Abstract
To quantify the adaptive significance of insect learning, we documented the behavior and growth rate of grasshoppers (Schistocerca americana) in an environment containing two artificial food types, one providing a balanced diet of protein and carbohydrate, which maximizes growth, and the other being carbohydrate-deficient, which is unsuitable for growth. Grasshoppers in the Learning treatment experienced a predictable environment, where the spatial location, taste, and color of each food source remained constant throughout the experiment. In contrast, grasshoppers of the Random treatment developed in a temporally varying environment, where the spatial location, taste, and color of the balanced and deficient food types randomly alternated twice each day. Our results show that the grasshoppers that could employ associative learning for diet choice experienced higher growth rates than individuals of the Random treatment, demonstrating the adaptive significance of learning in a small short-lived insect.
Associative learning has been documented and subjected to intense research in various nonsocial insects, including flies (1–3), parasitoid wasps (4, 5), and grasshoppers (6, 7). Insect behavior, however, appears to be dominated by innate preferences and patterns (8), and it has not been clear whether insect learning significantly improves fitness (9, 10). As a first step in evaluating the adaptive significance of learning in an insect, we compared the growth rate of grasshoppers (Schistocerca americana) under environmental conditions that either allowed or prevented the employment of associative learning for diet choice. Specifically, we asked whether the growth rate of individual grasshoppers using associative learning would be higher than that of individuals prevented from employing associative learning.
Methods
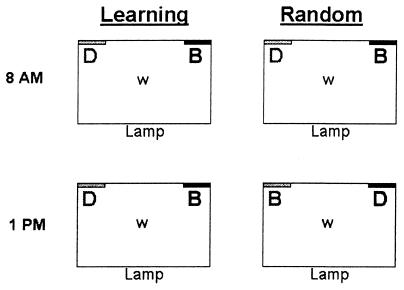
The subjects were newly molted sixth-instar nymphs from a laboratory colony of the S. americana grasshoppers maintained at the University of Arizona, Tucson. Pairs of nymphs were matched for sex and body mass, randomly assigned to the Learning and Random groups, and placed individually inside 20 × 30 cm plastic cages with screened tops, where they were maintained until they molted into adults. The room temperature was 24°C and a 40-W lamp near each cage on a 12:12 light:dark schedule provided light and additional heat (Fig. 1). A piece of wire mesh near the lamp provided a roosting place where the grasshoppers spent most of their time basking.
Figure 1.
A cage contained a water dish (w) and two food dishes, one consisting of a nutritionally balanced (B) and the other deficient (D) food. Twice a day, we removed the food dishes and introduced new dishes. The Learning grasshoppers experienced a predictable environment: the two food types were always placed at the same sides, near the same colored card, and with the same flavor. The Random grasshoppers had a temporally varying environment: the location, background color, and flavor of each diet type was randomly determined on each food change. The figure depicts two examples to illustrate the protocol; see Methods for full details.
Each cage contained two food dishes, one with 1 g of balanced diet of 14% (wt/wt) protein and 14% (wt/wt) complex carbohydrates, and the other with 1 g of deficient diet with 14% protein but no carbohydrates. Specifically, the balanced diet (11, 12) consisted of 50 g of indigestible cellulose, 10 g of dextrin, 6 g of casein, 2 g of peptone, 2 g of albumin, 1.8 g of salt mixture, 0.4 g of cholesterol, 0.2 g of ascorbic acid, 0.14 g of vitamin mix, 400 μl of linolenic acid, and 25 mg of a nonnutrient flavoring (secondary compound), either coumarin or citral. The proportions of carbohydrate and protein in the balanced diet allow maximal growth and survival in the closely related grasshopper Schistocerca gregaria (13). The deficient diet was identical to the balanced one, except that the dextrin was replaced with cellulose. Although grasshoppers do not possess taste receptors for complex carbohydrates, they can readily taste their simple byproducts created by salivary amylase (ref. 14 and unpublished data). Feeding on a carbohydrate-deficient diet is associated with lower growth rates (12, 15). Citral and coumarin stimulate both the taste and olfactory systems. At the low concentrations used, neither each compound alone nor a mixture of the two affects feeding and growth rate (12, 16).
