Abstract
Sex hormones are presumed to contribute to sexual dimorphism in the immune system. Estrogen, in particular, has been suggested to predispose women to systemic lupus erythematosus. We report here that estradiol (E2) can break B cell tolerance and induce a lupus-like phenotype in nonautoimmune mice transgenic for the heavy chain of a pathogenic anti-DNA antibody. E2 treatment resulted in a rise in anti-DNA serum titers and in Ig deposition in renal glomeruli. ELISPOT analysis confirmed a significant increase in the number of high-affinity anti-DNA antibody-secreting B cells in the spleens of E2-treated mice. Hybridomas generated from E2-treated mice express high-affinity, unmutated anti-DNA antibodies, indicating that naïve B cells that are normally deleted or anergized are rescued from tolerance induction. Finally, immunohistochemical studies revealed increased Bcl-2 expression in splenic B cells of E2-treated mice. These data demonstrate that estrogen interferes with tolerance induction of naïve autoreactive B cells and that the presence of these B cells in the periphery is associated with up-regulation of Bcl-2.
Previous studies have demonstrated a sexual dimorphism in the immune response. In general, females exhibit higher levels of serum Ig than males and mount a more vigorous humoral immune response (1–3). This enhanced activation of B cells may contribute to the greater susceptibility of women to autoimmune disease, including systemic lupus erythematosus (SLE), which occurs at a female-to-male ratio of 10:1 (3–5).
There is mounting evidence that estrogen has immunomodulatory effects (5, 6). Peripheral blood mononuclear cells derived from patients with SLE and stimulated in vitro with estrogen undergo polyclonal activation, secrete anti-double-stranded DNA (dsDNA) IgG, and display diminished apoptosis (7, 8). Data from many mouse models of SLE provide compelling evidence that estrogen can augment in vivo production of autoantibodies. In NZB/W F1 mice, which develop a lupus-like syndrome with spontaneous production of antibodies to DNA and glomerulonephritis, females manifest an earlier onset of disease and earlier mortality (9). Treatment with exogenous estrogen accelerates disease in both male and female mice, whereas ovariectomy or administration of testosterone to female mice ameliorates disease (10, 11). Similar effects of sex hormones have been demonstrated in MRL/lpr mice (12, 13) and in C57BL/10 × DBA/2 F1 mice in a graft-vs.-host disease model of lupus nephritis (14). Administration of 17β-estradiol (E2) also has been shown to augment production of autoantibodies in the nonautoimmune mouse strains BALB/c and C57BL/6 (15, 16), although it does not lead to disease. The molecular mechanisms whereby estrogens and androgens modulate the immune system have not been addressed.
We elected to analyze the effect of estrogen in nonautoimmune BALB/c mice transgenic for the γ2b heavy (H) chain of a nephritogenic anti-DNA antibody (17–19). This H chain associates with numerous endogenous light chains to generate antibodies with varying affinities for dsDNA, as well as nonautoimmune specificities. Serum autoantibody titers are negligible in these mice, yet detailed analysis demonstrates the existence of three populations of anti-dsDNA B cells. A nontolerized B cell population displays low affinity for dsDNA (20). An anergic population secretes high-affinity anti-dsDNA antibodies only after in vitro stimulation with lipopolysaccharide. This population displays somatic mutation that, in some cases, clearly accounts for high-affinity DNA binding (21). A high-affinity B cell population undergoes deletion, but can be found in BALB/c mice transgenic for both the R4A-γ2b H chain and Bcl-2 overexpressed in the B cell compartment (22) or in R4A-γ2b NZB/NZW F1 transgenic mice (23). Autoantibodies from both the anergic and deleted populations have an apparent affinity for dsDNA of 10−8 to 10−9 M and deposit in glomeruli of severe combined immunodeficient mice (23).
Because the R4A-γ2b BALB/c transgenic mice effectively tolerize high-affinity autoreactive B cells, it is possible to ask whether estrogen alters tolerance induction and, if so, at what stage of B cell development. The advantage of this transgenic model is that it offers an opportunity to study both B cells that arise in the bone marrow and produce high-affinity anti-DNA antibodies in their germ-line configuration, as well as B cells acquiring high affinity by somatic mutation in the periphery. The results from our studies demonstrate that E2 treatment blocks tolerance induction of high-affinity, naïve autoreactive B cells arising in the bone marrow. Furthermore, survival of these autoreactive B cells in the periphery associates with the up-regulation of the antiapoptotic Bcl-2 protein in B cells.
