Abstract
OBJECTIVE
In periodontitis, matrix metalloproteinase 3 (MMP-3, stromelysin 1) is present at increased levels in active disease sites compared to inactive or healthy sites, and the levels are correlated with clinical parameters and associated with progression of the disease. Interleukin 4 (IL-4) has been shown in other systems to suppress interleukin-1 (IL-1) induced expression of MMP-3, but this has not been shown in human gingival fibroblasts. The objective of this study is to determine the effects of IL-4 on the IL-1 induced expression of MMP-3 in human gingival fibroblasts isolated from patients with periodontitis.
METHODS
Northern blot analysis was performed to determine the effects of IL-4 on the IL-1 induction of MMP-3 mRNA. MMP-3 protein levels were determined by ELISA, and prostaglandin E2 (PGE2) levels were measured by enzyme immunoassay (EIA). DNA binding of AP-1 and NF-κB was assessed by electrophoretic mobility shift assay (EMSA).
RESULTS
Northern blot analysis revealed that co-incubation of gingival fibroblasts with IL-1 and IL-4 resulted in a significant decrease in MMP-3 mRNA levels compared to IL-1 alone, with a concomitant decrease in protein levels. This inhibition is dose dependent, and is apparent as early as 3 hours after stimulation. IL-1-induced production of PGE2 was not affected in 4 of 6 cultures isolated from different individuals. Addition of exogenous PGE2 had no effect on the suppressive effects of IL-4. DNA binding of transcription factors AP-1 and NF-κB was not affected by IL-4.
CONCLUSION
IL-4 inhibits the IL-1 induction of MMP-3 in human gingival fibroblasts isolated from patients with periodontitis. This effect is independent of PGE2 and is not due to inhibition of the DNA binding activity of known transcription factors binding to the MMP-3 promoter.
Keywords: Periodontitis, Stromelysin-1, Gene expression regulation, Interleukin-4, Interleukin-1
INTRODUCTION
Periodontitis is the most common cause of adult tooth loss in the U.S. 1, with an estimated 1 in 3 adults suffering from some form 2. In addition to its direct impact, periodontitis may also contribute to the development of several other diseases, including cardiovascular disease, pre-term low birth weight and diabetes 3,4. Bacteria are essential for initiation of periodontitis, but host factors are largely responsible for the development of a chronic inflammatory state leading to destruction of periodontal support structures 5.
Chronic inflammation in periodontitis is characterized by increased levels of IL-1β, TNFα and prostaglandin E2 (PGE2) 6–8. Interestingly, however, there seems to be a local T cell imbalance, with a relative absence of IL-4 producing T cells at sites of periodontal inflammation 9–11. This imbalance appears to be progressive, with decreasing levels of IL-4 correlated with loss of collagen and with increasing clinical severity 12. In addition, polymorphisms in the IL-4 promoter and intron that are associated with decreased serum levels of IL-4 are also associated with increased susceptibility to early onset periodontitis 13. It has been suggested that correcting this cytokine imbalance in chronic inflammatory conditions might be therapeutic. In fact, adenoviral transfer of IL-4 has been shown to be protective against cartilage degradation induced by injection of rheumatoid arthritis synovial tissue into joints of SCID mice 14 and against collagen-induced arthritis 15. However, a better understanding of the mechanisms of IL-4’s beneficial effects might make possible safer and more economical therapies.
MMP-3 (Stromelysin-1) is a metalloproteinase with broad substrate specificity, degrading proteoglycan, laminin, fibronectin, and the non-fibrillar collagens 16. Perhaps equally important, it is also capable of activating other pro-MMPs, including MMP-1, -8, -9 and –13 17–21, of inactivating plasminogen activator inhibitor I 22 and of cleaving FasL to produce sFasL 23. MMP-3 is produced by gingival and synovial fibroblasts, chondrocytes, macrophages, neutrophils, and endothelial cells in response to inflammatory cytokines and mitogens. In periodontitis, MMP-3 is present at increased levels in active disease sites compared to inactive or healthy sites 24–29, and the levels are correlated with clinical parameters and associated with progression of the disease 28.
IL-4 has been shown to inhibit the IL-1 induction of MMP-3 expression in human skin fibroblasts 30 and articular chondrocytes 31,32, as well as in human synovial fibroblasts 33. However, effects of IL-4 on MMP-3 expression have not been demonstrated in cells relevant to periodontitis. Here, we show that IL-4 also inhibits the IL-1-induced expression of MMP-3 mRNA and protein in human gingival fibroblasts (HGF) isolated from patients with periodontitis. This effect appears to be independent of any effects on production of PGE2 or DNA binding of transcription factors known to regulate MMP-3 expression.
MATERIALS AND METHODS
Cell culture
Human gingival tissue from patients undergoing periodontal surgery was obtained from Howard M. Sobel, D.D.S. and Kevan S. Green, D.M.D. of Sobel Periodontal Associates, P.C. The tissue was processed by enzymatic dispersion to produce primary cultures 34,35. Cells were maintained in Eagle’s Minimal Essential Medium (EMEM) supplemented with 10% fetal bovine serum and antibiotic/antimycotic (penicillin, streptomycin, amphotericin; Gibco BRL, Grand Island, NY). Cells between passages 3 and 5 were used for experiments. Cells were serum-deprived for 16 hours in serum-free EMEM supplemented with 10% ITS (insulin, transferrin, sodium selenite; Sigma, St. Louis, MO) prior to the addition of 100 ng IL-1β/ml (a gift of R. Newton, Wilmington, DE) in the presence or absence of various doses of IL-4 (Gibco BRL, Grand Island, NY).
RNA Isolation and Northern Blotting
Total RNA was isolated according to the acid-phenol method of Chomczynski and Sacchi 36 at various times after treatment, and run on 1% agarose-formaldehyde gels. Probes were made by random priming (Stratagene, La Jolla, CA) of cDNA fragments corresponding to rat stromelysin-1 (MMP-3, American Type Culture Collection, Rockville, MD) and glyceraldehyde 3 phosphate dehydrogenase (GAPDH, a gift of R. Newton, Wilmington, DE). Northern blots were quantitated by densitometric scanning and normalized to GAPDH.
Quantitation of MMP-3 Protein and Prostaglandin E2
MMP-3 protein levels were quantitated by ELISA (Amersham, Arlington Heights, IL) in conditioned media of human gingival fibroblasts (HGF) that were untreated, treated with 100 ng IL-1/ml alone or treated with both 100 ng IL-1/ml and 10 ng IL-4/ml for 24 hours. Levels of PGE2 were measured by EIA (Amersham) in 6 hour conditioned media.
Nuclear Extract Isolation and EMSA
Nuclear extracts were isolated according to the method of Schreiber et al. 37 and quantitated in mini-Bradford assays (Pierce, Rockford, IL). Synthetic oligonucleotides corresponding to the consensus binding sites for NF-κB and AP-1 (Santa Cruz, Biotechnology, Inc., Santa Cruz, CA) were labeled by T4 polynucleotide kinase in the presence of γ32P-ATP. Binding reactions contained 5 μg protein, 20 mM Hepes-OH pH 7, 50 mM NaCl, 0.2 M EDTA, 5% glycerol, 4 μg dIdC and 10,000 cpm probe.
RESULTS
IL-4 inhibits the IL-1 induction of MMP-3 mRNA and protein
In order to determine the effect of IL-4 on the IL-1 induction of MMP-3 mRNA expression in human gingival fibroblasts (HGF), total RNA was isolated at various times after addition of either IL-1 alone or IL-1 simultaneously with IL-4. Northern blot analysis revealed a significant reduction in the IL-1 induced expression of MMP-3 mRNA, approximately 70% at 6 hours (Figure 1). This inhibition was evident as early as 3 hours after stimulation.
