Abstract
We have previously reported on a carbohydrate-based vaccine program for immunotherapy in cancer patients. One such vaccine, based on the globo H antigen conjugated to the protein keyhole limpet hemocyanin (KLH), has been in clinical evaluation. Although this and other carbohydrate vaccines have been shown to induce antibody responses, there are currently no quantitative data on the antibody levels achieved in immunized patients by these or other anti-cancer vaccines. We report herein an efficient route to complex synthetic oligosaccharides attached to an affinity matrix for identifying and isolating antibodies elicited against such a carbohydrate-based vaccine in humans. Pre- and postvaccination profiles from serum samples of patients immunized with globo H-KLH were compared. All anti-globo H antibody activity was efficiently separated from other serum constituents. The isolated antibodies were readily quantified, and their specificities were analyzed. Since no comparable data were available on antibodies resulting from the vaccination of other cancer patients, we compared the observed levels with those quoted in studies with bacterial polysaccharide vaccines that had been quantified. Remarkably, cancer patients immunized with globo H-KLH produce anti-globo H antibody levels often exceeding those formed by immunization with bacterial polysaccharides. In addition, substantial quantities of both IgG and IgM antibodies were elicited, clearly indicating a class switch to IgG. Taken together, these analyses serve to clarify several aspects of the immune response to the vaccine and give several new insights to the carbohydrate-based vaccination strategy. Furthermore, antibodies so isolated could well have applications in clinical therapy.
Among the many structural and functional transformations that attend oncogenesis, altered expression of cell surface carbohydrates has recently emerged as an opportunity for the development of vaccine strategies. Various oligosaccharide motifs have been suggested to be either specific to or substantially over-expressed on the surface of certain tumor cells (1). Conceptually, the goal of a carbohydrate-based vaccine initiative would be to educate the immune system to identify certain glyco-patterns as cancer-related. An immune response thus stimulated might prove effective against cells bearing the tumor-associated carbohydrate, especially circulating cancer cells and micrometastases. Thus, active immunization (2) with carbohydrate tumor antigens is being explored as a therapeutic modality in a number of experimental tumor models and in cancer patients. In recent studies, we and others have designed and evaluated a number of carbohydrate-based cancer vaccines, including gangliosides, e.g., GM2, GD2, GD3, and FucGM1 (3, 4), mucin-core structures, e.g., TF, Tn, and S-Tn (5–8), and blood group-related antigens, e.g., globo H and Ley (9–11).
In the context of our total synthesis approach to the development of anti-cancer vaccines, synthetic constructs based on the globo H, Ley, Tn, and TF antigens have advanced on to the level of clinical evaluation, with others being tested in mice (12). Strictly, carbohydrate epitopes would not be expected to induce cytotoxic T cell responses. Therefore, clinically positive outcomes would most likely result from antibody-mediated effector mechanisms, such as complement-dependent cytotoxicity (CDC), antibody-dependent cellular cytotoxicity (ADCC), and induction of inflammation. Although all the carbohydrate vaccines mentioned above have been shown to induce antibody responses as measured by assays with defined carbohydrate antigens and whole tumor cells, and the antibody levels assessed by titer of reactivity, there is currently little reliable information on the actual antibody levels achieved in immunized patients. We sought a method to better evaluate the level and specificity of vaccine-induced antibodies and to accomplish enrichment and separation of those antibodies elicited through vaccination. In this paper, we report on the quantitative characterization of the antibody response generated in humans with the globo H carbohydrate-based vaccine. Localization of the antibodies was achieved with an affinity matrix bearing the totally synthetic immunizing antigen.
Demonstrated immunogenicity in preclinical models is an important milestone en route to the clinical setting. In such cases, vaccination of animals has supported the mechanistic framework described above. For example, we have reported CDC against tumors bearing the globo H epitope for mice vaccinated with a globo H-keyhole limpet hemocyanin (KLH) construct (9). Furthermore, in murine models where GD2 was the target antigen, both passively administered monoclonal and vaccine-induced polyclonal antibodies were strong enough to retard tumor formation from previously administered tumor cells and provided deterrence to micrometastases (13). These results have led to cautious optimism as to whether an induced and sufficiently potent antibody response could eliminate circulating cancer cells and micrometastases in cancer patients. However, tumor-associated carbohydrates, though aberrantly expressed, are not strictly foreign to the human subject. Therefore, tolerance must be broken for an immune response to be registered without provoking autoimmunity. While our vaccines are progressing through various stages of clinical evaluation (12), vaccine-induced antibodies against GM2 have correlated with improved disease-free and overall survival in melanoma patients (4, 14).
