Abstract
Multiple myelomas produce tumor-specific antigen (TSA) in the form of idiotype (Id) on monoclonal Ig. CD4+ T cells can recognize Id-peptide on MHC class II molecules and protect against challenges with MOPC315 cells, which are, as common for myelomas, class II-negative. The present study explains these previous results by demonstrating that Id can be transferred from myeloma cells to antigen-presenting cells (APC), which present processed Id-peptide on their class II molecules to Id-specific T cell receptor-transgenic (TCR-TG) CD4+ T cells. Id-primed tumor APC were heterogeneous, the majority being dendritic cells with class II+, CD11b+ CD11c+ CD40+ CD80+ CD86+ markers. The APC were localized beneath CD31+ endothelial cells of tumor microvessels, and their frequency declined with tumor progression. The APC could stimulate Id-specific naive TCR-TG, short-term polarized TCR-TG, and cloned CD4+ T cells to proliferate and produce cytokines in vitro. Furthermore, small MOPC315 tumors established in Id-specific TCR-TG mice contained clusters of activated (CD69+CD25+) and proliferating (BrdUrd+) Id-specific transgenic CD4+ blasts. The activated Id-specific T cells were located adjacent to Id-primed dendritic cells in the tumor. Thus, a TSA can be transferred in vivo from myeloma, and possibly other types of cancer cells to APC for MHC class II presentation to CD4+ T cells.
CD4+ as well as CD8+ cells are considered important in antitumor responses (1). Because tumor cells most often lack MHC class II and costimulatory molecules, CD4+ T cell activation might require transfer of tumor-specific antigen (TSA) before presentation by professional antigen-presenting cells (APC).
There is indirect genetic and functional evidence for transfer and presentation of TSA by professional APC (2–4). In one instance, priming of tumor APC could be directly demonstrated in a somewhat unphysiological model using a transfected pseudo-tumor antigen (ovalbumin), a cotransfected IL-3 gene, and a class-I-restricted T cell hybridoma as read-out (5). Similarly, when carcinoma cells were cotransduced in vitro with granulocyte/macrophage colony-stimulating factor and CD40 ligand genes, dendritic cells (DC) that infiltrate tumors were shown to take up and present endogenous viral tumor-associated antigen in class-I-restricted fashion (6).
There is as yet little evidence that TSA-specific T cells are activated in tumors. Tumor infiltrating T cells (TIL) possibly may have a tumor specificity because they display a limited TCR heterogeneity (7). More convincingly, an increased frequency of melanoma-specific CD8+ cells were found in metastatic lymph nodes (LN) (8). However, there is no direct evidence for activation and proliferation of TSA-specific T cells in the tumor itself.
The BALB/c MOPC315 myeloma secretes M315 monoclonal IgA with λ2315 L chains (9–11). Because of their extreme diversity, each monoclonal Ig contains in its variable regions a unique TSA called idiotype (Id). Immunization with M315 induces an Id-specific resistance to a challenge with MOPC315 cells (12). Subsequent work has demonstrated that an Id-peptide derived from the third hypervariable region (amino acids 91–101) of the λ2315 L chain, presented on the I-Ed class II molecule, is recognized by Id-specific CD4+ cells (13). Naive Id-specific CD4+ T cells from mice made transgenic (TG) for an Id-specific TCR conferred protection against the MOPC315 plasmacytoma (14).
MOPC315 cells, like myeloma cells in general (15), lack MHC class II molecules. How then could Id-specific CD4+ T cells have an antimyeloma effect (16–19)? Here, we describe that professional APC in the myeloma present Id to protective CD4+ cells, which become activated and proliferate.
Materials and Methods
Mice.
λ2315-specific, T cell receptor (TCR) (Vα1, Jα19; Vβ8.2, Jβ1.2)-TG mice on a BALB/c (20) or C.B-17 SCID (14) background were of specific pathogen-free (SPF) standard, bred in a heterozygous [TCR-TG × nontransgenic (N-TG)] state behind a barrier. Both euthymic and adult thymectomized mice were used with similar results. RAG2−/− (H-2b), C.B-17 SCID, and BALB/c mice were from Bomholtgaard Breeding and Research Center (Ry, Denmark).
Cell Lines and Injection of Tumor Cells.
MOPC315.4 (IgA, λ2315) (21) and J558 (IgA,λ1) are transplantable BALB/c plasmacytomas (American Type Culture Collection). Mice (6–18 wk old) were injected s.c. in the interscapular region with 2 × 106 MOPC315.4 or 5 × 105 J558 cells. M315 and J558 myeloma protein concentrations in serum were assayed by ELISA (21).
APC.
DC-like (APC-I) and macrophage (MΦ)-like (APC-II) cells were isolated essentially as described for spleen DC and MΦ, respectively (22). Briefly, tissue fragments were digested with collagenase (400 units/ml; Sigma) and DNase (0.33 mg/ml; Sigma) for 45 min at 22°C. Released cells were pooled and washed, EDTA (pH 7.2) was added to 6 mM, and the cells were centrifuged over 54% Percoll (Amersham Pharmacia) at 1,500 × g for 20 min at room temperature. The interphase cells (pellets contained the plasmacytoma cells) were cultured at 37°C. After 1.5–2 h, nonadherent cells were discarded. After an additional 12–18 h, detached cells (denoted APC-I) and remaining adherent (detached with 20 mM EDTA in PBS at 4°C for 15 min) cells (APC-II) were collected. APC I- and APC-II contained 1.3% and 0.8% of CD138+ tumor cells, respectively. For T cell-stimulation assays, APC were irradiated with 2,000 rads, a dose that inhibited growth of MOPC315 and J558 cell lines by 98% and 94%, respectively (data not shown).