Figure 1.
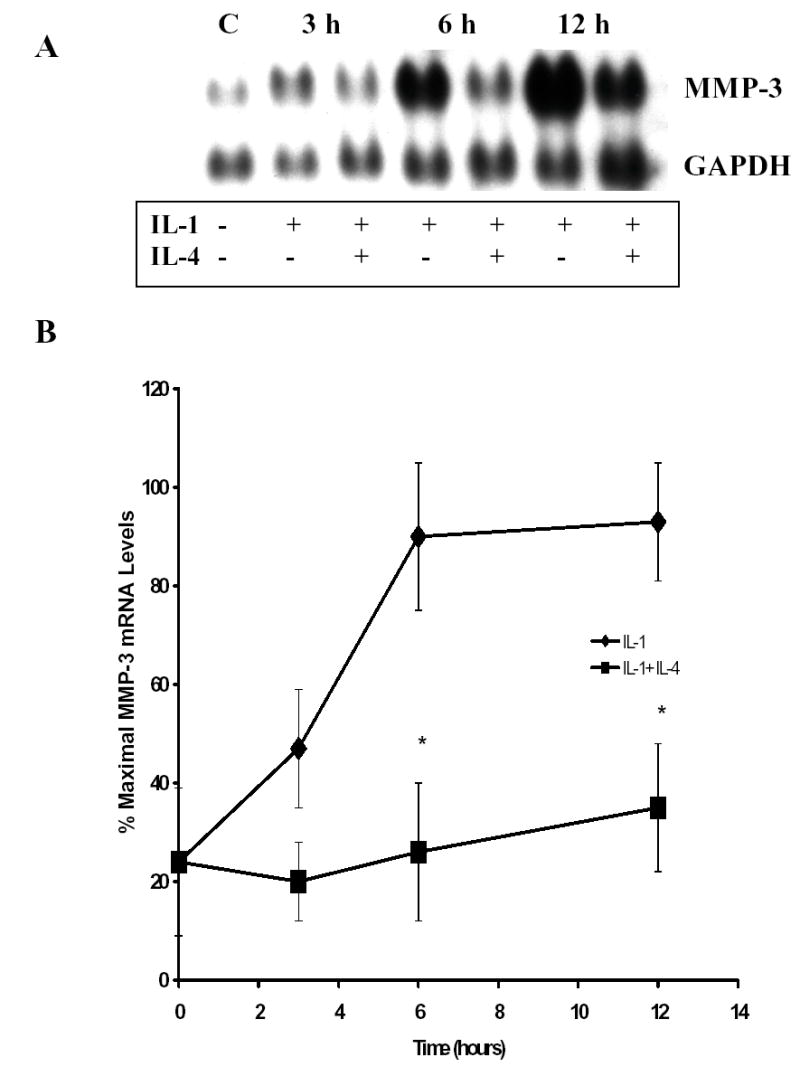
Inerleukin-4 (IL-4) inhibits IL-1 induction of MMP-3 mRNA in human gingival fibroblasts (HGF). Total RNA was isolated from untreated HGF and cells treated for the indicated times with IL-1β alone (100 ng/ml) or in combination with 10 ng/ml IL-4. A. Northern blots were hybridized to cDNA probes corresponding to MMP-3 and GAPDH. B. Blots were quantitated by scanning densitometry and normalized to levels of GAPDH. Shown are data from three independent experiments, each of which utilized RNA isolated from three pooled HGF cultures established from three different donors. (* p< 0.05 vs. IL-1 alone).
Incremental doses of IL-4 were added simultaneously with a constant dose of IL-1 for dose curve analysis. Total RNA was isolated 6 hours after stimulation, and Northern blot analysis was performed to determine the effects of each dose on the IL-1 induction of MMP-3. Figure 2 shows that the suppressive action of IL-4 is dose dependent, with some inhibition seen with 0.1 ng IL-4/ml, and maximal inhibition was seen with 50 ng IL-4/ml. The IC50, as determined by regression analysis of this data, was approximately 0.3 ng/ml. These results are very similar to those seen in synovial fibroblasts 33.
Figure 2.
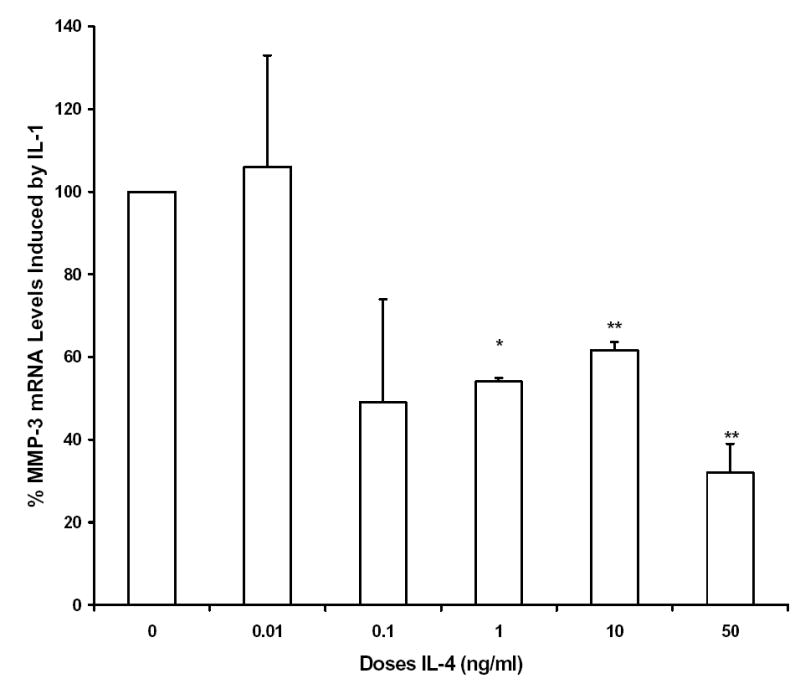
Interleukin-4 (IL-4) inhibition of IL-1-induced expression of MMP-3 mRNA is dose dependent. Total RNA was isolated from HGF cultures 6 hours after addition of 100 ng/ml IL-1β alone or together with the indicated doses of IL-4. Shown are data from three independent experiments, each of which utilized RNA isolated from three pooled HGF cultures established from three different donors. (* p < 0.05; ** p<0.01 vs. IL-1 alone)
Levels of MMP-3 protein were measured in conditioned media from 6 different HGF cultures derived from different donors, which were treated with IL-1 alone or co-incubated with 10 ng/ml IL-4 for 24 hours. There was variation among the 6 cultures in both basal and IL-1 induced levels of MMP-3. The extent of IL-4 suppression of the IL-1 induction of MMP-3 protein varied as well, from essentially no inhibition to over 90% (Figure 3). Taken together, however, there was an average inhibition of ~ 54% (p<0.01), and this rises to 65% (p < 0.01) if culture #2 is excluded as an outlier.
Figure 3.
