Abstract
Background
Fatigue is one of the most common and distressing complaints among cancer patients, not only during radiation and chemotherapy, but also for months to years after the completion of treatment. Fatigue interferes with patients’ daily lives, reduces their quality of life, and is often a significant reason why patients discontinue treatment. We hypothesized that some of the fatigue may be related to disrupted circadian rhythms and low light exposure. The main objective of this study therefore was to investigate the association between fatigue and light exposure among patients with breast cancer.
Methods
As part of a larger, ongoing prospective study on fatigue, sleep, and circadian rhythms in patients with breast cancer, an analysis of 63 women newly diagnosed with stage I–IIIA breast cancer and scheduled to receive four cycles of adjuvant or neoadjuvant anthracycline-based chemotherapy was conducted. Data were collected before and during weeks 1, 2, and 3 of cycle 1 and cycle 4. Fatigue was assessed using the Short Form of Multidimensional Fatigue Symptom Inventory. Light exposure was recorded with a wrist actigraph.
Results
There were significant correlations between fatigue levels and light exposure (r=−0.28 to −0.45) within both cycle 1 and cycle 4, such that higher levels of fatigue were associated with less light exposure. There were also significant correlations between changes in light exposure and changes in fatigue within the first 2 weeks of each cycle (r=−0.28 to −0.52).
Conclusions
Increased fatigue was significantly correlated with decreased light exposure among patients with breast cancer. Although the cause and effect of exacerbated fatigue and decreased light exposure cannot be confirmed by the current study, and lower light exposure may just in part be due to the fatigued patients spending less time outdoors in bright light, two hypotheses are proposed about the mechanisms by which light may alleviate the fatigue of patients with breast cancer. These results suggest the need for prospective intervention studies of light therapy for breast-cancer-related fatigue.
Keywords: Breast cancer, Fatigue, Light, Chemotherapy
Introduction
Fatigue is one of the most common and distressing complaints among cancer patients, occurring in more than 75% of patients undergoing chemotherapy [1, 2]. The fatigue is experienced not only during chemotherapy [1, 3, 4], and for months to years after the completion of treatment [5–7], but at times also before the administration of any treatment [8, 9]. Fatigue interferes with daily life activities, reduces quality of life [10, 11], and is often a significant reason why patients discontinue treatment [1].
The causes of cancer-related fatigue are multifactorial and likely include physiological factors (e.g., pain, anemia, endocrine and cytokine changes), psychological factors (e.g., depression, anxiety), sociocultural factors (e.g., education, cognitive and behavioral responses), and chronobiological factors (e.g., sleep and circadian rhythms) [2, 12, 13]. Richardson demonstrated that biological, psychological, social, and personal factors might influence the onset, impact, expression, duration, and severity of fatigue in patients with cancer [4]. Morrow et al. further raised four hypotheses for the development of cancer-related fatigue that focused primarily on physiological and pathophysiological aspects [2]. Ancoli-Israel et al. also hypothesized that increased bright light exposure might decrease fatigue in patients with breast cancer [12].
Bright light treatment has been shown to be effective for seasonal and nonseasonal depression, delayed and advanced sleep phase syndromes, jet lag syndrome, and shift work syndrome [14–20]. Fatigue has also been associated with these same disorders, but little is known about the association between bright light and fatigue. To our knowledge, no previous studies have focused on the relationship between fatigue and light exposure in any group of patients. This paper describes the association found between fatigue and light exposure in patients with breast cancer and discusses possible mechanisms of their interaction.
Patients and methods
Patients
As part of a larger prospective study on fatigue, sleep, and circadian rhythms in patients with breast cancer, data from 63 women (mean age 52.0 years, SD=10.5 years, range 34–79 years) were analyzed for fatigue and light exposure. Only those women who had light data available for all time points were included. All women were newly diagnosed with stage I–IIIA breast cancer (34% with stage I, 24% with stage II, 23% with stage IIIA) and had not previously received chemotherapy. All participants were scheduled to receive at least four cycles of adjuvant or neoadjuvant anthracycline-based chemotherapy with each cycle 3 weeks apart [92% with doxorubicin and cyclophosphamide (AC), or AC plus fluorouracil, AC plus docetaxel, or AC plus paclitaxel; 8% with cyclophosphamide, epirubicin, and fluorouracil (CEF)]. Among the 63 women, 75% were Caucasian, 65% were married, 78% had at least some college, and 71% reported an annual income of more than $30,000. Pregnant women, those undergoing bone marrow transplants, and those with metastatic or stage IIIB (including inflammatory) breast cancer, with confounding underlying medical illnesses, with significant preexisting anemia, or with other physical or psychological impairments were excluded.