In these contexts, we have concentrated on exploring the therapeutic potential of several tumor antigens, and globo H is one of our most advanced candidates. The hexasaccharide designated globo H was identified on human prostate, breast, and small cell lung carcinomas, as well as in a restricted number of normal epithelial tissues (15, 16). Originally isolated in ceramide form from the human breast cancer cell line MCF-7 (17, 18), it is also suggested to exist in the form of a glycoprotein (19). The enhanced expression of antigens assumed to be globo H on both primary and metastatic prostate cancer specimens (20), along with the presence of PSA, a protein biomarker specific to prostate cancer, drew us to the possibility of initiating a globo H-based vaccine strategy in prostate cancer.
After completing a total synthesis (see Scheme S1) that provided suitable quantities of globo H for evaluation, we formulated a vaccine wherein the globo H hexasaccharide was conjugated to KLH and administered with the adjuvant QS-21 (21). Studies in mice demonstrated carbohydrate immunogenicity, and initial clinical evaluation in relapsed prostate cancer patients demonstrated the successful and safe induction of antibodies specifically focused against globo H, i.e., tolerance was broken but no autoimmune reactions occurred (9, 10). These antibodies were able to react with globo H naturally expressed on cancer cells and to induce CDC. We are beginning phase II trials, and as chemical synthesis allowed us to attain strict control over the structure of our antigens, we were uniquely poised to initiate an effort to determine in detail additional properties of the antibodies generated in cancer patients in response to our vaccine. To this end, we have manufactured an affinity column matrix bearing the globo H antigen. For control purposes, and for serological evaluation in other clinical trials (22), we have also prepared a Ley pentasaccharide-based affinity column. We know of no previous use of affinity chromatography in the context of a total synthesis-driven carbohydrate-based vaccination strategy in clinical trials. As we will show, the study of purified human polyclonal antibodies promises to be valuable in the clinical evaluation of cancer patients by enhancing the insight into the immunological response to vaccination.
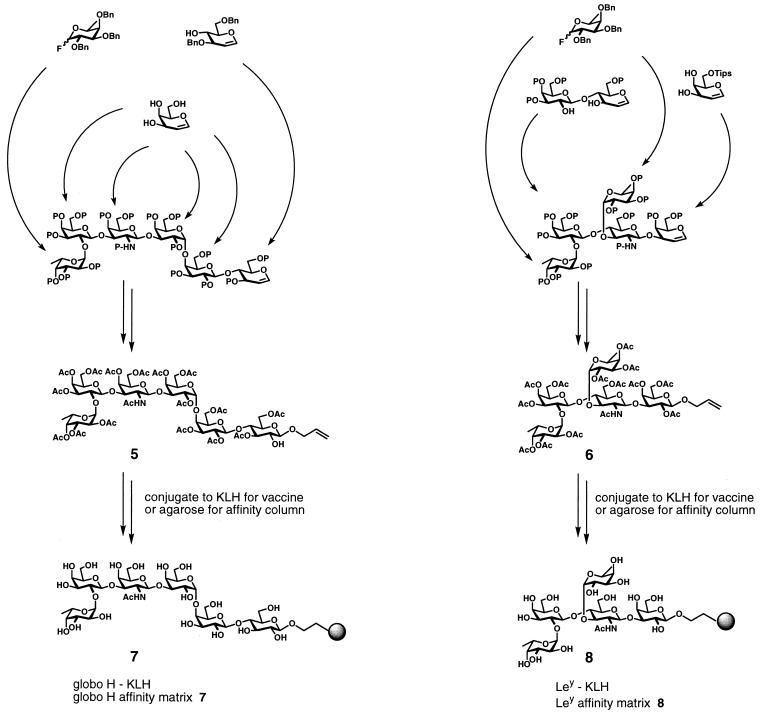
Scheme 1.
Materials and Methods
Preparation of Globo H Affinity Column.
Protected allyl glycoside 5 was prepared as described (21). Ozone was passed through a solution of 5 (52 mg, 0.030 mmol) in MeOH (10 ml) at −78°C. The reaction was monitored by TLC using CH2Cl2/MeOH (10:1) as eluent. The reaction was purged at −78°C with a stream of N2 upon disappearance of the starting material to remove excess ozone, usually after 5 min, followed by the addition of dimethyl sulfide (10 ml). The resulting solution was allowed to warm to room temperature and stirred for a total of 3 h. The crude material was concentrated with a stream of N2 and was used immediately for conjugation to agarose.
