proline.
Glutamate and glutamine not only are compatible solutes but are also involved in nitrogen metabolism and part of the nitrogen regulatory network. The regulation of gene expression and activities of the biosynthetic enzymes by nitrogen availability has been studied to a great extent in the gram-negative bacterium E. coli and the gram-positive bacterium B. subtilis. H. halophilus contains two gene clusters which both potentially encode glutamine synthetases. glnA1 and glnR form an operon, and the structure of the operon is identical to that of the nitrogen-regulated glnRA operon of B. subtilis (6). Analysis of potential promoter sequences revealed a sequence 19 bp upstream of the start codon with 100% identity to the vegetative σA-dependent promoters from B. subtilis (28) and a GlnR binding site upstream of glnA1. These sequence similarities suggest, but do not prove, an involvement of the glnRA operon of H. halophilus in nitrogen metabolism. In contrast, the salt-induced gene glnA2 is not preceded by a glnR homologue, and GlnA2 is less conserved than GlnA1. The sequence of glnA2 is preceded by a putative promoter and followed by a putative transcription terminator. Together with the finding that the up- and downstream genes are not obviously related to solute formation, it is speculated that glnA2 is not part of an operon. Conserved GlnR binding sites were not found. However, we found well-conserved sequences with similarity to σB promoters of B. subtilis that are involved in stress-activated gene expression. Together with fliC (25) and luxS (Sewald et al., unpublished data), glnA2 is the third gene whose expression is stimulated by chloride. How this is achieved is currently unknown, but one possibility would be by means of a coactivator of gene expression, such as YvyD (19).
The activity of the glutamine synthetase was strictly dependent on the presence of chloride in the assay buffer. This finding may be interpreted to reflect a direct activation of glutamine synthetase by chloride. Internal chloride concentrations in H. halophilus have been determined and shown to vary depending on the extracellular chloride concentration. At a 1.0 M extracellular chloride concentration, the internal chloride concentration was 400 to 500 mM (24). It is noteworthy that a sequence alignment revealed seven positively charged residues (arginine and lysine) only in GlnA2 from H. halophilus (see Fig. S8 in the supplemental material). These might be involved in chloride signaling. However, stimulation by chloride of an upstream component in a signal transduction chain leading to activation of glutamine synthetase cannot be excluded. Future work using purified enzymes will need to answer this question.
Glutamine synthetase GlnA2 of H. halophilus is apparently involved in the biosynthesis of glutamine as a compatible solute. This leaves us with the question of how glutamate is produced. The genome revealed two glutamate synthase genes, but expression of the two genes was not salt dependent. For B. subtilis, it is known that the transcription of gltAB depends on the positive regulator GltC (26). In B. subtilis, the corresponding gene is in close proximity to gltAB (1). This is not the case for H. halophilus. However, several putative binding sites for this regulator could be identified that show identities of up to 67% to the sequence from B. subtilis (data not shown). In addition to the GltC binding sites, a putative binding site for the positive regulator TnrA was identified between positions −71 and −55 upstream of gltAB1. Even if the GltC and TnrA regulators are not encoded in proximity to gltAB1 (ORFs encoding these regulators were found elsewhere in the genome [not shown]), the identified binding sites support the hypothesis that gltAB1 expression is not regulated by salinity but by nitrogen availability.
The amount of glutamate synthase activity was close to the detection limit. Therefore, the effect of salinity or anions on activity was not tested. However, its low activity and the apparent independence of gene expression on salinity do not rule out the involvement of glutamate synthase in the production of glutamate from glutamine. Future experiments will address a possible regulation of glutamate synthase by the growth phase and/or glutamine in H. halophilus. Differential regulation of glutamine synthetase and glutamate synthase might be the basis for the observed shift of the glutamine/glutamate ratio at higher salt concentrations. Furthermore, the shift may also reflect the involvement of glutamine as an amino group donor in the production of other solutes, such as ectoine, at high salinities.