One of the two food dishes was on the right side, in front of a 10 × 10 cm brown plastic card and contained the secondary compound citral, and the other dish was on the left side, in front of a 10 × 10 cm green plastic card and contained coumarin. In a preliminary test of innate preference between the two food types used in the experiment (brown card + citral on the right side versus green card + coumarin on the left side), grasshoppers chose each food type at similar proportions: 53% chose the left dish with the green background and coumarin, and 47% chose the right dish with the brown background and citral (χ2 = 0.1, df = 1, P > 0.5, n = 34 grasshoppers).
The Learning treatment was designed to allow grasshoppers to learn to associate the food qualities with various cues, and the Random treatment was designed to make such long-term association between quality and cues impossible. At the start of the experiment, each Learning grasshopper was randomly assigned to receive the balanced food on one side with one color and flavor and the deficient food on the other side with the other color and flavor. That is, half the Learning grasshoppers received the balanced food with citral and brown background on the right side and the deficient food with coumarin and green background on the left side, and the other half received the balanced food with coumarin and green background on the left side and the deficient food with citral and brown background on the right side. At the start of each half-day period, the food dishes were replaced with new ones, but the association between food type and stimuli remained the same for the remainder of the experiment, creating a fully predictable environment (Fig. 1). In contrast, at the start of each half-day period, each Random grasshopper was randomly assigned to receive the balanced food associated with one set of stimuli and the deficient food with the other (Fig. 1), producing an unpredictable environment, which prevents associative learning (10, 17).
Because grasshoppers are not active immediately after molting, we commenced observations on the second day of the sixth instar and continuously recorded the grasshoppers' behavior for 8 h each day through day 7. This period is the main activity and feeding phase of the last instar (16, 18). We recorded feeding bouts of each grasshopper at each food dish by using a laptop computer. A single visit and a meal were defined as a single or series of feeding bouts in which a grasshoppers remained within 5 cm from the food dish.
The behavioral information from the 6-day observation period was summarized to include for each grasshopper and each day the number and type of meals, the proportion of balanced-food meals, and the proportion of time spent feeding on the balanced food. Proportional data were arcsine transformed, and the data set was analyzed with repeated-measures ANOVAs (19). Besides treatment effects, the models also included effects of replicate and sex. Growth rate was calculated as the gain in dry mass from the start of the sixth instar until molting into the adult stage, over the instar duration. The initial dry mass was estimated from the wet mass by using a conversion factor derived in previous studies with grasshoppers from the same colony (12, 16), and the final dry mass was measured directly after freeze drying. Fat content was measured by comparing adult dry mass before and after double extraction with chloroform. Because initial body mass is highly correlated with mass increase, growth rate and fat mass were analyzed with an ANCOVA (analysis of covariance), with dry mass at the start of the sixth instar as a covariate, and treatment and replicate as main effects. Each of the two replicates initially included 12 grasshoppers, but 5 individuals failed to feed on the novel artificial diets, 1 died on day 3, and another on day 10, leaving n = 18 and n = 17 for the analyses of behavior and growth rate, respectively. There were no between-treatment differences in either initial body mass (ANOVA, F1,13 = 0.4, P > 0.5) or time of first meal (F1,13 = 1.3, P > 0.25).