Of the women referred to the study, seven were ineligible because they were put on a different chemotherapy regimen and six dropped out because they were overwhelmed with their diagnosis.
Instruments
Fatigue
Fatigue was assessed using the 30-item Multidimensional Fatigue Symptom Inventory–Short Form (MFSI-SF) [21, 22], which has been shown to be a valid and reliable tool for the multidimensional assessment of cancer-related fatigue for both clinical and research applications. The items of the MFSI-SF collapse into five subscales of fatigue dimensions: General, Emotional, Physical, Mental, and Vigor. Each subscale includes six items and each item is rated on a 5-point scale indicating how true the statement was during the last week (0=not at all, 4=extremely). Higher scores indicate more severe fatigue, except for the Vigor subscale, where a higher score indicates less fatigue (more vigor). The sum of General, Physical, Emotional, and Mental subscale scores minus the Vigor subscale score generates a total fatigue score. The range of possible scores for each subscale is 0 to 24, and the range for total fatigue score is −24 to 96. Normative data suggest a score above 1 indicates fatigue.
Light
Light exposure was recorded using the Actillume wrist actigraph recorder (Ambulatory Monitoring Inc, Ardsley, New York) [23]. The Actillume recorder is a small device, approximately 1×3×6 cm, worn on the wrist. It contains a log-linear photometric transducer (sensitive from <0.01 to >100,000 lux: below moonlight to the brightest summer day at noon), a piezoelectric linear accelerometer (which records movement sensitive to 0.003 g and above), a microprocessor, 32K RAM memory, and associated circuitry. The log lux measurements at the wrist and at the forehead had a correlation of r=0.93, so wrist placement seems quite satisfactory to reflect light exposure near the eyes [24]. As an example of how bright lux levels are, looking at the horizon on a clear day at noon would yield a light exposure of about 10,000 lux. Most indoor rooms are rarely over 500–700 lux.
Actillume data were downloaded onto a desktop computer and analyzed using Action 3 (Ambulatory Monitoring Inc). The activity data were used to distinguish wake from sleep (data that will be presented in a subsequent publication). Light exposure data (lux levels) were exported to a text file first, and duration (minutes of exposure >1,000 lux/day) and intensity (mean lux levels) were then calculated. Since few indoor lights emit light levels above 700 lux, minutes of exposure of >1,000 lux/day was chosen because that would suggest that the women had been outdoors.
Procedure
The study was approved by the University of California Committee on Protection of Human Subjects and by the Protocol Review and Monitoring Committee of the UCSD Rebecca and John Moores Cancer Center. After consent forms were signed, medical records were abstracted for medical history and current medication use.
The eight time points of data collection were before the start of the first cycle of chemotherapy (cycle 1 week −1 or C1W−1), during the 3 weeks of cycle 1 [week 1 (C1W1)= chemotherapy administration; week 2 (C1W2)=point of nadir of blood count; week 3 (C1W3)=recovery], before the start of cycle 4 [cycle 4 week −1 (C4W−1); note: this is usually the third week of cycle 3], and during the 3 weeks of cycle 4 (C4W1, C4W2, and C4W3). Table 1 presents the number of days that data collection occurred pre- or postchemotherapy. In general, baseline data collection began the week before chemotherapy, followed by data collection on the morning after chemotherapy administration. Data collected in each subsequent week (weeks 2 and 3) were collected on the same day of the week as during week 1.
Table 1.