T Cell Assays.
Stimulators were the indicated numbers per well of 2,000 rad-irradiated APC I and II. Responders were 2 × 104 Id-responsive CD4+ cells per well, corresponding to 5 × 104 TCR-TG SCID or 105 TCR-TG LN cells; 1.6 × 105 TCR-TG SCID or 3 × 105 TCR-TG spleen cells; 2 × 104 short-term cultured Th1- or Th2-polarized TCR-TG SCID LN cells (23); 2 × 104 cloned 7A10B2 cells. Positive control was addition of an optimal concentration of 91–101 (λ2315) synthetic peptide (4 μg/ml). Assays for IL-2, IL-4, and IFN-γ were determined in supernatants withdrawn at 60 h (16). Cultures then were pulsed and harvested 16 h later, and [3H]TdR incorporation was counted.
Flow Cytometry.
Tumor tissue was treated with DNase and collagenase (as described above) and the released cells were used for staining. Unspecific stainings were blocked by incubation with heat-aggregated 30% normal rat serum in PBS for 10 min at 0°C. The following commercially available mAbs were used, conjugated with either FITC, phycoerythrin, allophycocyanin, or biotin: CD1d (1B1), CD4 (RM4.5), CD8 (53–6.7), CD11b (M1/70), CD11c (HL3), CD13 (R3–242), CD19 (1D3), CD25 (3C7), CD40 (3/23), CD69 (H1.2F3), CD80 (16-10A1), CD86 (GL-1), CD95 (Jo2), CD138 (281–2), CD154 (CD40Ligand; MR1), Ly-6G (Gr-1; RB6–8C5), DX-5 and various fluorochrome-conjugated, isotype-matched control mAbs (PharMingen). Purified MΦ-specific F4/80 (C1:A3–1) and anti-scavenger receptor (2F8) mAbs were from ImmunoKontact (Bioggio, Switzerland). Human CTLA4Ig-IgG1 was a gift from P. Linsley (Immunex, Seattle). mAbs against the following specificities were affinity-purified and conjugated with FITC or biotin by using in-house procedures: TG-TCR-clonotype (GB113, ref. 24), Vβ8 (F23.1, ref. 25), αβTCR (H57), CD11c (HB224; ATCC), CD95L (MFL-1), F4/80 (HB198; ATCC), DEC205 (NLDC145), hamster IgG (UC8), pan-MHC class II (TIB120; ATCC) and I-Ed class II (13/4, ref. 26). Biotinylated Abs were detected with streptavidin (SA)-CyChrome (PharMingen), purified mAbs with phycoerythrin-labeled goat anti-rat-IgG Ig (Southern Biotechnology Associates). Combinations of the mAbs were used for double, triple, or quadruple stainings of cells. Triple stainings for CD4, CD8, and BrdUrd were carried out as described (27). Cells were acquired and analyzed on a FACScalibur (Becton Dickinson) instrument with cellquest software.
Immunofluorescence.
Biopsies were embedded in OCT compound. Frozen sections were mounted on l-polylysine-coated glass slides, air-dried overnight, prefixed in acetone for 10 min, and then stored at −20°C. Single and double stainings of sections were done in several steps with avidin/biotin blocking before each staining with unlabeled, FITC-conjugated or biotinylated mAbs against CD11b (M1/70), CD31 (MEC 13.3), CD138 (281–2) (PharMingen), MHC class II (TIB120; 14.4.4S; ATCC), BrdUrd (Zymed BrdU Staining Kit), and TCR-TG clonotype (GB113). To detect unlabeled or FITC-conjugated mAbs, sections were incubated with biotinylated rabbit anti-rat IgG (Vector Laboratories) or biotinylated anti-FITC mAb (Sigma), respectively. For each mAb, a differently labeled reagent was used [SA-Texas Red (GIBCO), SA-Cy2, SA-Cy3 (Molecular Probes), or SA-peroxidase (ABC Vectastain kit, Vector)] to detect biotinylated Ab(s) from the previous step. For detection of BrdUrd, sections were fixed for 10 min in 4% paraformaldehyde. Nuclei were stained blue with 4,6-diamino-2-phenylindoldihydrochloride (DAPI) or hematoxylin. Pictures were made with a charge-coupled device camera (Hamamatsu, Ichinocho, Japan) and adobe photoshop software.
Results
APC Purified from MOPC315 Tumors Spontaneously Present Id.
