Abstract
In this paper, we present evidence that activation of 5-hydroxytryptamine 2B (5-HT2B) receptors by serotonin (5-HT) leads to cell-cycle progression through retinoblastoma protein hyperphosphorylation and through activation of both cyclin D1/cdk4 and cyclin E/cdk2 kinases by a mechanism that depends on induction of cyclin D1 and cyclin E protein levels. The induction of cyclin D1 expression, but not that of cyclin E, is under mitogen-activated protein kinase (MAPK) control, indicating an independent regulation of these two cyclins in the 5-HT2B receptor mitogenesis. Moreover, by using the specific platelet-derived growth factor receptor (PDGFR) inhibitor AG 1296 or by overexpressing a kinase-mutant PDGFR, we show that PDGFR kinase activity is essential for 5-HT2B-triggered MAPK/cyclin D1, but not cyclin E, signaling pathways. 5-HT2B receptor activation also increases activity of the Src family kinase, c-Src, Fyn, and c-Yes. Strikingly, c-Src, but not Fyn or c-Yes, is the crucial molecule between the Gq protein-coupled 5-HT2B receptor and the cell-cycle regulators. Inhibition of c-Src activity by 4-amino-5-(4-methylphenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine (PP1) or depletion of c-Src is sufficient to abolish the 5-HT-induced (i) PDGFR tyrosine kinase phosphorylation and MAPK activation, (ii) cyclin D1 and cyclin E expression levels, and (iii) thymidine incorporation. This paper elucidates a model of 5-HT2B receptor mitogenesis in which c-Src acts alone to control cyclin E induction and in concert with the receptor tyrosine kinase PDGFR to induce cyclin D1 expression via the MAPK/ERK pathway.
Keywords: cyclins D1 and E, G protein-coupled receptors, platelet-derived growth factor receptor, receptor signaling, c-Src
Serotonin (5-hydroxytryptamine, 5-HT) is involved in regulating cellular functions of central and peripheral nervous systems, endocrine and exocrine organs, as well as vascular and hematopoietic systems (1). These actions are mediated by numerous cognate receptors. For instance, 5-HT acts as a growth factor in a variety of cells, a function that is mediated by 5-HT1A, 5-HT1B, 5-HT2A, 5-HT2B, or 5-HT2C receptor subtypes (2–5). The 5-HT2B receptor belongs to the G protein-coupled receptor (GPCR) family. Binding of 5-HT to 5-HT2B receptor activates Gq protein, thereby stimulating phospholipase C, which initiates a rapid release of inositol trisphosphate and results in a rise in intracellular calcium levels (6). The signal transduction pathways activated downstream of the 5-HT2B receptor include the Ras and mitogen-activated protein kinase (MAPK of the MAPK/ERK subfamily) pathways (3). Despite numerous studies of 5-HT signal transduction pathways, the critical steps from signal integration to its proliferative action have not been elucidated.
The regulation of cell proliferation is a complex process controlled by external mitogenic factors. Once cells are induced to proliferate, passage through the mitotic cell cycle is directed by activation of a series of cyclins and their catalytic subunits, cyclin-dependent kinases (cdks) (7). Mitogenic factors act by stimulating both the proliferation of quiescent cells arrested in G0 and the progression of the cell cycle through the restriction point in late G1. Progression through G1 and the G1/S transition in mammalian cells is regulated by cdk4 and cdk6, which form complexes with cyclin D1, and by cdk2, which forms a complex with cyclin E (7). The G1 cyclin/cdk complexes drive the phosphorylation of retinoblastoma protein (pRb) that represents the limiting event in G1 progression (8). However, the mechanism whereby 5-HT triggers G1 progression has not been examined.
The link between upstream signaling circuitry and the cell-cycle machinery is of interest. Identified candidates for this link are Ras and MAPK (8, 9). Cyclin D1 and cyclin E expression are induced by growth factors, including estrogen and heregulin, possibly through Ras/MAPK pathways (10, 11). The functional link between receptor tyrosine kinase (RTK) pathways and cdks seems established; however, little is known about the G1 effectors of mitogenic signaling triggered by GPCRs. Recently, GPCRs have been shown to stimulate rapid tyrosine phosphorylation of RTKs, a phenomenon known as transactivation (12). Cytoplasmic tyrosine kinases of the Src family have been independently implicated in activation of the MAPK pathway by GPCR (13–15). However, the downstream targets and the role in biological responses of the cross-talk between GPCRs and tyrosine kinase activation have not been elucidated in the cell-cycle context.
We previously reported that expression of 5-HT2B receptors in the nontransformed mouse fibroblast LMTK− cells is mitogenic and transforming, because these cells form foci and induce tumors in nude mice. Also, the same receptor is overexpressed with a similar coupling to Ras in human carcinoid tumor cells (3). Because uncontrolled cell proliferation is the hallmark of cancer cells, studying of the complete signal-transduction system from the membrane to the cell-cycle machinery is essential to understand 5-HT-dependent transformation of this cell line.
In this study, the mechanism by which 5-HT binding to the 5-HT2B receptor leads to activation of the cell-cycle machinery is examined. We present evidence for the differential involvement of Src and the platelet-derived growth factor receptor (PDGFR) in 5-HT2B-mediated cell-cycle progression.
Materials and Methods
Antibodies.
All antibodies were from Santa Cruz Biotechnology except the anti-active MAPK from Promega, the anti-pRb from PharMingen, and the anti-phosphotyrosine PY20 from Upstate Biotechnology.
Cell Culture and Transfection.