In summary, the data presented here identified the glutamine synthetase as a component of the chloride regulon of H. halophilus. The glutamine synthetase is a key enzyme in the production of the compatible solutes glutamate and glutamine, but also in the production of ectoine and proline. An inactive glutamine synthetase would reduce or diminish the cells' ability to synthesize compatible solutes, and therefore growth of the moderate halophile would be impaired in the absence of chloride. This interpretation is in line with the observation that the cell yield is chloride dependent, indicating that an essential, chloride-induced component(s), such as a compatible solute, is diluted out during growth in the absence of chloride. The data presented here give for the first time a rationale for the chloride dependence of H. halophilus growth and are in line with the assumption that H. halophilus “senses” salinity via the chloride concentration of its environment.
Supplementary Material
Acknowledgments
This study was supported by a grant from the German-Israel Foundation for Scientific Research and Development (GIF) to V.M.
Footnotes
Present address: Institut de Biologia Molecular de Barcelona (CSIC) and Institut de Recerca Biomedica, Parc Cientific de Barcelona, Barcelona, Spain.
REFERENCES
- 1.Belitsky, B. R., L. V. Wray, Jr., S. H. Fisher, D. E. Bohannon, and A. L. Sonenshein. 2000. Role of TnrA in nitrogen source-dependent repression of Bacillus subtilis glutamate synthase gene expression. J. Bacteriol. 182:5939-5947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bender, R. A., K. A. Janssen, A. D. Resnick, M. Blumenberg, F. Foor, and B. Magasanik. 1977. Biochemical parameters of glutamine synthetase from Klebsiella aerogenes. J. Bacteriol. 129:1001-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bohannon, D. E., M. S. Rosenkrantz, and A. L. Sonenshein. 1985. Regulation of Bacillus subtilis glutamate synthase genes by the nitrogen source. J. Bacteriol. 163:957-964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonete, M. J., F. Perez-Pomares, S. Diaz, J. Ferrer, and A. Oren. 2003. Occurrence of two different glutamate dehydrogenase activities in the halophilic bacterium Salinibacter ruber. FEMS Microbiol. Lett. 226:181-186. [DOI] [PubMed] [Google Scholar]
- 5.Bradford, M. M. 1976. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 6.Brown, S. W., and A. L. Sonenshein. 1996. Autogenous regulation of the Bacillus subtilis glnRA operon. J. Bacteriol. 178:2450-2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Claus, D., F. Fahmy, H. J. Rolf, and N. Tosunoglu. 1983. Sporosarcina halophila sp. nov., an obligate, slightly halophilic bacterium from salt marsh soils. Syst. Appl. Microbiol. 4:496-506. [DOI] [PubMed] [Google Scholar]
- 8.Dinnbier, U., E. Limpinsel, R. Schmid, and E. P. Bakker. 1988. Transient accumulation of potassium glutamate and its replacement by trehalose during adaptation of growing cells in Escherichia coli K-12 to elevated sodium chloride concentrations. Arch. Microbiol. 150:348-357. [DOI] [PubMed] [Google Scholar]
- 9.Dohrmann, A. B., and V. Müller. 1999. Chloride dependence of endospore germination in Halobacillus halophilus. Arch. Microbiol. 172:264-267. [DOI] [PubMed] [Google Scholar]
- 10.Fisher, S. H. 1999. Regulation of nitrogen metabolism in Bacillus subtilis: vive la différence! Mol. Microbiol. 32:223-232. [DOI] [PubMed] [Google Scholar]
- 11.Grant, W. D. 2004. Life at low water activity. Philos. Trans. R. Soc. Lond. B 359:1249-1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hecker, M., W. Schumann, and U. Völker. 1996. Heat-shock and general stress response in Bacillus subtilis. Mol. Microbiol. 19:417-428. [DOI] [PubMed] [Google Scholar]
