Abstract
We have analyzed the movement of single 22S dynein molecules from Tetrahymena cilia by using a nanometer measuring system equipped with optical tweezers. Statistical analysis proved that a single molecule of 22S dynein can move processively and develop force at low concentrations of ATP (<20 μM). The maximum force was ≈4.7 pN, and the force-velocity curve was convex down. During force development, dynein molecules showed stepwise displacement of ≈8 nm and frequently exhibited backward steps of ≈8 nm. At higher concentrations of ATP (≧20 μM) single molecules of 22S dynein were not observed to move processively. Twenty-two S dynein seems to switch over from a processive mode to a nonprocessive mode, sensing a subtle change of ATP concentrations. These observations indicate that the processivity, maximum force, and step size of dynein are similar to those of kinesin, but the ATP concentration-dependence, force-velocity relationship, and backward steps are clearly distinct from kinesin.
Dynein is the first microtubule motor protein to be discovered and functions as a molecular engine for ciliary and flagellar movement (1). Dynein constitutes the outer and inner arms of axonemes, and multiple forms (monomer, dimer, and trimer) of dyneins have been isolated from axonemes, even in a single species. More than 20 years after the first discovery of axonemal dynein, cytoplasmic dynein was identified (2) and found to be involved in transport of organelles and vesicles (3) and in spindle assembly (4, 5) and chromosome segregation (6, 7). Both axonemal and cytoplasmic dynein consist of multiple subunits referred to as heavy chains (>500 kDa), intermediate chains (60–150 kDa), and light chains (<50 kDa). Each dynein heavy chain comprises a globular motor domain that hydrolyzes ATP and interacts with microtubules.
Molecular genetics revealed that there are more than 10 different dynein heavy chain genes in eukaryotes that produce ciliated or flagellated cells (8, 9). From the amino acid sequence similarity, dyneins are classified into three groups: namely, axonemal outer arms, axonemal inner arms, and cytoplasmic dynein (10). Each dynein heavy chain contains four conserved ATP binding sites, P1–P4. The P1 site is thought to be an ATP catalytic site, but the functions of the P2-P4 sites are unknown. Because of its large size and complexity, the structure and function of the dyneins are not well characterized yet, compared with myosin and the kinesins.
Among the dyneins, Tetrahymena ciliary 22S dynein is relatively well characterized. 22S dynein is derived from the outer arms of ciliary axonemes. The molecular weight of 22S dynein is about two million, and it comprises three different heavy chains and has a three-headed “flower bouquet” structure (11, 12). The kinetics of ATP hydrolysis by 22S dynein is thought to be similar to that of actomyosin (13). In microtubule gliding assays, 22S dynein translocates microtubules at ≈8 μm/s directed toward the minus end of microtubules (14).
Recently, very high resolution systems have been developed to observe aspects of the process of force generation by individual motor proteins. Myosin and kinesin have now been extensively investigated in these systems, and the force, displacement, and step size of single molecules have been measured (15–21). Processive motors are especially suitable for analysis of cyclic and continuous movement because a single molecule moves through many catalytic and associated mechanical cycles while remaining attached to its track. Findings using these high-resolution systems have improved our understanding of the molecular behavior of myosin and kinesin during force generation.
We have now examined the motility of 22S dynein from Tetrahymena cilia to reveal single molecule behavior of dynein. Our observation indicated that 22S dynein can move processively like kinesin (22, 23), sea urchin flagellar inner arm dynein (24), Chlamydomonas flagellar dynein c (25), and myosin V (26). However, this property of 22S dynein was restricted only at low concentrations of ATP and was not observed at high concentrations of ATP.
Materials and Methods
Preparation of 22S Dynein from Tetrahymena.
22S dynein was purified from Tetrahymena thermophila (strain B-255) cilia as described (12). In brief, cilia were obtained by dibucaine treatment of the cells and then were demembranated with Nonidet-P40. The isolated axonemes were extracted with high salt buffer (0.6 M NaCl/10 mM Hepes⋅NaOH, pH 7.4/4 mM MgCl2/0.1 mM EGTA/1 mM DTT/1 mM phenylmethylsulfonyl fluoride). The dynein extract was purified by sucrose density gradient centrifugation [5–25% sucrose in buffer A (10 mM Tris⋅acetate, pH 7.5/50 mM K-acetate/4 mM MgSO4/1 mM EGTA/1 mM DTT)], and a protein peak at around 22S was pooled as 22S dynein, was stored in liquid N2, and was used within a month.