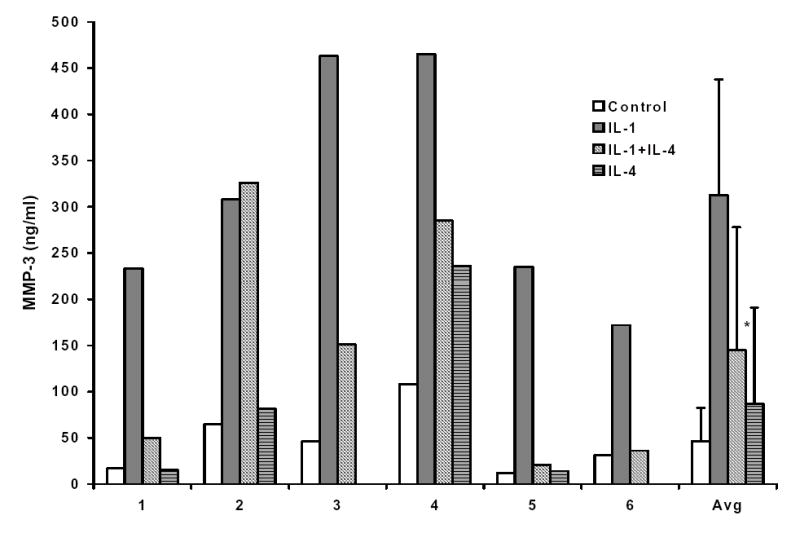
Interleukin-4 (IL-4) inhibits the IL-1 induction of MMP-3 protein. Conditioned medium was harvested from HGF cultures incubated for 24 hours with 100 ng/ml IL-1β alone or in the presence of 10 ng/ml IL-4. Levels of MMP-3 protein were measured in triplicate by enzyme-linked immunosorbent assay. HGF cultures derived from six different individuals (numbered 1 through 6 on the graph) were used. (* p< 0.05 vs. IL-1 alone).
IL-4 inhibition of IL-1 induced MMP-3 production is not always associated with decreased production of prostaglandin E2
IL-4 has been reported to inhibit IL-1 induced production of PGE2 in several model systems 33,38–41, including human gingival fibroblasts and periodontal ligament fibroblasts isolated from individuals with healthy periodontia 42, and in some cases the inhibition of PGE2 production has been linked to IL-4’s suppressive effects on gene expression. However, there are conflicting reports on the role of PGE2 in IL-1 induced production of MMP-3 43–50. In order to determine whether IL-4 inhibition of MMP-3 production is associated with changes in PGE2 levels in HGF, levels of PGE2 were measured in 6 hour conditioned media from 6 different HGF cultures (derived from 6 different donors), stimulated with IL-1 in the presence or absence of IL-4. Interestingly, IL-4 inhibited the IL-1 induced production of PGE2 in only 2 of the 6 HGF cultures (Figure 4), and on average, there was no effect. Furthermore, addition of exogenous PGE2 along with IL-1 and IL-4 had no effect on the ability of IL-4 to inhibit the IL-1 induction of MMP-3 (Figure 5).
Figure 4.
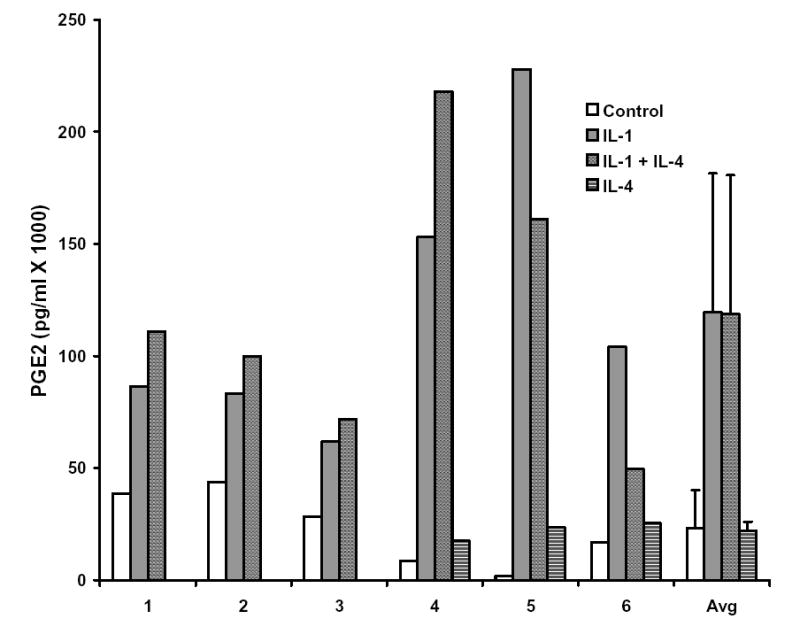
Interleukin-4 (IL-4) does not inhibit IL-1 induced production of prostaglandin E2 in human gingival fibroblasts isolated from patients with periodontitis. Levels of PGE2 were measured in triplicate in conditioned medium from HGF cultures treated for 6 hours with 100 ng/ml IL-1 alone or IL-1 and 10 ng/ml IL-4. HGF cultures isolated from 6 different individuals (numbered 1 through 6 on the graph) were used.
Figure 5.
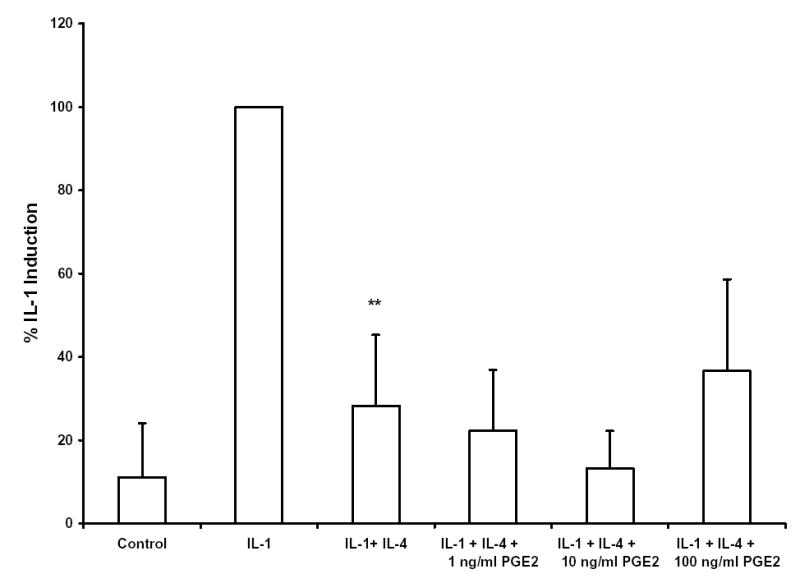
Interleukin-4 (IL-4) inhibition of IL-1-induced expression of MMP-3 mRNA is independent of PGE2. Indicated amounts of PGE2 were added to cultures of HGF simultaneously with 100 ng/ml IL-1β and 10 ng/ml IL-4. Total RNA was isolated after 6 hours, and Northern blots were hybridized with cDNA probes corresponding to MMP-3 and GAPDH. Blots were quantitated by scanning densitometry and normalized to levels of GAPDH. Shown are data from seven independent experiments, utilizing HGF cultures derived from seven different donors. (** p<0.01 vs. IL-1 alone)
Binding of transcription factors AP-1 and NF-κB is not affected by IL-4
Transcription factor AP-1 is an important factor in the regulation of the MMP-3 gene 51–54 and there is evidence that NF-κB may also play a role 55–57. Since IL-4 has been shown in other systems to inhibit gene expression by interfering with AP-1 58,59 and NF-κB activation 60–62, the effects of IL-4 on AP-1 and NF-κB DNA binding were investigated. Nuclear extracts were isolated from HGF cultures one hour after addition of IL-1 alone, IL-4 alone, or IL-1 in the presence of IL-4. As shown in Figure 6, IL-4 had no effect on AP-1 or NF-κB DNA binding activity. In addition, binding to other promoter elements, the polyoma virus enhancer 3 (PEA-3) site 63 and the stromelysin IL-1 responsive element (SIRE) 64, was also unaffected by IL-4 (data not shown).
Figure 6.