The crude ozonolysis product was taken up in MeOH (20 ml) and transferred to a flask charged with amino agarose (Bio-Rad; 10 ml gel, 16.29 μmol/ml, 160.29 μmol), which had been preequilibrated with MeOH (twice with 10 ml). The resultant slurry was treated with NaBH3CN (1 M, 120 μl, 4 eq) and mixed by vigorous agitation overnight at room temperature. The solvent was removed by filtration, and the polymer was washed with MeOH (twice with 10 ml). The derivatized agarose was treated with acetic anhydride (50 μl) in MeOH (10 ml) for 30 min to ensure that all remaining amine functions were capped. Negative ninhydrin test on <1 mg of material was used to verify lack of free amine functionality. The solid matrix was washed with MeOH (twice with 10 ml) and treated with NaOMe (25% in MeOH, 300 μl) in MeOH (10 ml) for 12 h with vigorous agitation at room temperature. The polymer was washed with MeOH (three times with 10 ml), isopropyl alcohol (three times with 10 ml), and finally with 0.05% aqueous NaN3 (twice with 10 ml), providing 10 ml of affinity column material 7. The loading was determined to be 0.6 μmol of globo H per ml of gel as determined by fucose analysis of the functionalized gel (23).
Preparation of Ley Affinity Column.
Protected Ley-allyl glycoside 6 was prepared as described (24). Ley-agarose was prepared following the same procedure as above with 20 mg of 6 and 4 ml of amino agarose gel. The loading of 8 was determined to be 0.15 μmol of Ley per ml of gel as determined by fucose analysis of the functionalized gel (23).
Sera and Antibodies.
Sera from prostate cancer patients immunized with a globo H-KLH/QS21 vaccine have been described (9, 10). Sera from patients immunized with 3, 10, 30, and 100 μg of globo H-KLH showing maximum titers (usually after three to four immunizations) corresponding to patients 2, 4, 8, 13, 14, and 17 in ref. 10 were selected. Monoclonal antibody VK9 for the detection of globo H has been described (25).
Isolation of Antibodies by Affinity Chromatography.
Globo H- or Ley-agarose column (3.0 ml) was first equilibrated in PBS [20 ml, 0.15 M NaCl, 0.02 M sodium phosphate buffer (pH 7.2)]. The serum to be analyzed (1.0 ml) was then added to the column and allowed to react for 1 h by agitating gently at 4°C. Subsequently, the column was eluted with (i) PBS (10 ml), (ii) 1 M NaCl in PBS (5 ml), and (iii) 0.05 M glycine⋅HCl (pH 2.5) (10 ml), and fractions (1.0–2.0 ml) were collected. The samples eluted with the third buffer were collected directly into 75 μl of saturated Na2HPO4 to give a final pH of 6.5–7.5. The fractions were assayed for the presence of protein by monitoring optical density at 280 nm and for antibody activity by ELISA (see below). Fractions showing antibody activity were pooled and used for further analysis. The columns were used repeatedly after washing in the glycine buffer (30 ml) and re-equilibration in PBS. To remove human serum albumin (HSA) from the eluted fractions, the samples were reapplied to a globo H-agarose column and washed with PBS (10 ml), PBS containing 1% Nonidet P-40 (10 ml), and PBS (10 ml) before eluting the antibody with glycine⋅HCl buffer (10 ml) as above.
ELISA.
ELISA was performed as described (25), except that in some experiments, reactivity was assayed with fluorescein phosphate (Molecular Probes) [0.05 mM in 0.1 M Tris (pH 9.9), 50 mM NaCl, 10 mM MgCl2, and 0.1 mM ZnCl2] and quantification in a fluorescence plate reader with excitation at 485 nm and emission at 535 nm (Wallac, Gaithersburg, MD; model 1420). For determination of IgG subclass, alkaline phosphatase-coupled anti-IgG1, -IgG2, - IgG3, and -IgG4 specific antibodies (Southern Biotechnology Associates) were used in the final step.
SDS/PAGE.
SDS/PAGE in 7% acrylamide gels was carried out under nonreducing condition as described (26).
Results
Globo H hexasaccharide (5) armed with a terminal allyl group was synthesized by using the glycal assembly approach to oligosaccharide synthesis (21) (Scheme S1). The allyl group was converted to the corresponding aldehyde by ozonolysis and linked to amine functionalized agarose by reductive amination, followed by capping of residual amine functionality to give globo H-bound agarose (7). On-resin deprotection provided a fully functional globo H antigen-bound affinity matrix. In much the same way, the Ley pentasaccharide (6), also as the allyl glycoside (24), was converted to the antigen-specific affinity matrix (8). For comparison, the unprotected globo H allyl glycoside (not shown) was ozonized and reductively coupled to amine-functionalized agarose to give affinity material that was identical in all respects to 7 (data not shown). Column material, for both globo H and Ley, could be routinely produced on a 10-ml scale beginning with approximately 50 mg of protected carbohydrate.