Preparation of Dynein-Coated Beads.
22S dynein-coated beads were prepared according to the method of Wang et al. (27) and Higuchi et al. (21). Six microliters of 5% (vol/vol) (=140 pM) fluorescent latex beads (1.0 μm in diameter, carboxylate-modifed latex; blue L5280; Molecular Probes) in buffer A were incubated with four microliters of various concentrations (2–23 nM) of 22S dynein solution on ice for 2 min, and then one microliter of BSA solution (5 mg/ml) was added.
Preparation of Rhodamine-Labeled and Polarity-Marked Microtubules.
Tubulin was purified from porcine brain by three cycles of polymerization-depolymerization followed by phosphocellulose column chromatography (28). The purified tubulin was labeled with X-rhodamine succinimidyl ester (C-1309; Molecular Probes) by the method of Hyman et al. (29). Fluorescent rhodamine-labeled microtubules were prepared by co-polymerizing purified tubulin and rhodamine-labeled tubulin at a 20:1 ratio in 80 mM Pipes⋅NaOH, 1 mM MgCl2, 0.2 mM EGTA, and 1 mM GTP at 37°C for 20 min, followed by addition of 20 μM taxol. Asymmetrically labeled fluorescent microtubules containing a bright rhodamine seed at the minus end were prepared according to Hyman et al. (29).
Bead Motility Assay.
Motility assays of beads and the apparatus used for bead nanometry were as described previously (20). Experiments were performed in a buffer (50 mM K⋅acetate/4 mM Mg2SO4/1 mM EGTA/0.5 mg/ml casein/10 mM Tris⋅acetate, pH 7.5) containing an oxygen scavenging system (18 μg/ml catalase/100 μg/ml glucose oxidase/12 mM glucose) (30) at 25–27°C.
For the statistical analysis of binding and moving beads, beads mixed with 22S dynein at different molar ratio of dynein to beads were tested in assays by placing a trapped bead in contact with a microtubule on the glass surface. Binding and movement by the bead was judged after placing the bead at nine different positions of three microtubules for 10 s each. Data for displacements of the beads were passed through a 50-Hz low pass filter. The sampling time of each point was 2.5 ms.
Results
Demonstration of Bead Movement by Single 22S Dynein Molecules.
The 22S dynein-coated beads moved along microtubules in an optical trap and showed repeated attachment, force development, and detachment. Although these features resemble those of kinesin-coated beads, the direction of 22S dynein movement as determined by bead movement on asymmetrically labeled fluorescent microtubules was toward the minus end of microtubules, opposite to that of kinesin.
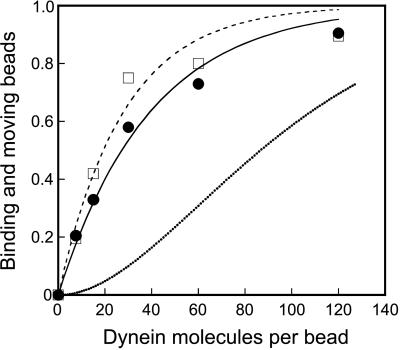
To determine whether a single molecule of 22S dynein can move a bead for a long distance (>50 nm), we prepared 22S dynein-coated beads at various molar ratios of dynein to beads at the mixing and examined bead binding and moving in the absence and presence of ATP, respectively. As 22S dynein molecules exist as single heterotrimer molecules in solution (12) and it is unlikely that they make oligomers by diluting them with a buffer used for mixing with beads, 22S dynein molecules are considered to behave as single molecules at the binding to beads. In the absence of ATP, single dynein molecules are expected to bind tightly to microtubules (31). Fig. 1 shows the fraction of binding and moving beads. Both the binding and moving plots fit better to the curves predicted for single molecule behavior (χ2 = 0.738 and 0.321, respectively) than to the curves predicted for two or more molecules being required for binding and moving (χ2 = 1.19 and 5.04, respectively) (not shown) when we assume a Poisson probability of 22S dynein molecules attaching to the beads (23). The curve fits are given by 1 − exp(−x/27.9) for binding beads, and 1 − exp (−x/39.3) for moving beads, where x is the molar ratio of dynein to a bead at the mixing. These results indicate that the bead binding and the bead movement can be explained as single molecular events, respectively. The slightly lower values for the moving beads may reflect the fraction of 22S dynein molecules, which can bind to a microtubule but cannot move on it because of an unfavorable attachment. Alternatively, the number of moving beads might be underestimated because the period of movement was too short to detect the movement.