Methods
Transgenic Mice.
Female BALB/c mice (2–6 months old) transgenic for the R4A-γ2b H chain were bred and housed in a barrier facility and moved to a nonbarrier facility during experiments.
Estradiol Treatment.
Mice were anesthetized with metofane and pellets containing E2 or placebo (P) (Innovative Research of America) were implanted s.c. The E2 pellets are designed to release 17β-estradiol over a 2-month period to achieve a constant serum level of 75–100 pg/ml. Mice were analyzed after 5 weeks of treatment.
Serum Estradiol Concentrations.
Serum samples were collected every week and stored at −70°C. E2 concentrations were determined by RIA (Diagnostic Products, Los Angeles) by using the manufacturer's protocol.
Anti-dsDNA Antibody ELISA.
Immulon-2 plates (Dynex Technologies, Chantilly, VA) were coated with sonicated and filtered calf thymus DNA at 100 μg/ml (23). Supernatants from cloned hybridomas normalized for Ig concentration or with serum diluted 1:500 to 1:2,000 were added for 1.5 hr at 37°C, followed by a 1:1,000 dilution of goat anti-mouse γ2b antibody conjugated to alkaline phosphatase (Southern Biotechnology Associates). The reaction was developed with p-nitrophenylphosphate sodium (Sigma). The optical density at 405 nm was determined in a Titertek Multiscan ELISA reader.
Analysis of Glomerular Ig Deposition.
Formalin-fixed, paraffin-embedded kidneys were stained with biotinylated-anti-mouse IgG and developed with the alkaline phosphatase ABC detection kit (Vector Laboratories) (24).
FACS Analysis.
Splenocytes were isolated from E2- and P-treated mice. After lysis of red blood cells, cells were stained with 0.5 μg of FITC- or phycoerythrin-conjugated antibodies specific for γ2b (Southern Biotechnology Associates), CD3 (PharMingen), or B220 (Sigma) for 30 min at 4°C, washed, then fixed with 2% paraformaldehyde and analyzed on a FACScan flow cytometer (Becton Dickinson).
ELISPOT Assay.
Immulon-2 plates were coated with 50 μl of calf thymus DNA at 10 or 100 μg/ml. Plates were blocked with 1% BSA-PBS, and 106 splenocytes per well were added at 37°C for 4 hr. Biotin-conjugated goat anti-mouse γ2b (Southern Biotechnology Associates) was added at a 1:1,000 dilution. After overnight incubation at 4°C or 4 hr at room temperature, plates were washed and incubated at room temperature for 1 hr with alkaline phosphatase-conjugated streptavidin (Southern Biotechnology Associates) at a 1:1,000 dilution. 5-Bromo-4-chloro-3 indolyl phosphate substrate (Sigma) was added for 1–3 hr.
Generation of B Cell Hybridomas.
Splenocytes were isolated from E2-treated R4A-γ2b transgenic BALB/c mice and fused to the NSO cell line by using standard methodology at a spleen cell-to-myeloma cell ratio of 2:1 (20). Cells were plated in 96-well U-bottom plates (Costar) at 2 × 105 splenocytes/ml in HAT medium (hypoxanthine/aminopterin/thymidine) with 20% FCS (HyClone).
RNA Dot Blots.
To detect transgene expression, an RNA dot blot was performed (20). Dot blot-positive hybridomas were cloned on soft agar or by limiting dilution. Selected clones were screened for expression of a VK1 light chain by RNA dot blot by using a 230-bp probe for members of the VK1 family (23).
Analysis of Vκ Gene Usage.
Total RNA was extracted from both VK1 and non-VK1 clones, and reverse transcription–PCR was performed with primers specific for the mouse VK1 framework 1 (FR1) or with degenerate FR1 VK primers and a κ constant region primer (23). PCR products were purified by using Qiaquick spin columns (Qiagen), and V gene sequences were determined by using an ABI 377 automated sequencer (Perkin–Elmer, Applied Biosystems Division). Sequences were analyzed by using gcg software.
Quantitation of γ2b.
Quantitation of antibodies in supernatants from cloned hybridomas was performed as described (20).
Measurement of Apparent Affinities.
Determination of apparent affinities of anti-DNA antibodies was calculated based on the concentration of soluble DNA resulting in 50% inhibition of antigen binding as described (20, 25).
Immunofluorescence Staining.