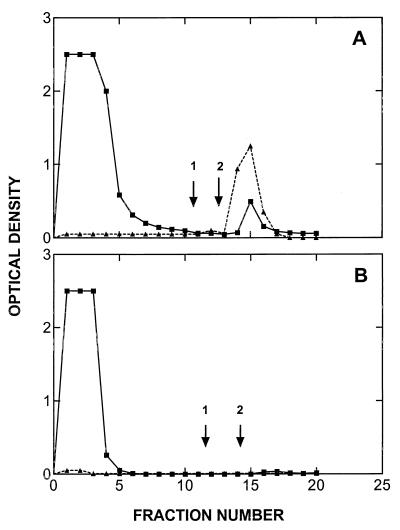
Sera from six patients who had been immunized with a globo H-KLH vaccine and sera from three normal individuals were fractionated on a globo H-agarose affinity as described in Materials and Methods. As assayed by ELISA, all anti-globo H antibody activity in the sera of immunized patients was retained by the column. Elution with high salt (1 M NaCl) did not remove antibody from the column, but elution with a glycine⋅HCl (pH 2.5) buffer resulted in the elution of anti-globo H antibody (Fig. 1A). Sera obtained from the same patients before immunization, or from normal individuals, contained undetectable, or only trace quantities, of antibody (Fig. 1B). As a control of specificity, no antibody from globo H-KLH immunized patients bound to Ley-agarose column, while antibody from patients immunized with Ley-KLH (22) did react with the Ley-agarose column (data not shown).
Figure 1.
Affinity chromatography of sera from a patient (no. 4) on globo H-agarose column. (A) Postvaccination serum. (B) Prevaccination serum. Fractions were assayed for protein levels (■, OD280) and reactivity with globo H-ceramide by ELISA (▴). Initial wash buffer: PBS; second wash (arrow 1): 1 M NaCl/PBS; final elution buffer (arrow 2): 0.05 M glycine⋅HCl (pH 2.5).
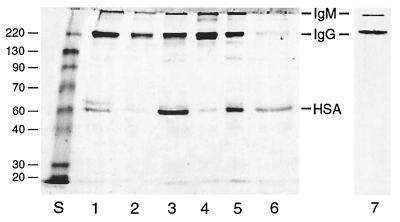
Pooled fractions from the six anti-globo H sera were analyzed for protein content by using the Lowry assay (Table 1) and by SDS/PAGE electrophoresis for purity (Fig. 2). The total protein content of the eluted fractions ranged from 50 to 370 μg/ml of serum. However, SDS/PAGE analysis showed that in addition to IgG and IgM immunoglobulins some of these samples contained substantial amounts of a component migrating with HSA. The identity of this component was confirmed to be HSA by Western blotting with anti-HSA antibody (data not shown). The proportion of IgM and IgG immunoglobulins in the sera was estimated by scanning the Coomassie blue-stained gel in a Bio-Rad GS700 scanner and Quantity One data analysis system (Table 1).
Table 1.
Yields of immunoglobulins isolated from human sera by affinity chromatography on globo H-agarose
| Sera | Eluted protein, μg*
|
Eluted immunoglobulin, μg†
|
||
|---|---|---|---|---|
| Preimmune | Immune | IgM | IgG | |
| Patients | ||||
| 1 | <10 | 150 | 35 | 65 |
| 2 | <10 | 110 | 27 | 49 |
| 3 | <10 | 180 | 37 | 75 |
| 4 | <10 | 370 | 76 | 210 |
| 5 | <10 | 140 | 29 | 64 |
| 6 | <10 | 50 | 14 | 11 |
| Normal individuals | ||||
| 1 | <10 | — | — | — |
| 2 | <10 | — | — | — |
| 3 | <10 | — | — | — |
Yield from 1.0 ml of serum. All eluted samples contained albumin and other unidentified serum proteins.
† Values obtained by scanning a Coomassie blue-stained gel of the total eluted protein sample.
Figure 2.