Results
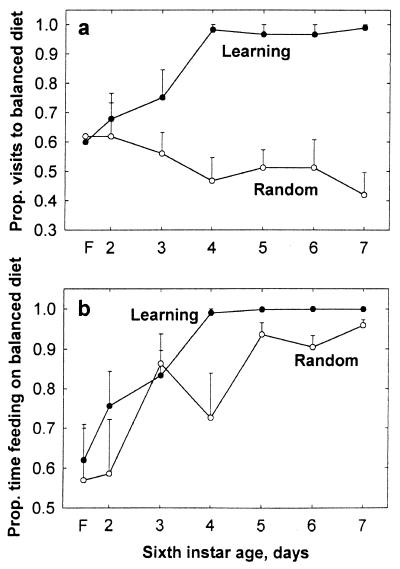
The grasshoppers spent most of the time basking near the lamps at their preferred temperature of above 30°C (20), and occasionally visited the food and water dishes. The proportion of visits to the balanced-diet dish increased to almost 1 for the Learning grasshoppers but remained at random level for the Random treatment (repeated-measures ANOVA, F1,10 = 56, P < 0.001, and F5,50 = 5.8, P < 0.001 for between-treatment differences in means and slopes, respectively; within the Learning treatment, there was a significant increase in the proportion of visits to the full diet, F5,45 = 8, P < 0.001, whereas within the Random treatment, there was no significant change, F5,35 = 0.7, P > 0.5, Fig 2a).
Figure 2.
The proportion (mean + SE) of visits to (a) and time spent feeding on (b) the dish containing nutritionally balanced food during the first 6 days of feeding. In addition, “F” on the abscissa refers to choice of the first observed meal (in a) or duration of the first meal on each diet type (in b).
Grasshoppers from both treatments showed an increase in the proportion of time spent feeding on the balanced diet, but the mean and rate of increase were higher for the Learning than Random treatment (F1,10 = 16.9, P < 0.005, and F5,50 = 2.7, P < 0.05 for between-treatment differences in means and slopes, respectively; Fig. 2b). The effects of replicate and sex were nonsignificant (P > 0.25). Within the learning treatment, there was no significant difference in performance between the grasshoppers that received the balanced diet in association with brown color and citral on the right side and those that received the balanced diet with green color and coumarin on the left side (P > 0.8).
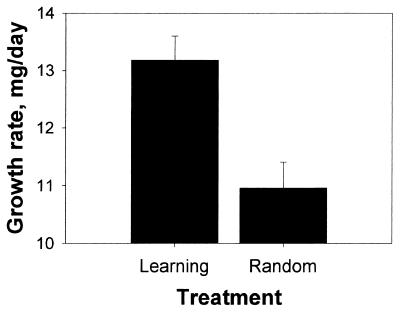
The growth rate of the Learning group was 20% higher than that of the Random group (ANCOVA, F1,12 = 13.1, P < 0.005, Fig. 3). The growth rate data can be separated into three components, fat mass, fat-free mass, and instar duration. The fat content of newly molted adults of the Learning treatment was 15% higher (23.8 ± 0.9 vs. 20.7 ± 1 mg), and the fat-free mass was 11% higher (131 ± 6.1 vs. 118 ± 7 mg) than that of the Random treatment. Finally, the instar duration in the Learning treatment was 7% shorter than in the Random treatment (282 ± 4 vs. 302 ± 5 h).
Figure 3.
The average growth rate (adjusted least-square means + SE) of grasshoppers from the Learning and Random treatments.
How did learning improve growth rate? The Learning and Random treatments did not differ in the total time spent feeding (repeated-measures ANOVA, F1,10 = 0.9, P > 0.3) although the total time spent feeding on the balanced diet was slightly larger for the Learning treatment (F1,10 = 4, P = 0.075). The overall proportion of time spent feeding on the balanced diet was 99.4% for the Learning treatment and 88.6% for the Random treatment (ANOVA, F1,14 = 56.5, P < 0.001). This corresponds to protein-to-carbohydrate consumption ratios of 0.501:0.499 by the Learning treatment and 0.53:0.47 by the Random treatment.