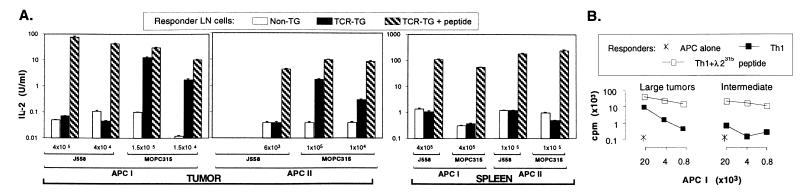
APC were isolated from large s.c. Id+ MOPC315 tumor tissue of BALB/c mice, according to a procedure for isolation of DC and MΦ from the spleen. APC I and APC II, anticipated to be enriched for DC and MΦ, respectively, were irradiated to inhibit proliferation of any contaminating tumor cells (1.3%) and tested for their ability to activate various types of Id-specific CD4+ T cells in the absence of added antigen (Fig. 1). Activation was measured both as proliferation and lymphokine production (IL-2 for naive cells, IFN-γ for Th1, IL-4 for Th2), and results were always concordant (data not shown).
Figure 1.
APC purified from MOPC315 tumors are spontaneously primed with Id and stimulate T cells. (A) Transiently (APC I) and permanently (APC II) plastic-adherent cells were isolated from tumors and spleens of BALB/c mice with large s.c. MOPC315 (1.4–2.5 cm, serum M315 140–420 μg/ml) or J558 tumors, irradiated, cultured with LN cells from either N-TG or TCR-TG mice, and IL-2 in supernatants determined. As a positive control, 91-101 (λ2315) synthetic peptide was added to cultures. (B) APC I, isolated from intermediate (0.5–1.5 cm) and large (1.5–2.5 cm) size MOPC315 tumors established in TCR-TG mice, were tested for their ability to stimulate Th1-polarized TCR-TG SCID LN cells. Isolated APC II gave similar results.
Both types of tumor APC could spontaneously stimulate T cells from TCR-TG mice (Fig. 1A). T cell responses were weaker than those obtained by adding an optimal concentration of synthetic Id-peptide to cultures. The responses were transgene specific because T cells from N-TG mice did not react. Responses were Id specific because APC isolated from an Id-negative tumor (J558) failed to elicit any response, although they could present synthetic Id-peptide. APC from the tumor were more efficiently primed than spleen APC, which were nonstimulatory.
APC I and APC II isolated from MOPC315 tumors of TCR-TG mice gave similar results demonstrating that Id-specific T cells do not extinguish Id-primed APC (Fig. 1B). APC isolated from large MOPC315 tumors (>1.5 cm, serum M315 >140 μg/ml) stimulated T cells to a greater extent than their counterparts from intermediate-size (0.5–1.5 cm; serum M315 50–140 μg/ml) tumors (Fig. 1B). [Serum M315 correlate with tumor size (weight, diameter); ref. 28.] Responses could clearly be detected with 2,500 APC per well (Fig. 2), corresponding to 250–625 class II+ CD80/86+ cells (see later).
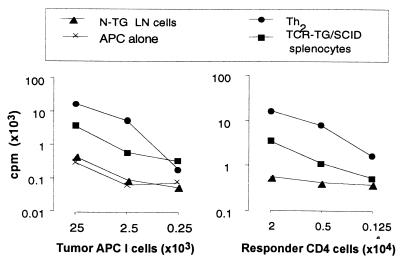
Figure 2.
APC I, isolated from large MOPC315 tumors in BALB/c, were titrated against a constant number of Id-specific CD4+ responders (2 × 104/w; Left), and Id-specific CD4+ cells were titrated against a constant number of APC I (2.5 × 104/w; Right).
Many Id-specific TCR-TG LN cells additionally express an endogenous α-chain, and thus an unknown second reactivity (29). To exclude this confounding factor, we compared the ability of Id-primed APC to stimulate naive CD4+ cells and Th2-polarized cells, both obtained from recombination-deficient TCR-TG SCID mice of barrier-bred, specific pathogen-free standard. APC-I cells stimulated proliferation of naive CD4+ cells, although to a lesser extent than Th1- (Fig. 1B) or Th2-polarized cells (Fig. 2). Responses of Id-specific naive CD4+ T cell were blocked by two different anti-class II mAbs and by a human CTLA4Ig hybrid molecule that binds to mouse CD80 and CD86 (data not shown).
MOPC315 Cells Isolated from s.c. Tumors Do Not Themselves Stimulate Class II-Restricted, Id-Specific T Cells.
Freshly isolated ex vivo MOPC315 cells, identified by their expression of a plasma cell marker CD138 (Syndecan-1), did not express class II molecules as detected by immunohistochemistry (Fig. 3A) or flow cytometry (data not shown). Because either transient or physically undetectable levels of class II expression might have functional consequences, it was important to test Id-presentation capability of MOPC315 in functional T cell assays. First, APC function of pellet cells from the 54% Percoll gradient, containing about 70% MOPC315 cells, was compared with that of APC-I and -II preparations (1.3% MOPC315 cells). The results strongly argue against MOPC315 cells themselves being able to present Id (Fig. 4A).
