Abstract
Mammalian cytosolic thioredoxin reductase (TrxR) has a redox center, consisting of Cys59/Cys64 adjacent to the flavin ring of FAD and another center consisting of Cys497/selenocysteine (SeCys)498 near the C terminus. We now show that the C-terminal Cys497-SH/SeCys498-Se− of NADPH-reduced enzyme, after anaerobic dialysis, was converted to a thioselenide on incubation with excess oxidized Trx (TrxS2) or H2O2. The Cys59-SH/Cys64-SH pair also was oxidized to a disulfide. At lower concentrations of TrxS2, the Cys59-SH/Cys64-SH center was still converted to a disulfide, presumably by reduction of the thioselenide to Cys497-SH/SeCys498-Se−. Specific alkylation of SeCys498 completely blocked the TrxS2-induced oxidation of Cys59-SH/Cys64-SH, and the alkylated enzyme had negligible NADPH-disulfide oxidoreductase activity. The effect of replacing SeCys498 with Cys was determined by using a mutant form of human placental TrxR1 expressed in Escherichia coli. The NADPH-disulfide oxidoreductase activity of the purified Cys497/Cys498 mutant enzyme was 6% or 11% of that of wild-type rat liver TrxR1 with 5,5′-dithiobis(2-nitrobenzoic acid) or TrxS2, respectively, as substrate. Disulfide formation induced by excess TrxS2 in the mutant form was 12% of that of the wild type. Thus, SeCys has a critical redox function during the catalytic cycle, which is performed poorly by Cys.
The thioredoxin reductase (TrxR) enzymes are homodimeric flavoproteins that catalyze the reduction of Trx by NADPH. TrxRs characterized from a wide variety of species show significant amino acid sequence similarity, although they differ in size, structure, and catalytic mechanism. TrxRs from Escherichia coli (1) and Saccharomyces cerevisiae (2) are dimers of 35-kDa subunits, whereas TrxRs from higher eukaryotes, including mammals (3–5), Caenorhabditis elegans (6), and Plasmodium falciparum (malarial parasite trophozoite; ref. 7), are dimers of 55- to 58-kDa subunits. The major difference between the lower and higher molecular mass enzymes is that the latter contain an additional redox center preceding their C-terminal Gly; mammalian and C. elegans TrxRs have Cys and selenocysteine (SeCys) residues in the conserved sequence (-Gly-Cys-SeCys-Gly), and P. falciparum TrxR has two Cys residues in the sequence (-Cys-X-X-X-Cys-Gly).
Removal of the SeCys residue by limited proteolysis (8), by oxidative selenium elimination, or by specific alkylation of the SeCys residue with bromoacetate at pH 6.5 (9, 10) led to a loss in catalytic activity, indicating an essential role for the SeCys. The Cys/SeCys pair was proposed earlier to undergo redox change during the catalytic cycle (11). This suggestion followed the observation that complete reduction of mammalian TrxR required three equivalents of dithionite per subunit, whereas only two equivalents are required to reduce the N-terminal FAD and the disulfide center.
Mutational studies with P. falciparum TrxR (7) suggested that its C-terminal Cys residues are essential for catalytic activity. They form a disulfide intermediate that is then reduced by the N-terminal region Cys pair during the catalytic cycle. If a similar reaction sequence occurs in mammalian TrxR, the C-terminal adjacent Cys/SeCys residues should be oxidized to a thioselenide (-S-Se-, the analogue of a disulfide). Formation of a disulfide bond between two adjacent Cys residues in a polypeptide is unfavorable because of the distance between sulfur atoms (12) and also because it requires constraining the intracysteinyl peptide bond to the cis rather than the preferred trans orientation (13, 14). The larger atomic size of selenium is thought to obviate these unfavorable characteristics.
Using cytosolic TrxR [also called TrxR1 to distinguish it from mitochondrial TrxR2 purified from rat liver (15) and another isoenzyme termed TrxR3 (16)], we now show that the Cys/SeCys pair of the reduced enzyme is indeed converted to a thioselenide on oxidation with either hydrogen peroxide or TrxS2. Further, the SeCys residue is required both for NADPH-disulfide oxidoreductase activity and for TrxS2-mediated oxidation of the N-terminal region Cys59/Cys64 pair to the disulfide, conclusions supported by results from studies with TrxR1 selectively alkylated at the SeCys. The low activities of a SeCys → Cys substitution mutant in these two processes illustrate the ineffectiveness of sulfur as a selenium replacement in TrxR.
Experimental Procedures
Materials.