Spleens from E2- and P-treated mice were embedded in paraffin and sectioned. Sections were blocked with 5% goat serum for 30 min at room temperature. For B220/Bcl-2 dual staining, sections were rinsed in PBS and incubated with 10 μg/ml rabbit anti-mouse Bcl-2 IgG (Santa Cruz Biotechnology) for 1.5 hr at room temperature. Sections were incubated with 10 μg/ml rat anti-mouse B220 (PharMingen) and biotinylated-goat anti-rabbit IgG (1:100 dilution) for 1 hr at room temperature and then in the dark for 15–30 min with rabbit anti-rat IgG-FITC diluted 1:10 (Vector Laboratories) and streptavidin-Cy3 diluted 1:20 (Sigma). PNA/Bcl-2 dual staining was performed as above with PNA-FITC diluted 1:10 (Vector Laboratories).
Statistical Analysis.
Statistical analysis was carried out by using ANOVA and unpaired Student's t test. Statistically significant values are P < 0.05.
Results
Production of Anti-dsDNA Antibodies in E2-Treated Mice.
To determine whether E2 increases survival of anti-DNA B cells and up-regulates production of anti-DNA antibodies, female R4A-γ2b transgenic BALB/c mice were implanted with P or E2 pellets designed to release between 75 and 100 pg/ml of 17β-estradiol into the circulation for up to 60 days. Sera assayed by RIA displayed average E2 concentrations of 16 pg/ml (range, 6–35) in both E2- and P-treated mice at week 0 and 94 pg/ml (range, 50–130) in E2-treated mice and 24 pg/ml (range, 16–35) in P-treated mice by 5 weeks of treatment.
Titers of transgene-encoded γ2b anti-dsDNA titers rose significantly in mice treated with E2 at 5 months (n = 6) (P < 0.0001) or 2 months of age (n = 6) (P = 0.0001), but not in age-matched, P-treated mice (Fig. 1). Furthermore, E2-treated mice displayed glomerular Ig deposition, indicating that increased levels of E2 lead to activation of pathogenic anti-dsDNA B cells (Fig. 2). No glomerular Ig deposition was observed in P-treated R4A-γ2b mice, consistent with our earlier findings that R4A-γ2b BALB/c mice effectively regulate the production of pathogenic anti-dsDNA antibodies. No IgM antibody was detected in kidneys of E2-treated mice, demonstrating that only transgene encoded anti-DNA antibodies were present. After 6 weeks of E2 treatment, histologic changes other than Ig deposition were not present.
Figure 1.
Serum anti-dsDNA antibody titers from E2- and P-treated mice. Sera were tested for anti-dsDNA activity by ELISA. (A) Sera diluted 1:2,000 from 5- to 6-month-old female mice [E2 (n = 6) and P (n = 4)]. (B) Sera diluted 1:500 from 2- to 3-month-old female mice [E2 (n = 6) and P (n = 5)]. Both 5- to 6-month-old and 2- to 3-month-old E2-treated transgenic mice displayed a significant increase in anti-dsDNA titers (P < 0.0001 and P = 0.0001, respectively). Data are presented as the mean ± SD. In some P-treated mice, the SD is so small that no bars are shown.
Figure 2.
Kidney sections stained for glomerular Ig deposition. Formalin-fixed kidneys were embedded in paraffin and sectioned. Ig was stained with alkaline phosphatase-conjugated anti-mouse IgG. Glomerular Ig deposition was detected in the kidneys of five E2-treated mice, but not five P-treated mice. A representative kidney is shown for each group. (×100.)
Analysis of Peripheral B Cells.
Spleens of E2-treated R4A-γ2b transgenic mice consistently contained approximately 2-fold more cells than spleens of P-treated mice. Flow cytometry showed no difference in the percentage of B220+ cells in either the bone marrow or spleen of E2- and P-treated mice (data not shown). The percentage of splenic B cells that express γ2b, however, was increased significantly in E2-treated mice, with no increase seen in bone marrow B cells (Fig. 3A). In our experience, essentially all γ2b+ B cells in these mice express the transgene (21, 23). Thus, the major expansion of transgene expressing B cells in E2-treated mice appears to occur after they exit the bone marrow and move into the periphery.
Figure 3.