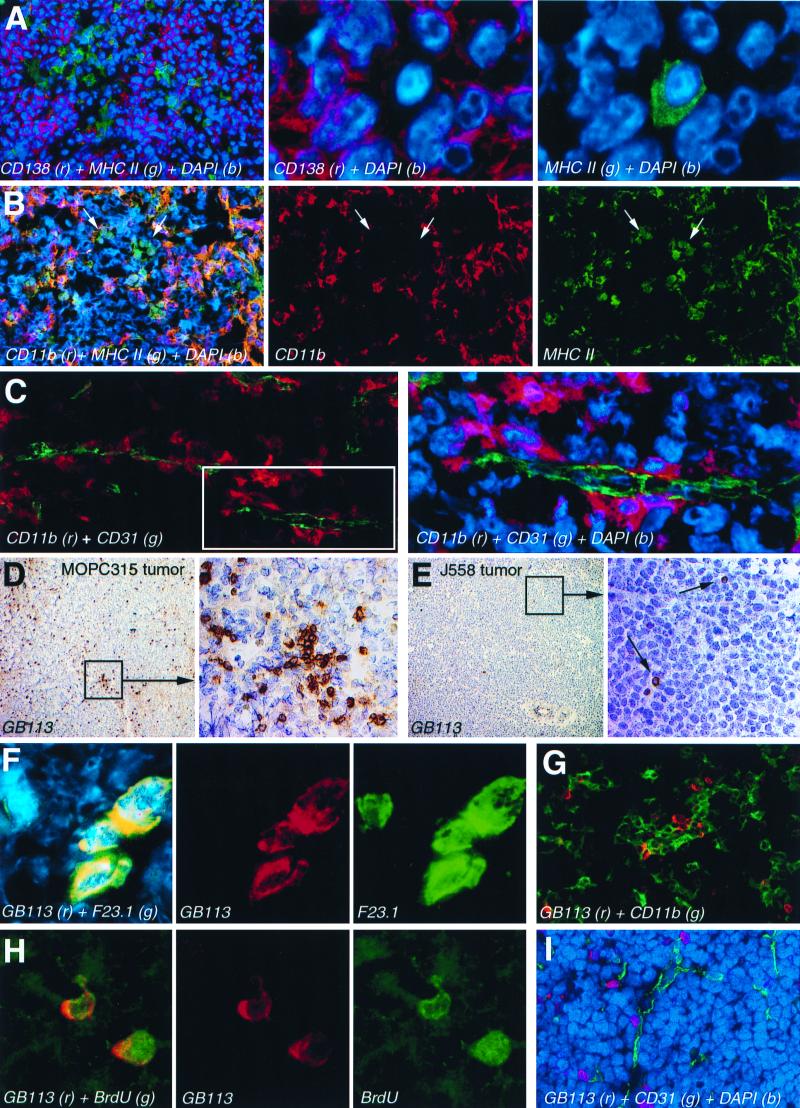
Figure 3.
Immunohistochemical characterization of APC and T cells in small MOPC315 tumors. (A) Staining with anti-CD138 (Syndecan-1) and anti-class II (I-Ed) and DAPI (nuclei). (B) Staining with anti-CD11b (Mac-1) and anti-class II. Arrows indicate clusters of cells that are class II+ CD11b−. (C) (Left) Staining with anti-CD31 (green) and anti-CD11b (red). (Right) Two-fold magnification of the framed field on the left with DAPI-stained nuclei. (D and E) (Left) Single staining with TCR-TG- clonotype specific GB113 mAb (brown) of small Id+ MOPC315 (D) or Id− J558 (E) tumors, with hematoxilin-stained nuclei. (Right) Five-fold magnifications of framed fields on the left. (F) Staining with GB113 and Vβ8-specific F23.1 mAbs, and DAPI. (G) Staining with GB113 and anti-CD11b mAbs. (H) Staining of tumor sections, from mice given BrdUrd, with GB113 and anti-BrdUrd mAbs. (I) Staining with GB113 mAb (red), anti-CD31 mAb (green), and DAPI.
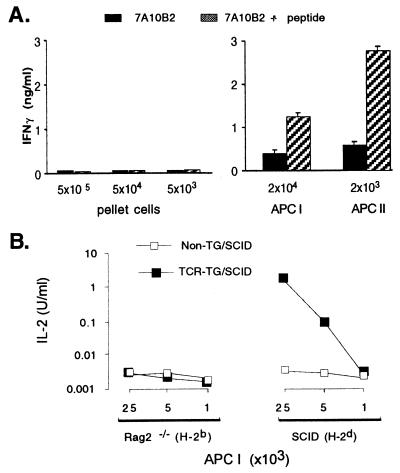
Figure 4.
MOPC315 cells do not present Id themselves. (A) Ex vivo tumor cell suspensions were spun over 54% Percoll, and pellet cells (enriched for MOPC315 cells) and APC I and II tested for their ability to induce IFN-γ production by Id-specific cloned Th1 cells (7A10B2). (B) Large MOPC315 tumors were established in Rag2−/− (H-2b) and SCID (H-2d) mice. APC I cells were tested for their ability to induce IL-2 production by TCR-TG SCID splenocytes.
Secondly, APC from MOPC315 tumors established in Rag2−/−(H-2b) mice did not stimulate naive Id-specific T cells, whereas the APC isolated from tumors in C.B-17 SCID mice (H-2d) did (Fig. 4B). This excludes MOPC315 as the Id-presenting cell and demonstrates, as expected, that H-2d haplotype of APC is required for stimulation of I-Ed-restricted, Id-specific CD4+ cells. Moreover, the Id-primed H-2d APC do not need to be B cells (as the latter are absent in SCID mice).
Surface Markers of Tumor APC.