TrxR1 was purified from rat liver as described (15). Human TrxR1 gene (TRR clone 30B from human placenta; ref. 17) was kindly provided by Pamela Gasdaska (Arizona Cancer Center, Tucson, AZ). Rabbit anti-serum to TrxR1 was produced by standard procedures, and monospecific anti-TrxR1 antibodies were prepared from the serum with the use of purified TrxR absorbed to a nitrocellulose membrane. Recombinant rat Trx was prepared as described (15). Horseradish peroxidase-conjugated antibody to rabbit IgG was obtained from Amersham Pharmacia, and biotin-conjugated iodoacetamide (BIAM) was from Molecular Probes.
Determination of Protein Concentration.
The concentration of TrxR1 subunit was determined spectrophotometrically with ɛ463 of 11,300 M−1⋅cm−1 subunit as described (11). The concentration of recombinant Trx was also determined spectrophotometrically with ɛ280 of 8,610 M−1⋅cm−1, which was calculated based on its amino acid composition.
Preparation of Reduced TrxR.
Purified TrxR1 was incubated for 30 min in PBS without Ca2+ and Mg2+ at pH 7.2 (PBS) containing 1 mM EDTA and 200 μM NADPH. The reduced enzyme was then dialyzed for 8 h against the same buffer devoid of NADPH in an anaerobic chamber.
HPLC-MS.
Peptides were separated by reverse-phase HPLC with both spectrophotometric and MS detection (Hewlett–Packard model 1100) by using a Vydac narrow-bore C18 column (218TP5205, Vydac, Hesperia, CA). The initial solvent was 0.05% trifluoroacetic acid with elution by acetonitrile/0.05% trifluoroacetic acid at 1%/min gradient and a flow rate of 0.2 ml/min. The effluent from the spectrophotometric detector was mixed in a T-shaped device with 100 μl/min acetic acid pumped by another model 1100 pump, and the mixture was introduced into the mass spectrometer (18). The capillary voltage was 4,500 V, and the fragmentor was programmed to ramp from 50 V at 50 mass units (mu), 80 V at 1,500 mu, and 140 V at 2,500 mu. Data were collected from 550 to 2,000 mu.
E. coli Expression of Cys497/Cys498 TrxR1 Mutant.
To generate the recombinant TrxR1 in which SeCys498 is replaced by Cys, the full-length human placental TrxR1 gene was used. The PCR amplified DNA was cloned into the pQE-30 vector (Qiagen, Chatsworth, CA) to encode a polyhistidine peptide at the N terminus, and the resulting construct was expressed in E. coli strain M15[pREP4].
Purification of Mutant TrxR1.
The mutant enzyme was purified from bacterial extracts by successive chromatography on Ni-NTA column (Qiagen), 2′,5′-ADP-Sepharose 4B column (Amersham Pharmacia), and phenyl-Sepharose HPLC column (Toya Soda, Tokyo; ref. 15). Elution of TrxR1 protein was monitored by immunoblot analysis with antibodies to rat TrxR1 (15).
Results
Selective Alkylation and Identification of SeCys498 as a Redox-Sensitive Site.
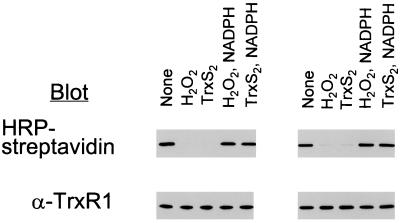
Recently, it was shown that the SeCys residue in the C-terminal redox center of human TrxR1 (9) and rat liver TrxR2 (15) can be alkylated selectively at pH 6.5 with bromo[1-14C]acetate and BIAM, respectively. In the present study, selective BIAM labeling was used to monitor the oxidation state of SeCys in oxidized and reduced preparations of TrxR. Reduced TrxR1 oxidized either with H2O2 or with excess TrxS2 failed to react with BIAM (Fig. 1 Left). However, after aliquots of the oxidized enzymes were again treated with NADPH, strong labeling with the biotinyl group was observed. In fact, the labeling intensities of the rereduced enzymes were similar to the labeling intensity of control enzyme, indicating that the oxidation was fully reversible. We considered the possibility that a conformational change rather than a true oxidation of the SeCys was responsible for the lack of labeling with BIAM after treatment with H2O2 or TrxS2. Such a change had not occurred, however, because biotin-labeling of denatured enzyme (Fig. 1 Right) was the same as that of native enzyme (Fig. 1 Left).
Figure 1.