Figure 1.
Fraction of beads binding (□) and moving (●) in the absence and the presence of 3 μM ATP, respectively, at various molar ratios of 22S dynein molecules to beads at the mixing. The plots are fitted to 1 − exp(−x/27.9) (broken line) and 1 − exp(−x/39.2) (solid line), where x is the molar ratio of 22S dynein to beads at the mixing. The dotted line indicates the probability, including geometric considerations, of a bead adsorbing two or more 22S dynein molecules that can simultaneously interact with a microtubule.
Even if the fitting of the moving curve was not enough to show single molecular events, we have another explanation to show that a single molecule of 22S dynein can move a bead. As the bead rotates in the optical trap because of Brownian motion, any active single 22S dynein molecule can contact the microtubule at some point in time and bind to it in the absence of ATP. Any bead that does not bind in the absence of ATP is assumed to have no functional dynein molecules attached to it. From this unbound fraction, the fraction of the beads that has two or more 22S dynein molecules was calculated. For two dynein molecules to interact simultaneously with a microtubule, we estimate that the two molecules must be within 440 nm of each other on a 1-μm bead because a dimension of dynein molecule is ≈50 nm (12, 32). This corresponds to 5% of the total surface area of a 1-μm bead. Taking this into account, only a part of the beads can bind to microtubules in the absence of ATP via two or more 22S dynein molecules (dotted line in Fig. 1). The observed fraction of moving beads (filled circle in Fig. 1) was far greater than this, indicating that a single molecule is sufficient to move a bead under our experimental conditions.
At mixing ratios of dynein to beads of 10:1 and 30:1, more than 97 and 90%, respectively, of the moving beads are estimated to be driven by a single dynein molecule if the geometry was considered. On the other hand, more than 90% of the moving beads showed force generation profiles similar to those of force generation as shown in Figs. 2a and 3a. We therefore concluded that these profiles of the bead movement were attributable to a single molecule of 22S dynein and that a single molecule of 22S dynein can move processively.
Figure 2.
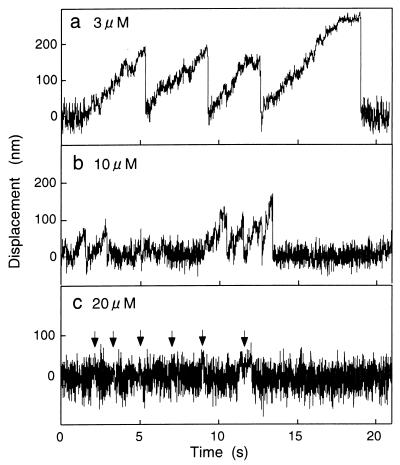
Time courses of bead displacement driven by single 22S dynein molecules at various concentrations of ATP. ATP concentrations were as indicated. The molar ratio of 22S dynein to beads was 30:1 at the mixing, and the trap stiffness was 0.010 pN/nm. The arrows indicate signals of active movements, judging from the decrease of Brownian noise.
Figure 3.