Mouse fibroblast LMTK− cells were transfected stably with an expression vector (pSG5) carrying the mouse 5-HT2B receptor cDNA by using Lipofectamine (GIBCO) as described previously (16) to generate LM6 cells. The plasmid (pcDNA3) carrying a mutant PDGFR-β (K634A) at the kinase activity site (17) was introduced into LM6 cells by using high-efficiency transferrin receptor-mediated Lipofectamine transfection (18). LM6 cells grown in 90-mm dishes were treated with 4 ml of serum- and antibiotic-free medium, including 50 μM Lipofectamine, 10 μg of total plasmid DNA, and 16 μM transferrin overnight, then medium including 10% serum was added for 24 h. At 48 h after transfection, cells were serum-starved for 36 h, then treated and lysed. With this protocol, transfection efficiency in LM6 cells reached 85% as measured by cotransfection of a β-galactosidase-containing construct.
To reduce endogenous protein expression, LM6 cells were transfected with phosphorothioate-protected oligonucleotides also by using transferrin receptor-mediated Lipofectamine transfection (19). After transfection in similar conditions of oligonucleotides corresponding to mouse p42 and p44MAPK (20), c-Src (21), or Fyn (22), cells were serum-starved for 36 h, then treated and lysed. The efficiency of transfection was verified by Western blot analysis. For example, at least 70% of cells became transfected for MAPK antisense oligonucleotides within 5 h, and transfection reached ≈95% 16 h after adding medium that included serum.
Cell-Cycle Analysis.
Progression through the cell cycle was monitored by flow cytometric analysis of the DNA content of cell populations stained with propidium iodide and was carried out with a fluorescence-activated cell sorter (FACScan flow cytometer; Becton Dickinson). The percentage of cells within G1, S and G2, and M phases was determined by using cellquest software (Becton Dickinson).
Thymidine Incorporation.
Cells were seeded on 24-well plates at a density of 103 cells per well, grown overnight, and serum-starved for 36–48 h. Quiescent cells were treated with 5-HT at different concentrations and time points, and 0.5 μCi (1 Ci = 37 GBq) of [3H]thymidine was added to the culture during the last 4 h of incubation. The free radioactive thymidine was washed away in 5% trichloroacetic acid, and the incorporated radioactive thymidine was quantified by scintillation counting.
Cell Lysis, Immunoprecipitation, and Immunoblotting.
After treatment of quiescent cells with 5-HT, cells were lysed in an immunoprecipitation buffer containing protease inhibitors. Antibody/antigen complexes were detected with an enhanced chemiluminescence kit (Amersham Pharmacia) per manufacturer's instructions. For immunoprecipitations, lysates containing equal amounts of protein were incubated with the appropriate antibody overnight at 4°C, 25 μg/ml of protein A/G-Sepharose beads (Amersham Pharmacia) were added for 1 h, and the immune complexes were washed two times with immunoprecipitation buffer. Loading homogeneity was verified by stripping and reprobing the blots according to the enhanced chemiluminescence kit's recommendations. Densitometric analysis was performed with an image analyzer (GS-700; Bio-Rad).
Cyclin/cdk-Associated Kinase Assay.
For cyclin E/cdk2 kinase activity, the lysate (500 μg) was immunoprecipitated with cyclin E antibody, and histone H1 (2.5 μg) was used as a substrate. For cyclin D1/cdk4 kinase assay, the lysate (500 μg) was immunoprecipitated with cyclin D1 antibody, and glutathione S-transferase–retinoblastoma protein (GST-Rb; 10 μg) was used as a substrate according to the information found in ref. 23.
Kinase Assay for Src Family Kinases.
The enzyme assay designed to measure the phosphotransferase activity of Src family kinases in immunoprecipitates was used. The assay kit is based on phosphorylation of a specific substrate peptide derived from p34-cdc2 (24) by Src kinase. The enzymatic activity is exhibited as -fold increase (basal activity is 32.73 pmol/min per cell).
Data Analysis and Statistics.
Statistical analyses were performed according to the Kruskal–Wallis test. The chosen significance criterion was P < 0.05. All values given are representative of at least two duplicated independent experiments.
Results
5-HT2B Receptor Activation Stimulates S Phase Entry, pRb Phosphorylation, and G1 Cyclin Kinase Activity.
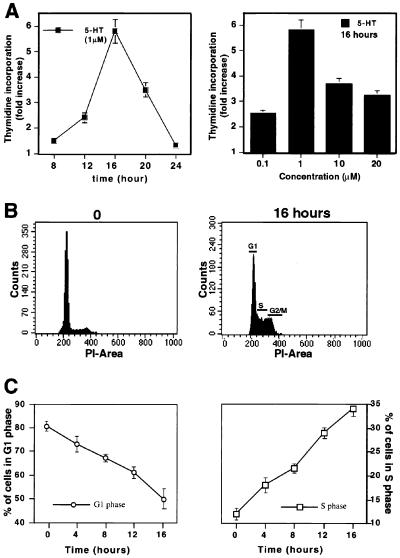
We determined the kinetics of thymidine incorporation of quiescent fibroblasts expressing 5-HT2B receptor (LM6). A peak of DNA synthesis was observed after 16 h of 5-HT treatment, at which time the rate of thymidine incorporation was increased 5-fold over uninduced cells (Fig. 1A). Activation of thymidine incorporation reached a maximum at 1 μM 5-HT and could be blocked by ritanserin (1 μM), a selective antagonist of 5-HT2B receptors (data not shown). Flow cytometric analysis of serum-starved cells stimulated with 5-HT for 0–16 h showed that >60% of cells had begun replicating their DNA by the time the peak of thymidine incorporation occurred (Fig. 1B). The proportion of cells in S phase increased >30% after 16 h, concomitant with a decrease in the number of cells in G1 phase (from 80% to 50%; Fig. 1C).
Figure 1.