Effect of exposure of NADPH-reduced TrxR1 to H2O2 or TrxS2 on labeling with BIAM. NADPH-reduced TrxR1 was dialyzed anaerobically to remove NADPH and then incubated for 10 min at room temperature with 200 μM H2O2 or 12 equivalents of TrxS2 per subunit to produce E-H2O2 and E-TrxS2. The H2O2 reaction was stopped by adding catalase, and the E-TrxS2 mixture was adjusted to pH 5.2. One-half of each oxidized enzyme sample was incubated for 20 min with 200 μM NADPH at pH 7.2 to produce E-H2O2-NADPH or E-TrxS2-NADPH. An aliquot (5 μg) of each was incubated with 50 μM BIAM for 10 min in the absence (Left) or presence (Right) of 6 M guanidine-HCl in PBS buffer (pH 7.2) containing 1 mM EDTA and then with 1 mM IAM for 5 min at pH 8.8. The samples shown at Right were dialyzed against 20 mM Tris⋅HCl (pH 8.0) buffer containing 1 mM EDTA to remove guanidine. All samples were subjected to SDS/PAGE on a 10% gel and then transferred to a nitrocellulose membrane. The biotinyl carboxamidomethyl [(B)CAM]-labeled proteins were detected by streptavidin blotting with horseradish peroxidase (HRP)-conjugated streptavidin and enhanced chemiluminescence detection. Equal application of protein among gel lanes was confirmed by immunoblot analysis with antibodies to TrxR1.
Thioselenide Bond Formation Between Cys497 and SeCys498.
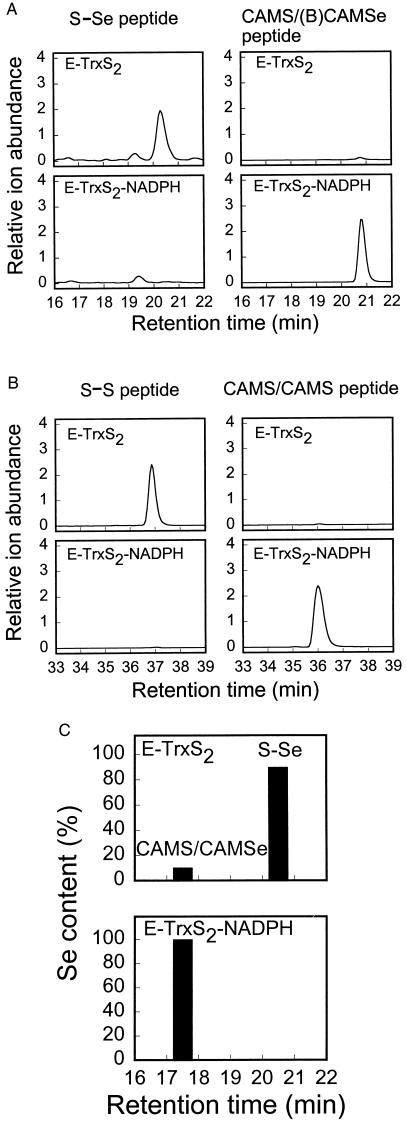
To characterize the chemical nature of the Cys59/Cys64 and Cys497/SeCys498 centers in oxidized TrxR1, TrxS2-treated TrxR1 was reacted with BIAM at pH 7.2, denatured, and then treated with iodoacetamide (IAM) under denaturing conditions to alkylate residues that had not reacted with BIAM.§ The derivatized proteins were digested with proteinase Lys-C, and the resultant peptides were analyzed by HPLC-MS. The chromatograms (Fig. 2A) showed a peak at 20.5 min with a mass of 1,297.7 mu, in excellent agreement with 1,297.3 mu calculated for the 13 residue C-terminal fragment (R487SGGDILQSGCUG499) in which Cys497 and SeCys498 (U) are bridged by a thioselenide (S-Se) bond. When the oxidized TrxR1 was reduced before exposure to BIAM, a new peak appeared at 20.9 min with a mass of 1,682.8 mu. The calculated mass for the C-terminal peptide with CAM-Cys497 and (B)CAM-SeCys498 was 1,682.4 mu. Thus, the C-terminal Cys and SeCys center is converted to a thioselenide that is fully reduced when the enzyme reacts with NADPH.
Figure 2.