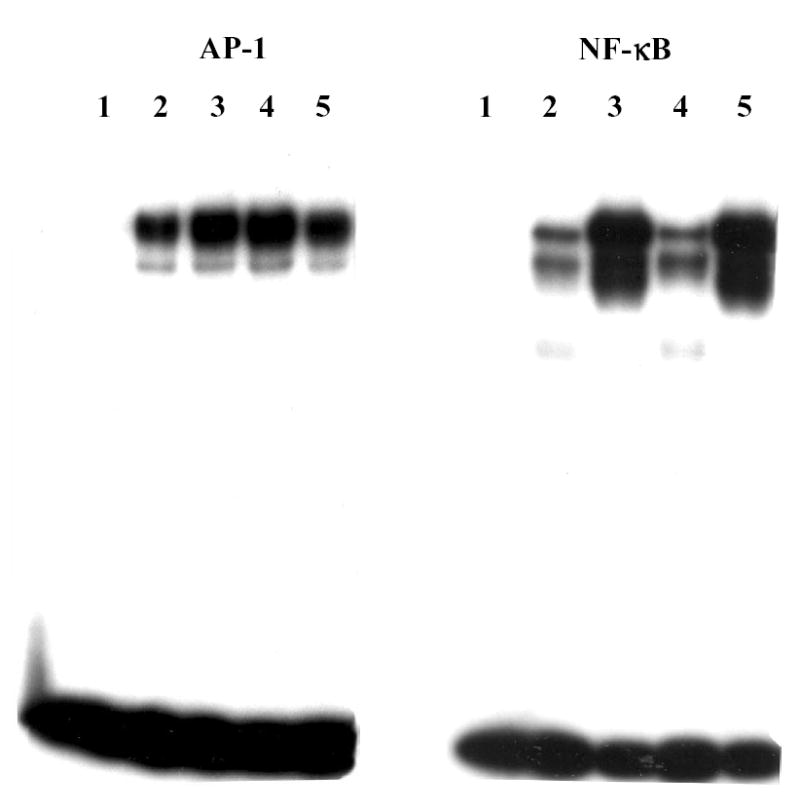
DNA binding of transcription factors activator protein-1 (AP-1) and nuclear factor-κB (NF-κB) is not affected by IL-4. Nuclear extracts were isolated from HGF cultures treated for one hour with 100 ng/ml IL-1β (lane 3), 10 ng/ml IL-4 (lane 4) or both IL-1 and IL-4 (lane 5), as well as from control cultures (lane 2). Lane 1, probe alone, no nuclear extract. Binding of 5 μg nuclear extract to 32P-labeled oligo(dT) probes corresponding to consensus AP-1 and NF-κB binding sites were determined by electrophoretic mobility shift assay.
DISCUSSION
Chronic inflammatory conditions such as periodontitis and rheumatoid arthritis result in tissue destruction due in large part to local over-expression of inflammatory mediators, including MMPs and prostaglandins. Here we show that the anti-inflammatory cytokine IL-4 has a dose-dependent inhibitory effect on the IL-1 induction of MMP-3. Although similar results have been reported in human skin and synovial fibroblasts 30,33,65 and articular chondrocytes 31,32, this to our knowledge is the first report of such findings in cells relevant to periodontitis.
Although the present data do not address the issue of whether or not the inhibition takes place at the transcriptional level, they are consistent with that conclusion. MMP-3 expression is regulated primarily at the transcriptional level, and previous results showed that IL-4 inhibited IL-1 induced transcription from the MMP-3 promoter in transiently transfected human foreskin fibroblasts 33. However, IL-4 has also been shown to suppress gene expression by decreasing mRNA stability 66, and that possibility cannot be excluded.
Several previous reports showing IL-4 inhibition of gene expression have focused on inhibition of prostaglandin synthesis, presumably leading to decreased production of cAMP 40,41,67,68. However, Sugiyama et al. 41 found that IL-4 inhibition of IL-1α induced cyclooxygenase II mRNA and PGE2 production was cell-type specific, occurring in PMA-differentiated U937 cells and freshly prepared adherent synoviocytes, but not in rheumatoid synovial fibroblasts. In addition, there are several conflicting reports concerning the role of prostaglandins in the IL-1 induction of MMP-3. Inhibition of prostaglandin synthesis by indomethacin or other inhibitors of cyclooxygenase has been shown to both augment 50 and inhibit 47,69 MMP-3 expression, as has exogenous addition of PGE2 46,48–50 and alterations in levels of cAMP 48–50.
Hayashi et al. 42 found that IL-4 inhibits IL-1 induced production of PGE2 in three different types of normal fibroblasts, including periodontal ligament and gingival fibroblasts. Our results, in contrast, show failure of IL-4 to inhibit PGE2 production by 4 of 6 gingival fibroblast cultures (Figure 4). There is currently no clear explanation of these results. However, it must be reiterated that our HGF cultures were derived from patients with periodontitis, whereas those of Hayashi et al. were derived from tissue from healthy periodontia. Although simple variation among individuals cannot be excluded based on these small sample sizes, it is possible that cells isolated from chronically inflamed tissue have been altered in a way that interferes with normal responses to IL-4. One example of cells from diseased tissue exhibiting altered responses comes from the work of Millward-Sadler et al. 70. They found that mechanical stimulation of normal chondrocytes results in decreased expression of MMP-3 via an integrin-mediated, IL-4 dependent mechanism, but this was not the case in chondrocytes isolated from donors with osteoarthritis. Further studies suggest that IL-4 signaling in OA chondrocytes is preferentially through the type I (IL4α/cγ) receptor rather than via the type II (IL4α/IL13R) receptor 71. The make-up of the IL-4 receptor on gingival fibroblasts, and whether or not it is altered in periodontitis is not known. It is clear however, that at least some aspects of IL-4 signaling are still intact, since IL-4 is able to inhibit MMP-3 expression in most, if not all, cultures.
It is also possible that some of the individual variation observed might be due to characteristics of the tissue donors. Our tissue samples were supplied without any information about the donors. However, both smoking and diabetes are strong risk factors for periodontitis 72, and could conceivably have effects on gingival cell properties and their response to cytokines. For example, exposure to volatile components of cigarette smoke alters the cytoskeleton and reduces cell adhesions of HGF 73, and nicotine interferes with the normal localization of β1 integrin to the plasma membrane 74. Both smoking and diabetes can increase oxidant stress 75,76, which can activate transcription factors such as AP-1 and NF-κB 60–62, and treatment of human skin fibroblasts with tobacco smoke extract increased MMP-3 mRNA expression 77.
Our results further show that addition of exogenous PGE2 has no effect on IL-4’s ability to suppress the IL-1 induction of MMP-3. These results are consistent with our earlier results with human synovial fibroblasts 33 and those of Prontera et al. 30, who showed that the IL-4 inhibition of IL-1 induced MMP-3 expression in human skin fibroblasts is independent of protein kinase A or cAMP levels. Taken together, these data suggest that even when IL-4 can inhibit production of PGE2, this is not causally related to its ability to inhibit expression of MMP-3.
The mechanisms involved in IL-4 suppression of MMP-3 expression are not known. Transcription factor AP-1 plays an important role in regulating transcription from the MMP-3 promoter in response to a variety of cytokines and mitogens, including IL-1 51–54, as do members of the Ets family of transcription factors 63. There is also some evidence that NF-κB may play a role 55–57. IL-4 has been shown to affect gene expression by interfering with the DNA binding activity of AP-1 58,59 and NF-κB 60–62 in other systems; however our results suggest that this is not the case in HGF. Interestingly, AP-1 binding activity seemed to be constitutively activated in these HGF. Similar results have been found in rheumatoid arthritis synovial fibroblasts 78,79. IL-4 had no effect on AP-1 binding, either basal or in the presence of IL-1. NF-κB had a lower basal level of DNA binding, but IL-4 had no effect on basal or IL-1 induced binding. IL-1 induced binding of nuclear proteins to PEA3/Ets and stromelysin IL-1 responsive element (SIRE) binding sites was also not affected by IL-4 (data not shown).
