Abstract
We have reported that fusions of murine dendritic cells (DCs) and murine carcinoma cells reverse unresponsiveness to tumor-associated antigens and induce the rejection of established metastases. In the present study, fusions were generated with primary human breast carcinoma cells and autologous DCs. Fusion cells coexpressed tumor-associated antigens and DC-derived costimulatory molecules. The fusion cells also retained the functional potency of DCs and stimulated autologous T cell proliferation. Significantly, the results show that autologous T cells are primed by the fusion cells to induce MHC class I-dependent lysis of autologous breast tumor cells. These findings demonstrate that fusions of human breast cancer cells and DCs activate T cell responses against autologous tumors.
Keywords: breast cancer, fusion cells, heterokaryons, DF3A, MUC1 antigen
Dendritic cells (DCs) are potent antigen-presenting cells capable of initiating primary immune responses (1, 2). DCs derive their potency from the expression of MHC class I, class II, costimulatory, and adhesion molecules that provide secondary signals for the stimulation of naïve T cell populations (3–5). Various strategies have been developed to introduce tumor-specific antigens into DCs and thereby to generate cytotoxic T lymphocyte (CTL) responses against malignant cells. DCs pulsed with tumor-associated peptides or proteins have led to the induction of antitumor immunity and disease regression in animal and clinical studies (6–11). Tumor vaccines have also been generated by viral transduction of DCs with tumor-specific genes or through transfection with liposomal DNA or RNA (12–18). Approaches designed to circumvent the facts that few tumor-specific antigens have been identified, that their immunogenicity is uncertain, and that tumor cells may evade recognition through the down-regulation of single antigens have included the loading of DCs with tumor-cell lysates, peptides eluted from tumor-cell membranes, and tumor-cell RNA (19, 20).
Another approach to the induction of primary antitumor immunity is through the generation of fusions between tumor cells and DCs (21). In this strategy, multiple tumor antigens, including those yet unidentified, are processed endogenously and presented by MHC class I pathways in the context of costimulatory signals. In an animal model, fusions between murine carcinoma cells and syngeneic DCs coexpressed the MUC1 tumor-associated antigen and DC-derived MHC class II, B7–1, and B7–2 molecules. Animals with established pulmonary metastases and vaccinated with the fusion cells were rendered disease-free (21). Similar results have been obtained by vaccinating mice with fusions of DCs and other tumor-cell types (22, 23).
In this paper, we investigate whether functionally active fusions can be prepared from human breast cancer cells and human DCs. We demonstrate the generation of human heterokaryons that express both breast tumor-associated antigens and DC-derived costimulatory molecules. The fusion cells are functionally active in stimulating autologous T cell proliferation and, importantly, induce CTL activity directed against autologous breast tumor cells.
Materials and Methods
Breast Carcinoma Cell Culture.
Human MCF-7 breast carcinoma cells (American Type Culture Collection) were grown in DMEM supplemented with 10% heat-inactivated FCS, 2 mM l-glutamine, 100 units/ml penicillin, and 100 μg/ml streptomycin. Human breast carcinoma cells were obtained with Institutional Review Board approval from biopsies of primary tumors and metastatic lesions to skin, lungs, and bone marrow. The cells were separated by incubation in Ca2+/Mg2+-free Hanks' balanced salt solution containing 1 mg/ml collagenase, 0.1 mg/ml hyaluronidase, and 1 mg/ml DNase. Breast tumor cells also were isolated from malignant pleural effusions by centrifugation and lysis of contaminating red blood cells. The breast tumor cells were maintained in RPMI medium 1640 (GIBCO/BRL) supplemented with 10% heat-inactivated autologous human serum, 2 mM l-glutamine, 100 units/ml penicillin, 100 μg/ml streptomycin, and 1 μg/ml insulin (Sigma).
Preparation of DCs, Monocytes, and T Cells.
Peripheral blood mononuclear cells (PBMC) were isolated from patients with metastatic breast cancer by Ficoll-Hypaque density-gradient centrifugation. The PBMC were suspended in RPMI medium 1640 supplemented with 10% human serum (Sigma) for 1 h. The nonadherent cells were removed, and T cells were isolated by nylon-wool separation. The adherent cells were cultured for 1 wk in RPMI medium 1640/10% human serum containing 1000 units/ml granulocyte–macrophage colony-stimulating factor (GM-CSF; Genzyme) and 500 units/ml IL-4 (Genzyme). The GM-CSF/IL-4-stimulated DCs expressed MHC classes I and II, B7–1, B7–2, intercellular adhesion molecule, CD40, and variable amounts of CD83, but did not express CD14, CD19, cytokeratin, or MUC1. Nonadherent and loosely adherent cells were harvested by repeated washes to generate the DC population. Firmly adherent monocytes were released from the plates with trypsin.