Identification and quantitation of the Cys497/SeCys498 thioselenide and the Cys59/Cys64 disulfide in E-TrxS2 and E-TrxS2-NADPH. (A and B) E-TrxS2 and E-TrxS2-NADPH were prepared from the NADPH-reduced TrxR1 as described in Fig. 1. The resulting enzymes (10 μg) were labeled with 50 μM BIAM for 20 min in oxygen-free PBS buffer (pH 7.2) containing 1 mM EDTA and then with 1 mM IAM for 5 min in 50 mM Tris⋅HCl (pH 8.8) containing 6 M guanidine-HCl. After adjustment to pH 5.2, the samples were dialyzed for 4 h against 10 mM sodium acetate buffer (pH 5.2) and then for 2 h against 20 mM Tris⋅HCl buffer (pH 8.0). The dialyzed samples were diluted to 10% (vol/vol) in acetonitrile and then incubated with endoproteinase Lys-C at 37°C overnight. The resulting peptide mixtures were analyzed by HPLC-MS. (A) Extracted ion chromatograms for m/z = 649.6 (1,297.7 mu) of the Cys497/SeCys498-containing peptide (Left) and for m/z = 842.5 (1,682.8 mu) of the CAM-Cys497/(B)CAM-SeCys498-containing peptide (Right). (B) Extracted ion chromatograms for m/z = 1,043.9 (3,129.3 mu) of the Cys59/Cys64-containing peptide (Left) and for m/z = 1,082.6 (3,245.5 mu) of the CAM-Cys59/CAM-Cys64-containing peptide (Right). (C) E-TrxS2 and E-TrxS2-NADPH (20 μg) prepared from the NADPH-reduced TrxR1 as described in Fig. 1 were reacted with 1 mM IAM for 5 min in 50 mM Tris⋅HCl (pH 8.8) containing 6 M guanidine-HCl. Lys-C peptides were prepared and fractionated on a C18 HPLC column. Fractions (0.2 ml) were collected manually, and each was analyzed for selenium with a Perkin–Elmer model 4100 ZL atomic absorption spectrometer. The amount of selenium in the total soluble peptide mixture was 100%. The identity of the selenium-containing peptide eluting at 17.5 min was confirmed by matrix-assisted laser desorption ionization time-of-flight MS (measured = 1,413.8 mu; calculated = 1,413.4 mu). When the oxidized peptide eluting at 20.5 min was reduced with DTT, alkylated, and rechromatographed, it eluted at 17.5 min as expected (not shown).
The chromatograms also included peaks of 3,129.3 mu at 37.0 min and 3,245.5 mu at 36.0 min (Fig. 2B). The mass difference of 116 mu matches that expected for a peptide with two Cys residues in disulfide linkage compared with that containing two CAM-Cys derivatives. However, the absolute mass of each peptide is 42 mu higher than that calculated from the reported sequence (19). Direct sequencing of the 36-min peptide (CAM-Cys/CAM-Cys) showed the presence of Arg-Trp instead of Asn-Gly as originally reported, and this discrepancy accounts for the 42-mu difference. Thus, the corrected sequence for the 36-min peptide is V38 MVLDFVTPTPLGTRWGLGGTCVNVGCIPK67. The determined sequence of the first 18 residues of the 37.0-min peptide matched those of the 36-min peptide, establishing that the disulfide bond was formed between Cys59 and Cys64. Thus, it can be concluded that a thioselenide bond is formed between Cys497 and SeCys498 in the C-terminal redox center when TrxR1 is oxidized with excess TrxS2 and that a disulfide bond is formed between Cys59 and Cys64. Both of these bonds are reduced when the oxidized enzymes are incubated subsequently with NADPH, and full reactivity with alkylating agents is restored. Similar results were obtained with enzyme oxidized with 0.2 mM H2O2 (data not shown).
To characterize the SeCys-containing peptides further, Lys-C-generated peptide fragments were purified by reverse-phase HPLC and sequenced by automated Edman degradation. The readable sequence of the peptide at 20.5 min (Fig. 2A) from TrxS2 oxidized enzyme was RSGGDILQSG, as expected for the C-terminal peptide with a thioselenide bond linking Cys497 and SeCys498. The peptide from reduced enzyme eluted at 20.9 min, and this peptide contained over 95% of the biotin label in the peptide digest, as determined by ELISA (15). This peptide also contained about 95% of the total selenium present in the digest, as determined by atomic absorption spectrometry. After further purification of this (B)CAM-labeled, selenium-containing peptide on a Neutravidin affinity column (15), the sequence was determined to be RSGGDILQSGCXG, matching the known sequence for residues 487 to 499 of rat TrxR1 (19).¶ The residue in position 497 was identified as CAM-Cys, whereas that at 498 could not be matched to a standard residue, consistent with it being (B)CAM-SeCys.
Levels of Cys59/Cys64 Disulfide and Cys497/SeCys498 Thioselenide in TrxR1 Oxidized by TrxS2.