Mean number of days postchemotherapy of data collection
| Mean number of days | SD | Range | |
|---|---|---|---|
| Pre-cycle 1 chemotherapya | |||
| Cycle 1 week −1 | −7.7 | −5.9 | −1 to −28 |
| Cycle 1 chemotherapyb | |||
| Cycle 1 week 1 | 1.0 | 0.4 | 0 to 3 |
| Cycle 1 week 2 | 8.9 | 1.6 | 7 to 15 |
| Cycle 1 week 3 | 15.8 | 1.7 | 14 to 22 |
| Pre-cycle 4 chemotherapyc | |||
| Cycle 4 week −1 | −6.1 | −1.3 | −3 to −10 |
| Cycle 4 chemotherapyd | |||
| Cycle 4 week 1 | 1.0 | 0.6 | 0 to 3 |
| Cycle 4 week 2 | 8.6 | 1.7 | 7 to 14 |
| Cycle 4 week 3 | 15.9 | 2.4 | 12 to 23 |
Number of days before administration of cycle 1 chemotherapy
Number of days after administration of cycle 1 chemotherapy
Number of days before administration of cycle 4 chemotherapy
Number of days after administration of cycle 4 chemotherapy
During each of eight points of data collection, the women wore the Actillume recorder for three consecutive 24-h periods (i.e., 72 h) and completed an accompanying sleep log on which bedtime, wake time, and any napping behavior were recorded. MFSI-SF was completed once during the 3 days of data collection at each of the same eight time points.
Data analysis
Light data were separated into two periods: out-of-bed time and in-bed time, with bedtime based on the time reported on sleep logs. Since average light exposure of in-bed time suggested women were appropriately in the dark during all eight data collection time points (mean 1.9 lux, SD=5.7 lux, range 0–71.4 lux), only out-of-bed light data were used for analyses.
Spearman rank correlations were computed to examine the associations between fatigue (MFSI-SF total and subscale scores) and duration of light exposure (minutes >1,000 lux/day) and intensity of light (mean lux levels). Spearman correlations were also performed between the changes in fatigue and changes in light exposure between week −1 and week 1, between week 1 and week 2, and between week 2 and week 3 within both cycle 1 and cycle 4.
For descriptive purposes and to examine changes in fatigue and changes in light over time, multivariate analysis of variance with weeks of chemotherapy (W−1, W1, W2, W3) as the within-subject factor was computed for each cycle for fatigue (total and subscale scores) and duration and intensity of light exposure (minutes >1,000 lux/day and mean lux), followed by preplanned contrasts between baseline and each week of C1 and C4 and between each of the treatment weeks within C1 and C4.
In order to examine the changes of fatigue and changes of light exposure from before the start of chemotherapy (C1W−1) to each week of C4, paired t tests were performed. To compare mean levels of fatigue between C1 and C4, paired t tests were also performed.
All analyses were performed using version 8.02 of SAS [25]. All statistical tests with p values <0.05 are reported as statistically significant.
Results
Fatigue
The mean fatigue scores for each week of each cycle are shown in Table 2. In general, fatigue significantly increased from baseline (W−1) to each week of cycle 1 (p value range <0.0001–0.02), but remained constant from week 1 to week 3.
Table 2.
Fatigue measures (mean±SD) (n) by week for cycle 1 and cycle 4
| Cycle 1
|
Cycle 4
|
|||||||
|---|---|---|---|---|---|---|---|---|
| MFSI-SF | Week −1 | Week 1 | Week 2 | Week 3 | Week −1 | Week 1 | Week 2 | Week 3 |
| General | 6.13±5.88 (59) | 10.06±6.35 (52) | 9.62±6.1 (53) | 9.04±6.81 (53) | 10.98±6.64 (50) | 11.73±6.47 (53) | 11.72±7.33 (50) | 12.44±6.89 (51) |
| Physical | 2.94±3.95 (59) | 4.31±4.87 (52) | 4.40±4.77 (53) | 3.93±4.46 (53) | 4.87±6.02 (50) | 5.90±5.38 (53) | 5.08±5.46 (50) | 4.64±5.22 (51) |
| Mental | 3.70±3.60 (59) | 5.02±4.27 (52) | 5.09±5.52 (53) | 4.49±4.34 (53) | 5.76±5.90 (49) | 6.19±5.48 (53) | 5.98±5.90 (50) | 5.75±5.17 (51) |
| Emotional | 5.06±4.20 (58) | 5.24±4.75 (52) | 5.55±5.43 (53) | 5.48±5.60 (53) | 5.72±6.20 (49) | 6.49±5.48 (50) | 6.12±5.91 (50) | 5.74±5.09 (51) |
| Vigor | 11.85±5.53 (59) | 9.63±5.23 (52) | 10.18±5.86 (53) | 10.02±6.33 (53) | 9.27±6.19 (49) | 8.40±5.95 (53) | 8.88±6.11 (50) | 9.15±5.98 (51) |
| Total | 6.85±18.59 (58) | 14.99±20.38 (52) | 14.48±24.31 (53) | 12.92±23.62 (53) | 18.23±27.47 (49) | 21.91±24.55 (53) | 20.03±26.02 (50) | 19.43±22.89 (51) |
Fatigue within each week of cycle 4 was significantly higher than during baseline (C1W−1) (p value range, 0.0005–0.045) and significantly higher than each week of cycle 1 (p value range, 0.0005–0.045) (see Fig. 1).