Cell Fusion.
DCs were mixed with MCF-7 or primary breast cancer cells at a 10:1 ratio and were incubated in serum-free RPMI medium 1640 containing 50% polyethylene glycol for 5 min. After slowly diluting with serum-free RPMI medium 1640, the cells were washed, resuspended in RPMI medium 1640 supplemented with 10% autologous human serum and 500 units/ml GM-CSF, and incubated at 37°C for 7–14 d.
Flow Cytometry.
Cells were washed with PBS and incubated with murine antibodies directed against MUC1 (DF3; ref. 24), MHC class I (W6/32), MHC class II (HLA-DR), B7–1 (CD80), B7–2 (CD86), or ICAM (CD54) (PharMingen) for 1 h on ice. After washing with PBS, the cells were incubated with fluorescein-conjugated goat anti-mouse IgG for 30 min on ice. The cells were washed again and then incubated with phycoerythrin-conjugated anti-MHC class II or anti-B7.1 for 1 h at 4°C. Samples were then washed, fixed with 2% paraformaldehyde, and subjected to bidimensional analysis by fluorescence-activated cell sorter analysis using FACScan (Becton Dickinson).
Immunohistochemistry.
Cytospin preparations of the cell populations were fixed in acetone for 10 min. The slides were incubated with mAb DF3 (anti-MUC1) or anti-cytokeratin antibody (AE1/AE3, Boehringer Mannheim) for 30 min at room temperature, and then they were washed with biotinylated horse anti-mouse Ig for an additional 30 min. Reactivity was detected with avidin–biotin complex (ABC) solution (Vector Laboratories). The cells were then incubated with murine anti-MHC class II for 30 min and alkaline phosphatase-labeled anti-mouse Ig for an additional 30 min. Alkaline phosphatase-ABC solution (Vector Laboratories) was used to generate a blue counterstain.
Autologous T Cell Stimulation.
DCs, breast tumor cells, and fusion cells were exposed to 30 Gy of ionizing radiation and added to autologous T cells in 96-well flat-bottom culture plates for 5 d. [3H]Thymidine uptake by T cells was measured at 12 h after a pulse of 1 μCi per well (New England Nuclear; 1 Ci = 37 GBq).
CTL Assays.
PBMC were cocultured with autologous breast tumor or fusion cells for 10 d in the presence of 20 units/ml human IL-2 (HuIL-2). The stimulated T cells were harvested by nylon-wool separation and used as effector cells in CTL assays with cell targets. Primary breast tumor cells, monocytes, MCF-7 cells, primary ovarian cancer cells, and K562 cells were labeled with 51Cr for 60 min at 37°C. After washing to remove unincorporated isotope, the targets (2 × 104) were cocultured with effector cells for 5 h at 37°C. In the indicated experiment, labeled target cells were incubated with mAb W6/32 (anti-MHC class I) for 30 min at 37°C before being added to the effector cells. The supernatants were assayed for 51Cr release in a γ counter. Spontaneous release of 51Cr was assessed by incubation of targets in the absence of effectors, whereas maximum or total release of 51Cr was determined by incubation of targets in 0.1% Triton X-100. Percentage of specific 51Cr release was determined by the following equation: percent specific release = [(experimental − spontaneous)/(maximum − spontaneous)] × 100.
Results and Discussion
Phenotype of Human Breast Tumor Cell/DC Fusions.