Analysis of peripheral B cells. (A) Spleen and bone marrow cells from E2- and P-treated mice were analyzed for B220 and γ2b expression by flow cytometry. Mean percentages (±SD) are shown. The percentage of γ2b (transgene-positive) B cells in the spleen is greater in E2-treated mice (n = 5) than in P-treated mice (n = 4) (P < 0.03), but not in bone marrow. (B) The number of anti-dsDNA γ2b B cells was enumerated by ELISPOT. The mean number (±SD) of high-affinity anti-DNA B cells in 105 splenocytes of E2-treated (n = 6) and P-treated (n = 6) mice is shown. E2-treated mice contain more high-affinity anti-dsDNA-secreting B cells in the spleen (P < 0.02).
To enumerate transgene-encoded anti-dsDNA-producing B cells, we performed ELISPOT assays with splenocytes from E2- and P-treated mice. When DNA was absorbed to plates at a concentration of 100 μg/ml, permitting detection of both high- and low-affinity anti-dsDNA B cells, there was no significant difference between the number of anti-dsDNA-producing B cells in spleens of E2- and P-treated mice (data not shown). An ELISPOT assay performed using 10 μg/ml dsDNA to enumerate only those B cells with high affinity for dsDNA demonstrated significantly more high-affinity anti-dsDNA B cells in the spleens of E2-treated mice (P < 0.02) (Fig. 3B). These data demonstrate that there is increased survival of high-affinity anti-DNA-secreting B cells in the spleens of E2-treated mice. Consistent with the study by Verthelyi and Ahmed (16), the spots generated by anti-dsDNA B cells from E2-treated mice were larger than the spots produced from P mice, indicating that E2 treatment may enhance antibody secretion.
Analysis of B Cell Hybridomas from E2 Mice.
To characterize the anti-dsDNA B cell populations generated in E2-treated mice, B cell hybridomas were generated. Approximately 600 wells were screened from six fusions, and 83 γ2b anti-dsDNA antibody-producing hybridomas were obtained. Fifty hybridomas were cloned, all of which expressed the transgene, and 29 secreting greater than 5 μg/ml Ig per 106 cells per 24 hr were selected for further analysis.
Fifteen hybridomas representing the weakest and strongest dsDNA-binding antibodies revealed apparent affinities ranging from 10−6 M to 10−9 M, with six antibodies displaying apparent affinities of 10−8 M to 10−9 M (Table 1). Such high-affinity anti-dsDNA B cells could not be derived from P-treated mice and have been immortalized as hybridomas from this mouse model only when splenocytes are stimulated with lipopolysaccharide (21) or when derived from mice that also overexpress Bcl-2 in a B cell-specific manner (22). It appears, therefore, that E2 treatment led to the existence of a population of high-affinity anti-DNA B cells that usually undergoes deletion or anergy induction.
Table 1.
Apparent affinity and light chain usage of hybridomas from E2-treated mice
| Clone | Apparent affinity, M | Vκ gene | GenBank accession no. | Jκ gene |
|---|---|---|---|---|
| R4A* | 9.1 × 10−8 | Vκ1-A | M28131 | Jκ2 |
| 11-K2C | 4.5 × 10−9 | Vκ1-A | M28131 | Jκ4 |
| 9-11B9 | 3.6 × 10−9 | Vκ1-A | M28131 | Jκ4 |
| E3-B10 | 8.4 × 10−9 | Vκ1-A | M28131 | Jκ4 |
| E6-3D9 | 3.7 × 10−7 | Vκ1-A | M28131 | Jκ5 |
| 11-G5D4 | 7.4 × 10−6 | Vκ-RF | AJ235936 | Jκ1 |
| A10-1E4 | 5.4 × 10−6 | Vκ-RF | AJ235936 | Jκ1 |
| B15-3C6 | 6.6 × 10−9 | Vκ-RF | AJ235936 | Jκ1 |
| C11-1A4 | 2.2 × 10−6 | Vκ-21 | ND | Jκ1 |
| E5-3B2 | 1.0 × 10−6 | Vκ-Ser | X02816 | Jκ2 |
| A7-1D9 | 2.3 × 10−8 | Vκ-Gx1 | AF021868 | Jκ5 |
| B11-1B7 | 4.3 × 10−8 | Vκ-8 | L17135 | Jκ1 |
| A7-4(39) | 9.0 × 10−6 | Vκ-21 | ND | Jκ2 |
| 11-A3C6 | 1.7 × 10−7 | ND | ND | ND |
| A7-2G1 | 5.7 × 10−7 | ND | ND | ND |
| B1-1(28) | 6.2 × 10−6 | ND | ND | ND |
Clones were obtained from six individual fusions. ND, not determined.
*Original R4A mAb.