Fig. 1.
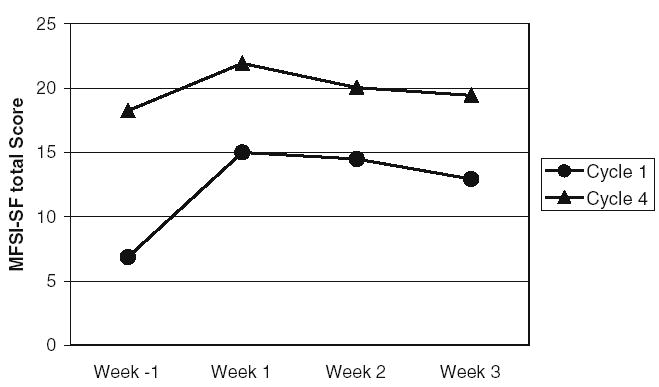
Mean total fatigue (MFSI-SF) by week of cycle 1 and cycle 4. Total fatigue was significant higher during cycle 4 compared with cycle 1 (p=0.0005)
There were no significant differences in fatigue between women with different stages of cancer or between those with different chemotherapy regimens in any of the analyses.
Light exposure
Table 3 lists the mean light exposure levels before and during chemotherapy. In general, both intensity (p< 0.0001) and duration (p<0.0001) of light exposure significantly decreased from baseline (C1W−1) to W1 in both cycle 1 and cycle 4. There were no differences in light exposure levels between baseline and any other weeks, or between the other weeks of treatment in either cycle, except for cycle 4, in which the intensity (p=0.006) and duration (p=0.007) were both significantly higher during week 2 than during week 1. There were no significant differences in mean levels of intensity or duration of light exposure between cycle 4 and cycle 1.
Table 3.
Light exposure measures (mean±SD) (n) by Week of Cycle 1 and Cycle 4
| Cycle 1
|
Cycle 4
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Light exposure | Week −1 (n=63) | Week 1 (n=57) | Week 2 (n=54) | Week 3 (n=55) | Week −1 (n=50) | Week 1 (n=54) | Week 2 (n=52) | Week 3 (n=52) |
| Intensitya | 475.39±470.71 | 282.00±346.25* | 490.71±600.10 | 380.42±372.35 | 395.68±439.09 | 313.29±446.23** | 418.39±496.60 | 338.94±371.88 |
| Durationb | 54.75±48.61 | 33.23±43.45* | 53.04±51.09 | 48.21±46.06 | 47.92±43.73 | 38.36±12.38** | 52.54±51.87 | 44.99±44.92 |
Mean lux
Minutes >1,000 lux/day
p<0.0001 when compared with pretreatment (cycle 1 week −1),
p<0.05 when compared with pretreatment (cycle 4 week −1)
There were no significant differences in light exposure between women with different stages of cancer or between those with different chemotherapy regimens in any of the analyses.
Correlations between fatigue and light exposure
In general, during W1 of cycle 1 and of cycle 4, more fatigue and less vigor were associated with lower light intensity (lower mean lux) and shorter duration of bright light exposure (fewer minutes at >1,000 lux/day). During C1W1, the General fatigue scale was significantly correlated with the duration of bright light exposure (minutes >1,000 lux/day, r=−0.40, p=0.003) (see Fig. 2). During C4W1, the total MFSI-SF score (r=−0.38, p=0.006), General (r=−0.45, p=0.0006), Mental (r=−0.31, p=0.026), and Physical (r=−0.35, p=0.011) subscale scores were all significantly correlated with duration of bright light exposure. During C4W3, significant correlations were found between duration of bright light exposure and the total MFSI-SF (r=−0.30, p=0.033), as well as General (r=−0.30, p=0.031), Emotional (r=−0.35, p=0.012), and Vigor (r=0.28, p=0.045) subscale scores.