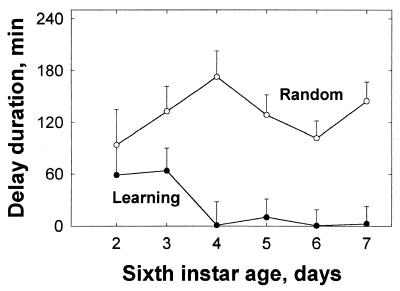
In addition to the difference in the quality of food ingested, there was a major difference in the temporal pattern of feeding on the balanced food. By day 4, the Learning grasshoppers almost always approached the dish of balanced food directly. By contrast, the Random grasshoppers approached the deficient food first on approximately 50% of their feeding attempts (Fig. 2a). The Random grasshoppers spent little time on the deficient food (Fig. 2b), usually returning more or less immediately to the lamp, and then, on subsequent foraging attempts, they encountered the balanced food. We assumed that the grasshoppers were physiologically prepared to ingest a meal upon their first feeding attempt, and that the persistent occurrence of delays was detrimental to growth rate. We then calculated the sum of all delays between the first feeding attempt and the commencement of feeding on the balanced food. The Random grasshoppers averaged a total time delay of 93–173 min per day. By contrast, the Learning grasshoppers experienced only negligible delays after day 3 (repeated measures ANOVA, F1,16 = 27.7, P < 0.001 for between-treatment differences in mean delay; Fig. 4).
Figure 4.
The time delay (mean + SE) between a first feeding attempt and the start of feeding on the balanced food dish during the first 6 days of feeding.
Discussion
Associative learning has been documented in various insects (9, 10, 21). While it seemed intuitive that such learning should contribute to fitness, experimental data have not directly shown improved fitness from learning. In our experiment, the employment of associative learning by the Learning grasshoppers enabled them to attain higher growth rates than the Random grasshoppers during the last nymphal stage. Such grasshoppers typically take a minimum of 5 weeks to reach adulthood and may live several months as adults (20). Hence the benefits of learning documented here over a single nymphal stage may translate into greater benefits over the whole grasshopper lifetime. Growth rate may be positively associated with grasshopper fitness because it is positively correlated with the number and size of eggs laid and number of generations per year (22, 23). Two factors could contribute to the higher growth rates of the Learning grasshoppers. First, the Learning grasshoppers achieved a dietary balance closer to the optimal than the Random grasshoppers (11, 24). Second, the Learning grasshoppers probably maintained a better temporal spacing of meals than the Random grasshoppers (Fig. 4).
During the experiment, the Learning grasshoppers quickly developed a preference for the balanced food (Fig. 2), a behavior that can readily be attributed to associative learning (6, 7). In contrast, the Random grasshoppers could not learn to restrict visits to the balanced food (Fig. 2a). The Random grasshoppers were able to reject the unbalanced food after a brief feeding period, however, allowing them to achieve a more balanced diet over time (Fig. 2b). Presumably changes in the sensitivity of the maxillary palp chemoreceptors under carbohydrate deprivation enhanced the Random grasshoppers' ability to detect the presence or absence of carbohydrates (25). In addition, it is also possible that the Random grasshoppers learned to ignore the color, secondary compound, and location information (which varied twice a day) and focus instead on sensing carbohydrates (which they could probably sense once the complex carbohydrate, dextrin, was broken by salivary amylase).
The Learning grasshoppers could learn to orient to their preferred food dish (Fig. 2a), but grasshoppers of either treatment gradually increased the proportion of time feeding on the balanced diet (Fig. 2b). This result perhaps indicates that a major benefit of learning is in reducing the time spent traveling to and initiating feeding on suboptimal food plants. In natural settings, reduced travel distance would also decrease predation if predation rate is higher during travel than during rest.
The data presented here illustrate how we can reconcile traditional views of the insects as driven by instincts and recent research on insect learning. A combination of mechanisms is used in maximizing the quality of the diet in generalist insect herbivores such as grasshoppers. Innate physiological mechanisms direct acceptability and ingestion of food, modulated by changes in taste receptors (25). In addition, associative learning allows the generalist herbivore to feed more efficiently when environmental conditions, such as the persistence of association between resource quality and cues, and length of experience, permit the use of learning (10, 17).
In conclusion, even although the Random grasshoppers were able to modify some of their behavior over time even without using associative learning, the employment of associative learning by the Learning grasshoppers allowed them to perform significantly better and achieve higher growth rates, illustrating the adaptive significance of learning in a small short-lived insect.