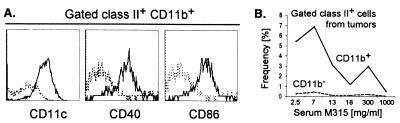
The APC-I and APC-II preparations were similar and contained 10–25% of cells that expressed class II and CD80/86 molecules required for stimulation of naive Id-specific CD4+ cells. The majority of these Id-primed APC were CD11b+, CD11c+, CD40+, CD80+, and CD86+ (Fig. 5A, and data not shown), but negative for markers of T-(CD4−, CD8−), B-(CD19−), NK-(DX-5−), granulocyte-(Ly-6G−) cell lineages, lymphoid-dendritic cells (CD1d−, CD4−, CD8α−, CD13−, DEC205−), and macrophages (F4/80−, scavenger receptor−). They were also CD95− (Fas), CD95L− (FasL), and CD138− (data not shown). A small fraction of class II+ tumor APC cells lacked CD11b expression (Fig. 3B, arrows). The CD11b+ APC were located beneath CD31+ endothelial cells of tumor vessels (Fig. 3C). The frequency of APC was 5–7% in minute (1–2 mm) tumors, but dropped rapidly with increase of the tumor size, which indicates that tumor cells outgrow APC as tumor enlarges (Fig. 5B).
Figure 5.
Phenotype profiles of APC isolated from MOPC315 tumors. (A) APC I cells were triple-stained, and gated class II+ CD11b+ cells were analyzed for CD11c, CD40, or CD86 expression, compared with isotype-matched control Abs (dashed lines). (B) MOPC315 tumors of various sizes were double-stained, and frequencies of class II+ CD11b+ and class II+ CD11b− populations were plotted as a function of serum M315 concentration [categorical (nonlinear) abscissa].
Id-Specific CD4+ Cells Accumulate in Small Id+ Tumors.
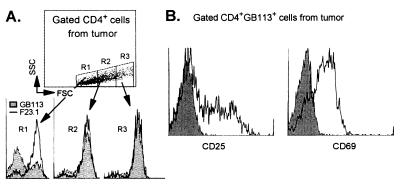
The TCR-TG mice have both Id-specific T cells [≈70% of the CD4+ cells express TG TCR (αTβT)] and nonspecific T cells [≈30% of the CD4+ cells express endogenous TCR α-chains (αEβT)] (29). The two populations can readily be identified with anti-TCR mAbs because Id-specific cells simultaneously bind clonotype (αTβT)-specific GB113 and Vβ8 (βT)-specific F23.1 mAbs, whereas nonspecific cells only stain with F23.1 (20, 29), as illustrated in Figs. 3F and 7A.
Figure 7.
Id-specific CD4+ T cells are activated blasts in small MOPC315 tumors in TCR-TG mice. (A) Single tumor cell suspensions were triple-stained with anti-CD4 and anti-CD8 mAbs, and either GB113 or F23.1 mAbs. A combined CD4+ and forward/side scatter gate was used, and cells of various sizes (R1, R2, and R3) were analyzed for TCR composition with TCR-TG clonotype-specific (αTβT) GB113 and βT (Vβ8)-specific F23.1 mAbs. (B) Tumor cells were triple-stained, and expression of CD25 and CD69 on gated Id-specific CD4+ cells is shown, together with isotype-matched control mAbs (shaded areas).
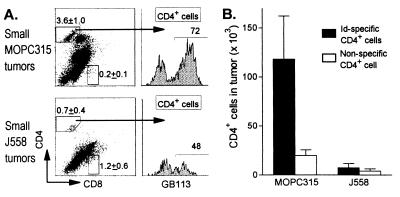
TCR-TG mice were injected with high loads of MOPC315 cells to partially overcome their tumor protection (28) and analyzed when MOPC315 tumors were still small (<0.5 g, serum M315 < 50 μg/ml), before deletion of Id-specific CD4+ cells (28). TIL were strongly skewed toward CD4+ in MOPC315 tumors (Fig. 6), compared with lymphoid organs in TCR-TG mice (14). Moreover, Id-specific CD4+ TIL were much more frequent in Id+ MOPC315 than in Id− J558 tumors (Figs. 3 D and E and 6), and often occurred as blasts in clusters (Fig. 3D).
Figure 6.
Id-specific CD4+ cells accumulate in small Id+ tumors. Small MOPC315 and J558 tumors were triple-stained with the clonotype-specific GB113, anti-CD4, and anti-CD8 mAbs [MOPC315 (n = 5, 0.2 ± 0.1 g, 3.8 ± 0.6 × 106 cells, serum M315: 29 ± 14 μg/ml); J558 (n = 4, 0.6 ± 0.4 g, 4.1 ± 2.8 cells, serum J558: 95 ± 54 μg/ml)]. (A) CD4 and CD8 cells were analyzed for TG TCR expression (GB113 mAb). The CD4 and CD8 gates were set on LN cells analyzed in parallel. A considerable number of tumor cells, outside the indicated CD4 and CD8 gates, stained variably for CD4 and CD8, but these cells were not bona fide T cells because they did not express TCR. By contrast, >80–90% of cells within the indicated CD4 gate were genuine T cells because they stained with the β-transgene (Vβ8)-specific F23.1 mAb (see Fig. 7A). Representative examples are shown; numbers represent mean frequencies ± SD. (B) Absolute number of Id-specific (GB113+) and nonspecific (GB113−) CD4+ cells in MOPC315 and J558 tumors.