Fig. 2.
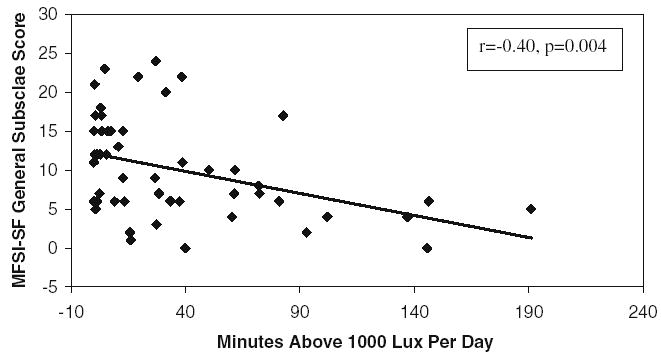
Scatterplot of general fatigue (MFSI-SF) and duration of bright light exposure (minutes >1,000 lux/day) during cycle 1 week 1. More general fatigue was significantly correlated with fewer minutes of >1,000 lux light exposure
In order to better understand the relationship between fatigue and light exposure, correlations were also computed between changes in fatigue and changes in light exposure between week −1 and week 1, between week 1 and week 2, and between week 2 and week 3 within cycle 1 and within cycle 4. During both cycle 1 and cycle 4, there were significant negative correlations from week −1 to week 1 for changes in total fatigue and bright light duration and in total fatigue and intensity (both r=−0.33, p=0.031) and from week 1 to week 2 for changes in total fatigue and bright light duration (r=−0.29, p=0.048). There were similar correlations for each of the fatigue subscales.
Discussion
The results of this study suggested that increased fatigue was associated with decreased intensity and duration of bright light exposure. We found significant correlations between fatigue and light exposure during the chemotherapy administration weeks of both cycles, as well as during week 3 of cycle 4. We also explored the relationship between the changes in fatigue levels and changes in light exposure from one week to the next and found significant correlations in both cycles from the pretreatment period to week 1, as well as from week 1 to week 2, suggesting that as the amount of light exposure increased, the amount of fatigue decreased. By week 3 of each cycle, both light and fatigue were stable. This association between fatigue and light exposure may suggest an existence of a negative feedback loop, with side effects of chemotherapy causing more severe fatigue, more severe fatigue causing less outdoor activity, less time spent outdoors causing lower bright light exposure, and lower light exposure then further exacerbating the fatigue. On the contrary, increased light exposure may break this negative feedback loop and mitigate the fatigue. These results need to be replicated and the casual relationship between light and fatigue needs to be examined more thoroughly. However, the results are sufficiently suggestive to warrant clinical trials of light treatment to test this hypothesis.
The results of this study also suggested that fatigue was stable within the cycles, but was significantly higher than before the start of treatment. In addition, fatigue not only persisted but was exacerbated by the fourth cycle of chemotherapy. The pattern of fatigue getting worse during and after chemotherapy confirms reports of other studies [8, 26]. However, the current data show a different pattern of fatigue during the second week of each cycle than previously reported. The so-called “roller-coaster” pattern, with significantly lower fatigue at the midpoint of each cycle, reported by Berger [26] and by Schwartz [27], was not confirmed in this study. Instead, we found that fatigue essentially remained at the same level within cycle 1 and within cycle 4, but got worse from cycle 1 to cycle 4. To our knowledge, this is the first study to report systematically recorded fatigue during cycle 4 of chemotherapy in patients with breast cancer, and to report increased fatigue during the fourth cycle relative to the first cycle. One question that could arise is whether the amount of fatigue reported by the women in this study differed from reports in other studies. Although there are no data reported in the literature during cycle 4, the fatigue levels reported during cycle 1 by the women in this study did not differ from those reported by cancer patients used in the validation report of the MFSI-SF [21]. The different pattern of fatigue found in this study may therefore be a result of the longer period studied and the more extensive definition of fatigue employed. Our results may also be specific to anthracycline-based chemotherapy, as different types of chemotherapy were used in the studies by Berger [36% with cyclophosphamide, methotrexate, and fluorouracil (CMF); 28% with doxorubicin and cyclophosphamide (A/C); 39% with cyclophosphamide, doxorubucin, and fluorouracil (CAF)] [26] and Schwartz [70% with doxorubicin and cyclophosphamide (A/C), 23% with cyclophosphamide, methotrexate, and fluorouracil (CMF), and 3% with vinorelbine] [27].