5-HT2B receptor activation stimulates S phase entry. (A) 5-HT increases thymidine incorporation. Quiescent cells were treated with 5-HT (1 μM) for the indicated time (Left) or with different concentrations of 5-HT for 16 h (Right). (B) 5-HT increases the percentage of cells in S phase. Representative cell-cycle profiles are presented at 0 h (Left) and 16 h (Right) after 5-HT treatment. PI, propidium iodide. (C) Progression from G1 phase (Left) to S phase (Right) after 5-HT (1 μM) treatment. Thymidine incorporation and the percentage of cells in G1, S, and G2/M phases were determined as described in Materials and Methods. Values represent the mean ± SEM for duplicate samples obtained from three independent experiments.
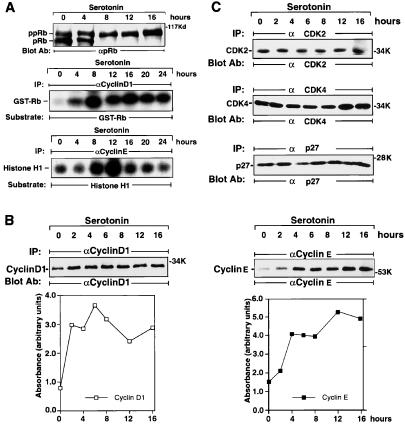
In growth-arrested LM6 cells, 70% of pRb was hypophosphorylated (Fig. 2A Top). 5-HT2B receptor stimulation resulted in an increase of hyperphosphorylated pRb within 4 h and in the almost complete disappearance of the hypophosphorylated form of pRb by 8 h. A maximum was reached between 12 and16 h, when over 80% of pRb was hyperphosphorylated. The kinetics of pRb phosphorylation preceded the time of entry into S phase. Cyclin D1/cdk4 activity increased significantly after 4 h of 5-HT treatment (1 μM), reaching a maximum at 8 h (Fig. 2A Middle). After stimulation, cyclin E/cdk2 activity increased between 4 and 8 h, peaking at 12 h (Fig. 2A Bottom). The time point when cyclin E/cdk2 activity peaks corresponds to the G1/S phase transition. The initial hyperphosphorylation of pRb is coincident with the peak in cyclin D1/cdk4 activation at 8 h. Subsequent progressive hyperphosphorylation of pRb could be caused by cyclin E/cdk2 activation, which reached a maximum at 12 h. This finding is consistent with progressive hyperphosphorylation of pRb by cyclin D1- and cyclin E-dependent kinase complexes.
Figure 2.
5-HT2B receptor activation stimulates pRb phosphorylation and G1 cyclin kinase activity. (A) Stimulation of pRb hyperphosphorylation and G1 cyclin levels by 5-HT. Membranes were probed with pRb antibody, which recognizes both hypo- and hyperphosphorylated states of protein. The positions of hypophosphorylated pRb and hyperphosphorylated ppRb are indicated (Top). For cyclin D1/cdk4 kinase assay (Middle) or for cyclin E/cdk2 kinase assay (Bottom), the 5-HT-dependent phosphorylation of both complexes was assayed as described in Materials and Methods. IP, immunoprecipitation; α, anti-. (B) 5-HT increases the protein levels of G1 cyclins D1 (Left) and E (Right). (C) Protein levels of cdk4, cdk2, or cdk inhibitor p27kip are not affected by 5-HT. The positions of cyclin D1, cyclin E, cdk4, cdk2, and p27kip are indicated. These data are representative of three individual experiments.
5-HT2B Receptor Stimulation Increases Cyclin D1 and Cyclin E Expression Levels.
Expression of cyclin D1 protein increased within 2 h after stimulation and achieved its maximum level between 6 and 8 h (Fig. 2B Left). Cyclin E expression levels increased more slowly, beginning at 2 h after stimulation and peaking at 12 h (Fig. 2B Right). The profiles of cyclin D1 and cyclin E expression levels induced by 5-HT correlate with the kinetics of cyclin D1- and cyclin E-associated kinase activity (Fig. 2A). The levels of other cyclins, such as cyclin A (data not shown), or cdk2, cdk4, and p27kip protein levels remained unchanged during this period (Fig. 2C).
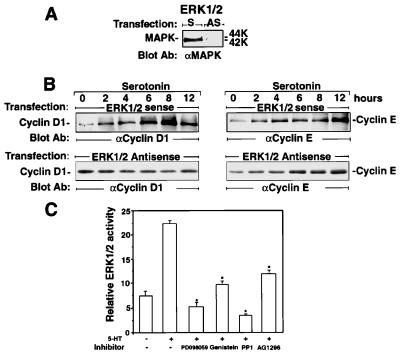
MAPK Regulates Cyclin D1 but Not Cyclin E Levels in the 5-HT2B Receptor Mitogenic Signaling and Is Regulated by Tyrosine Kinases.
In MAPK (ERK1/ERK2) antisense oligonucleotide-transfected cells (Fig. 3A), 5-HT (1 μM) was able to increase cyclin E expression to the same extent as seen in the sense oligonucleotide-transfected cells, whereas cyclin D1 stimulation was abolished (Fig. 3B). PD 098059, a specific inhibitor of the enzyme MEK-1 (25), at a concentration of 15 μM, which maximally inhibits MAPK activation (Fig. 3C), significantly inhibited 5-HT-induced cyclin D1 but not cyclin E levels. Genistein, a general tyrosine kinase inhibitor (15 μM) inhibited 5-HT-induced MAPK activity as assessed by the relative ERK1/ERK2 activity (Fig. 3C). In the same assay, both the PDGFR-specific inhibitor tyrphostin AG 1296 (0.1 μM) (26) and the Src family kinase inhibitor 4-amino-5-(4-methylphenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine (PP1; 1 μM) (27) reduced 5-HT-induced MAPK activity (Fig. 3C). By measuring thymidine incorporation, in addition to genistein (15 μM), AG 1296 (0.1 μM) significantly reduced 5-HT-induced DNA synthesis by 85% and 65%, respectively, indicating that activation of PDGFR could regulate cell-cycle machinery (data not shown).