SDS/PAGE analysis of fractions isolated from sera from six patients, immunized with globo H-KLH, by affinity chromatography on a globo H-agarose column. Lanes 1–6, low pH-eluted fractions from patients 1–6. Lane 7, eluted fraction from patient 3 further purified to remove HSA. Lane S, protein standards (220, 130, 90, 70, 60, 40, 30, and 20 kDa). The migration rates of IgM, IgG, and HSA are indicated. The samples were separated on a 7% polyacrylamide gel under nonreducing conditions and then stained with Coomassie blue.
Removal of the contaminating HSA from the eluted antibody samples proved to be surprisingly difficult. After reapplication of the sample to a second globo H-agarose column, the albumin was retained by the column and again eluted with the antibody. Similar coelution was obtained when the sample was applied to an N-acetylated amino-agarose column and even when fractionation by ion-exchange chromatography on a DEAE-agarose column was attempted (data not shown). Successful purification of the antibody was achieved by applying the sample to a globo H-agarose column and washing the column with PBS and then PBS/1% Nonidet P-40 (which removed the albumin) before eluting the antibody in glycine (pH 2.5) buffer. SDS/PAGE analysis of a typical purified sample is shown in Fig. 2, lane 7.
The presence of both IgM and IgG antibodies in the purified antibody samples, as detected by SDS/PAGE (Fig. 2), was confirmed by ELISA using specific antibodies (Table 2). Subclass analysis with anti-IgG subclass antibodies revealed that IgG1 antibodies were detected in all six samples and that IgG2, IgG3, and IgG4 antibodies were detected in one or more of the samples (Table 2).
Table 2.
Class and subclass typing of purified antibodies from patients' sera
| Patient | Titer*
|
|||||
|---|---|---|---|---|---|---|
| IgM | IgG | IgG1 | IgG2 | IgG3 | IgG4 | |
| 1 | 1 :640 | 1 :1280 | 1 :40 | ND | ND | ND |
| 2 | 1 :2560 | 1 :5120 | 1 :80 | ND | ND | ND |
| 3 | 1 :640 | 1 :2560 | 1 :20 | ND | ND | 1:20 |
| 4 | 1 :2560 | 1 :10240 | 1 :80 | 1:160 | ND | 1:20 |
| 5 | 1 :640 | 1 :5120 | 1 :1280 | ND | ND | 1:40 |
| 6 | 1 :640 | 1 :640 | 1 :20 | ND | 1:80 | 1:20 |
The titer is the lowest dilution of antibody showing ELISA value at least 1.5 greater than background. Titers can be compared only within a given class/subclass for each antibody sample. ND, not detected.
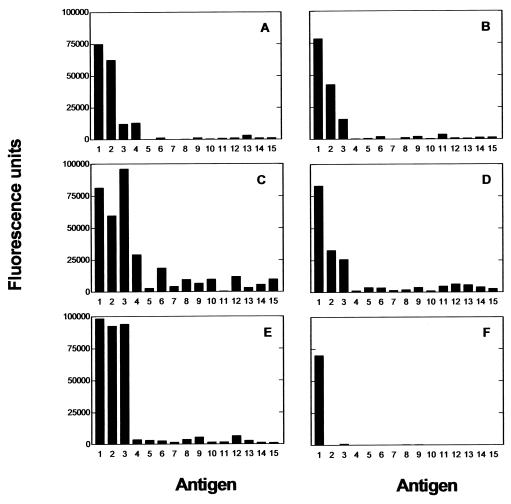
The specificity of the eluted antibodies from 5 of the 6 patients was analyzed by direct ELISA on a panel of 15 glycoconjugates (Fig. 3). As expected, antibody fractions from the five patients reacted with globo H. Significant reactivity with different globo H-related compounds, which differed substantially between the patients, was also evident. The antibodies from all five patients crossreacted to some extent with galactosyl-globoside (SSEA-3) and globoside. Furthermore, in two patients, the reactivity with these two structures was as high, or almost as high, as with globo H itself (Fig. 3 C and E). Although Ley-ceramide showed weak reactivity with the sera from three patients, the other antigens tested were essentially unreactive. As a positive control, an anti-globo H mouse monoclonal antibody (VK-9) reacted exclusively with globo H (Fig. 3E).
Figure 3.