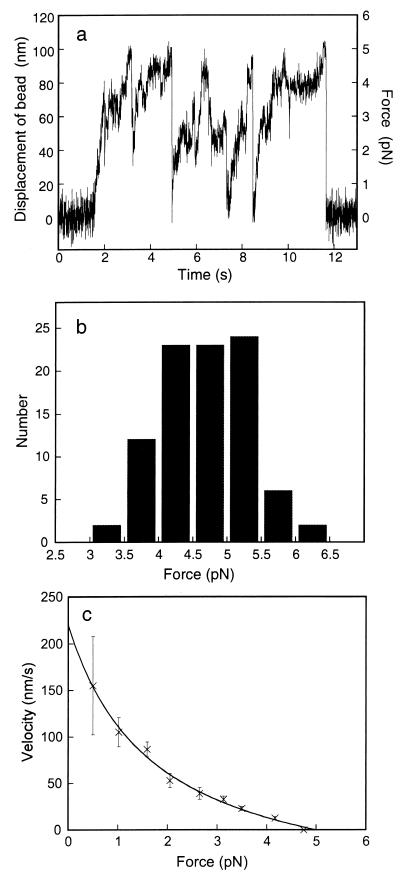
Displacement and force of beads coated with single 22S dynein molecules at high trap stiffness. (a) Time course of displacement of a 22S dynein-coated bead at 3 μM ATP. The bead was prepared by mixing 22S dynein molecules and beads at 10:1 molar ratio. The trap stiffness was 0.050 pN/nm. Force (right-hand scale) was calculated from the displacement multiplied by the trap stiffness (20). (b) Histogram of the maximum force developed by single molecules of 22S dynein. The maximum force was measured where the bead stayed more than 0.2 s at the maximal level of displacement without taking further steps. Trap stiffness was 0.050–0.20 pN/nm. The mean and standard deviation are 4.7 and 0.64 pN, respectively (n = 92). (c) Force-velocity relationship of single 22S dynein molecules. Twelve runs of bead displacements were averaged, and the displacement of 22S dynein molecules was calculated according to Kojima et al. (20). The velocities were calculated from the slopes of the displacement. Error bars indicate confidence intervals of the velocities at a confidence level of 95%. The curve fit (v + 1.70)(y + 74.9) = 504 refers to Hill (33).
Processive Movement of Single 22S Dynein Molecules Depends on ATP Concentration.
To analyze the movement of single 22S dynein molecules, we prepared the bead by mixing 22S dynein and beads at 30:1 molar ratio for all of the experiments described below. Fig. 2a shows a typical example of a time course of bead displacement driven by a single 22S dynein molecule at 3 μM ATP. As the ATP concentration increased, the pattern of bead displacement changed. At 10 μM ATP, the beads detached from the microtubules and were pulled back to the center of the trap before they reached maximum force (plateau) and the profile of the movement was truncated (Fig. 2b).
At 20 μM ATP, the beads did not show clear displacement, as shown in Fig. 2c. Active movement (arrows in Fig. 2c) was verified by a decrease in Brownian movement of the bead (17). The signals were spike-like, but they were almost buried in noise. The profile of the movement was observed for almost all of the bead that showed some interaction with microtubules, and the profile was not much affected by ATP concentrations higher than 20 μM ATP.
These experiments were done under low trap stiffness (0.010 pN/nm) so that 22S dynein can travel long distances at low force. The traveling distances (the distances between the center of the optical trap and the plateau) reduced as ATP concentrations increased. These results show that single molecules of 22S dynein move processively at lower concentrations of ATP, but they don't move processively at higher concentrations of ATP.
Force Development and Displacement of a Single 22S Dynein Molecule.
To estimate the maximum force of single 22S dynein molecules, we observed the bead movement at 3 μM ATP under relatively high trap stiffness (0.050 pN/nm) (Fig. 3a). Under these conditions, the bead traveled ≈100 nm and stayed for a while at the maximal level. Fig. 3b shows a histogram of the maximum force of 22S dynein measured from the maximal level of the displacement where the bead stayed more than 0.2 s at trap stiffness of 0.050–0.20 pN/nm. The mean value was 4.7 ± 0.64 pN (mean ± SD, n = 92). Under relatively low trap stiffness (0.010 pN/nm), the beads were able to travel to the maximal level to show the maximum force of 4–5 pN, although many runs showed detachment before the bead reached to the plateau.
Next, we examined the force-velocity relationship for 22S dynein. The displacement corresponding to 22S dynein measurement is obtained by correcting the bead displacement by an attenuation factor (20). The force-velocity relationship of single 22S dynein molecules was not linear but instead was convex down (Fig. 3c).