- 13.Hemmila, I. A., and P. I. Mantsala. 1978. Purification and properties of glutamate synthase and glutamate dehydrogenase from Bacillus megaterium. Biochem. J. 173:45-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kunte, H. J., E. A. Galinski, and H. G. Trüper. 1993. A modified FMOC-method for the detection of amino acid-type osmolytes and tetrahydropyrimidines (ectoines). J. Microbiol. Methods 17:129-136. [Google Scholar]
- 15.Livak, K. J., and T. D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−DDCt method. Methods 25:402-408. [DOI] [PubMed] [Google Scholar]
- 16.Lowry, O. H., N. J. Rosebrough, A. L. Farr, and R. J. Randall. 1951. Protein measurement with the folin-phenol reagent. J. Biol. Chem. 193:265-275. [PubMed] [Google Scholar]
- 17.Mei, B. G., and R. S. Jiao. 1988. Purification and properties of glutamate synthase from Nocardia mediterranei. J. Bacteriol. 170:1940-1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller, R. E., and E. R. Stadtman. 1972. Glutamate synthase from Escherichia coli. An iron-sulfide flavoprotein. J. Biol. Chem. 247:7407-7419. [PubMed] [Google Scholar]
- 19.Müller, V., and S. H. Saum. 2005. The chloride regulon of Halobacillus halophilus: a novel regulatory network for salt perception and signal transduction in bacteria, p. 303-310. In N. Gunde-Cimerman, A. Oren, and A. Plemenitas (ed.), Adaption to life at high salt concentrations in Archaea, Bacteria, and Eukarya, vol. 9. Springer, Dordrecht, The Netherlands. [Google Scholar]
- 20.Ratti, S., B. Curti, G. Zanetti, and E. Galli. 1985. Purification and characterization of glutamate synthase from Azospirillum brasilense. J. Bacteriol. 163:724-729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roberts, M. F. 2005. Organic compatible solutes of halotolerant and halophilic microorganisms. Saline Systems 1:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roessler, M., and V. Müller. 2001. Chloride dependence of glycine betaine transport in Halobacillus halophilus. FEBS Lett. 489:125-128. [DOI] [PubMed] [Google Scholar]
- 23.Roessler, M., and V. Müller. 2002. Chloride, a new environmental signal molecule involved in gene regulation in a moderately halophilic bacterium, Halobacillus halophilus. J. Bacteriol. 184:6207-6215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roessler, M., and V. Müller. 1998. Quantitative and physiological analyses of chloride dependence of growth of Halobacillus halophilus. Appl. Environ. Microbiol. 64:3813-3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roessler, M., G. Wanner, and V. Müller. 2000. Motility and flagellum synthesis in Halobacillus halophilus are chloride dependent. J. Bacteriol. 182:532-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schell, M. A. 1993. Molecular biology of the LysR family of transcriptional regulators. Annu. Rev. Microbiol. 47:597-626. [DOI] [PubMed] [Google Scholar]
- 27.Schreier, H. J., and R. W. Bernlohr. 1984. Purification and properties of glutamate synthase from Bacillus licheniformis. J. Bacteriol. 160:591-599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schreier, H. J., C. A. Rostkowski, J. F. Nomellini, and K. D. Hirschi. 1991. Identification of DNA sequences involved in regulating Bacillus subtilis glnRA expression by the nitrogen source. J. Mol. Biol. 220:241-253. [DOI] [PubMed] [Google Scholar]
- 29.Severin, J. 1993. Kompatible Solute und Wachstumskinetik bei halophilen aeroben heterotrophen Eubakterien. Ph.D. thesis. University of Bonn, Bonn, Germany.
- 30.Woolfolk, C. A., B. Shapiro, and E. R. Stadtman. 1966. Regulation of glutamine synthetase. I. Purification and properties of glutamine synthetase from Escherichia coli. Arch. Biochem. Biophys. 116:177-192. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.