In this mouse model, the anergic population primarily utilizes Vκ1 light chains (21), whereas the deleted population utilizes a broad spectrum of light chains (23). RNA dot blot analysis of the 29 hybridomas revealed that four expressed Vκ1 genes and the rest expressed a variety of non-Vκ1 genes. All four Vκ1-expressing hybridomas expressed Vκ1-A-Jκ rearrangements without somatic mutation, with three displaying high affinity for DNA. These observations demonstrate that E2 rescues high-affinity autoreactive B cells arising in the bone marrow and can rescue B cells that usually undergo deletion.
The non-Vκ1 encoded high-affinity anti-dsDNA antibodies B15–3C6, A7–1D9, and B11–1B7 display evidence for somatic mutation. For B15–3C6, it is clear that somatic mutation increases the affinity of DNA binding, because two other hybridomas, 11-G5D4 and A10–1E4, use the same light chain as B15–3C6 but are low affinity and lack somatic mutation (Fig. 4). This single example of rescue of a B cell acquiring high affinity for DNA in the periphery suggests that E2 also rescues mature B cells, but does not permit us to determine whether this is a routine event.
Figure 4.
Vκ-RF light chain nucleotide and amino acid sequences. Nucleotide and amino acid sequences of the germ-line gene are shown in the top line: dashed lines below that denote identical sequences, nonbold letters denote silent mutations, and bolded letters denote replacement mutations; *, missing bases. 11-G5D4 and A10–1E4 utilize the same Vκ-RF and Jκ genes as B15–3C6, but are unmutated and have low apparent affinity for DNA (10−6 M). B15–3C6 is somatically mutated and has high apparent affinity for DNA (10−9 M). All three hybridomas were obtained from separate fusions. The B15–3C6 Vκ sequence is available from GenBank (accession no. AF217806).
Bcl-2 Expression in E2-Treated Mice.
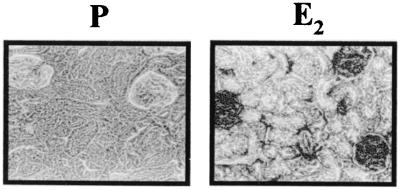
Bcl-2 expression is known to increase survival of autoreactive B cells (22, 23). Because it also has been demonstrated that E2 can augment Bcl-2 expression in cells expressing estrogen receptors (24, 25), we analyzed Bcl-2 expression in the spleens of E2-treated mice. The level of Bcl-2 expression in germinal center [peanut agglutinin (PNA)+] B cells (B220+) was higher in the spleens of E2-treated mice (Fig. 5 Center). This is best seen in Fig. 5 Right, where colocalization of Bcl-2 and B220 or PNA is shown. This level of Bcl-2 is equivalent to that seen in Bcl-2 transgenic mice (data not shown), showing that E2 leads to an up-regulation of Bcl-2 and providing a potential mechanism for mediating the survival of autoreactive B cells that are normally subject to deletion and anergy.
Figure 5.

Immunohistochemistry of spleen sections for Bcl-2, B220, and PNA. Formalin-fixed spleen sections from E2-treated (n = 5) and P-treated (n = 5) mice were dual-stained for Bcl-2 (Cy-3) and B220 (FITC) or PNA (FITC). Cells stained with both reagents appear yellow. A representative spleen is shown from each group. (×100.)
Discussion
It has been speculated that estrogen contributes to the greater incidence of lupus in women. The clinical data to support this theory are mainly anecdotal, and in those animal models of lupus in which the role of estrogen in the exacerbation of disease is well documented, the dose of E2 used was much greater than in this study (9, 11) and there have been no mechanistic hypotheses put forward. The results from our studies provide two observations concerning the role of estrogen in autoimmunity: E2 blocks tolerance induction of naïve autoreactive B cells arising in the bone marrow, and the rescue of autoreactive B cells associates with up-regulation of Bcl-2.
The R4A-γ2b transgenic mouse model offers an opportunity to study both B cells that arise in the bone marrow and produce high-affinity anti-DNA antibodies in their germ-line configuration as well as B cells acquiring high-affinity DNA binding by somatic mutation in the periphery. Using this model, we could ask whether either anti-DNA B cell subset is rescued in the presence of increased levels of estrogen. Titers of transgene-encoded γ2b anti-dsDNA antibodies from E2-treated mice were significantly higher than titers from P-treated animals, and histologic analysis of kidneys revealed the presence of IgG2b deposition in glomeruli of E2-treated mice. An enhanced percentage of transgene-expressing B cells was found in spleens of E2-treated mice, consistent with the selective expansion of transgenic B cells that normally would be deleted or would display a diminished half-life as occurs with anergic cells. ELISPOT assays confirmed the presence of high-affinity anti-DNA B cells in E2-treated mice. Together, these data show enhanced survival and activation of high-affinity anti-DNA B cells in the presence of increased serum estrogen levels.