The effects of IL-4 on gene expression are generally mediated through activation of STAT6, a member of the “signal transducers and activators of transcription” family 80. STAT6 has been shown to exist in human synovial fibroblasts, and to be capable of activation by IL-4 81, however it has not been studied in HGF. STAT6 induces transcription through interactions with the co-activator p300/CBP 82. AP-1, NF-κB and Ets 1 and 2, as well as other transcription factors, also require p300/CBP, and their activity can be inhibited by factors such as STATs that compete for the co-activator 83–89. Thus, IL-4 could be inhibiting transactivation of MMP-3 via a transcription factor without necessarily affecting its DNA binding activity. It is also possible that the inhibitory effects of IL-4 are mediated through other transcription factors or elements that have not yet been identified or characterized.
In summary, we have presented evidence that co-incubation of HGF with IL-4 and IL-1 results in dose-dependent reduction in the IL-1 induced production of MMP-3. Surprisingly, this inhibition was not consistently associated with decreased production of PGE2, and did not involve inhibition of DNA binding activity of transcription factors known to be involved in regulation of MMP-3 regulation. Further study is needed to address the mechanism of this inhibition and to address issues of individual variation in the cell cultures.
Acknowledgments
The authors would like to thank Howard M. Sobel, D.D.S. and Kevan S. Greene, D.M.D. for providing gingival tissue samples, and Bill Laidlaw and Willie Mae Johnson for their tissue culture expertise.
Footnotes
This work was supported by grant R29DE12096 from the NIH/NIDCR to RCB.
References
- 1.Williams RC. Periodontal disease. N Engl J Med. 1990;322:373–382. doi: 10.1056/NEJM199002083220606. [DOI] [PubMed] [Google Scholar]
- 2.Brown LJ, Brunelle JA, Kingman A. Periodontal status in the United States, 1988–1991: prevalence, extent, and demographic variation. J Dent Res. 1996;75 doi: 10.1177/002203459607502S07. Spec No:672–683. [DOI] [PubMed] [Google Scholar]
- 3.Li X, Kolltveit KM, Tronstad L, Olsen I. Systemic diseases caused by oral infection. Clin Microbiol Rev. 2000;13:547–558. doi: 10.1128/cmr.13.4.547-558.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fowler EB, Breault LG, Cuenin MF. Periodontal disease and its association with systemic disease. Mil Med. 2001;166:85–89. [PubMed] [Google Scholar]
- 5.Position paper: epidemiology of periodontal diseases. American Academy of Periodontology. J Periodontol. 1996;67:935–945. [PubMed] [Google Scholar]
- 6.Stashenko P, Fujiyoshi P, Obernesser MS, Prostak L, Haffajee AD, Socransky SS. Levels of interleukin 1 beta in tissue from sites of active periodontal disease. J Clin Periodontol. 1991;18:548–554. doi: 10.1111/j.1600-051x.1991.tb00088.x. [DOI] [PubMed] [Google Scholar]
- 7.Stashenko P, Jandinski JJ, Fujiyoshi P, Rynar J, Socransky SS. Tissue levels of bone resorptive cytokines in periodontal disease. J Periodontol. 1991;62:504–509. doi: 10.1902/jop.1991.62.8.504. [DOI] [PubMed] [Google Scholar]
- 8.Honig J, Rordorf-Adam C, Siegmund C, Wiedemann W, Erard F. Increased interleukin-1 beta (IL-1 beta) concentration in gingival tissue from periodontitis patients. J Periodontal Res. 1989;24:362–367. doi: 10.1111/j.1600-0765.1989.tb00883.x. [DOI] [PubMed] [Google Scholar]
- 9.Yamamoto M, Fujihashi K, Hiroi T, McGhee JR, Van Dyke TE, Kiyono H. Molecular and cellular mechanisms for periodontal diseases: role of Th1 and Th2 type cytokines in induction of mucosal inflammation. J Periodontal Res. 1997;32:115–119. doi: 10.1111/j.1600-0765.1997.tb01391.x. [DOI] [PubMed] [Google Scholar]
- 10.Ukai T, Mori Y, Onoyama M, Hara Y. Immunohistological study of interferon-gamma- and interleukin-4-bearing cells in human periodontitis gingiva. Arch Oral Biol. 2001;46:901–908. doi: 10.1016/s0003-9969(01)00057-7. [DOI] [PubMed] [Google Scholar]
- 11.Shapira L, van Dyke TE, Hart TC. A localized absence of interleukin-4 triggers periodontal disease activity: a novel hypothesis. Med Hypotheses. 1992;39:319–322. doi: 10.1016/0306-9877(92)90056-i. [DOI] [PubMed] [Google Scholar]
- 12.Ejeil AL, Gaultier F, Igondjo-Tchen S, Senni K, Pellat B, Godeau G, Gogly B. Are cytokines linked to collagen breakdown during periodontal disease progression? J Periodontol. 2003;74:196–201. doi: 10.1902/jop.2003.74.2.196. [DOI] [PubMed] [Google Scholar]
- 13.Michel J, Gonzales JR, Wunderlich D, Diete A, Herrmann JM, Meyle J. Interleukin-4 polymorphisms in early onset periodontitis. J Clin Periodontol. 2001;28:483–488. doi: 10.1034/j.1600-051x.2001.028005483.x. [DOI] [PubMed] [Google Scholar]
- 14.Jorgensen C, Apparailly F, Couret I, Canovas F, Jacquet C, Sany J. Interleukin-4 and interleukin-10 are chondroprotective and decrease mononuclear cell recruitment in human rheumatoid synovium in vivo. Immunology. 1998;93:518–523. doi: 10.1046/j.1365-2567.1998.00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lubberts E, Joosten LA, van Den Bersselaar L, Helsen MM, Bakker AC, van Meurs JB, Graham FL, Richards CD, van Den Berg WB. Adenoviral vector-mediated overexpression of IL-4 in the knee joint of mice with collagen-induced arthritis prevents cartilage destruction. J Immunol. 1999;163:4546–4556. [PubMed] [Google Scholar]
- 16.Parsons SL, Watson SA, Brown PD, Collins HM, Steele RJ. Matrix metalloproteinases. Br J Surg. 1997;84:160–166. [PubMed] [Google Scholar]
- 17.Knauper V, Wilhelm SM, Seperack PK, DeClerck YA, Langley KE, Osthues A, Tschesche H. Direct activation of human neutrophil procollagenase by recombinant stromelysin. Biochem J. 1993;295:581–586. doi: 10.1042/bj2950581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knauper V, Lopez-Otin C, Smith B, Knight G, Murphy G. Biochemical characterization of human collagenase-3. J Biol Chem. 1996;271:1544–1550. doi: 10.1074/jbc.271.3.1544. [DOI] [PubMed] [Google Scholar]
- 19.Murphy G, Cockett MI, Stephens PE, Smith BJ, Docherty AJ. Stromelysin is an activator of procollagenase. A study with natural and recombinant enzymes. Biochem J. 1987;248:265–268. doi: 10.1042/bj2480265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ogata Y, Enghild JJ, Nagase H. Matrix metalloproteinase 3 (stromelysin) activates the precursor for the human matrix metalloproteinase 9. J Biol Chem. 1992;267:3581–3584. [PubMed] [Google Scholar]