Analysis of specificity of antibody fractions isolated from five immunized patients: patient 2 (A), patient 4 (B); patient 8 (C), patient 13 (D); and patient 14 (E) (as listed in ref. 10). Reactivity was measured with a direct ELISA using rabbit anti-human IgG (H and L chains) conjugated to alkaline phosphatase as the second antibody. (F) Anti-globo H monoclonal antibody VK-9. Test compounds: 1, globo H-Cer; 2, gal-globoside (SSEA-3 antigen); 3, globoside; 4, Ley (Fucα1–2Galβ1–4[Fucα1–3]GlcNAcβ1–3Gal)-BSA; 5, Leb (Fucα1–2Galβ1–3[Fucα1–4]GlcNAcβ1–3Gal)-BSA; 6, Lex (Galβ1–4[Fucaα1–3]GlcNAc)-PAA; 7, Lex-Cer; 8, Lea (Galβ1–3[Fucaα1–4]GlcNAc)-PAA; 9, H type 2 (Fucα1–2Galβ1–4GlcNAc)-PE; 10, H type 2-PAA; 11, H type 1 (Fucα1–2Galβ1–3GlcNAcβ1–3Gal)-BSA; 12, H type 1-PAA; 13, lactose-CETE; 14, Galα1–4Galβ1–4Glc-CETE; 15, Galα1–4GlcNAc-CETE-BSA. Abbreviations: Cer, ceramide; BSA, bovine serum albumin; PAA, polyacrylamide; PE, phosphatidylethanolamine; CETE, 2-(carbomethoxyethylthio)ethyl.
Discussion
We first set out to develop an efficient route to manufacture complex synthetic oligosaccharides attached to a solid support. The protected oligosaccharides 5 (21) and 6 (24) were smoothly converted into the functional affinity matrices 7 and 8 (Scheme S1). The syntheses are scaleable, general, and efficient even for molecules of this sophistication. Both columns very efficiently separated human polyclonal antibodies directed at the corresponding carbohydrates from other serum immunoglobulins and other serum proteins. The specificity of anti-globo H binding was demonstrated with the Ley column, in that no “cross talk” occurred. Thus, antibodies from patients vaccinated with globo H-KLH bound to the globo H column; however, the anti-globo H antibodies did not bind to the Ley affinity column. Preimmune sera and sera from individuals with no history of cancer did not contain antibodies binding to the globo H column. Thus, the affinity columns were operational and proved useful for identifying and isolating polyclonal antibodies directed against the carbohydrate portion of the parent vaccine.
Assessment of antibody response to vaccination with carbohydrates, or other antigens, commonly relies on titers given as unitless quantities. Typically, the titer values are assessed by comparison to those given by other samples. A number of studies with bacterial polysaccharide vaccines determined the level of the antibody response in weight units (e.g., μg/ml of serum), but no published anti-cancer vaccine studies seem to provide such data. In this study, we show that patients immunized with a globo H-KLH conjugate vaccine responded with the production of 25–280 μg of antibody per ml of serum (Table 1). As no comparable data are available on antibodies resulting from the vaccination of other cancer patients, we compare these levels with those quoted in a number of studies with bacterial polysaccharide vaccines in which antibody levels had been quantified. In a study on antibodies elicited in adults with a pneumococcal conjugate vaccine, Soininen et al. (27) reported IgG levels of only 0.58–1.33 μg/ml of serum (they did not report IgM levels), whereas Anttila et al. (28) reported the induction of 7.8–57.8 μg/ml IgG in 15-mo-old children. Kabat and Berg (29) reported anti-dextran antibodies, mainly in the range of 1.9–97.5 μg/ml of serum in adults immunized with dextran polysaccharide (with two individuals with levels >250 μg/ml). Remarkably, these comparisons show that the immunization of cancer patients with a carbohydrate conjugate vaccine resulted in the production of antibody levels similar to, and often exceeding, those formed by immunization with bacterial polysaccharide vaccines. The use of affinity-purified antibodies also revealed that substantial quantities of IgG, as well as IgM antibodies, were produced in response to the vaccine. Thus, even though the globo H epitope is apparently expressed to a small extent on normal tissues, it is possible to break tolerance by using the conjugate vaccine together with adjuvant and to generate a potent immune response focused against this constellation.
The IgG subclass distribution of the anti-globo H antibodies was surprisingly heterogeneous. It is frequently stated that anti-carbohydrate antibody responses are restricted to the IgG2 subclass (30), though exceptions to this rule have been noted (30, 31). Our results are encouraging in that all patients responded with the production of IgG1 antibodies, and whereas only one produced detectable levels of IgG2, three patients also produced IgG4 antibodies (Table 2).
Testing of the purified antibodies by ELISA against a panel of 15 glycoconjugates confirmed the ability of all the postvaccine antibodies to react with globo H. A number of the antibody samples, however, showed an unanticipated degree of reactivity with galactosylgloboside and globoside (Fig. 3). Crossreaction of antibodies with related antigen structures is commonly observed. For anti-carbohydrate antibodies, these reactivities are normally focused on the nonreducing terminal moieties. When assessed by using whole sera, patients immunized with the globo H-KLH conjugate seemed to have produced antibodies that mainly recognize an epitope area encompassing five nonreducing terminal carbohydrate units (9); however, when purified antibodies from the same patients were examined, binding of internal carbohydrate sequences was revealed as well.