To determine the ratio of oxidized to reduced SeCys present in the various treated enzymes, purified peptides were assayed for selenium by atomic absorption spectrometry. For this purpose, CAM-Cys/CAM-SeCys derivatives of oxidized and reduced enzyme preparations were prepared by reaction with IAM, because the resulting alkylated peptides in Lys-C digests could be separated completely from the oxidized S-Se peptides by HPLC (Fig. 2C), whereas the CAM-Cys/(B)CAM-SeCys labeled peptide peak overlapped the oxidized thioselenide peptide peak (Fig. 2A). Analysis of a reduced TrxR1 preparation that had been oxidized by exposure to 12 equivalents of TrxS2 for 10 min showed that 90% of the SeCys was in the oxidized thioselenide form and that 10% was in the reduced form (Fig. 2C Upper). After reduction of the oxidized enzyme sample with NADPH, all of the SeCys was reduced and fully reactive with IAM (Fig. 2C Lower).
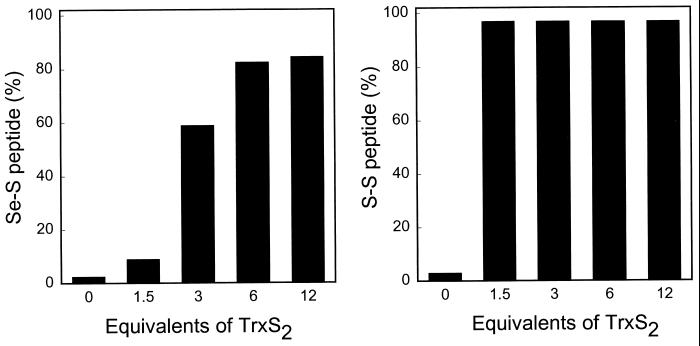
A ratio of 1.5 TrxS2 to TrxR was sufficient to oxidize the Cys/Cys redox pair completely to the disulfide, but under these conditions, only about 10% of the Cys/SeCys pair was in the oxidized thioselenide form (Fig. 3). More extensive oxidation of the Cys/SeCys pair required a ratio of at least 3.0. The preferential reduction of the Cys/SeCys at the expense of Cys/Cys is consistent with the lower redox potential of a Cys/SeCys pair compared with that of a Cys/Cys.
Figure 3.
Effect of varying the concentration of TrxS2 on the formation of the Cys497/SeCys498 thioselenide and the Cys59/Cys64 disulfide. NADPH-reduced TrxR1 (20 μg) was incubated for 10 min at room temperature with the indicated amount of TrxS2 in oxygen-free PBS buffer (pH 7.2) containing 1 mM EDTA. The samples were then reacted with 1 mM IAM at pH 8.8 in 6 M guanidine-HCl as described in the legend to Fig. 2C. The reaction mixtures after dialysis were digested with Lys-C; peptides were purified by HPLC; and the selenium contents of the S-Se peptide and the CAM-Cys/CAM-SeCys peptide were determined by atomic absorption spectroscopy. The amounts of Cys/Cys peptide and CAM-Cys/CAM-Cys peptide were estimated from the HPLC peak areas. (Left) The formation of the oxidized, thioselenide-containing peptide, expressed as a percentage of the sum of the oxidized (S-Se) and alkylated (CAM-Cys/CAM-SeCys) derivatives of reduced Cys497/SeCys498. (Right) The formation of the disulfide-containing peptide, expressed as a percentage of the sum of the oxidized (S-S) and alkylated (CAM-Cys/CAM-Cys) derivatives of the reduced Cys59/Cys64 pair.
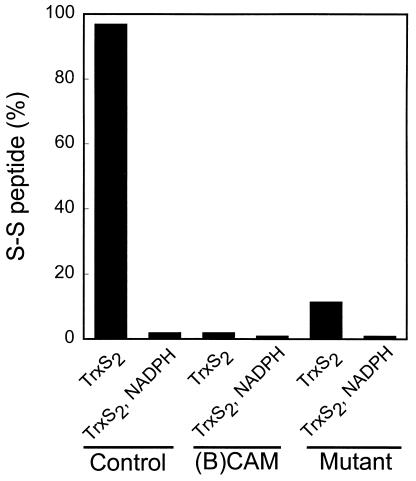
Electron transfer from the redox centers of reduced TrxR1 to the disulfide substrate, TrxS2, requires the presence of the unmodified SeCys498 residue (Fig. 4). Incubation of reduced TrxR1 with 1.5 equivalents of TrxS2 resulted in complete oxidation of the Cys59/Cys64 pair to the disulfide. However, when enzyme that was specifically alkylated on the SeCys498 residue with BIAM was treated with TrxS2 under the same condition, only 2% of the Cys/Cys pair was oxidized. Similar results were obtained with a mutant TrxR1 form in which SeCys was replaced with Cys. Only 12% of the Cys59/Cys64 pair in the mutant enzyme was oxidized to the disulfide. The large rate enhancement exerted by a selenolate anion in dithiol–disulfide interchange reactions (20) can explain the requirement of the C-terminal SeCys residue for efficient reduction of the TrxS2 substrate. Subsequent transfer of reducing equivalents from the Cys59-SH/Cys64-SH pair regenerates the C-terminal redox center and forms a disulfide between Cys59 and Cys64.