To characterize the rescued population of anti-dsDNA B cells and to determine whether pathogenic B cells acquired high affinity for DNA in the bone marrow by Ig gene rearrangement or in the periphery by somatic mutation, we generated B cell hybridomas from E2-treated mice. It was not possible to generate hybridomas making high-affinity anti-DNA antibodies from unstimulated splenocytes of R4A-γ2b transgenic mice by using conventional hybridoma technology (18). This is consistent with data from other studies showing that, in the absence of serum titers of anti-DNA antibodies, it is not possible to recover high-affinity anti-DNA hybridomas from unstimulated B cells. Several of the hybridomas generated from E2-treated mice displayed high affinity for dsDNA, confirming the survival of B cells that normally are tolerized in this mouse model. Furthermore, several high-affinity antibodies were encoded by germ-line genes without somatic mutation, demonstrating that estrogen acts on autoreactive B cells that arise as part of a naïve repertoire. We also have identified high-affinity anti-DNA B cells displaying evidence of somatic mutation. Because we can be sure that high-affinity DNA binding was a consequence of somatic mutation in one hybridoma, it is possible the E2 may also permit survival and activation of cells acquiring autoreactivity by somatic mutation, but we do not know whether this is a routine event.
It has been reported recently that E2 treatment of another nonautoimmune strain, C57BL/6, results in the production of autoantibodies displaying multiple autospecificities (15, 16). It was concluded that E2 treatment results in the polyclonal activation and expansion of both autoreactive and nonautoreactive plasma cells. The increase in IgG2b anti-dsDNA antibody titer observed in E2-treated R4A-γ2b mice reflects the specificity of the heavy chain transgene and its enhanced probability of forming an anti-DNA antibody. This is consistent with the absence of IgM in the kidneys of E2-treated mice. We also observed, however, selective expansion of high-affinity, autoreactive transgenic B cells, accounting for the increased percentage of γ2b B cells in the periphery of E2-treated mice. In the presence of E2, antigen exposure may lead to proliferation of autoreactive B cells.
We and others have shown that apoptotic pathways are important in the death of autoreactive B cells (26, 27), and we have reported that overexpression of Bcl-2 leads to survival of autoreactive B cells in R4A-γ2b transgenic mice (22). The immunohistochemical studies reported here revealed the up-regulation of Bcl-2 expression in B220+ B cells and germinal center B cells in spleens of E2-treated mice. Bcl-2 has been demonstrated to be up-regulated by estrogen in a breast cancer cell line and to protect against apoptosis (28, 29). The bcl-2 promoter contains putative estrogen-responsive elements and could, therefore, be subject to a direct effect of E2 (29). In addition, estrogen activates a mitogen-activated protein kinase cascade that may lead to bcl-2 expression (30, 31). It recently has been shown that B cells express estrogen receptors (32), so it is possible that estrogen might exert some effects by acting directly on B cells. Alternatively, the up-regulation of Bcl-2 in B cells is mediated through interaction with another estrogen-responsive cell type.
Bcl-2 has been shown to increase survival of autoreactive B cells arising in the bone marrow, permitting them to populate periphery lymphoid structures (33, 34). In the R4A-γ2b, bcl-2 double-transgenic mice, Bcl-2 also has been shown to enhance survival of cells acquiring autoreactivity in the periphery (22). The overexpression of Bcl-2 is likely to contribute significantly to the autoimmune phenotype. It is, however, unlikely to be the only effect of estrogen on the B cells in these mice because bcl-2, R4A-γ2b double-transgenic mice display elevated serum titers of anti-DNA antibody but no glomerular deposition. Thus, estrogen may lead to a selective up-regulation of high-affinity anti-DNA antibody, but it could also lead to alterations in antigen display in the kidney.