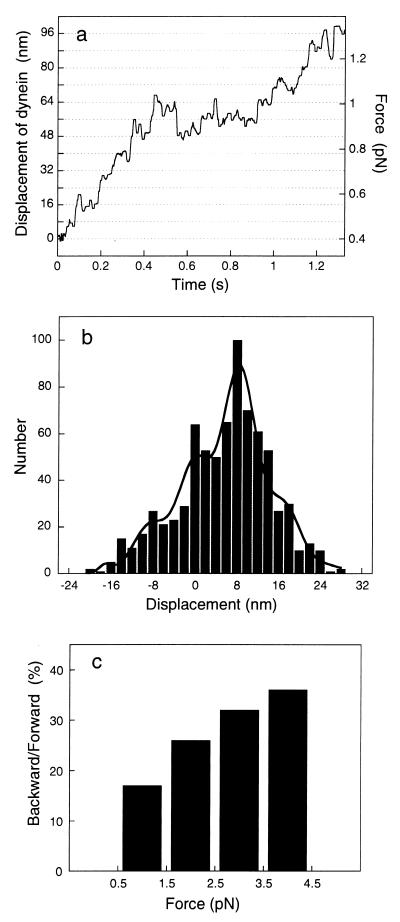
During force development, the bead moved stepwise (Fig. 4a). The bead displacement was converted to displacement of 22S dynein molecules, as described above. Single molecules of 22S dynein moved and paused briefly at around every 8-nm displacement, suggesting that dynein has a unitary step size of ≈8 nm. To estimate the step size of 22S dynein molecules, the net displacements was scored at every 0.1-s interval (Fig. 4b). The 0.1-s interval was chosen because it corresponded to approximately half a dwell period at the same position. There was a distinguishable peak at around 8 nm as well as small peaks. These peaks were found to be at multiples of 8.5 nm from multiple Gaussian fitting. Pairwise distance analysis (34) of the same data (Fig. 4a) revealed that there is a 7.9-nm periodicity in the histogram of pairwise distances between all pairs of data points (not shown). From these results, the unitary step size of 22S dynein molecule was estimated to be around 8 nm.
Figure 4.
Stepwise movement of 22S dynein molecules. (a) Time course of displacement of 22S dynein at 3 μM ATP. The bead was prepared by mixing 22S dynein molecules and beads at a 30:1 molar ratio. The trap stiffness was 0.010 pN/nm, and the stiffness of the proteins was 0.20 pN/nm. The displacement corresponding to 22S dynein movement is obtained by correcting the bead displacement multiplied by the elastic correction factor, 1.05, due to the compliance of proteins (20) and passed through a median filter with 12.5-ms window. The displacement of 22S dynein was calculated at a force of more than 0.4 pN to avoid a large amount of noise in the attenuation factor at low force. (b) Histogram of step size. The window of 100-ms interval was moved every 5 ms on a and another similar example, and the net displacements in a given interval were scored. The histogram was fitted to multiple Gaussian curves as indicated by integer of i = −2 to 3 of Ai ⋅ exp{−(x − i⋅D)2/(2σ2)}, where D is the elementary step size and Ai and σ are the amplitude and the standard deviation. D and σ are 8.5 and 3.2 nm, respectively. (c) Ratio of backward steps to forward steps at different levels of force.
The peak at around −8 nm indicates that backward steps occur frequently. The ratio of backward steps to forward steps was calculated at the 1-pN force levels from 0.5 to 4.5 pN (Fig. 4c). The results showed that the backward steps occur more frequently at higher load.
Discussion
Under our experimental conditions, the average number of functional 22S dynein molecules on a beads was estimated to be 1 when the molar ratio of 22S dynein to bead was about 40 to 1 at the mixing. This value is about 20 to 40 times higher than the values for kinesin-coated beads (20, 23, 35), suggesting that efficiency of binding of functional 22S dynein to beads is lower than that of kinesin. This is supported by the observation of the surface density of functional dynein c molecules using fluorescent ATP analogue (25). Nonetheless, several lines of evidence from the statistical analysis showed that single molecules of 22S dynein were able to move the bead in an optical trap (see Results).
The bead displacements of greater than 100 nm by a single molecule of 22S dynein mean that 22S dynein can move processively like kinesin. The maximum force of 4.7 pN for a single molecule of 22S dynein was comparable to that of a single inner arm on a doublet microtubule of flagellar axonemes from sea urchin sperm [6 pN (24)]. Because 22S dynein did not pause long at the maximal level and it was difficult to judge whether the bead reached the maximal force level or not, our value could be an underestimate. This value is in the same range as those of kinesin [5–7pN (20, 36)] and myosin [3–6pN (15, 16)].