Figure 4.
TrxS2-induced oxidation of the Cys59-SH/Cys64-SH pair in native, (B)CAM-modified, and SeCys498 → Cys498 mutant TrxR1 enzymes. (B)CAM-labeled enzyme was prepared by labeling NADPH-reduced TrxR1 with 50 μM BIAM alone. E. coli-expressed SeCys498 → Cys498 mutant TrxR1 was prepared as described in Experimental Procedures. Control, (B)CAM-modified, and mutant enzymes (30 μg) were incubated with three equivalents of Tris(2-carboxyethyl)phosphine per subunit to assure complete reduction of redox centers (9) and then oxidized by exposure for 2 min at 25°C to six equivalents of TrxS2 per subunit in PBS buffer (pH 7.2) containing 1 mM EDTA. One-half of each TrxS2-treated enzyme sample was reduced with 200 μM NADPH. Each of the resulting enzyme samples was labeled with IAM, digested with Lys-C, and subjected to HPLC analysis for the Cys59/Cys64 containing-peptide as described in the legend to Fig. 3.
Determination of TrxR1 specific activities for the overall NADPH-disulfide oxidoreductase reaction confirmed the requirement for SeCys for maximal catalytic activity (Table 1). With the artificial substrate 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB), enzyme containing the (B)CAM-modified SeCys had very low activity (1.4%) compared with native enzyme and was virtually inactive with Trx or the coupled Trx-oxidized insulin substrate pair. The mutant enzyme in which Cys replaced SeCys had low activity on DTNB and TrxS2. Thus, the significant catalytic advantage imparted by selenium over sulfur in this enzyme is evident from the 9- to 15-fold enhancement of catalytic activity observed with the SeCys-containing enzyme.
Table 1.
Catalytic activities of Trx Rs
| Substrate | Specific activities of TrxR enzymes,
μmol per min per mg
|
||
|---|---|---|---|
| Control TrxR1 | (B)CAM-TrxR1 | SeCys498 → Cys498 TrxR1 | |
| DTNB | 42 | 0.6 | 2.7 |
| TrxS2 | 13 | 0.07 | 1.5 |
| Oxidized insulin, TrxS2 | 15 | Not detectable | Not detectable |
DTNB reduction was monitored spectrophotometrically at 412 nm in a 1-ml reaction mixture containing 100 mM potassium phosphate (pH 7.4), 2 mM EDTA, 1.5 mM DTNB, 0.2 mg of BSA, 0.2 mM NADPH, and 100–500 ng of native, 1–5 μg of (B)CAM-modified, or 1–5 μg of SeCys → Cys mutant TrxR1. NADPH-dependent reduction of TrxS2 was monitored spectrophotometrically at 340 nm in reaction mixtures containing 120 μM TrxS2, 50 mM potassium phosphate (pH 7.0), 1 mM EDTA, and 0.2 mM NADPH. Activities of TrxR were also measured by coupling the NADPH-dependent reduction of Trx and oxidized insulin in reaction mixtures containing 7.5 μM TrxS2, 80 μM oxidized insulin, 50 mM potassium phosphate (pH 7.0), 1 mM EDTA, 0.2 mM NADPH, and 1 μg of TrxR1.
Discussion
We have presented direct evidence for the formation of a thioselenide bond between Cys497 and SeCys498 in TrxR when reduced enzyme reacts with excess oxidized Trx. The oxidized enzyme also contains a disulfide bond between Cys59 and Cys64. Identification of these structures was based on MS analyses, Edman sequence determination of isolated peptides, measurement of selenium content by atomic absorption, reduction of oxidized TrxR by NADPH or DTT to yield a form that reacted with alkylating reagents, and quantitative detection of the biotinylated alkyl group by streptavidin blot analysis. When lower TrxS2 concentrations were used, the Cys59/Cys64 pair again was converted completely to the disulfide form, but substantially less of the C-terminal Cys/SeCys thioselenide linkage was formed (Fig. 3). After termination of the reaction in these experiments, reducing equivalents still present in the enzyme are redistributed among the redox centers based on the relative redox potentials of each accessible center. The center with the lowest redox potential will be reduced preferentially at equilibrium. In TrxR, the Cys/SeCys center will be reduced under these conditions.