This brings up the question of whether the type of chemotherapy or the stage of disease might influence the amount of fatigue. In our study, the women were at different stages of severity and received different chemotherapies. However, there were no significant differences in reports of fatigue when analyzed by stage of disease or by type of chemotherapy.
Although the cause and effect of exacerbated fatigue and decreased light exposure cannot be confirmed by the current study, and lower light exposure may just in part be due to the fatigued patients spending less time outdoors in bright light, two hypotheses can be proposed about the mechanisms by which light may alleviate the fatigue of patients with breast cancer.
The first hypothesis is that light may improve fatigue via the entrainment of circadian rhythms. Examples of circadian rhythms, i.e., 24-h rhythms, include changes or alternations of hormone secretion, body temperature, and sleep–wake cycles. The circadian rhythm in humans is approximately 24 h and is regulated by neural (e.g., superchiasmatic nuclei, SCN) and hormonal (e.g., melatonin) processes. The synchronization of this rhythm with the solar day and night is maintained mainly through entrainment by light [28]. Data suggest that fatigue among patients with breast cancer may be related to disrupted circadian rhythms [29, 30]. Studies have shown that light therapy is effective in the treatment of circadian-rhythm related disorders (e.g., seasonal affective disorder, delayed or advanced sleep phase syndrome, jet lag syndrome, and shift work syndrome) and in consolidating sleep and improving the strength of circadian rhythms [31, 32].
A second hypothesis by which light may alleviate breast cancer fatigue is by improving mood. Treatment of seasonal affect disorder (SAD) with bright light improves symptoms of depression [33]. Fatigue is an independent symptom of SAD that differs from depression [34], but that also diminishes when SAD is alleviated. In this study, Emotional fatigue, which was already worse in cancer than noncancer subjects before the start of treatment (S. Ancoli-Israel et al., unpublished data), remained consistent within both cycles and was even worse during cycle 4 than cycle 1, suggesting that the affective dimension of fatigue may play an important role in chronic cancer-related fatigue. Increasing light exposure might alleviate the mood and consequently the fatigue.
There were some limitations to this study. Although data were rarely collected on a weekend since data collection always began on the same day of the week as chemotherapy, some of the women worked outside the home and some did not, which may have effected how much time they were able to spend outdoors in bright light. In addition, only limited information was available on how much these women exercised and whether they exercised outdoors. Future studies may wish to control for occupations and exercise levels.
In summary, our results showed that in patients with breast cancer, increased fatigue was associated with decreased light exposure during the first 2 weeks of the first and fourth cycles of chemotherapy. Although the cause and effect of fatigue and light exposure have not yet been confirmed, increased bright light exposure has been shown to improve sleep and mood in other populations and may therefore decrease fatigue among patients with breast cancer. These results suggest that prospective studies to evaluate the usefulness of light therapy in mitigating fatigue in patients with breast cancer might be warranted.
Acknowledgments
This study was supported by NCI CA85264, NIA AG08415, NCI R25 CA 65745, the UCSD Rebecca and John Moores Cancer Center (NCI P30 CA-23100), the UCSD General Clinical Research Center (MO1-RR00827), the Department of Veterans Affairs VISN-22 Mental Illness Research, Education and Clinical Center (MIRECC), and the Research Service of the Veterans Affairs San Diego Healthcare System.