Besides the direction of the movement on microtubules, the profile of movement of 22S dynein is different from that of kinesin with regard to several points. First, processive movement of 22S dynein can be observed only at low ATP concentrations (≦10 μM). At higher concentrations of ATP (≧20 μM), the 22S dynein-coated beads did not move more than 40 nm from the center of the optical trap, and the signal showed spikes like myosin movement (15). We tentatively describe this feature as nonprocessive, although we cannot discriminate between nonprocessive and less-processive from the record, as the dimension of 22S dynein molecule expand ≈40 nm and the exact step size coupled to crossbridge cycle is unknown. Axonemal outer dynein arms are known to accelerate sliding velocity between adjacent doublet microtubules in flagellar movement (37, 38). To achieve very fast sliding movement (≈15 μm/s), outer arm dynein probably has a low duty ratio at high concentrations of ATP, so that multiple arms in tandem on a doublet microtubule do not interfere each other. As the ATP concentration in living flagella and cilia is on the order of mM, the outer arm dynein seem to be designed to move nonprocessively at higher concentrations of ATP. Probably, outer arm dyneins seem to have a processive ancestor common to inner arm dyneins and cytoplasmic dyneins, and the processivity at low ATP concentrations may be vestiges of it. Alternatively, the processivity of 22S dynein might be incidental due to very slow ATP binding to multiple heads. In any case, 22S dynein can be switched between processive and nonprocessive modes by a subtle change of ATP concentrations. We suppose that this ATP concentration-dependence is a unique property of dynein molecules that may be related to the multiple ATP-binding sites within each motor domain, although the role of this property in vivo is speculative and as yet unknown.
The second difference between 22S dynein and kinesin is that the force-velocity relationship of single 22S dynein molecules was convex down whereas that of single kinesin measured in the same system was linear (20). Recent study of the kinesin force-velocity curve obtained with a force-feedback system showed that the curves were concave down at 2 mM ATP and nearly linear at 5 μM ATP (39). These observations suggest differences in the mechanochemical cycles of 22S dynein and kinesin. The convex down curve for 22S dynein could be partly explained by increasing frequencies of backward steps at higher loads. The convex down force-velocity curve is similar to that of muscle fibers (33).
The third difference is that the profile of 22S dynein movement shows backward steps during force development (Fig. 4a). The unitary size of these backward steps was about 8 nm, the same as forward steps, and higher frequencies of backward steps were observed at higher load. These frequencies are significantly higher than those for kinesin movement [2–8% at 3–6 pN (20)] and similar to those for inner-arm dynein c of Chlamydomonas (25) measured in the same optical trap assay system. Higher frequencies of backward steps suggest large detachment rates of dynein molecules, and this property may be important for axonemal dyneins that move fast in living cilia and flagella.
In the optical trap we used, the 22S dynein-coated bead did not show lateral movement and the long-range backward movement (100 nm) observed for cytoplasmic dynein in a similar bead movement assay without optical trapping (27). Shingyoji et al. (24) reported force oscillations by flagellar dynein arms using an assay in which dynein sits on a doublet microtubule. We did not observe such an oscillatory movement for 22S dynein derived from ciliary outer arms, supporting the idea that the oscillatory movement is caused by inner arms. The backward steps during the course of directional movement suggests that a common property of dynein molecules is to move backward to some extent.
Although the dynein step size of 8 nm is the same as kinesin step size, dynein and kinesin probably walk on a microtubule in different ways because the dimensions of these molecules are quite different. A single kinesin head measures 70 × 45 × 45 Å (40) whereas the diameter of a spherical motor domain of 22S dynein molecule is ≈130–160 Å (12, 32). The dynein motor domain has an extended structure: a stalk and a stalk-head, and stalk-heads can bind to microtubules (41, 42). The stalk structure may help multiple dynein heads to bind microtubules at 8-nm periodicity of tubulin dimers. However, it is difficult to envision that a dynein motor domain interacts with every tubulin dimer along a single protofilament in a hand-over-hand fashion, as has been proposed for kinesin (43), especially for three-headed 22S dynein. Each of the three heads might interact with a microtubule by turns like an inchworm, and/or they could follow multiple protofilaments in a single microtubule.