- 21.Suzuki K, Enghild JJ, Morodomi T, Salvesen G, Nagase H. Mechanisms of activation of tissue procollagenase by matrix metalloproteinase 3 (stromelysin) Biochemistry. 1990;29:10261–10270. doi: 10.1021/bi00496a016. [DOI] [PubMed] [Google Scholar]
- 22.Lijnen HR, Arza B, Van Hoef B, Collen D, Declerck PJ. Inactivation of plasminogen activator inhibitor-1 by specific proteolysis with stromelysin-1 (MMP-3) J Biol Chem. 2000;275:37645–37650. doi: 10.1074/jbc.M006475200. [DOI] [PubMed] [Google Scholar]
- 23.Matsuno H, Yudoh K, Watanabe Y, Nakazawa F, Aono H, Kimura T. Stromelysin-1 (MMP-3) in synovial fluid of patients with rheumatoid arthritis has potential to cleave membrane bound Fas ligand. J Rheumatol. 2001;28:22–28. [PubMed] [Google Scholar]
- 24.Aiba T, Akeno N, Kawane T, Okamoto H, Horiuchi N. Matrix metalloproteinases-1 and -8 and TIMP-1 mRNA levels in normal and diseased human gingivae. Eur J Oral Sci. 1996;104:562–569. doi: 10.1111/j.1600-0722.1996.tb00142.x. [DOI] [PubMed] [Google Scholar]
- 25.Haerian A, Adonogianaki E, Mooney J, Docherty JP, Kinane DF. Gingival crevicular stromelysin, collagenase and tissue inhibitor of metalloproteinases levels in healthy and diseased sites. J Clin Periodontol. 1995;22:505–509. doi: 10.1111/j.1600-051x.1995.tb00797.x. [DOI] [PubMed] [Google Scholar]
- 26.Ingman T, Sorsa T, Michaelis J, Konttinen YT. Immunohistochemical study of neutrophil- and fibroblast-type collagenases and stromelysin-1 in adult periodontitis. Scand J Dent Res. 1994;102:342–349. doi: 10.1111/j.1600-0722.1994.tb01481.x. [DOI] [PubMed] [Google Scholar]
- 27.Kubota T, Nomura T, Takahashi T, Hara K. Expression of mRNA for matrix metalloproteinases and tissue inhibitors of metalloproteinases in periodontitis-affected human gingival tissue. Arch Oral Biol. 1996;41:253–262. doi: 10.1016/0003-9969(95)00126-3. [DOI] [PubMed] [Google Scholar]
- 28.Alpagot T, Bell C, Lundergan W, Chambers DW, Rudin R. Longitudinal evaluation of GCF MMP-3 and TIMP-1 levels as prognostic factors for progression of periodontitis. J Clin Periodontol. 2001;28:353–359. doi: 10.1034/j.1600-051x.2001.028004353.x. [DOI] [PubMed] [Google Scholar]
- 29.Reynolds JJ, Hembry RM, Meikle MC. Connective tissue degradation in health and periodontal disease and the roles of matrix metalloproteinases and their natural inhibitors. Adv Dent Res. 1994;8:312–319. doi: 10.1177/08959374940080022701. [DOI] [PubMed] [Google Scholar]
- 30.Prontera C, Crescenzi G, Rotilio D. Inhibition by Interleukin-4 of stromelysin expression in human skin fibroblasts: role of PKC. Exp Cell Res. 1996;224:183–188. doi: 10.1006/excr.1996.0126. [DOI] [PubMed] [Google Scholar]
- 31.Shingu M, Miyauchi S, Nagai Y, Yasutake C, Horie K. The role of IL-4 and IL-6 in IL-1-dependent cartilage matrix degradation. Br J Rheumatol. 1995;34:101–106. doi: 10.1093/rheumatology/34.2.101. [DOI] [PubMed] [Google Scholar]
- 32.Nemoto O, Yamada H, Kikuchi T, Shinmei M, Obata K, Sato H, Seiki M, Shimmei M. Suppression of matrix metalloproteinase-3 synthesis by interleukin-4 in human articular chondrocytes. J Rheumatol. 1997;24:1774–1779. [PubMed] [Google Scholar]
- 33.Borghaei RC, Rawlings PL, Jr, Mochan E. Interleukin-4 suppression of interleukin-1-induced transcription of collagenase (MMP-1) and stromelysin 1 (MMP-3) in human synovial fibroblasts. Arthritis Rheum. 1998;41:1398–1406. doi: 10.1002/1529-0131(199808)41:8<1398::AID-ART8>3.0.CO;2-B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mochan E, Uhl J, Newton R. IL-1 stimulation of synovial plasminogen activator production. J Rheum. 1986;13:15–19. [PubMed] [Google Scholar]
- 35.Mochan E, Uhl J, Newton R. Evidence that interleukin 1 induction of synovial cell plasminogen activator is mediated via prostaglandin E2 and cyclic AMP. Arthritis Rheum. 1986;29:1078–1084. doi: 10.1002/art.1780290904. [DOI] [PubMed] [Google Scholar]
- 36.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid-guanidinium-thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 37.Schreiber E, Matthais P, Muller MM, Schaffner W. Rapid detection of octamer binding protein with ‘mini-extracts’ prepared from a small number of cells. Nuc Acids Res. 1989;17:6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dechanet J, Rissoan MC, Banchereau J, Miossec P. Interleukin 4, but not interleukin 10, regulates the production of inflammation mediators by rheumatoid synoviocytes. Cytokine. 1995;7:176–183. doi: 10.1006/cyto.1995.1024. [DOI] [PubMed] [Google Scholar]
- 39.Endo T, Ogushi F, Sone S, Ogura T, Taketani Y, Hayashi Y, Ueda N, Yamamoto S. Induction of cyclooxygenase-2 is responsible for interleukin-1 beta-dependent prostaglandin E2 synthesis by human lung fibroblasts. Am J Respir Cell Mol Biol. 1995;12:358–365. doi: 10.1165/ajrcmb.12.3.7873203. [DOI] [PubMed] [Google Scholar]
- 40.Seitz M, Loetscher P, Dewald B, Towbin H, Ceska M, Baggiolini M. Production of interleukin-1 receptor antagonist, inflammatory chemotactic proteins, and prostaglandin E by rheumatoid and osteoarthritic synoviocytes--regulation by IFN-gamma and IL-4. J Immunol. 1994;152:2060–2065. [PubMed] [Google Scholar]
- 41.Sugiyama E, Taki H, Kuroda A, Mino T, Yamashita N, Kobayashi M. Interleukin-4 inhibits prostaglandin E2 production by freshly prepared adherent rheumatoid synovial cells via inhibition of biosynthesis and gene expression of cyclo-oxygenase II but not of cyclo-oxygenase I. Ann Rheum Dis. 1996;55:375–382. doi: 10.1136/ard.55.6.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hayashi Y, Kobayashi M, Kuwata H, Atsumi G, Deguchi K, Feng Wei X, Kudo I, Hasegawa K. Interferon-gamma and interleukin 4 inhibit interleukin 1beta-induced delayed prostaglandin E(2)generation through suppression of cyclooxygenase-2 expression in human fibroblasts. Cytokine. 2000;12:603–612. doi: 10.1006/cyto.1999.0622. [DOI] [PubMed] [Google Scholar]
- 43.Yamada H, Kikuchi T, Nemoto O, Obata K, Sato H, Seiki M, Shinmei M. Effects of indomethacin on the production of matrix metalloproteinase-3 and tissue inhibitor of metalloproteinases-1 by human articular chondrocytes. J Rheumatol. 1996;23:1739–1743. [PubMed] [Google Scholar]
- 44.Nishikawa M, Yamaguchi Y, Yoshitake K, Saeki Y. Effects of TNFalpha and prostaglandin E2 on the expression of MMPs in human periodontal ligament fibroblasts. J Periodontal Res. 2002;37:167–176. doi: 10.1034/j.1600-0765.2002.00656.x. [DOI] [PubMed] [Google Scholar]