Figure 3.
MAPK regulates cyclin D1 but not cyclin E levels in the 5-HT2B receptor mitogenic signaling and is regulated by tyrosine kinases. (A) Depletion of MAPK by antisense transfection. LM6 cells were transfected transiently with antisense (AS) or sense (S) oligonucleotides of mouse MAPK (ERK1/2) mRNA and probed with MAPK antibody. (B) 5-HT-induced cyclin D1 expression, but not that of cyclin E, is abolished in MAPK-depleted cells. After visualization with anti-cyclin D1 antibody, the membranes were washed and reprobed with cyclin E antibody. The positions of cyclin E and cyclin D1 are indicated. (C) MAPK activation is regulated by tyrosine kinases. After pretreatment with inhibitors for MAPK kinase (15 μM PD 098059), tyrosine kinase (15 μM genistein), Src (1 μM PP1), and PDGFR (0.1 μM AG 1296), the cells were incubated with 5-HT (1 μM) for 10 min, then revealed with anti-active MAPK antibody. After being revealed by enhanced chemiluminescence, the relative active ERK1 and ERK2 protein levels were assessed by using a luminometer. The stars indicate significant inhibition of the parameters as compared with 5-HT stimulation alone (P < 0.05; n > 3).
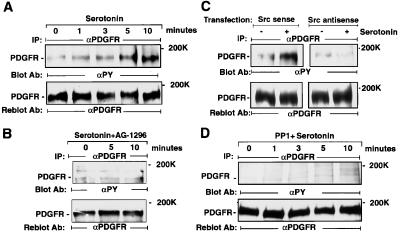
5-HT2B Receptor Activation Triggers Tyrosine Phosphorylation of PDGFR.
Time-course experiments revealed that in the absence of PDGF, 5-HT (1 μM) induces PDGFR-β tyrosine phosphorylation, reaching its maximum within 10 min in the absence of variation in the level of PDGFR protein (Fig. 4A). Ligand-independent phosphorylation of PDGFR by 5-HT could be blocked completely by AG 1296 at 0.1 μM (Fig. 4B). Overexpression of a kinase mutant PDGFR-β (K634A) diminished PDGFR phosphorylation by 5-HT (data not shown), indicating that 5-HT2B receptor stimulation transactivates PDGFR. c-Src depletion (Fig. 4C) or c-Src inhibition by PP1 (1 μM) (Fig. 4D) abolished 5-HT-induced PDGFR-β phosphorylation without changing PDGFR protein levels.
Figure 4.
5-HT2B receptor activation triggers tyrosine phosphorylation of PDGFR. (A) 5-HT stimulates PDGFR phosphorylation. Cells were incubated with 5-HT (1 μM) for the indicated times. (B) AG 1296 inhibits 5-HT-induced PDGFR phosphorylation. Cells were pretreated with AG 1296 (0.1 μM) for 15 min before stimulation with 5-HT (1 μM) for the indicated times. (C) c-Src depletion inhibits 5-HT-induced PDGFR phosphorylation. After transfection with c-Src sense or antisense oligonucleotides, serum-starved cells were stimulated with vehicle (−) or 5-HT (1 μM; +) for 5 min. (D) PP1 inhibits 5-HT-induced PDGFR phosphorylation. Cells were pretreated with PP1 (1 μM) for 15 min before stimulation with 5-HT (1 μM) for the indicated times. After cell lysis, 250 μg of samples was immunoprecipitated with anti-β-PDGFR antibody. The induction of PDGFR tyrosine phosphorylation was analyzed after immunoblotting with anti-phosphotyrosine antibody (Upper) and, after stripping, the same blot was reprobed with anti-PDGFR antibody (Lower). The position of PDGFR-β is indicated. These data are representative of at least two individual experiments.
5-HT2B Receptor Stimulation Activates Src Family Kinase Activity, and c-Src Activation Is Required for Thymidine Incorporation.
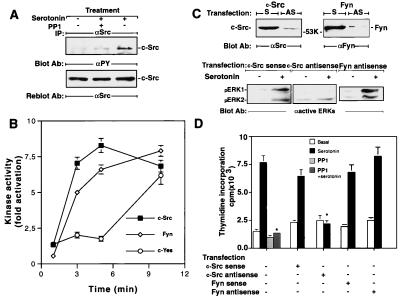
Stimulation of LM6 cells by 5-HT (1 μM) raises the phosphorylation level of c-Src within 5 min (Fig. 5A), whereas this phosphorylation was completely blocked by PP1 (1 μM), which also blocked PDGFR phosphorylation induced by 5-HT (Fig. 4D). No change in the level of Src protein was observed during this assay (Fig. 5A). In a Src kinase assay (24), 5-HT provoked a 1.5-fold increase in c-Src kinase activity at 1 min, reaching to the maximum (8-fold increase) at 3 min, which was sustained after 10 min of stimulation (Fig. 5B). This increase could be blocked by PP1 (1 μM) but not AG 1296 (1 μM) (data not shown). Other Src family kinases (Fyn and c-Yes) expressed in fibroblast cells could substitute for c-Src. Therefore, we also assayed Fyn and c-Yes activity in response to 5-HT. Fyn activity rose when 5-HT2B activation reached the maximum at 10 min (Fig. 5B). c-Yes activity was elevated later than c-Src activity was. c-Src-antisense transfection (but not sense) inhibited MAPK activity and thymidine incorporation, whereas Fyn antisense or sense transfection did not alter either activity (Fig. 5 C and D).
Figure 5.