References
- 1.Winningham ML, Nail LM, Burke MB, Brophy L, Cimprich B, Jones LS, Pickard-Holley S, Rhodes V, Pierre B, St, Beck S, Glass EC, Mock VL, Mooney KH, Piper B. Fatigue and the cancer experience: the state of the knowledge. Oncol Nurs Forum. 1994;21:23–36. [PubMed] [Google Scholar]
- 2.Morrow GR, Andrews PLR, Hickok JT, Roscoe JA, Matteson S. Fatigue associated with cancer and its treatment. Support Care Cancer. 2002;10:389–398. doi: 10.1007/s005200100293. [DOI] [PubMed] [Google Scholar]
- 3.Smets EMA, Garssen B, Schuster-Uitterhoeve AL, de Haes JC. Fatigue in cancer patients. Br J Cancer. 1993;68:220–224. doi: 10.1038/bjc.1993.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Richardson A. Fatigue in cancer patients: a review of the literature. Eur J Cancer Care. 1995;4:20–32. doi: 10.1111/j.1365-2354.1995.tb00049.x. [DOI] [PubMed] [Google Scholar]
- 5.de Jong N, Courtens AM, Abu-Saad HH, Schouten HC. Fatigue in patients with breast cancer receiving adjuvant chemotherapy: a review of the literature. Cancer Nurs. 2002;25:283–297. doi: 10.1097/00002820-200208000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Lindley C, Vasa S, Sawyer WT, Winer EP. Quality of life and preferences for treatment following systemic adjuvant therapy for early-stage breast cancer. J Clin Oncol. 1998;16:1380–1387. doi: 10.1200/JCO.1998.16.4.1380. [DOI] [PubMed] [Google Scholar]
- 7.Bower JE, Ganz PA, Desmond KA, Rowland JH, Meyerowitz BE, Belin TR. Fatigue in breast cancer survivors: occurrence, correlates, and impact on quality of life. J Clin Oncol. 2000;18:743–753. doi: 10.1200/JCO.2000.18.4.743. [DOI] [PubMed] [Google Scholar]
- 7a.Ancoli-Israel S, Parker BA, Marler M, Sadler GR, Jones V, Liu L. Fatigue, sleep and circadian rhythms prior to chemotherapy in breast cancer. Breast Cancer Res Treat. 2004;8(Suppl 1) doi: 10.1007/s00520-005-0861-0. abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacobsen PB, Hann DM, Azzarello LM, Horton J, Balducci L, Lyman GH. Fatigue in women receiving adjuvant chemotherapy for breast cancer: characteristics, course, and correlates. J Pain Symptom Manage. 1999;18:233–242. doi: 10.1016/s0885-3924(99)00082-2. [DOI] [PubMed] [Google Scholar]
- 9.Cimprich B. Pretreatment symptom distress in women newly diagnosed with breast cancer. Cancer Nurs. 1999;22:185–194. doi: 10.1097/00002820-199906000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Visser MRM, Smets EMA. Fatigue, depression and quality of life in cancer patients: how are they related? Support Care Cancer. 1998;6:101–108. doi: 10.1007/s005200050142. [DOI] [PubMed] [Google Scholar]
- 11.Tchen N, Juffs HG, Downie FP, Yi Q-L, Hu H, Chemerynsky I, Clemons M, Crump M, Goss PE, Warr D, Tweedale ME, Tannock IF. Cognitive function, fatigue, and menopausal symptoms in women receiving adjuvant chemotherapy for breast cancer. J Clin Oncol. 2003;21:4175–4183. doi: 10.1200/JCO.2003.01.119. [DOI] [PubMed] [Google Scholar]
- 12.Ancoli-Israel S, Moore P, Jones V. The relationship between fatigue and sleep in cancer patients: a review. Eur J Cancer Care. 2001;10:245–255. doi: 10.1046/j.1365-2354.2001.00263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacobsen PB, Stein K. Is fatigue a long-term side effect of breast cancer treatment? Cancer Control. 1999;6:256–263. doi: 10.1177/107327489900600304. [DOI] [PubMed] [Google Scholar]
- 14.Remick RA. Diagnosis and management of depression in primary care: a clinical update and review. CMAJ. 2002;167:1253–1260. [PMC free article] [PubMed] [Google Scholar]
- 15.Prasko J, Horacek J, Klaschka J, Kosova J, Ondrackova I, Sipek J. Bright light therapy and/or imipramine for inpatients with recurrent non-seasonal depression. Neuroendocrinol Lett. 2002;23:109–113. [PubMed] [Google Scholar]
- 16.Kripke DF. Light treatment for nonseasonal depression: speed, efficacy, and combined treatment. J Affect Disord. 1998;49:109–117. doi: 10.1016/s0165-0327(98)00005-6. [DOI] [PubMed] [Google Scholar]