- 45.Tung JT, Arnold CE, Alexander LH, Yuzbasiyan-Gurkan V, Venta PJ, Richardson DW, Caron JP. Evaluation of the influence of prostaglandin E2 on recombinant equine interleukin-1beta-stimulated matrix metalloproteinases 1, 3, and 13 and tissue inhibitor of matrix metalloproteinase 1 expression in equine chondrocyte cultures. Am J Vet Res. 2002;63:987–993. doi: 10.2460/ajvr.2002.63.987. [DOI] [PubMed] [Google Scholar]
- 46.Mauviel A, Halcin C, Vasiloudes P, Parks WC, Kurkinen M, Uitto J. Uncoordinate regulation of collagenase, stromelysin, and tissue inhibitor of metalloproteinases genes by prostaglandin E2: selective enhancement of collagenase gene expression in human dermal fibroblasts in culture. J Cell Biochem. 1994;54:465–472. doi: 10.1002/jcb.240540413. [DOI] [PubMed] [Google Scholar]
- 47.Domeij H, Yucel-Lindberg T, Modeer T. Signal pathways involved in the production of MMP-1 and MMP-3 in human gingival fibroblasts. Eur J Oral Sci. 2002;110:302–306. doi: 10.1034/j.1600-0722.2002.21247.x. [DOI] [PubMed] [Google Scholar]
- 48.DiBattista JA, Pelletier JP, Zafarullah M, Fujimoto N, Obata K, Martel-Pelletier J. Coordinate regulation of matirx metalloprtoeinases and tissue inhibitor of metalloproteinase expression in human synovial fibroblasts. J Rheumatol Suppl. 1995;43:123–128. [PubMed] [Google Scholar]
- 49.DiBattista JA, Martel-Pelletier J, Fujimoto N, Obata K, Zafarullah M, Pelletier JP. Prostaglandins E2 and E1 inhibit cytokine-induced metalloprotease expression in human synovial fibroblasts. Mediation by cyclic-AMP signalling pathway. Lab Invest. 1994;71:270–278. [PubMed] [Google Scholar]
- 50.Case JP, Lafyatis R, Kumkumian GK, Remmers EF, Wilder RL. IL-1 regulation of transin/stromelysin transcription in rheumatoid synovial fibroblasts appears to involve two antagonistic transduction pathways, an inhibitory, prostaglandin-dependent pathway mediated by cAMP, and a stimulatory, protein kinase C-dependent pathway. J Immunol. 1990;145:3755–3761. [PubMed] [Google Scholar]
- 51.Buttice G, Quinones S, Kurkinen M. The AP-1 site is required for basal expression but is not necessary for TPA-response of the human stromelysin gene. Nucleic Acids Res. 1991;19:3723–3731. doi: 10.1093/nar/19.13.3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Quinones S, Buttice G, Kurkinen M. Promoter elements in the transcriptional activation of the human stromelysin-1 gene by the inflammatory cytokine, interleukin 1. Biochem J. 1994;302:471–477. doi: 10.1042/bj3020471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Quinones S, Saus J, Otani Y, Harris ED, Jr, Kurkinen M. Transcriptional regulation of human stromelysin. J Biol Chem. 1989;264:8339–8344. [PubMed] [Google Scholar]
- 54.Sirum-Connolly K, Brinckerhoff CE. Interleukin-1 or phorbol induction of the stromelysin promoter requires an element that cooperates with AP-1. Nucleic Acids Res. 1991;19:335–341. doi: 10.1093/nar/19.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bond M, Baker AH, Newby AC. Nuclear factor kappaB activity is essential for matrix metalloproteinase-1 and -3 upregulation in rabbit dermal fibroblasts. Biochem Biophys Res Commun. 1999;264:561–567. doi: 10.1006/bbrc.1999.1551. [DOI] [PubMed] [Google Scholar]
- 56.Bond M, Chase AJ, Baker AH, Newby AC. Inhibition of transcription factor NF-kappaB reduces matrix metalloproteinase-1, -3 and -9 production by vascular smooth muscle cells. Cardiovasc Res. 2001;50:556–565. doi: 10.1016/s0008-6363(01)00220-6. [DOI] [PubMed] [Google Scholar]
- 57.Chase AJ, Bond M, Crook MF, Newby AC. Role of nuclear factor-kappa B activation in metalloproteinase-1, -3, and -9 secretion by human macrophages in vitro and rabbit foam cells produced in vivo. Arterioscler Thromb Vasc Biol. 2002;22:765–771. doi: 10.1161/01.atv.0000015078.09208.92. [DOI] [PubMed] [Google Scholar]
- 58.Dokter WH, Esselink MT, Halie MR, Vellenga E. Interleukin-4 inhibits the lipopolysaccharide-induced expression of c-jun and c-fos messenger RNA and activator protein-1 binding activity in human monocytes. Blood. 1993;81:337–343. [PubMed] [Google Scholar]
- 59.Dokter WHA, Koopmans SB, Vellenga E. Effects of IL-10 and IL-4 on LPS-induced transcription factors (AP-1, NF-IL6, and NF-kappa B) which are involved in IL-6 regulation. Leukemia. 1996;10:1308–1316. [PubMed] [Google Scholar]
- 60.Donnelly RP, Crofford LJ, Freeman SL, Buras J, Remmers E, Wilder RL, Fenton MJ. Tissue-specific regulation of IL-6 production by IL-4. Differential effects of IL-4 on nuclear factor-kappa B activity in monocytes and fibroblasts. J Immunol. 1993;151:5603–5612. [PubMed] [Google Scholar]
- 61.Beppu M, Ikebe T, Shirasuna K. The inhibitory effects of immunosuppressive factors, dexamethasone and interleukin-4, on NF-kappaB-mediated protease production by oral cancer. Biochim Biophys Acta. 2002;1586:11–22. doi: 10.1016/s0925-4439(01)00080-1. [DOI] [PubMed] [Google Scholar]
- 62.Abu-Amer Y. IL-4 abrogates osteoclastogenesis through STAT6-dependent inhibition of NF-kappaB. J Clin Invest. 2001;107:1375–1385. doi: 10.1172/JCI10530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wasylyk C, Gutman A, Nicholson R, Wasylyk B. The c-Ets oncoprotein activates the stromelysin promoter through the same elements as several non-nuclear oncoproteins. Embo J. 1991;10:1127–1134. doi: 10.1002/j.1460-2075.1991.tb08053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Borghaei RC, Sullivan C, Mochan E. Identification of a cytokine-induced repressor of interleukin-1 stimulated expression of stromelysin 1 (MMP-3) J Biol Chem. 1999;274:2126–2131. doi: 10.1074/jbc.274.4.2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Oriente A, Fedarko NS, Pacocha SE, Huang SK, Lichtenstein LM, Essayan DM. Interleukin-13 modulates collagen homeostasis in human skin and keloid fibroblasts. J Pharmacol Exp Ther. 2000;292:988–994. [PubMed] [Google Scholar]
- 66.Suk K, Erickson KL. Differential regulation of tumour necrosis factor-alpha mRNA degradation in macrophages by interleukin-4 and interferon-gamma. Immunology. 1996;87:551–558. doi: 10.1046/j.1365-2567.1996.500561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Corcoran ML, Stetler-Stevenson WG, Brown PD, Wahl LM. Interleukin 4 inhibition of prostaglandin E2 synthesis blocks interstitial collagenase and 92-kDa type IV collagenase/gelatinase production by human monocytes. J Biol Chem. 1992;267:515–519. [PubMed] [Google Scholar]