The results of this study differ in several significant ways from those previously reported using whole sera from these immunized patients (10, 11). In particular, the levels of IgG induction were found to be considerably higher than were apparent from the titers obtained on whole sera. Our results show that significant class-switching to IgG antibodies had occurred. These findings support the concept of using a protein carrier, such as KLH, to increase the immunogenicity of carbohydrate antigens, thereby broadening the spectrum of Ig classes produced. In addition, the use of purified antibodies enabled the clear determination of the IgG subclass of the responses. Finally, the present study demonstrated the wide spectrum of specificities induced to structures related to globo H (galactosyl-globoside and globoside), indicating the presence of antibodies, not apparent in the earlier study, reacting with internal sequences as well as with terminal sugar residues. These differences may reflect the difficulties inherent in analyzing the reactivities of whole sera by ELISA in which the levels of unrelated serum constituents (particularly IgG) substantially dwarf the specific antibodies reacting with the immunizing antigen. Whether this suffices to explain the previous finding that only 2 of the 19 patients showed significant IgG reactivity, whereas 10 of 18 patients showed IgM reactivity, is uncertain. It is also possible that the low avidity of IgG in comparison with pentameric IgM could also explain these findings.
In summary, the development of a method for the isolation of antibodies elicited in response to our vaccination protocol has allowed the characterization of significant amounts of purified anti-globo H antibodies from individual patients. Such antibodies could be used in several ways. It is interesting to consider, for example, a therapeutic study in which subjects are retreated with their own purified antibodies as radiolabeled or drug-substituted conjugates. Such an approach would simplify the technical difficulties involved in “humanizing” mouse monoclonal antibodies and the regulatory limitations of using human antibodies derived from other sources, as well as provide new insights into cancer and anti-cancer vaccine therapy.
Acknowledgments
This work was supported by the National Institutes of Health (Grants AI-16943, CA-28824, CA-71506, and CA-08748), Cancer Research Institute, CaP CURE, Swim Across America, and the PepsiCo Foundation.
Abbreviations
- CDC
complement-dependent cytotoxicity
- ADCC
antibody-dependent cellular cytotoxicity
- KLH
keyhole limpet hemocyanin
- HSA
human serum albumin
References
- 1.Hakomori S, Zhang Y. Chem Biol. 1997;4:97–104. doi: 10.1016/s1074-5521(97)90253-2. [DOI] [PubMed] [Google Scholar]
- 2.Ragupathi G, Livingston P O. Cancer Immunol Immunother. 1997;45:10–19. doi: 10.1007/s002620050395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Livingston P O, Natoli E J, Calves M J, Stockert E, Oettgen H F, Old L J. Proc Natl Acad Sci USA. 1987;84:2911–2915. doi: 10.1073/pnas.84.9.2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Livingston P O, Wong G Y C, Adluri S, Tao Y, Padavan M, Parente R, Hanlon C, Calves M J, Helling F, Ritter G, et al. J Clin Oncol. 1994;12:1036–1044. doi: 10.1200/JCO.1994.12.5.1036. [DOI] [PubMed] [Google Scholar]
- 5.MacLean G D, Reddish M, Koganty R R, Wong T, Gandhi S, Smolenski M, Samuel J, Nabholtz J M, Longenecker B M. Cancer Immunol Immunother. 1993;36:215–222. doi: 10.1007/BF01740902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.MacLean G D, Reddish M A, Koganty R R, Longenecker B M. J Immunother. 1996;19:59–68. doi: 10.1097/00002371-199601000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Singhal A, Fohn M, Hakomori S. Cancer Res. 1991;51:1406–1411. [PubMed] [Google Scholar]
- 8.Ragupathi G, Howard M, Cappello S, Koganty R R, Qiu D, Longenecker B M, Reddish M A, Lloyd K O, Livingston P O. Cancer Immunol Immunother. 1999;48:1–8. doi: 10.1007/s002620050542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ragupathi G, Slovin S F, Adluri S, Sames D, Kim I J, Kim H M, Spassova M, Bornmann W G, Lloyd K O, Scher H I, et al. Angew Chem Int Ed Engl. 1999;38:563–566. doi: 10.1002/(SICI)1521-3773(19990215)38:4<563::AID-ANIE563>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 10.Slovin S F, Ragupathi G, Adluri S, Ungers G, Terry K, Kim S, Spassova M, Bornmann W G, Fazzari M, Dantis L, et al. Proc Natl Acad Sci USA. 1999;96:5710–5715. doi: 10.1073/pnas.96.10.5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kudryashov V, Kim H M, Ragupathi G, Danishefsky S J, Livingston P O, Lloyd K O. Cancer Immunol Immunother. 1998;45:281–286. doi: 10.1007/s002620050444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Danishefsky, S. J. & Allen, J. (2000) Angew. Chem. Int. Ed. Engl., in press.