CD4+Blasts in Id+ Tumors Are Id Specific and Proliferate.
As analyzed by flow cytometry, CD4+ blasts in MOPC315 tumors were enriched for Id-specific CD4+ cells, whereas small CD4+ cells were mainly of the nonspecific type (Fig. 7). These results are consistent with the finding of many GB113+ blasts in sections of small MOPC315 tumors (Fig. 3 D and F). The sparse CD8+ cells in MOPC315 tumors (Fig. 6A) were all of a small size (data not shown).
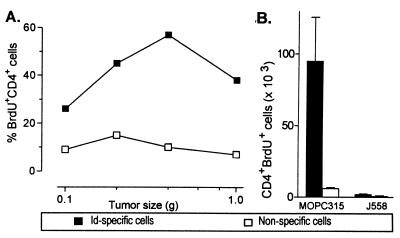
To investigate in vivo proliferation of Id+-specific CD4+ cells, TCR-TG mice with small MOPC315 tumors were given BrdUrd for the last 3 days before analysis. Id-specific T cells, analyzed in sections, had incorporated BrdUrd, and thus had proliferated (Fig. 3G). As quantified by flow cytometry, a considerable fraction of Id-specific CD4+ cells were BrdUrd+, whereas incorporation into nonspecific CD4+ cells was negligible (Fig. 8A). Id-specific CD4+ cells incorporated BrdUrd even in larger MOPC315 tumors (>0.5 g, serum M315 > 50 μg/ml) (Fig. 8A), suggesting that they proliferate until they become deleted (data not shown, and ref. 28). BrdUrd incorporation in Id-specific CD4+ cells was much more frequent in MOPC315 tumors than in J558 tumors, demonstrating the Id specificity of the proliferation (Fig. 8B).
Figure 8.
Id-specific CD4+ cells in small and intermediate-sized MOPC315 tumors in TCR-TG mice incorporate BrdUrd. (A) Tumor-bearing mice were given BrdUrd for the last 3 days before analysis. Tumor single cell suspensions were triple-stained with anti-CD4, GB113, and anti-BrdUrd mAbs. Id-specific and nonspecific CD4+ T cell were analyzed for BrdUrd incorporation. (B) Comparison of BrdUrd incorporation in CD4+ T cells in small MOPC315 and J558 tumors (see Fig. 6).
Id-Specific CD4+ Cells in Id+ Tumors Have Activated Phenotype.
Id-specific CD4+ cells in small MOPC315 tumors displayed an activated phenotype with elevated surface expression of CD69 and CD25 (Fig. 7B). Activation was Id specific, both at the T cell (Id specific vs. nonspecific) and tumor cell (Id+ vs. Id−) levels.
Id-Specific CD4+Cells Colocalize with APC in the MOPC315 Tumors.
Id-specific CD4+ cells were intermingled with class II+CD11b+ APC in tumor sections (Fig. 3H). Moreover, Id-specific T cells (Fig. 3I) and the APC (Fig. 3C) were close to CD31 (PECAM-1)+ tumor vessels. This proximity suggests that Id-specific T cells are interacting with the Id-primed APC in the tumor.
Discussion
In this study, we show that TSA (Id)-specific CD4+ T cells in TCR-TG mice are activated and proliferate in small extramedullary Id+ plasmacytomas: (i) The CD4+/CD8+ ratio was skewed toward CD4 in tumor tissue; (ii) CD4+ blasts within tumors were selectively enriched for cells expressing Id-specific TCR; (iii) Id-specific CD4+ TIL incorporated BrdUrd and proliferated locally in clusters; (iv) Id-specific CD4+ cells in tumors had an activated phenotype (CD69+CD25+); (v) Activation required Id-expression by the tumor; and (vi) Activation required the Id-specific TCR, because nonspecific CD4+ TIL with endogenous TCR α-chains were few and nonactivated.
The class II-negative MOPC315 plasmacytoma cells themselves cannot present the Id to the class II-restricted CD4+ cells. However, Id-primed APC directly purified from the tumor could stimulate costimulation-dependent naive Id-specific CD4+ cells. The APC were heterogeneous, but the large majority expressed class II+,CD11b+,CD11c+,CD40+,CD80+, and CD86+, consistent with most characteristics of DC of a myeloid origin (30). Importantly, the APC and Id-specific T cells coclustered beneath endothelium of CD31+ tumor microvessels. These data suggest that angiogenesis and diapedesis of APC in tumor may be related phenomena. It has been suggested that monocytes that traverse the endothelium by reverse transmigration differentiate into veiled DC (31).
APC could become primed in situ by endocytosis of myeloma protein in the extracellular tumor fluid, e.g., by means of Fc receptors. In fact, Id priming of APC may depend on secretion of Ig by tumor cells, as suggested by experiments in which TG mice were protected against an A20 B lymphoma transfectant that secretes λ2315, but succumb to another A20 transfectant that does not secrete λ2315 (16). Experiments with nonsecretory hemagglutinin A20 transfectants may be similarly interpreted (32). These studies point to a significance of secretion of TSA, which probably reflects the need for uptake and processing by costimulation-competent class II+ host APC. Similar results have been obtained in two different class I-restricted TCR-TG tumor models using pseudo-TSA-like lymphocytic choriomeningitis virus (LCMV) glycoprotein (33) and an allo class I MHC molecule (Ld) (34). CD8+ cells remained unaware of the tumors until powerful antigenic stimuli on costimulation-competent APC, such as infection with LCMV virus (33) or skin transplantation (34), induced CD8+ cells to attack the tumor.