Our motility assays of single 22S dynein molecules have revealed that 22S dynein shows a unique property of ATP concentration-dependence, such that the motor can move processively at lower concentrations of ATP but not at high ATP concentrations. There have been no reports describing such a property for any motor proteins. Recent structural studies of dynein (41, 42) have led to the proposal that the mechanism of force production by dynein is different from those of kinesin and myosin (44). Further studies on the motility of single dynein molecules, as well as studies on molecular structure and kinetics, should elucidate the mechano-chemical transduction of dynein molecules.
Acknowledgments
We thank J. Yajima and K. Shiroguchi for discussions. We are also grateful to Dr. S. Endow for reading through the manuscript and comments. This work was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Science and Culture of Japan.
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.050585297.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.050585297
References
- 1.Gibbons I R. Proc Natl Acad Sci USA. 1963;50:1002–1010. doi: 10.1073/pnas.50.5.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paschal B M, Vallee R B. Nature (London) 1987;330:181–183. doi: 10.1038/330181a0. [DOI] [PubMed] [Google Scholar]
- 3.Schnapp B J, Reese T S. Proc Natl Acad Sci USA. 1989;86:1548–1552. doi: 10.1073/pnas.86.5.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verde F, Berrez J M, Antony C, Karsenti E. J Cell Biol. 1991;112:1177–1187. doi: 10.1083/jcb.112.6.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walczac C E, Vernos I, Mitchison T J, Karsenti E, Heald R. Curr Biol. 1998;8:903–913. doi: 10.1016/s0960-9822(07)00370-3. [DOI] [PubMed] [Google Scholar]
- 6.Dujardin D, Wacker U I, Moreau A, Schroer T A, Rickard J E, De Mey J R. J Cell Biol. 1998;141:849–862. doi: 10.1083/jcb.141.4.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Starr D A, Williams B C, Hays T S, Goldberg M L. J Cell Biol. 1998;142:763–774. doi: 10.1083/jcb.142.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gibbons B H, Asai D J, Tang W-J, Hays T S, Gibbons I R. Mol Biol Cell. 1994;5:57–70. doi: 10.1091/mbc.5.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tanaka Y, Zhang Z, Hirokawa N. J Cell Sci. 1995;108:1883–1893. doi: 10.1242/jcs.108.5.1883. [DOI] [PubMed] [Google Scholar]
- 10.Gibbons I R. Cell Motil Cytoskeleton. 1995;32:136–144. doi: 10.1002/cm.970320214. [DOI] [PubMed] [Google Scholar]
- 11.Johnson K A, Wall J S. J Cell Biol. 1983;96:669–678. doi: 10.1083/jcb.96.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Toyoshima Y Y. J Cell Biol. 1987;105:887–895. doi: 10.1083/jcb.105.2.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson J A. Annu Rev Biophys Biophys Chem. 1985;14:161–188. doi: 10.1146/annurev.bb.14.060185.001113. [DOI] [PubMed] [Google Scholar]
- 14.Vale R D, Toyoshima Y Y. Cell. 1988;52:459–469. doi: 10.1016/s0092-8674(88)80038-2. [DOI] [PubMed] [Google Scholar]
- 15.Finer J T, Simmons R M, Spudich J A. Nature (London) 1994;368:113–119. doi: 10.1038/368113a0. [DOI] [PubMed] [Google Scholar]
- 16.Ishijima A, Kojima H, Higuchi H, Harada Y, Funatsu T, Yanagida T. Biophys J. 1996;70:383–400. doi: 10.1016/S0006-3495(96)79582-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Molloy J E, Burns J E, Kendrick-Jones J, Tregear R T, White D C. Nature (London) 1995;378:209–212. doi: 10.1038/378209a0. [DOI] [PubMed] [Google Scholar]
- 18.Svoboda K, Schmidt C F, Schnapp B J, Block S M. Nature (London) 1993;365:721–727. doi: 10.1038/365721a0. [DOI] [PubMed] [Google Scholar]