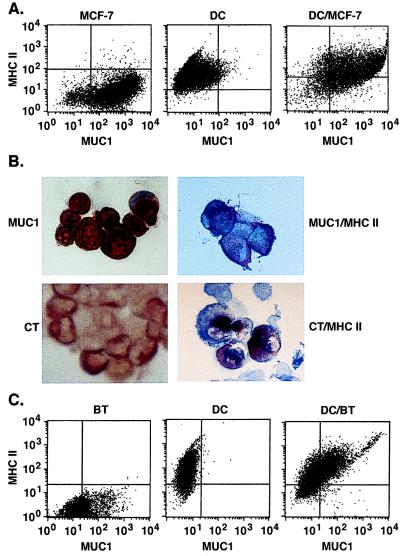
To determine whether human DCs can be used in the generation of heterokaryons with tumor cells, we prepared DCs from PBMC of patients with metastatic breast cancer. The DCs were first fused to human MCF-7 breast carcinoma cells. Bidimensional flow cytometry demonstrated that MCF-7 cells express the MUC1 carcinoma-associated antigen (24) and MHC class I, but not MHC class II, B7–1, B7–2, or ICAM (Fig. 1A and data not shown). By contrast, DCs expressed MHC class I, class II, and costimulatory molecules, but not MUC1. After fusion of MCF-7 cells and DCs, the resulting heterokaryons coexpressed MUC1 and MHC class II (Fig. 1A). Similar patterns of coexpression with MUC1 were obtained with B7.1, B7.2, and ICAM (data not shown). Because these findings support the formation of MCF-7/DC fusions, human breast cancer cells were isolated from patients with primary or metastatic tumors. Immunostaining of short-term cultures demonstrated that the breast carcinoma cells are positive for MUC1 (Fig. 1B, Upper Left) and cytokeratin (Fig. 1B, Lower Left). The breast tumor cells had no detectable expression of MHC class II, costimulatory, or adhesion molecules. The tumor cells were fused with autologous DCs and, after culturing for 7 days, the resultant population was analyzed for the presence of fusion cells. Fusion of the tumor cells to autologous DCs resulted in the generation of heterokaryons that dually expressed MUC1 and MHC class II (Fig. 1B, Upper Right) or cytokeratin and MHC class II (Fig. 1B, Lower Right). Analysis by bidimensional flow cytometry confirmed that the breast tumor cells expressed MUC1, and not MHC class II, whereas the autologous DCs were positive for MHC class II, but not MUC1, expression (Fig. 1C). By contrast, after fusion, >40% of the cells expressed both MUC1 and MHC class II (Fig. 1C). Similar results obtained with histochemical staining and bidimensional flow cytometry supported the generation of fusion cells and not aggregates. Based on assessment by both methods, the efficiency of autologous fusions prepared from six separate breast cancer patients ranged from 30% to 50% of the tumor-cell population.
Figure 1.
Phenotype of human DC/breast tumor cell fusions. (A) MCF-7 cells, human DCs, and fused DCs/MCF-7 cells were analyzed by bidimensional flow cytometry for expression of MUC1 and MHC class II. (B) Cytocentrifuge preparations of primary human breast tumor cells were stained with anti-MUC1 (Upper Left, red) or anti-cytokeratin (CT; Lower Left, red). Human DC/breast tumor fusion cells were incubated with anti-MUC1 or anti-cytokeratin and then with anti-MHC class II to generate a blue counterstain. The double-stained fusion cells are shown on the Right. (×40.) (C) Human breast tumor (BT) cells, autologous DCs, and fused DC/BT cells were analyzed by bidimensional flow cytometry for expression of MUC1 and MHC class II.
Function of the Breast Tumor Cell/DC Fusions.
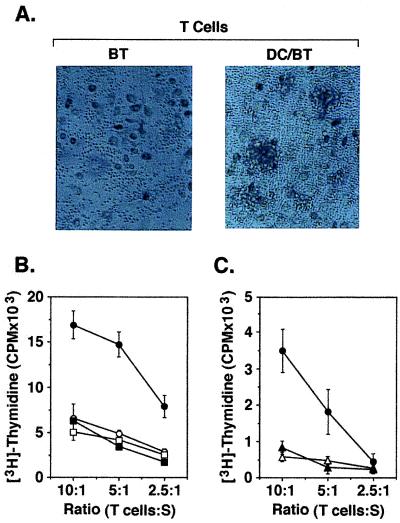
To determine whether the autologous fusion cells are effective in stimulating autologous T cells, the heterokaryons were cocultured with T cells isolated from nonadherent PBMC. As a control, the T cells were also cocultured with autologous tumor cells (Fig. 2A). Although there was no evidence for a T cell response to autologous tumor, the fusion cells stimulated T cell proliferation and the formation of T cell/fusion cell clusters (Fig. 2A). To assess the selectivity of this response, autologous T cells were incubated with DCs, irradiated breast tumor cells, or a mixture of unfused DCs and breast tumor cells. The results demonstrated little if any evidence for T cell stimulation by autologous DCs, tumor cells, or a mixture of the two cell types (Fig. 2B). By contrast, incubation of T cells with autologous fusion cells was associated with T cell proliferation (Fig. 2B). As additional controls, autologous T cells exhibited little if any response to polyethylene glycol-treated DCs or DCs fused to monocytes as compared with that obtained with DCs/tumor fusion cells (Fig. 2C). These findings demonstrate that fusion of breast tumor cells and DCs results in stimulation of a specific T cell response.