Aberrant Bcl-2 expression has been implicated in SLE; however, the role of Bcl-2 in autoimmunity remains controversial. Studies performed with intragenic markers revealed that particular polymorphisms in the bcl-2 locus appear to be associated with susceptibility for SLE (35). It has also been reported that bcl-2 transcripts are increased in bone marrow cells from patients with SLE (36). A failure to down-regulate Bcl-2 expression has been observed in thymic germinal centers of patients with the autoimmune disorder myasthenia gravis (37). Our data support the suggestion that estrogenic stimulation of the immune system leads to increased levels of Bcl-2 and may contribute to disturbances in negative selection of autoreactive B cells.
These observations provide a mechanistic insight into the effect of estrogen on the B cell repertoire. Estrogen blocks deletion of naïve autoreactive B cells that occur either in the bone marrow or in peripheral lymphoid organs and permits their activation in the periphery. Furthermore, increased estrogen leads to up-regulation of Bcl-2, which is probably responsible for the enhanced survival of autoreactive B cells. Continued studies may lead to a better understanding of how elevated estrogen levels contribute to increased susceptibility for SLE, whether estrogen levels must remain elevated to maintain a lupus-like phenotype, and, ultimately, to the development of antiestrogen therapies for lupus patients.
Acknowledgments
We thank Daniel Michael for computer graphics and Sylvia Jones for preparation of this manuscript. This work was supported by grants from National Institute of Allergy and Infectious Diseases and National Institute of Arthritis and Musculoskeletal and Skin Diseases (B.D.). M.S.B. is supported by a grant from National Institute of Arthritis and Musculoskeletal and Skin Diseases; C.M.G. is a fellow of the Irvington Institute for Immunological Research.
Abbreviations
- E2
17β-estradiol
- P
placebo
- dsDNA
double-stranded DNA
- SLE
systemic lupus erythematosus
Footnotes
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. AF217806).
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.040577497.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.040577497
References
- 1.Butterworth M, McClellan B, Allansmith M. Nature (London) 1967;13:483–488. [PMC free article] [PubMed] [Google Scholar]
- 2.Grossman C. Science. 1985;227:257–261. doi: 10.1126/science.3871252. [DOI] [PubMed] [Google Scholar]
- 3.Grossman C, Roselle G, Mendenhall C. J Steroid Biochem Mol Biol. 1991;40:649–659. doi: 10.1016/0960-0760(91)90287-f. [DOI] [PubMed] [Google Scholar]
- 4.Lahita R, Bradlow H, Ginzler E, Pang S, New M. Arthritis Rheum. 1987;30:241–248. doi: 10.1002/art.1780300301. [DOI] [PubMed] [Google Scholar]
- 5.Ahmed S, Penhale W, Talal N. Am J Pathol. 1985;121:531–551. [PMC free article] [PubMed] [Google Scholar]
- 6.Schuurs A, Verheul H. Steroid Biochem. 1990;35:157–172. doi: 10.1016/0022-4731(90)90270-3. [DOI] [PubMed] [Google Scholar]
- 7.Evans M, MacLaughlin S, Marvin R, Abdou N. Clin Immunol Immunopathol. 1997;82:258–262. doi: 10.1006/clin.1996.4300. [DOI] [PubMed] [Google Scholar]
- 8.Kanda N, Tsuchida T, Tamaki K. Arthritis Rheum. 1999;42:328–337. doi: 10.1002/1529-0131(199902)42:2<328::AID-ANR16>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 9.Roubinian J, Talal N, Siiteri P, Sadakian J. Arthritis Rheum. 1979;22:1162–1169. doi: 10.1002/art.1780221102. [DOI] [PubMed] [Google Scholar]
- 10.Melez K, Boegel W, Steinberg A. Arthritis Rheum. 1980;23:41–47. doi: 10.1002/art.1780230108. [DOI] [PubMed] [Google Scholar]
- 11.Roubinian J, Talal N, Greenspan J, Goodman J, Siiteri P. J Exp Med. 1978;147:1568–1583. doi: 10.1084/jem.147.6.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steinberg A, Roths J, Murphy D, Steinberg R, Raveche E. J Immunol. 1980;125:871–873. [PubMed] [Google Scholar]