Even with excess TrxS2, the maximal conversion of the Cys/SeCys pair to the thioselenide was about 80–90%, rather than 100%. This observation suggests the possibility that our enzyme preparation contained two forms, with the minor component unable to form the thioselenide. The nature of this heterogeneity is speculative at present. The enzyme was reduced by treatment with NADPH, followed by dialysis, and therefore, it is possible that bound pyridine nucleotide remaining on a fraction of the protein prevented formation of the thioselenide. In previous studies (9), inactivation and loss of selenium from reduced enzyme on exposure to oxygen were shown to be prevented by addition of 0.5 to 1 mM NADP+ or NADPH. Under these conditions, bound pyridine nucleotide-induced enzyme conformational changes had occurred.
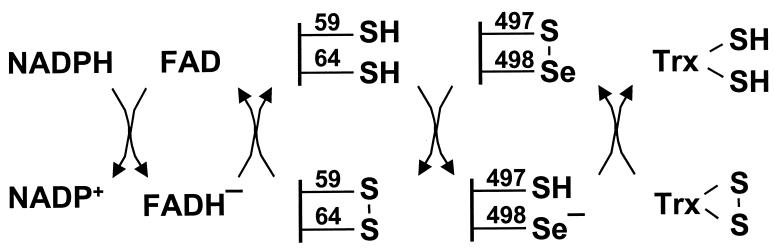
While this work was in progress, Gasdaska et al. (21) reported that a Cys497/Cys498 mutant of TrxR1 and a truncated mutant lacking residues 498 and 499 had decreased or no catalytic activity, respectively, compared with native enzyme. Such studies serve to underscore the importance of the SeCys residue for catalytic activity of mammalian TrxR. Based on our observations and previous studies, it is reasonable to propose that the reduced Cys497/SeCys498 pair is the primary site that undergoes oxidation by a disulfide substrate. Reduction of the resulting thioselenide by the Cys59-SH/Cys64-SH pair then could be achieved as shown in Fig. 5. As shown, the penultimate SeCys residue has a critical role in the ultimate transport of reducing equivalents from the Cys59-SH/Cys64-SH center to the disulfide substrate. Consistent with this view are the observations that the N-terminal region dithiol center is poorly oxidized to the disulfide when enzyme lacking SeCys498 or enzyme containing alkylated SeCys is reacted with a disulfide substrate. Although the wild-type TrxR of P. falciparum has considerably higher catalytic activity, in spite of the fact that its C-terminal redox center consists of Cys535 and Cys540, this difference may be a reflection of the more favorable disulfide bond formation when two Cys residues are separated by four residues. In the mutant human SeCys → Cys TrxR1, the two Cys residues are adjacent.
Figure 5.
Proposed reaction mechanism for TrxR1.
Based on the known rate enhancement effects of selenols on dithiol–disulfide interchange reactions, an essential role of SeCys in the reduction of disulfide substrates by mammalian TrxR enzymes is not surprising. Several studies, including those reported herein, show that selenium in the form of a SeCys residue is required for efficient catalysis of disulfide reductions. There is considerable evidence to suggest that, under enzyme turnover conditions with bound NADPH serving as ultimate reductant, reaction of the Cys-SH/SeCys-Se− pair with a disulfide substrate involves intermediate formation of a selenosulfide between the SeCys residue and one of the sulfur atoms of the substrate disulfide. Reduction of this Se-S bond by a reactive thiol group such as that of Cys497 then would form reduced Trx and an enzyme thioselenide linkage. Although the SeCys → Cys mutant can catalyze the reduction of disulfide substrates, it is much less efficient. The decreased efficiency would be expected from the requirement to form a strained disulfide between the adjacent Cys residues. At present, no structures for TrxR are available, and therefore, the actual distances between the selenium and sulfur atoms of the Cys/SeCys pair are unknown. Clarification of the electron transport pathway of the catalytic cycle of this interesting enzyme clearly requires additional detailed studies.
Abbreviations
- Trx
thioredoxin
- TrxR
Trx reductase
- SeCys
selenocysteine
- IAM
iodoacetamide
- BIAM
biotin-conjugated IAM
- mu
mass unit
- CAM
carboxamidomethyl
- (B)CAM
biotinyl CAM
- DTNB
5,5′-dithiobis(2-nitrobenzoic acid)
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.050579797.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.050579797
In a previous study, we were able to alkylate stoichiometrically the SeCys498 of native HeLa TrxR by exposure to bromoacetate at pH 6.5 (9). Less than 5% of the adjacent Cys497 was alkylated. In the present studies, we therefore denatured the protein before reaction with IAM to achieve stoichiometric alkylation of Cys residues.