5-HT induces Src family kinase activation, and activation of c-Src is required for MAPK and thymidine incorporation. (A) 5-HT induces phosphorylation of c-Src. Cells were pretreated with either vehicle or PP1 (1 μM) for 15 min before stimulation with 5-HT (1 μM) for 5 min. After immunoprecipitation with anti-Src antibody, the induction of c-Src tyrosine phosphorylation was analyzed after immunoblotting with anti-phosphotyrosine antibody (Upper) and, after stripping, reprobed with anti-Src antibody (Lower). The position of c-Src is indicated. (B) 5-HT induces Src family kinase activity. Quiescent cells were treated with 5-HT (1 μM) for the indicated times, washed, and lysed, and proteins were extracted. After immunoprecipitation with c-Src, Fyn, or c-Yes specific antibody, in vitro kinase activity was assessed as described in Materials and Methods. (C) Depletion of Src but not Fyn by antisense oligonucleotide transfection reduces MAPK activity induced by 5-HT. Cells were transfected transiently with antisense (AS) or sense (S) oligonucleotides of mouse c-Src or Fyn mRNA. Quiescent cell extract was treated with vehicle (Upper, −) or 5-HT (+) for 5 min, then lysed, and protein extracts were probed with corresponding antibodies (c-Src or Fyn, Upper) or anti-active MAPK antibody (Lower). The positions of c-Src, Fyn, and active ERK1 or ERK2 are indicated. (D) c-Src but not Fyn depletion abolishes 5-HT-induced thymidine incorporation. After the transfection of either c-Src or Fyn sense or antisense oligonucleotides, quiescent cells were treated with 5-HT (1 μM) for 16 h, thymidine incorporation was detected in these transfected cell lines or in PP1 (1 μM)-pretreated (for 15 min) cells. All values represent the mean ± SEM for duplicate samples obtained from at least two independent experiments.
c-Src Is Required for Both Cyclin D1 and Cyclin E Induction in 5-HT2B Receptor Mitogenic Signaling.
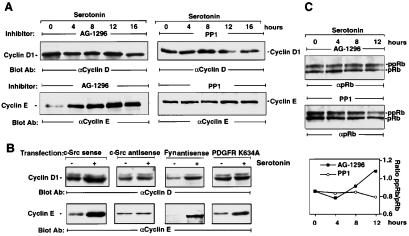
AG 1296 at the concentration that totally inhibits PDGFR-β phosphorylation (0.1 μM) reduced cyclin D1 expression levels induced by 5-HT stimulation, whereas induction in cyclin E expression remained unchanged (Fig. 6A). Similar data were obtained when LM6 cells were pretreated with a higher concentration of AG 1296 (1 μM). Moreover, when the kinase mutant PDGFR (K634A) was overexpressed, the cyclin D1, but not cyclin E, expression level was significantly reduced on stimulation (Fig. 6B). These data indicate that the signaling pathway downstream of PDGFR does not regulate cyclin E expression; however, PDGFR plays a key role in regulating 5-HT-induced cyclin D1 expression. PP1 at the concentration that totally inhibits c-Src kinase activity (1 μM) abolished both cyclin D1 and cyclin E levels induced by stimulation (Fig. 6A). Similar results in response to 5-HT were obtained in a c-Src-depleted cell line. However, in the Fyn-depleted cell line, neither cyclin E nor cyclin D1 expression levels were changed in response to 5-HT (Fig. 6B). Supporting evidence for these findings is the inhibition by AG 1296 of the initial hyperphosphorylation of pRb within the first 8 h after 5-HT2B receptor stimulation, a period in which pRb mainly is regulated by activation of cyclin D1/cdk4 kinase. However, 12 h after stimulation, progressive hyperphosphorylation of pRb through cyclin E/cdk2 kinase activity was blocked by PP1 but not by AG 1296 (Fig. 6C).
Figure 6.
c-Src is required for both cyclin D1 and cyclin E induction in 5-HT2B receptor mitogenic signaling. (A) Effect of tyrosine kinase inhibition on 5-HT-induced cyclin levels in LM6 cells. After pretreatment with AG 1296 (0.1 μM) or PP1 (1 μM) for 15 min, the cells were incubated with 5-HT at different time points, then revealed with cyclin D1 (R-124) antibody or with cyclin E antibody. The positions of cyclin E and cyclin D1 are indicated. (B) Effect of depletion of c-Src and Fyn or overexpression of a kinase-mutant PDGFR on 5-HT-induced cyclin D1 and cyclin E levels. Cells were transfected transiently with antisense or sense oligonucleotides of mouse c-Src or Fyn mRNA or plasmid-carrying kinase domain-mutated PDGFR (K634A). Quiescent cells were treated with vehicle (−) or 5-HT (+) for 12 h, then protein extracts were revealed with cyclin D1 antibody (72-12C). After visualization of immunocomplexes, the membranes were washed and reprobed with cyclin E antibody. The positions of cyclin E and cyclin D1 are indicated. (C) The effect of AG 1296 or PP1 on 5-HT-induced pRb hyperphosphorylation. Cells were pretreated with vehicle, PP1 (1 μM), or AG 1296 (0.1 μM) for 10 min before stimulation with 5-HT (1 μM) for the indicated times. Total cell lysates were analyzed by using pRb antibody. The positions of pRb and ppRb are indicated. These data are representative of at least two individual experiments. (Bottom) Ratios between ppRb and pRb that were evaluated by densitometry are plotted.
Discussion
In the absence of growth factors, 5-HT is potent at inducing cell-cycle progression of L cells expressing the 5-HT2B receptor (LM6) (Fig. 1). Stimulation of 5-HT2B receptor induces pRb hyperphosphorylation by activating both cyclin D1/cdk4 and cyclin E/cdk2 complexes through its ability to increase the cyclin D1 and cyclin E protein levels (Fig. 2). The induction of cyclin D1 and cyclin E proteins, which is important for progression of G1 phase and for transition to S phase, is, therefore, a critical target for 5-HT2B receptor-induced proliferation in G1 phase. The GPCR angiotensin II receptor activation has been shown to stimulate G1 phase progression through the induction of cyclin D1 (28). We elucidated that the regulation of G1 cyclin/cdk complex activity by 5-HT is independent of the cdk inhibitor p27kip, because p27kip did not shift from its association with cyclin D1/cdk4 to cyclin E/cdk2 (data not shown). Activation of the RTK ErbB-2 by heregulin in T-47D human breast cancer cells (10) or of the estrogen receptor in MCF-7 human breast cancer cells (23) triggers cell-cycle progression without significant decrease in p27kip expression levels. This study demonstrates that 5-HT2B receptor activation regulates cell-cycle progression by inducing cyclin E and cyclin D1 expression levels.