We and others (16–19, 21) have reported that Id-specific CD4+ cells have an antimyeloma effect. However, although Id-specific CD4+ cells can protect against challenges with small numbers of MOPC315 cells (14, 16), Id-specific CD4+ cells eventually become deleted within progressive plasmacytomas established by injection of high loads of tumor cells (28). The present results demonstrate that a phase of CD4+ T cell activation and proliferation within the tumor precedes the deletion. A similar activation and proliferation of Id-specific CD4+ T cells probably takes place when the MOPC315 challenge is successfully fought off (14, 16). The molecular mechanism by which activated CD4+ cells can eliminate MOPC315 cells is unknown.
It has been suggested that immunization with TSA-pulsed DC in patients with established disease may be beneficial (35). However, if TSA-primed host DC are already present in the tumor (present results), does it make sense to vaccinate with TSA-primed DC? Probably so, for a number of reasons. First, in a number of tumor diseases, host DC may in fact not be spontaneously TSA-primed, possibly because of lack of TSA transfer (16, 33, 34). Second, even though host DC might be primed with TSA, they could, in many tumors, fail to adequately stimulate T cells. For example, tumors could inhibit terminal maturation of DC. Third, DC incubated with TSA in vitro may have better loaded MHC molecules, and, therefore, a higher stimulatory potential, than spontaneously TSA-primed tumor DC. Activated Id-specific CD4+ cells become deleted with progressive myeloma disease in mice (28, 36). Therefore, Id vaccination of multiple myeloma patients is probably best performed after high-dose chemotherapy and stem-cell transplantation at a time when tumor burden is very low and new (Id-specific) T cells are being educated in the thymus. Likewise for other tumor types, it might be important to minimize the tumor load prior to DC vaccination immunotherapy.
Acknowledgments
We thank Peter Hofgaard, Hilde Omholt, and Olav Schreurs for providing outstanding technical assistance. This study is supported by grants from the Norwegian Research Council, the Norwegian Cancer Society, the Multiple Myeloma Research Foundation and McCarty Cancer Foundation.
Abbreviations
- APC
antigen-presenting cells
- DC
dendritic cells
- MΦ
macrophage
- N-TG
nontransgenic
- SA
streptavidin
- LN
lymph node
- Id
idiotype
- TCR
T cell receptor
- TG
transgenic
- TIL
tumor infiltrating T cells
- TSA
tumor-specific antigen
- DAPI
4,6-diamino-2-phenylindoldihydrochloride
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.050579897.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.050579897
References
- 1.Toes R E, Ossendorp F, Offringa R, Melief C J. J Exp Med. 1999;189:753–756. doi: 10.1084/jem.189.5.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang A Y, Golumbek P, Ahmadzadeh M, Jaffee E, Pardoll D, Levitsky H. Science. 1994;264:961–965. doi: 10.1126/science.7513904. [DOI] [PubMed] [Google Scholar]
- 3.Cayeux S, Richter G, Noffz G, Dorken B, Blankenstein T. J Immunol. 1997;158:2834–2841. [PubMed] [Google Scholar]
- 4.Gajewski T F, Fallarino F, Uyttenhove C, Boon T. J Immunol. 1996;156:2909–2917. [PubMed] [Google Scholar]
- 5.Pulaski B A, Yeh K Y, Shastri N, Maltby K M, Penney D P, Lord E M, Frelinger J G. Proc Natl Acad Sci USA. 1996;93:3669–3674. doi: 10.1073/pnas.93.8.3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiodoni C, Paglia P, Stoppacciaro A, Rodolfo M, Parenza M, Colombo MP. J Exp Med. 1999;190:125–133. doi: 10.1084/jem.190.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nitta T, Oksenberg J R, Rao N A, Steinman L. Science. 1990;249:672–674. doi: 10.1126/science.2382141. [DOI] [PubMed] [Google Scholar]
- 8.Romero P, Dunbar P R, Valmori D, Pittet M, Ogg G S, Rimoldi D, Chen J L, Lienard D, Cerottini J C, Cerundolo V. J Exp Med. 1998;188:1641–1650. doi: 10.1084/jem.188.9.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eisen H N, Simms E S, Potter M. Biochemistry. 1968;7:4126–4134. doi: 10.1021/bi00851a048. [DOI] [PubMed] [Google Scholar]