Acknowledgments
We thank A. Joern, S. J. Simpson, and A. Zera for helpful discussions, A. Joern and A. Zera for logistical help, and A. Kamil, D. Papaj, and B. Roitberg for comments on the manuscript. This work was supported by grants from the National Institutes of Health and the U.S. Department of Agriculture.
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.050461497.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.050461497
References
- 1.Quinn W G, Harris W A, Benzer S. Proc Natl Acad Sci USA. 1974;71:708–712. doi: 10.1073/pnas.71.3.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tully T. Proc Natl Acad Sci USA. 1996;93:13460–13467. doi: 10.1073/pnas.93.24.13460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dukas R. Behav Ecol Sociobiol. 1999;45:195–200. [Google Scholar]
- 4.Lewis W L, Takasu K. Nature (London) 1990;348:635–636. [Google Scholar]
- 5.Turlings T C J, Wackers F L, Vet L E M, Lewis W J, Tumlinson J H. In: Insect Learning. Papaj D R, Lewis A C, editors. London: Chapman and Hall; 1993. pp. 51–78. [Google Scholar]
- 6.Lee J C, Bernays E A. Anim Behav. 1990;39:163–173. [Google Scholar]
- 7.Raubenheimer D, Tucker D. Anim Behav. 1997;54:1449–1459. doi: 10.1006/anbe.1997.0542. [DOI] [PubMed] [Google Scholar]
- 8.Mayr E. Am Scientist. 1974;62:650–659. [PubMed] [Google Scholar]
- 9.Papaj D R, Prokopy R J. Annu Rev Entomol. 1989;34:315–350. [Google Scholar]
- 10.Dukas R. In: Cognitive Ecology. Dukas R, editor. Chicago: Univ. of Chicago Press; 1998. pp. 129–174. [Google Scholar]
- 11.Dadd R H. J Insect Physiol. 1960;4:319–347. [Google Scholar]
- 12.Bernays E A, Bright K L, Gonzales N, Angel J. Ecology. 1994;75:1997–2006. [Google Scholar]
- 13.Raubenheimer D, Simpson S J. Nutr Res Rev. 1997;10:151–179. doi: 10.1079/NRR19970009. [DOI] [PubMed] [Google Scholar]
- 14.Bernays E A, Chapman R F. In: Biochemical Aspects of Plant and Animal Coevolution. Harborne J B, editor. New York: Academic; 1978. pp. 99–141. [Google Scholar]
- 15.Raubenheimer D, Simpson S J. Anim Behav. 1993;45:953–964. [Google Scholar]
- 16.Bernays E A. Am Nat. 1998;151:451–464. doi: 10.1086/286132. [DOI] [PubMed] [Google Scholar]
- 17.Stephens D W. Behav Ecol. 1991;2:77–89. [Google Scholar]
- 18.Simpson S J. Entomol Exp Appl. 1982;31:265–275. [Google Scholar]
- 19.Systat. Statistics. Chicago: SPSS; 1999. [Google Scholar]
- 20.Uvarov B P. Grasshoppers and Locusts. London: Anti-Locust Research Centre; 1966. [Google Scholar]
- 21.Papaj D R, Lewis A C. Insect Learning. New York: Chapman and Hall; 1993. [Google Scholar]
- 22.Slansky F, Scriber J M. In: Comprehensive Insect Physiology, Biochemistry and Pharmacology. Kerkut G A, Gilbert L I, editors. Vol. 4. London: Academic; 1985. pp. 87–164. [Google Scholar]
- 23.Atkinson D, Begon M. Ecol Entomol. 1987;12:119–127. [Google Scholar]
- 24.Simpson S J, Simpson C L. In: Insect-Plant Interactions. Bernays E A, editor. Vol. 2. Boca Raton, FL: CRC; 1990. pp. 111–160. [Google Scholar]
- 25.Simpson S J, James S, Simmonds M S J, Blaney W M. Appetite. 1991;17:141–154. doi: 10.1016/0195-6663(91)90069-5. [DOI] [PubMed] [Google Scholar]