Preincubation of LM6 cells with PD 098059 at a concentration that completely inhibits MAPK activation also blocks 5-HT-induced thymidine incorporation. In MAPK-depleted cells, 5-HT still is able to induce cyclin E expression, whereas cyclin D1 induction is abolished, indicating that the MAPK pathway is necessary for 5-HT-dependent regulation of cyclin D1 but not of cyclin E levels (Fig. 3). Interestingly, MAPKs are required to pass the G1 restriction point in fibroblasts, and inhibition of MAPK activity abolishes cyclin D1 promoter activity (29). Inhibition of the MAPK by PD 098059 blocks heregulin-induced cell-cycle progression that is caused by cyclin D1 induction in T-47D human breast cancer cells (10). Therefore, our data indicate that, in 5-HT2B receptor mitogenic signaling, only cyclin D1 expression is regulated by MAPK; however, another pathway independent of MAPK activation regulates cyclin E expression.
Based on the initial observation that treatment of LM6 cells with a selective PDGFR kinase inhibitor, AG 1296, or a Src family kinase inhibitor, PP1, reduced 5-HT-induced MAPK activation, pRb hyperphosphorylation, and thymidine incorporation, tyrosine kinases seemed to be involved in the 5-HT2B mitogenic signaling. Tyrosine kinases were proposed to mediate MAPK activation by 5-HT1A5–HT2A (5, 30) and angiotensin II receptors. Furthermore, the fact that activation of 5-HT2B receptor stimulates PDGFR-β phosphorylation in the absence of PDGF demonstrates the existence of cross-talk between the Gq-coupled receptor and RTKs (Fig. 4). The requirement for the PDGFR kinase activity in the 5-HT2B receptor mitogenic pathway is demonstrated either by using the PDGFR kinase inhibitor AG 1296 or by overexpressing a kinase mutant PDGFR in LM6 cells in which PDGFR phosphorylation was abolished. Gi/Gq-coupled receptors activate the ERK pathway via both tyrosine kinase-dependent and -independent pathways. Receptors for endothelin, angiotensin II, lysophosphatidic acid, and muscarinic acetylcholine receptor have been shown to stimulate ligand-independent tyrosine phosphorylation of PDGFR (31), insulin-like growth factor-1 receptor-β subunit (32), epidermal growth factor receptor, and ErbB-2 (12). Lysophosphatidic acid and thrombin receptors have been shown to mediate ERK1/2 activation and transactivation of the epidermal growth factor receptor with pertussis toxin-sensitive G proteins (33). Because pertussis toxin was ineffective in blocking GTPase activation triggered by 5-HT in the same cell line (6), we excluded the Gi protein from 5-HT2B mitogenic signaling. In addition, activation of 5-HT2B receptor seems preferentially to transactivate PDGFR, because 5-HT does not phosphorylate the ErbB-2 receptor also present on LM6 cells, whereas endogenous epidermal growth factor receptor is not expressed (34). Therefore, ligand-independent activation of RTKs by GPCRs occurs in a cell-specific manner. Preferential coupling to or specific expression of the RTKs could explain this specificity. Treatment of LM6 cells with PDGFR kinase inhibitor AG 1296 or overexpression of a kinase mutant PDGFR abolished MAPK activity and cyclin D1 expression in response to 5-HT (Figs. 4 and 6). These data suggest the requirement of the PDGFR kinase activity for transduction of the 5-HT2B receptor-induced MAPK activity and cyclin D1 expression. Thus, in the 5-HT2B receptor mitogenic signaling, the PDGFR serves as a signal transducer of 5-HT-mediated regulation of MAPK activity and cyclin D1 but not cyclin E expression. This study demonstrates that PDGFR kinase activity is essential for 5-HT2B-triggered MAPK/cyclin D1 activation; however, this transactivation is not sufficient for cell-cycle progression.