Figure 2.
Stimulation of T cells by DC/autologous breast tumor fusion cells. (A) T cells isolated from PBMC were incubated with autologous breast tumor cells (BT; Left) or autologous DC/breast tumor fusion cells (DC/BT; Right) at a ratio of 10:1 for 5 d in the presence of 20 units/ml HuIL-2. Incubation with the fusion but not the tumor cells resulted in the formation of T cell clusters. (B) T cells were cultured with autologous DCs (○), autologous breast tumor cells (□), autologous breast tumor cells mixed with DCs (■), or autologous breast tumor/DC fusion cells (●) at the indicated ratios of T cells to stimulators (S). (C) T cells were cultured with polyethylene glycol-treated autologous DCs (▵), autologous DCs fused to autologous monocytes (▴), or autologous breast tumor/DC fusion cells (●) at the indicated ratios. After 7 d, uptake of [3H]thymidine was measured during a 12-h incubation. The results are expressed as mean ± SD of three replicates.
Generation of CTLs Against Human Breast Tumor.
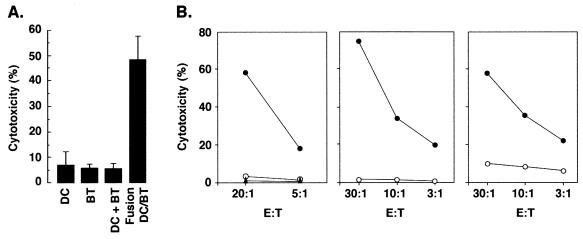
To assess the induction of tumor-specific CTLs, T cells were stimulated for 10 d and then isolated for assaying lysis of autologous tumor cells. T cells incubated with autologous DCs, irradiated breast tumor cells, or an unfused mixture of both exhibited a low level of autologous breast tumor cell lysis (Fig. 3A). Significantly, T cells stimulated with the fusion cells were effective in inducing cytotoxicity of autologous tumor (Fig. 3A). Similar results were obtained with T cells that had been stimulated with autologous DC/breast tumor cell fusions (Fig. 3B). Moreover, T cells that had been cocultured with autologous breast tumor cells alone failed to exhibit significant killing of tumor cells (Fig. 3B).
Figure 3.
Activation of anti-tumor CTLs by autologous fusion cells. (A) T cells were stimulated with autologous DCs, autologous breast tumor cells (BT), autologous DCs mixed with breast tumor cells (DC + BT), or autologous DC/breast tumor cell fusions (DC/BT) for 10 d in the presence of HuIL-2. The stimulated T cells were incubated with 51Cr-labeled autologous breast tumor cells at a ratio of 30:1. Percentage cytotoxicity (mean ± SD of three replicates) was determined by 51Cr release. (B) DCs from three patients were fused to autologous breast tumor cells. T cells were incubated with autologous breast tumor cells or autologous DC/breast tumor cell fusions in the presence of HuIL-2. 51Cr-labeled autologous breast tumor cells were incubated at the indicated effector-to-target cell ratios (E:T) with unstimulated T cells (▴), T cells stimulated with autologous tumor cells (○), or T cells stimulated with autologous DC/breast tumor cell fusions (●). CTL activity was determined by 51Cr release.