- 68.Mehindate K, al-Daccak R, Aoudjit F, Damdoumi F, Fortier M, Borgeat P, Mourad W. Interleukin-4, transforming growth factor beta 1, and dexamethasone inhibit superantigen-induced prostaglandin E2-dependent collagenase gene expression through their action on cyclooxygenase-2 and cytosolic phospholipase A2. Lab Invest. 1996;75:529–538. [PubMed] [Google Scholar]
- 69.Vignon E, Mathieu P, Louisot P, Richard M. In vitro effect of nonsteroidal antiinflammatory drugs on proteoglycanase and collagenase activity in human osteoarthritic cartilage. Arthritis Rheum. 1991;34:1332–1335. doi: 10.1002/art.1780341021. [DOI] [PubMed] [Google Scholar]
- 70.Millward-Sadler SJ, Wright MO, Davies LW, Nuki G, Salter DM. Mechanotransduction via integrins and interleukin-4 results in altered aggrecan and matrix metalloproteinase 3 gene expression in normal, but not osteoarthritic, human articular chondrocytes. Arthritis Rheum. 2000;43:2091–2099. doi: 10.1002/1529-0131(200009)43:9<2091::AID-ANR21>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 71.Salter DM, Millward-Sadler SJ, Nuki G, Wright MO. Differential responses of chondrocytes from normal and osteoarthritic human articular cartilage to mechanical stimulation. Biorheology. 2002;39:97–108. [PubMed] [Google Scholar]
- 72.Orbak R, Tezel A, Canakci V, Demir T. The influence of smoking and non-insulin-dependent diabetes mellitus on periodontal disease. J Int Med Res. 2002;30:116–125. doi: 10.1177/147323000203000203. [DOI] [PubMed] [Google Scholar]
- 73.Poggi P, Rota MT, Boratto R. The volatile fraction of cigarette smoke induces alterations in the human gingival fibroblast cytoskeleton. J Periodontal Res. 2002;37:230–235. doi: 10.1034/j.1600-0765.2002.00317.x. [DOI] [PubMed] [Google Scholar]
- 74.Snyder HB, Caughman G, Lewis J, Billman MA, Schuster G. Nicotine modulation of in vitro human gingival fibroblast beta1 integrin expression. J Periodontol. 2002;73:505–510. doi: 10.1902/jop.2002.73.5.505. [DOI] [PubMed] [Google Scholar]
- 75.Schmidt AM, Weidman E, Lalla E, Yan SD, Hori O, Cao R, Brett JG, Lamster IB. Advanced glycation endproducts (AGEs) induce oxidant stress in the gingiva: a potential mechanism underlying accelerated periodontal disease associated with diabetes. J Periodontal Res. 1996;31:508–515. doi: 10.1111/j.1600-0765.1996.tb01417.x. [DOI] [PubMed] [Google Scholar]
- 76.Takane M, Sugano N, Iwasaki H, Iwano Y, Shimizu N, Ito K. New biomarker evidence of oxidative DNA damage in whole saliva from clinically healthy and periodontally diseased individuals. J Periodontol. 2002;73:551–554. doi: 10.1902/jop.2002.73.5.551. [DOI] [PubMed] [Google Scholar]
- 77.Yin L, Morita A, Tsuji T. Alterations of extracellular matrix induced by tobacco smoke extract. Arch Dermatol Res. 2000;292:188–194. doi: 10.1007/s004030050476. [DOI] [PubMed] [Google Scholar]
- 78.Han Z, Boyle DL, Manning AM, Firestein GS. AP-1 and NF-kappaB regulation in rheumatoid arthritis and murine collagen-induced arthritis. Autoimmunity. 1998;28:197–208. doi: 10.3109/08916939808995367. [DOI] [PubMed] [Google Scholar]
- 79.Asahara H, Fujisawa K, Kobata T, Hasunuma T, Maeda T, Asanuma M, Ogawa N, Inoue H, Sumida T, Nishioka K. Direct evidence of high DNA binding activity of transcription factor AP-1 in rheumatoid arthritis synovium. Arthritis Rheum. 1997;40:912–918. doi: 10.1002/art.1780400520. [DOI] [PubMed] [Google Scholar]
- 80.Mikita T, Campbell D, Wu P, Williamson K, Schindler U. Requirements for interleukin-4-induced gene expression and functional characterization of Stat6. Mol Cell Biol. 1996;16:5811–5820. doi: 10.1128/mcb.16.10.5811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Muller-Ladner U, Judex M, Ballhorn W, Kullmann F, Distler O, Schlottmann K, Gay RE, Scholmerich J, Gay S. Activation of the IL-4 STAT pathway in rheumatoid synovium. J Immunol. 2000;164:3894–3901. doi: 10.4049/jimmunol.164.7.3894. [DOI] [PubMed] [Google Scholar]
- 82.Gingras S, Simard J, Groner B, Pfitzner E. p300/CBP is required for transcriptional induction by interleukin-4 and interacts with Stat6. Nucleic Acids Res. 1999;27:2722–2729. doi: 10.1093/nar/27.13.2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Delerive P, De Bosscher K, Besnard S, Vanden Berghe W, Peters JM, Gonzalez FJ, Fruchart JC, Tedgui A, Haegeman G, Staels B. Peroxisome proliferator-activated receptor alpha negatively regulates the vascular inflammatory gene response by negative cross-talk with transcription factors NF-kappaB and AP-1. J Biol Chem. 1999;274:32048–32054. doi: 10.1074/jbc.274.45.32048. [DOI] [PubMed] [Google Scholar]
- 84.Horvai AE, Xu L, Korzus E, Brard G, Kalafus D, Mullen TM, Rose DW, Rosenfeld MG, Glass CK. Nuclear integration of JAK/STAT and Ras/AP-1 signaling by CBP and p300. Proc Natl Acad Sci U S A. 1997;94:1074–1079. doi: 10.1073/pnas.94.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jayaraman G, Srinivas R, Duggan C, Ferreira E, Swaminathan S, Somasundaram K, Williams J, Hauser C, Kurkinen M, Dhar R, Weitzman S, Buttice G, Thimmapaya B. p300/cAMP-responsive element-binding protein interactions with ets-1 and ets-2 in the transcriptional activation of the human stromelysin promoter. J Biol Chem. 1999;274:17342–17352. doi: 10.1074/jbc.274.24.17342. [DOI] [PubMed] [Google Scholar]
- 86.Smits PHM, de Wit L, van der Eb AJ, Zantema A. The adenovirus E1A-associated 300 kDa adaptor protein counteracts the inhibition of the collagenase promoter by E1A and represses transformation. Oncogene. 1996;12:1529–1535. [PubMed] [Google Scholar]
- 87.Sheppard KA, Rose DW, Haque ZK, Kurokawa R, McInerney E, Westin S, Thanos D, Rosenfeld MG, Glass CK, Collins T. Transcriptional activation by NF-kappaB requires multiple coactivators. Mol Cell Biol. 1999;19:6367–6378. doi: 10.1128/mcb.19.9.6367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yang C, Shapiro LH, Rivera M, Kumar A, Brindle PK. A role for CREB binding protein and p300 transcriptional coactivators in Ets-1 transactivation functions. Mol Cell Biol. 1998;18:2218–2229. doi: 10.1128/mcb.18.4.2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wadgaonkar R, Phelps KM, Haque Z, Williams AJ, Silverman ES, Collins T. CREB-binding protein is a nuclear integrator of nuclear factor-kappaB and p53 signaling. J Biol Chem. 1999;274:1879–1882. doi: 10.1074/jbc.274.4.1879. [DOI] [PubMed] [Google Scholar]