- 19.Coppin C M, Pierce D W, Hsu L, Vale R D. Proc Natl Acad Sci USA. 1997;94:8539–8544. doi: 10.1073/pnas.94.16.8539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kojima H, Muto E, Higuchi H, Yanagida T. Biophys J. 1997;73:2012–2022. doi: 10.1016/S0006-3495(97)78231-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Higuchi H, Muto E, Inoue Y, Yanagida T. Proc Natl Acad Sci USA. 1997;94:4395–4400. doi: 10.1073/pnas.94.9.4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Howard J, Hudspeth A J, Vale R D. Nature (London) 1989;342:154–158. doi: 10.1038/342154a0. [DOI] [PubMed] [Google Scholar]
- 23.Block S M, Goldstein L S B, Schnapp B J. Nature (London) 1990;348:348–352. doi: 10.1038/348348a0. [DOI] [PubMed] [Google Scholar]
- 24.Shingyoji C, Higuchi H, Yoshimura M, Katayama E, Yanagida T. Nature (London) 1998;393:711–714. doi: 10.1038/31520. [DOI] [PubMed] [Google Scholar]
- 25.Sakakibara H, Kojima H, Sakai Y, Katayama E, Oiwa K. Nature (London) 1999;400:586–590. doi: 10.1038/23066. [DOI] [PubMed] [Google Scholar]
- 26.Mehta A D, Rock R S, Rief M, Spudich J A, Mooseker M S, Cheney R E. Nature (London) 1999;400:590–593. doi: 10.1038/23072. [DOI] [PubMed] [Google Scholar]
- 27.Wang Z, Khan S, Sheetz M P. Biophys J. 1995;69:2011–2023. doi: 10.1016/S0006-3495(95)80071-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weingarten M D, Lockwood A H, Hwo S Y, Kirschner M W. Proc Natl Acad Sci USA. 1975;72:1858–1862. doi: 10.1073/pnas.72.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hyman A, Drechsel D, Kellogg D, Salser S, Sawin K, Stefen P, Wordeman L, Mitchison T. Methods Enzymol. 1991;196:478–485. doi: 10.1016/0076-6879(91)96041-o. [DOI] [PubMed] [Google Scholar]
- 30.Kishino A, Yanagida T. Nature (London) 1988;334:74–76. doi: 10.1038/334074a0. [DOI] [PubMed] [Google Scholar]
- 31.Porter M E, Johnson K A. J Biol Chem. 1983;258:6575–6581. [PubMed] [Google Scholar]
- 32.Goodenough U, Heuser J. J Mol Biol. 1984;180:1083–1118. doi: 10.1016/0022-2836(84)90272-9. [DOI] [PubMed] [Google Scholar]
- 33.Hill A V. Proc R Soc London Ser B. 1938;126:139–195. [Google Scholar]
- 34.Schnitzer M J, Block S M. Nature (London) 1997;388:386–390. doi: 10.1038/41111. [DOI] [PubMed] [Google Scholar]
- 35.Coy D L, Wagenbach M, Howard J. J Biol Chem. 1999;274:3667–3671. doi: 10.1074/jbc.274.6.3667. [DOI] [PubMed] [Google Scholar]
- 36.Svoboda K, Block S M. Cell. 1994;77:773–784. doi: 10.1016/0092-8674(94)90060-4. [DOI] [PubMed] [Google Scholar]
- 37.Gibbons B H, Gibbons I R. Biochem Biophys Res Commun. 1976;73:1–6. doi: 10.1016/0006-291x(76)90488-5. [DOI] [PubMed] [Google Scholar]
- 38.Yano Y, Miki-Noumura T. J Cell Sci. 1981;48:223–239. doi: 10.1242/jcs.48.1.223. [DOI] [PubMed] [Google Scholar]
- 39.Visscher K, Schnitzer M J, Block S M. Nature (London) 1999;400:184–189. doi: 10.1038/22146. [DOI] [PubMed] [Google Scholar]
- 40.Kull F J, Sablin E P, Lau R, Fletterick R J. Nature (London) 1996;380:550–555. doi: 10.1038/380550a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gee M A, Heuser J E, Vallee R B. Nature (London) 1997;390:636–639. doi: 10.1038/37663. [DOI] [PubMed] [Google Scholar]
- 42.Koonce M P. J Biol Chem. 1997;272:19714–19718. doi: 10.1074/jbc.272.32.19714. [DOI] [PubMed] [Google Scholar]
- 43.Howard J. Annu Rev Physiol. 1996;58:703–729. doi: 10.1146/annurev.ph.58.030196.003415. [DOI] [PubMed] [Google Scholar]
- 44.Gee M A, Vallee R B. Eur Biophys J. 1998;27:466–473. doi: 10.1007/s002490050157. [DOI] [PubMed] [Google Scholar]