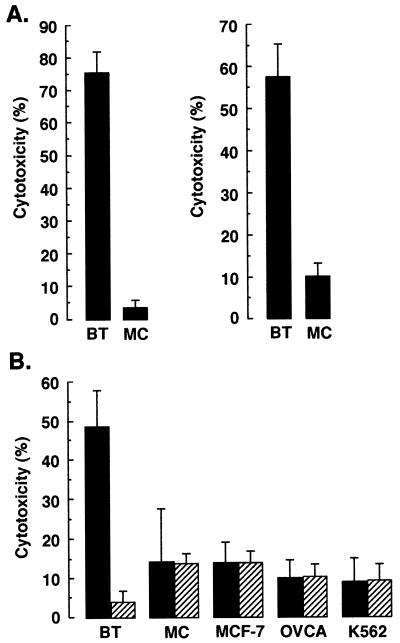
To define the specificity of the CTLs generated by incubation with fusion cells, we compared their function in the lysis of autologous tumor cells and other cell types. Incubation of fusion-stimulated T cells with autologous breast tumor cells or monocytes demonstrated selectivity for lysis of the tumor cells (Fig. 4A). In addition, T cells stimulated with autologous fusion cells demonstrated significant lysis of autologous breast tumor cells, whereas lysis of MCF-7 cells, primary ovarian cancer cells, and natural killer-sensitive K562 cells was similar to that obtained with autologous monocytes (Fig. 4B). The finding that preincubation of the targets with an anti-class I antibody resulted in abrogation of autologous breast tumor cell lysis indicated that the killing was MHC class I-restricted (Fig 4B). By contrast, the anti-class I antibody had little if any effect on lysis of the other cell types (Fig. 4B).
Figure 4.
Specificity of CTLs generated by autologous fusion cells. (A) T cells were incubated with autologous DC/breast tumor fusion cells in the presence of HuIL-2. The stimulated T cells were cultured with 51Cr-labeled autologous breast tumor cells (BT) or autologous monocytes (MC) for 5 h at a 30:1 ratio. (B) T cells stimulated with autologous DC/breast tumor fusion cells were incubated with 51Cr-labeled autologous breast tumor cells, autologous monocytes, MCF-7 cells, ovarian cancer cells (OVCA), or K562 cells at a 30:1 ratio (solid bars). The targets were also preincubated with an anti-MHC class I antibody (W6/32; 1:100 dilution) and then assayed for lysis (hatched bars). CTL activity was determined by 51Cr release. The results are expressed as mean ± SD of three replicates.
Conclusions.
The present findings demonstrate the generation of heterokaryons from human DCs and autologous breast carcinoma cells. In preclinical models, vaccination with DCs/tumor fusion cells has provided an approach in which both known and unidentified tumor antigens are presented in the context of costimulatory signals (21–23). Moreover, a fusion cell vaccine has been shown to reverse immunologic unresponsiveness to a tumor-associated antigen and to induce the rejection of established metastases (25). The results of the present studies extend these observations by demonstrating that human DC/breast tumor cell fusions stimulate autologous T cells, which are otherwise unresponsive to autologous breast tumor cells. Significantly, the fusion cells were effective in inducing anti-tumor CTLs, which lyse autologous carcinoma cells by an MHC class I-restricted mechanism. Characterization of the peptides recognized by these CTLs can be used to identify tumor-associated antigens that are the target of the immune response. In addition, the findings indicate that activation of immunity against human tumor-associated antigens by vaccination with DC/tumor cell fusions could have potential applicability to the field of antitumor immunotherapy.
Acknowledgments
We thank Drs. Mary Jane Houlihan and Susan Troyan for providing the primary breast carcinoma samples. This investigation was supported by Public Health Service Grant CA38493 awarded by the National Cancer Institute, Department of Health and Human Services, and by Komen Breast Grant 9825 awarded by the Susan G. Komen Breast Cancer Foundation.
Abbreviations
- DC
dendritic cell
- CTL
cytotoxic T lymphocyte
- PBMC
peripheral blood mononuclear cells
- HuIL-2
human IL-2
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.050587197.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.050587197
References
- 1.Steinman R M. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 2.Young J W, Steinman R M. Stem Cells (Dayton) 1996;14:376–387. doi: 10.1002/stem.140376. [DOI] [PubMed] [Google Scholar]