- 13.Zhang H, Zhang S, Cheung N-K V, Ragupathi G, Livingston P O. Cancer Res. 1998;58:2844–2849. [PubMed] [Google Scholar]
- 14.Livingston P O, Zhang S, Lloyd K O. Cancer Immunol Immunother. 1997;45:1–9. doi: 10.1007/s002620050394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Livingston P O. Cancer Biol. 1995;6:357–366. doi: 10.1016/1044-579x(95)90005-5. [DOI] [PubMed] [Google Scholar]
- 16.Zhang S, Cordon-Cardo C, Zhang H S, Reuter V E, Adluri S, Hamilton W B, Lloyd K O, Livingston P O. Int J Cancer. 1997;73:42–49. doi: 10.1002/(sici)1097-0215(19970926)73:1<42::aid-ijc8>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 17.Menard S, Tagliabue E, Canevari S, Fossati G, Colnaghi M I. Cancer Res. 1983;43:1295–1300. [PubMed] [Google Scholar]
- 18.Bremer E G, Levery S B, Sonnino S, Ghidoni R, Canevari S, Kannagi R, Hakomori S. J Biol Chem. 1984;259:14773–14777. [PubMed] [Google Scholar]
- 19.Adobati E, Panza L, Russo G, Colnaghi M, Canevari S. Glycobiology. 1997;7:173–178. doi: 10.1093/glycob/7.2.173. [DOI] [PubMed] [Google Scholar]
- 20.Zhang S, Zhang H S, Reuter V E, Slovin S F, Scher H I, Livingston P. Clin Cancer Res. 1998;4:295–302. [PubMed] [Google Scholar]
- 21.Park T K, Kim I J, Hu S, Bilodeau M T, Randolph J T, Kwon O, Danishefsky S J. J Am Chem Soc. 1996;118:11488–11500. [Google Scholar]
- 22.Sabbatini, P. J., Kudryashov, V., Govindaswami, R., Danishefsky, S. J., Livingston, P. O., Bornmann, W., Spassova, M., Spriggs, D., Aghajanian, C., Soignet, S., et al. (2000) Int. J. Cancer, in press. [PubMed]
- 23.Lloyd K O, Savage A. Glycoconjugate J. 1991;8:493–498. doi: 10.1007/BF00769849. [DOI] [PubMed] [Google Scholar]
- 24.Behar V, Danishefsky S J. Angew Chem Int Ed Engl. 1994;33:1468–1470. [Google Scholar]
- 25.Kudryashov V, Ragupathi G, Kim I J, Breimer M E, Danishefsky S J, Livingston P O, Lloyd K O. Cancer Immunol Immunother. 1998;45:281–286. doi: 10.1007/s002620050444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yin B W T, Finstad C L, Kitamura K, Federici M G, Welshinger M, Kudryashov V, Hoskins W J, Welt S, Lloyd K O. Int J Cancer. 1996;65:406–412. doi: 10.1002/(SICI)1097-0215(19960208)65:4<406::AID-IJC2>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 27.Soininen A, Seppala I, Nieminen T, Eskola J, Kayhty H. Vaccine. 1999;17:1889–1897. doi: 10.1016/s0264-410x(98)00475-7. [DOI] [PubMed] [Google Scholar]
- 28.Anttila M, Eskola J, Ahman H, Kayhty H. Vaccine. 1999;17:1970–1977. doi: 10.1016/s0264-410x(98)00458-7. [DOI] [PubMed] [Google Scholar]
- 29.Kabat E A, Berg D. J Immunol. 1953;70:514–532. [PubMed] [Google Scholar]
- 30.Normansell D E. Diagn Clin Immunol. 1987;5:115–128. [PubMed] [Google Scholar]
- 31.Livingston P O, Ritter G, Srivastava P, Padavan M, Calves M J, Oettgen H F, Old L J. Cancer Res. 1989;49:7045–7050. [PubMed] [Google Scholar]