Src family kinases have been suggested to mediate lysophosphatidic acid- or Gβγ-induced tyrosine phosphorylation of Src homology domain 2-containing protein and subsequent activation of MAPK, given that inhibition of the Src kinases markedly attenuated these responses (13, 15). Although, whether c-Src is “upstream or downstream” of RTKs is still in debate, there is supporting evidence that c-Src activation precedes RTK transactivation by GPCR. Overexpression of the Src kinase inhibitor kinase Csk has been shown to attenuate lysophosphatidic acid and α2-adrenergic receptor-mediated epidermal growth factor receptor phosphorylation in COS-7 cells (15). Recently, Src family kinases have been implicated in integrin but not PDGFR signal transduction in fibroblast cells obtained from knockout embryos where Src family kinases were nonfunctional (35). The results presented in this paper clearly indicate that Src family kinases are not downstream of PDGFR signaling, because PDGF could activate fully the mitogenic pathway in the absence of c-Src, Fyn, and c-Yes. Our data strongly suggest that c-Src plays an “upstream” role in the transactivation of PDGFR in the 5-HT2B mitogenic signaling, based on the observation that PP1 and, more convincingly, that c-Src depletion abolished 5-HT-induced PDGFR phosphorylation (Fig. 4). This finding indicates that c-Src targets phosphorylation sites of the PDGFR that modulate its activation. Moreover, inactivating PDGFR by a specific inhibitor, AG 1296, or overexpressing the kinase-mutant PDGFR did not affect Src kinase activation by 5-HT, confirming that it is not upstream of Src in 5-HT2B mitogenic signaling. The kinetics of c-Src kinase activation by 5-HT also show Src kinase activation earlier than PDGFR phosphorylation. The observed 5-HT-induced Src kinase activity suggests that c-Src acts as a kinase rather than as a scaffolding protein. Each of the Src family kinases (c-Src, Fyn, and c-Yes) that we examined was activated by 5-HT. However, c-Src activation in response to 5-HT is stronger and appears earlier than that of either c-Yes or Fyn (Fig. 5). Both the Fyn and c-Yes kinase activity after 5-HT2B stimulation do not correlate with PDGFR transactivation. Depletion of c-Src but not of Fyn abolished 5-HT2B receptor-induced thymidine incorporation, MAPK activation, and cyclins D1 and E expression. PP1 totally inhibited 5-HT-induced initial and progressive hyperphosphorylation of pRb (Fig. 6), which is regulated by cyclin D1- and cyclin E-dependent kinase activity, respectively. These data strongly support the notion that c-Src is the key factor in 5-HT2B receptor-induced cell-cycle progression. Fyn and c-Yes do not substitute for c-Src, but might be involved in other biological effects of the 5-HT2B receptor. Anti-pp60-c-Src but not c-Yes or Fyn antibodies blocked the Gq-coupled angiotensin II receptor-stimulated tyrosine phosphorylation (36), lending further support to a specialized role for c-Src in the Gq-dependent mitogenic process.
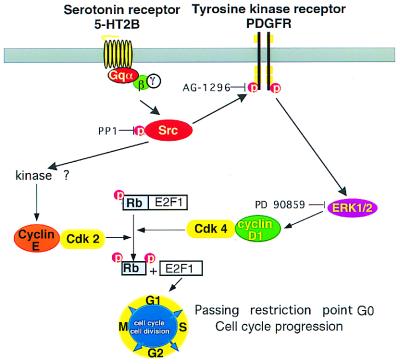
Our data support a model for the 5-HT mitogenic signaling in which c-Src is the crucial molecule that links the Gq-coupled 5-HT2B receptor and the cell-cycle regulators. c-Src alone controls cyclin E induction and transactivates RTK PDGFR to induce cyclin D1 expression via the MAPK/ERK pathway (Fig. 7).
Figure 7.
c-Src and PDGFR are connected functionally to each other and provide links to cell-cycle regulators in Gq-coupled 5-HT2B receptor mitogenic signaling. c-Src either acts alone to control cyclin E induction or in concert with a RTK PDGFR to induce cyclin D1 expression via the ERK/MAPK pathway. This action raises the level of pRb phosphorylation and triggers cell-cycle progression.
Acknowledgments
We thank Drs. P. Sassone-Corsi, Laurent Désaubry, J. Bosher, and C. Fode for critical reading of the manuscript and for stimulating discussions. We also thank Dr. L. Rönnstrand and Dr. C. H. Heldin for kindly providing a plasmid (pcDNA3) carrying a kinase-mutant PDGFR-β (K634A). This work has been supported by funds from the Centre National de la Recherche Scientifique, the Institut National de la Santé et de la Recherche Médicale, the Hôpital Universitaire de Strasbourg, the Université Louis Pasteur, and by grants from the Association Française Contre les Myopathies, from the Association pour la Recherche Contre le Cancer, and from the Ligue Nationale Contre le Cancer. C.G.N. is funded by an Eli Lilly–Université Louis Pasteur fellowship.
Abbreviations
- 5-HT
5-hydroxytryptamine
- 5-HT2B
5-hydroxytryptamine 2B
- cdk
cyclin-dependent kinase
- MAPK
mitogen-activated protein kinase
- PDGFR
platelet-derived growth factor receptor
- PP1
4-amino-5-(4-methylphenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine
- GPCR
G protein-coupled receptors
- pRb
retinoblastoma protein
- RTK
receptor tyrosine kinase
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.050282397.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.050282397
References
- 1.Wilkinson L O, Dourish C T. In: Serotonin Receptor Subtypes: Basic and Clinical Aspects. Peroutka S J, editor. Vol. 15. New York: Wiley–Liss; 1991. pp. 147–210. [Google Scholar]