- 3.Young J W, Koulova L, Soergel S A, Clark E A, Steinman R M, Dupont B. J Clin Invest. 1992;90:229–237. doi: 10.1172/JCI115840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Inaba K, Witmer-Pack M, Inaba M, Hathcock K S, Sakuta H, Azuma M, Yagita H, Okumura K, Linsley P S, Ikehara S, et al. J Exp Med. 1994;180:1849–1860. doi: 10.1084/jem.180.5.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caux C, Vanbervliet B, Massacrier C, Azuma M, Okumura K, Lanier L L, Banchereau J. J Exp Med. 1994;180:1841–1847. doi: 10.1084/jem.180.5.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Celluzzi C M, Mayordomo C I, Storkus W J, Lotze M T, Falo L D, Jr L D. J Exp Med. 1996;183:283–287. doi: 10.1084/jem.183.1.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mayordomo J I, Zorina T, Storkus W J, Zitvogel L, Celluzzi C, Falo L D, Jr, Melief C J, Ildstad S T, Kast W M, Deleo A B, Lotze M T. Nat Med. 1995;1:1297–1302. doi: 10.1038/nm1295-1297. [DOI] [PubMed] [Google Scholar]
- 8.Paglia P, Chiodoni C, Rodolfo M, Colombo M P. J Exp Med. 1996;183:317–322. doi: 10.1084/jem.183.1.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hsu F J, Benike C, Fagnoni F, Liles T M, Czerwinski D, Taidi B, Engleman E G, Levy R. Nat Med. 1996;2:52–58. doi: 10.1038/nm0196-52. [DOI] [PubMed] [Google Scholar]
- 10.Nestle F O, Alijagic S, Gilliet M, Sun Y, Grabbe S, Dummer R, Burg G, Schadendorf D. Nat Med. 1998;4:328–332. doi: 10.1038/nm0398-328. [DOI] [PubMed] [Google Scholar]
- 11.Nieda M, Nicol A, Kikuchi A, Kashiwase K, Taylor K, Suzuki K, Tadokoro K, Juji T. Blood. 1998;91:977–983. [PubMed] [Google Scholar]
- 12.Gong J, Chen L, Chen D, Kashiwaba M, Manome Y, Tanaka T, Kufe D. Gene Ther. 1997;4:1023–1028. doi: 10.1038/sj.gt.3300496. [DOI] [PubMed] [Google Scholar]
- 13.Specht J M, Wang G, Do M T, Lam J S, Royal R E, Reeves M E, Rosenberg S A, Hwu P. J Exp Med. 1997;186:1213–1221. doi: 10.1084/jem.186.8.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boczkowski D, Nair S K, Snyder D, Gilboa E. J Exp Med. 1996;184:465–472. doi: 10.1084/jem.184.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Condon C, Watkins S C, Celluzzi C M, Thompson K, Falo L D., Jr Nat Med. 1996;2:1122–1128. doi: 10.1038/nm1096-1122. [DOI] [PubMed] [Google Scholar]
- 16.Song W, Kong H L, Carpenter H, Torii H, Granstein R, Rafii S, Moore M A S, Crystal R G. J Exp Med. 1997;186:1247–1256. doi: 10.1084/jem.186.8.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ribas A, Butterfield L H, McBridge W H, Jilani S M, Bui L A, Vollmer C M, Lau R, Dissette V B, Hu B, Chen A Y, et al. Cancer Res. 1997;57:2865–2869. [PubMed] [Google Scholar]
- 18.Song E S, Lee V, Surh C D, Lynn A, Brumm D, Jolly D J, Warner J F, Chada S. Proc Natl Acad Sci USA. 1997;94:1943–1948. doi: 10.1073/pnas.94.5.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ashley D M, Faiola B, Nair S, Hale L P, Bigner D D, Gilboa E. J Exp Med. 1997;186:1177–1182. doi: 10.1084/jem.186.7.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zitvogel L, Mayordomo J I, Tjandrawan T, DeLeo A B, Clarke M R, Lotze M T, Storkus W J. J Exp Med. 1996;183:87–97. doi: 10.1084/jem.183.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gong J, Chen D, Kufe D. Nature Med. 1997;3:558–561. doi: 10.1038/nm0597-558. [DOI] [PubMed] [Google Scholar]
- 22.Lespagnard L, Mettens P, Verheyden A-M, Tasiaux N, Thielemans K, van Meirvenne S, Geldhof A, De Baetselier P, Urbain J, Leo O, Moser M. Int J Cancer. 1998;76:250–258. doi: 10.1002/(sici)1097-0215(19980413)76:2<250::aid-ijc13>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 23.Celluzzi C M, Falo L D., Jr J Immunol. 1998;160:3081–3085. [PubMed] [Google Scholar]
- 24.Kufe D, Inghirami G, Abe M, Hayes D, Justi-Wheeler H, Schlom J. Hybridoma. 1984;3:223–232. doi: 10.1089/hyb.1984.3.223. [DOI] [PubMed] [Google Scholar]
- 25.Gong J, Chen D, Kashiwaba M, Li Y, Takeuchi H, Qu H, Rowse G J, Gendler S J, Kufe D W. Proc Natl Acad Sci USA. 1998;95:6279–6283. doi: 10.1073/pnas.95.11.6279. [DOI] [PMC free article] [PubMed] [Google Scholar]