- 13.Apelgren L, Bailey D, Fouts R, Short L, Bryan N, Evans G, Sandusky G, Zuckerman S, Glasebrook A, Bumol V. Cell Immunol. 1996;173:55–63. doi: 10.1006/cimm.1996.0251. [DOI] [PubMed] [Google Scholar]
- 14.van Griensven M, Bergijk C, Baelde J, de Heer E. Clin Exp Immunol. 1997;107:254–260. doi: 10.1111/j.1365-2249.1997.261-ce1141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verthelyi D, Ahmed S. Endocrinology. 1994;135:2615–2622. doi: 10.1210/endo.135.6.7988450. [DOI] [PubMed] [Google Scholar]
- 16.Verthelyi D, Ahmed S. Cell Immunol. 1998;189:125–134. doi: 10.1006/cimm.1998.1372. [DOI] [PubMed] [Google Scholar]
- 17.Shefner R, Kleiner G, Turken A, Papazian L, Diamond B. J Exp Med. 1991;173:287–296. doi: 10.1084/jem.173.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Offen D, Spatz L, Escowitz H, Factor S, Diamond B. Proc Natl Acad Sci USA. 1992;89:8332–8336. doi: 10.1073/pnas.89.17.8332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katz J, Limpanasithikul W, Diamond B. J Exp Med. 1994;180:925–932. doi: 10.1084/jem.180.3.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bynoe M, Spatz L, Diamond B. Eur J Immunol. 1999;29:1304–1313. doi: 10.1002/(SICI)1521-4141(199904)29:04<1304::AID-IMMU1304>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 21.Iliev A, Spatz L, Ray S, Diamond B. J Immunol. 1994;153:3551–3556. [PubMed] [Google Scholar]
- 22.Kuo P, Bynoe M, Wang C, Diamond B. Eur J Immunol. 1999;29:3168–3178. doi: 10.1002/(SICI)1521-4141(199910)29:10<3168::AID-IMMU3168>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 23.Spatz L, Saenko V, Iliev A, Jones L, Geskin L, Diamond B. J Exp Med. 1997;185:1317–1326. doi: 10.1084/jem.185.7.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gaynor B, Putterman C, Valadon P, Spatz L, Scharff M, Diamond B. Proc Natl Acad Sci USA. 1997;94:1955–1960. doi: 10.1073/pnas.94.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nieto A, Gaya A, Jansa M, Moreno C, Vives J. Mol Immunol. 1984;21:537–543. doi: 10.1016/0161-5890(84)90070-1. [DOI] [PubMed] [Google Scholar]
- 26.Strasser A, Whittingham S, Vaux D, Bath M, Adams J, Cory S, Harris A. Proc Natl Acad Sci USA. 1991;88:8661–8665. doi: 10.1073/pnas.88.19.8661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ray S, Putterman C, Diamond B. Proc Natl Acad Sci USA. 1996;93:2019–2024. doi: 10.1073/pnas.93.5.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang T, Phang J. Cancer Res. 1995;55:2487–2489. [PubMed] [Google Scholar]
- 29.Teixeira C, Reed J, Pratt M. Cancer Res. 1995;55:3902–3097. [PubMed] [Google Scholar]
- 30.Migliaccio A, Di Domenico M, Castoria G, de Falco A, Bontempo P, Nola E, Auricchio F. EMBO J. 1996;15:1292–1300. [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Y, Boxer L, Latchman D. Nucleic Acids Res. 1999;27:2086–2090. doi: 10.1093/nar/27.10.2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suenaga R, Evans M, Mitamura K, Rider V, Abdou V. J Rheumatol. 1998;25:1305–1312. [PubMed] [Google Scholar]
- 33.Hartley S, Crosbie J, Brink R, Kantor A, Basten A, Goodnow C. Nature (London) 1991;353:765–769. doi: 10.1038/353765a0. [DOI] [PubMed] [Google Scholar]
- 34.Fulcher D, Lyons A, Korn S, Cook M, Koleda C, Parish C, Fazekas D, Basten A. J Exp Med. 1996;183:2313–2328. doi: 10.1084/jem.183.5.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mehrian R, Quismorio F J, Strassmann G, Stimmler M, Horwitz D, Kitridou R, Gauderman W, Morrison J, Brautbar C, Jacob C. Arthritis Rheum. 1998;41:596–602. doi: 10.1002/1529-0131(199804)41:4<596::AID-ART6>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 36.Alvarado-de la Barrera C, Alcocer-Varela J, Richaud-Patin Y, Alarcon-Segovia D, Llorente L. Scand J Immunol. 1998;48:551–556. doi: 10.1046/j.1365-3083.1998.00427.x. [DOI] [PubMed] [Google Scholar]
- 37.Shiono H, Fujii Y, Okumura M, Takeuchi Y, Inoue M, Matsuda H. Eur J Immunol. 1997;27:805–809. doi: 10.1002/eji.1830270402. [DOI] [PubMed] [Google Scholar]