- 2.Julius D. Annu Rev Neurosci. 1991;14:335–360. doi: 10.1146/annurev.ne.14.030191.002003. [DOI] [PubMed] [Google Scholar]
- 3.Launay J-M, Birraux G, Bondoux D, Callebert J, Choi D-S, Loric S, Maroteaux L. J Biol Chem. 1996;271:3141–3147. doi: 10.1074/jbc.271.6.3141. [DOI] [PubMed] [Google Scholar]
- 4.Seuwen K, Magnaldo I, Pouyssegur J. Nature (London) 1988;335:254–256. doi: 10.1038/335254a0. [DOI] [PubMed] [Google Scholar]
- 5.Garnovskaya M N, van Biesen T, Hawe B, Casanas Ramos S, Lefkowitz R J, Raymond J R. Biochemistry. 1996;35:13716–13722. doi: 10.1021/bi961764n. [DOI] [PubMed] [Google Scholar]
- 6.Loric S, Maroteaux L, Kellermann O, Launay J-M. Mol Pharmacol. 1995;47:458–466. [PubMed] [Google Scholar]
- 7.Jacks T, Weinberg R A. Science. 1998;280:1035–1036. doi: 10.1126/science.280.5366.1035. [DOI] [PubMed] [Google Scholar]
- 8.Peeper D S, Upton T M, Ladha M H, Neuman E, Zalvide J, Bernards R, DeCaprio J A, Ewen M E. Nature (London) 1997;386:177–181. doi: 10.1038/386177a0. [DOI] [PubMed] [Google Scholar]
- 9.Le Gall M, Grall D, Chambard J C, Pouyssegur J, Van Obberghen-Schilling E. Oncogene. 1998;17:1271–1277. doi: 10.1038/sj.onc.1202057. [DOI] [PubMed] [Google Scholar]
- 10.Fiddes R J, Janes P W, Sivertsen S P, Sutherland R L, Musgrove E A, Daly R J. Oncogene. 1998;16:2803–2813. doi: 10.1038/sj.onc.1201815. [DOI] [PubMed] [Google Scholar]
- 11.Prall O W J, Sarcevic B, Musgrove E A, Watts C K W, Sutherland R L. J Biol Chem. 1997;272:10882–10894. doi: 10.1074/jbc.272.16.10882. [DOI] [PubMed] [Google Scholar]
- 12.Daub H, Weiss F U, Wallasch C, Ullrich A. Nature (London) 1996;379:557–560. doi: 10.1038/379557a0. [DOI] [PubMed] [Google Scholar]
- 13.Dikic I, Tokiwa G, Lev S, Courtneidge S A, Schlessinger J. Nature (London) 1996;383:547–550. doi: 10.1038/383547a0. [DOI] [PubMed] [Google Scholar]
- 14.Thomas S M, Brugge J S. Annu Rev Cell Dev Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- 15.Luttrell L M, Hawes B E, van Biesen T, Luttrell D K, Lansing T J, Lefkowitz R J. J Biol Chem. 1996;271:19443–19450. doi: 10.1074/jbc.271.32.19443. [DOI] [PubMed] [Google Scholar]
- 16.Nebigil C G. Biochemistry. 1997;36:15949–15958. doi: 10.1021/bi971721m. [DOI] [PubMed] [Google Scholar]
- 17.Emaduddin M, Ekman S, Rönnstrand L, Heldin C H. Biochem J. 1999;341:523–528. [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng P W. Hum Gene Ther. 1996;7:275–282. doi: 10.1089/hum.1996.7.3-275. [DOI] [PubMed] [Google Scholar]
- 19.Shichiri M, Sedivy J M, Marumo F, Hirata Y. Mol Endocrinol. 1998;12:172–180. doi: 10.1210/mend.12.2.0064. [DOI] [PubMed] [Google Scholar]
- 20.Sale E M, Atkinson P G, Sale G J. EMBO J. 1995;14:674–684. doi: 10.1002/j.1460-2075.1995.tb07046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tanaka S, Amling M, Neff L, Peyman A, Uhlmann E, Levy J B, Baron R. Nature (London) 1996;383:528–531. doi: 10.1038/383528a0. [DOI] [PubMed] [Google Scholar]
- 22.Li Y, Chen B. J Leukocyte Biol. 1995;57:484–490. doi: 10.1002/jlb.57.3.484. [DOI] [PubMed] [Google Scholar]
- 23.Planas-Silva M D, Weinberg R A. Mol Cell Biol. 1997;17:4059–4069. doi: 10.1128/mcb.17.7.4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng H C, Nishio H, Hatase O, Ralph S, Wang J H. J Biol Chem. 1992;267:9248–9256. [PubMed] [Google Scholar]
- 25.Alessi D R, Cuenda A, Cohen P, Dudley D T, Saltiel A R. J Biol Chem. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- 26.Gazit A, App H, McMahon G, Chen J, Levitzki A, Bohmer F D. J Med Chem. 1996;39:2170–2177. doi: 10.1021/jm950727b. [DOI] [PubMed] [Google Scholar]
- 27.Hanke J H, Gardner J P, Dow R L, Changelian P S, Brissette W H, Weringer E J, Pollok B A, Connelly P A. J Biol Chem. 1996;271:695–701. doi: 10.1074/jbc.271.2.695. [DOI] [PubMed] [Google Scholar]
- 28.Watanabe G, Lee R J, Albanese C, Rainey W E, Batlle D, Pestell R G. J Biol Chem. 1996;271:22570–22577. doi: 10.1074/jbc.271.37.22570. [DOI] [PubMed] [Google Scholar]
- 29.Lavoie J N, L'Allemain G, Brunet A, Muller R, Pouyssegur J. J Biol Chem. 1996;271:20608–20616. doi: 10.1074/jbc.271.34.20608. [DOI] [PubMed] [Google Scholar]
- 30.Florian J A, Watts S W. J Pharmacol Exp Ther. 1998;284:346–355. [PubMed] [Google Scholar]
- 31.Linseman D A, Benjamin C W, Jones D A. J Biol Chem. 1995;270:12563–12568. doi: 10.1074/jbc.270.21.12563. [DOI] [PubMed] [Google Scholar]
- 32.Rao G N, Delafontaine P, Runge M S. J Biol Chem. 1995;270:27871–27875. doi: 10.1074/jbc.270.46.27871. [DOI] [PubMed] [Google Scholar]
- 33.Della Rocca G J, Maudsley S, Daaka Y, Lefkowitz R J, Luttrell L M. J Biol Chem. 1999;274:13978–13984. doi: 10.1074/jbc.274.20.13978. [DOI] [PubMed] [Google Scholar]
- 34.Herrlich A, Daub H, Knebel A, Herrlich P, Ullrich A, Schultz G, Gudermann T. Proc Natl Acad Sci USA. 1998;95:8985–8990. doi: 10.1073/pnas.95.15.8985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klinghoffer R A, Sachsenmaier C, Cooper J A, Soriano P. EMBO J. 1999;18:2459–2471. doi: 10.1093/emboj/18.9.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schieffer B, Paxton W G, Chai Q, Marrero M B, Bernstein K E. J Biol Chem. 1996;271:10329–10333. doi: 10.1074/jbc.271.17.10329. [DOI] [PubMed] [Google Scholar]