Edman sequencing of an HPLC-purified peptide from rat TrxR1 indicated that the previous report of the four-residue sequence A451LQP454 of the rat TrxR1 in ref. 19 was incorrect and that it is actually the five-residue sequence G451FAAA455. Thus, rat TrxR1 contains 499 amino acid residues, and the penultimate SeCys is the 498th, as in the case in human TrxR1 (5, 17).
References
- 1.Russel M, Model P. J Biol Chem. 1988;263:9015–9019. [PubMed] [Google Scholar]
- 2.Chae H Z, Chung S J, Rhee S G. J Biol Chem. 1994;269:27670–27678. [PubMed] [Google Scholar]
- 3.Luthman M, Holmgren A. Biochemistry. 1982;21:6628–6633. doi: 10.1021/bi00269a003. [DOI] [PubMed] [Google Scholar]
- 4.Tamura T, Stadtman T C. Proc Natl Acad Sci USA. 1996;93:1006–1011. doi: 10.1073/pnas.93.3.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gladyshev V N, Jeang K-T, Stadtman T C. Proc Natl Acad Sci USA. 1996;93:6146–6151. doi: 10.1073/pnas.93.12.6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buettner C, Harney J W, Berry M J. J Biol Chem. 1999;274:21598–21602. doi: 10.1074/jbc.274.31.21598. [DOI] [PubMed] [Google Scholar]
- 7.Wang P-F, Arscott L D, Gilberger T-W, Müller S, Williams C H., Jr Biochemistry. 1999;38:3187–3196. doi: 10.1021/bi982674g. [DOI] [PubMed] [Google Scholar]
- 8.Gromer S, Wissing J, Behne D, Ashman K, Schirmer R H, Flohé L, Becker K. Biochem J. 1998;332:591–592. doi: 10.1042/bj3320591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gorlatov S N, Stadtman T C. Proc Natl Acad Sci USA. 1998;95:8520–8525. doi: 10.1073/pnas.95.15.8520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gorlatov S N, Stadtman T C. Arch Biochem Biophys. 1999;369:133–142. doi: 10.1006/abbi.1999.1356. [DOI] [PubMed] [Google Scholar]
- 11.Arscott L D, Gromer S, Schirmer R H, Becker K, Williams C H., Jr Proc Natl Acad Sci USA. 1997;94:3621–3626. doi: 10.1073/pnas.94.8.3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schulz G E, Schirmer R H. Principles of Protein Structure. Heidelberg: Springer; 1979. [Google Scholar]
- 13.Capassoo S, Mattia C, Mazzarella L, Puliti R. Acta Crystallogr B. 1977;33:2080–2083. [Google Scholar]
- 14.Miller S M, Moore M J, Massey V, Williams C H, Jr, Disterfano M D, Ballou D P, Walsh C T. Biochemistry. 1989;28:1194–1205. doi: 10.1021/bi00429a037. [DOI] [PubMed] [Google Scholar]
- 15.Lee S-R, Kim J-R, Kwon K-S, Yoon H W, Levine R L, Ginsburg A, Rhee S G. J Biol Chem. 1999;274:4722–4734. doi: 10.1074/jbc.274.8.4722. [DOI] [PubMed] [Google Scholar]
- 16.Sun Q-A, Wu Y, Zappacosta F, Jeang K-T, Lee B J, Hatfield D L, Gladyshev V N. J Biol Chem. 1999;274:24522–24530. doi: 10.1074/jbc.274.35.24522. [DOI] [PubMed] [Google Scholar]
- 17.Gasdaska P Y, Gasdaska J R, Cochran S, Powis G. FEBS Lett. 1995;373:5–9. doi: 10.1016/0014-5793(95)01003-w. [DOI] [PubMed] [Google Scholar]
- 18.Apffel A, Fischer S, Goldberg G, Goodley P C, Kuhlmann F E. J Chromatogr A. 1995;712:177–190. doi: 10.1016/0021-9673(95)00175-m. [DOI] [PubMed] [Google Scholar]
- 19.Zhong L, Arnér E S J, Ljung J, Åslund F, Holmgren A. J Biol Chem. 1998;273:8581–8591. doi: 10.1074/jbc.273.15.8581. [DOI] [PubMed] [Google Scholar]
- 20.Singh R, Whitesides G M. J Org Chem. 1991;56:6931–6933. [Google Scholar]
- 21.Gasdaska J R, Harney J W, Gasdaska P Y, Powis G, Berry M J. J Biol Chem. 1999;274:25379–25385. doi: 10.1074/jbc.274.36.25379. [DOI] [PubMed] [Google Scholar]