- 10.Schulenburg E P, Simms E S, Lynch R G, Bradshaw R A, Eisen H N. Proc Natl Acad Sci USA. 1971;68:2623–2626. doi: 10.1073/pnas.68.11.2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eisen H N, Reilly E B. Annu Rev Immunol. 1985;3:337–365. doi: 10.1146/annurev.iy.03.040185.002005. [DOI] [PubMed] [Google Scholar]
- 12.Lynch R G, Graff R J, Sirisinha S, Simms E S, Eisen H N. Proc Natl Acad Sci USA. 1972;69:1540–1544. doi: 10.1073/pnas.69.6.1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bogen B, Malissen B, Haas W. Eur J Immunol. 1986;16:1373–1378. doi: 10.1002/eji.1830161110. [DOI] [PubMed] [Google Scholar]
- 14.Bogen B, Munthe L, Sollien A, Hofgaard P, Omholt H, Dagnaes F, Dembic Z, Lauritzsen G F. Eur J Immunol. 1995;25:3079–3086. doi: 10.1002/eji.1830251114. [DOI] [PubMed] [Google Scholar]
- 15.Muhlethaler-Mottet A, Otten L A, Steimle V, Mach B. EMBO J. 1997;16:2851–2860. doi: 10.1093/emboj/16.10.2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lauritzsen G F, Weiss S, Dembic Z, Bogen B. Proc Natl Acad Sci USA. 1994;91:5700–5704. doi: 10.1073/pnas.91.12.5700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kwak L W, Taub D D, Duffey P L, Bensinger W I, Bryant E M, Reynolds C W, Longo D L. Lancet. 1995;345:1016–1020. doi: 10.1016/s0140-6736(95)90757-2. [DOI] [PubMed] [Google Scholar]
- 18.Masaki H, Irimajiri K, Horiuchi A, Yamaguchi J, Toyosaki T, Suzuki R, Kurane I. Cell Immunol. 1996;174:180–189. doi: 10.1006/cimm.1996.0308. [DOI] [PubMed] [Google Scholar]
- 19.King C A, Spellerberg M B, Zhu D, Rice J, Sahota S S, Thompsett A R, Hamblin T J, Radl J, Stevenson F K. Nat Med. 1998;4:1281–1286. doi: 10.1038/3266. [DOI] [PubMed] [Google Scholar]
- 20.Bogen B, Gleditsch L, Weiss S, Dembic Z. Eur J Immunol. 1992;22:703–709. doi: 10.1002/eji.1830220313. [DOI] [PubMed] [Google Scholar]
- 21.Lauritzsen G F, Bogen B. Cell Immunol. 1993;148:177–188. doi: 10.1006/cimm.1993.1100. [DOI] [PubMed] [Google Scholar]
- 22.Stockinger B, Hausmann B. Int Immunol. 1994;6:247–254. doi: 10.1093/intimm/6.2.247. [DOI] [PubMed] [Google Scholar]
- 23.Munthe L A, Blichfeldt E, Sollien A, Dembic Z, Bogen B. Cell Immunol. 1996;170:283–290. doi: 10.1006/cimm.1996.0162. [DOI] [PubMed] [Google Scholar]
- 24.Bogen B, Lauritzsen G F, Weiss S. Eur J Immunol. 1990;20:2359–2362. doi: 10.1002/eji.1830201030. [DOI] [PubMed] [Google Scholar]
- 25.Staerz U, Rammensee H, Benedetto J, Bevan M. J Immunol. 1985;134:3994–4000. [PubMed] [Google Scholar]
- 26.Lemke H, Hammerling G J, Hammerling U. Immunol Rev. 1979;47:175–206. doi: 10.1111/j.1600-065x.1979.tb00293.x. [DOI] [PubMed] [Google Scholar]
- 27.Penit C, Vasseur F. Cytometry. 1993;14:757–763. doi: 10.1002/cyto.990140708. [DOI] [PubMed] [Google Scholar]
- 28.Bogen B. Eur J Immunol. 1996;26:2671–2679. doi: 10.1002/eji.1830261119. [DOI] [PubMed] [Google Scholar]
- 29.Munthe L A, Sollien A, Dembic Z, Bogen B. Scand J Immunol. 1995;42:651–661. doi: 10.1111/j.1365-3083.1995.tb03708.x. [DOI] [PubMed] [Google Scholar]
- 30.Salomon B, Cohen J L, Masurier C, Klatzmann D. J Immunol. 1998;160:708–717. [PubMed] [Google Scholar]
- 31.Randolph G J, Beaulieu S, Lebecque S, Steinman R M, Muller W A. Science. 1998;282:480–483. [PubMed] [Google Scholar]
- 32.Staveley-O'Carroll K, Sotomayor E, Montgomery J, Borrello I, Hwang L, Fein S, Pardoll D, Levitsky H. Proc Natl Acad Sci USA. 1998;95:1178–1183. doi: 10.1073/pnas.95.3.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Speiser D E, Miranda R, Zakarian A, Bachmann M F, McKall-Faienza K, Odermatt B, Hanahan D, Zinkernagel R M, Ohashi P S. J Exp Med. 1997;186:645–653. doi: 10.1084/jem.186.5.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wick M, Dubey P, Koeppen H, Siegel C T, Fields P E, Chen L, Bluestone J A, Schreiber H. J Exp Med. 1997;186:229–238. doi: 10.1084/jem.186.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Young J W, Inaba K. J Exp Med. 1996;183:7–11. doi: 10.1084/jem.183.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lauritzsen G F, Hofgaard P O, Schenck K, Bogen B. Int J Cancer. 1998;78:216–222. doi: 10.1002/(sici)1097-0215(19981005)78:2<216::aid-ijc16>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]