- 17.Dijk DJ, Boulos Z, Eastman CI, Lewy AJ, Campbell SS, Terman M. Light treatment for sleep disorders: consensus report. II. Basic properties of circadian physiology and sleep regulation. J Biol Rhythms. 1995;10:113–125. doi: 10.1177/074873049501000204. [DOI] [PubMed] [Google Scholar]
- 18.Terman M, Lewy AJ, Dijk DJ, Boulos Z, Eastman CI, Campbell SS. Light treatment for sleep disorders: consensus report. IV. Sleep phase and duration disturbances. J Biol Rhythms. 1995;10:135–147. doi: 10.1177/074873049501000206. [DOI] [PubMed] [Google Scholar]
- 19.Eastman CI, Boulos Z, Terman M, Campbell SS, Dijk DJ, Lewy AJ. Light treatment for sleep disorders: consensus report. VI. Shift work. J Biol Rhythms. 1995;10:157–164. doi: 10.1177/074873049501000208. [DOI] [PubMed] [Google Scholar]
- 20.Boulos Z, Campbell SS, Lewy AJ, Terman M, Dijk DJ, Eastman CI. Light treatment for sleep disorders: consensus report. VII. Jet lag. J Biol Rhythms. 1995;10:167–176. doi: 10.1177/074873049501000209. [DOI] [PubMed] [Google Scholar]
- 21.Stein KD, Martin SC, Hann DM, Jacobsen PB. A multidimensional measure of fatigue for use with cancer patients. Cancer Pract. 1998;6:143–152. doi: 10.1046/j.1523-5394.1998.006003143.x. [DOI] [PubMed] [Google Scholar]
- 22.Stein KD, Jacobsen PB, Blanchard CM, Thors C. Further validation of the multidimensional fatigue symptom inventory—short form. J Pain Symptom Manage. 2004;27:14–23. doi: 10.1016/j.jpainsymman.2003.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ancoli-Israel S, Cole R, Alessi CA, Chambers M, Moorcroft WH, Pollak C. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26:342–392. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- 24.Cole RJ, Kripke DF, Gruen W, Nava J. Ambulatory monitoring of light exposure: comparison of measurements at forehead and wrist. Sleep Res. 1990;19:364. [Google Scholar]
- 25.SAS Institute Inc (1999) SAS/STAT user’s guide, version 8. 1–3884
- 26.Berger AM. Patterns of fatigue and activity and rest during adjuvant breast cancer chemotherapy. Oncol Nurs Forum. 1998;25:51–62. [PubMed] [Google Scholar]
- 27.Schwartz AL. Daily fatigue patterns and effect of exercise in women with breast cancer. Cancer Pract. 2000;8:16–24. doi: 10.1046/j.1523-5394.2000.81003.x. [DOI] [PubMed] [Google Scholar]
- 28.Küller R. The influence of light on circarhythms in humans. J Physiol Anthropol Appl Hum Sci. 2002;21:87–91. doi: 10.2114/jpa.21.87. [DOI] [PubMed] [Google Scholar]
- 29.Berger AM, Farr L. The influence of daytime inactivity and nighttime restlessness on cancer-related fatigue. Oncol Nurs Forum. 1999;26:1663–1671. [PubMed] [Google Scholar]
- 30.Roscoe JA, Morrow GR, Hickok JT, Bushunow P, Matteson S, Rakita D, Andrews PLR. Temporal interrelationships among fatigue, circadian rhythm and depression in breast cancer patients undergoing chemotherapy treatment. Support Care Cancer. 2002;10:329–336. doi: 10.1007/s00520-001-0317-0. [DOI] [PubMed] [Google Scholar]
- 31.Ancoli-Israel S, Martin JL, Kripke DF, Marler M, Klauber MR. Effect of light treatment on sleep and circadian rhythms in demented nursing home patients. J Am Geriat Soc. 2002;50:282–289. doi: 10.1046/j.1532-5415.2002.50060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ancoli-Israel S, Gehrman PR, Martin JL, Shochat T, Marler M, Corey-Bloom J, Levi L. Increased light exposure consolidates sleep and strengthens circadian rhythms in severe Alzheimer’s disease patients. Behav Sleep Med. 2003;1:22–36. doi: 10.1207/S15402010BSM0101_4. [DOI] [PubMed] [Google Scholar]
- 33.Magnusson A, Boivin D. Seasonal affective disorder: an overview. Chronobiol Int. 2003;20:189–207. doi: 10.1081/cbi-120019310. [DOI] [PubMed] [Google Scholar]
- 34.Zubieta JK, Engleberg NC, Yargic LI, Pande AC, Demitrack MA. Seasonal symptom variation in patients with chronic fatigue—comparison with major mood disorders. J Psychiatr Res. 1994;28:13–22. doi: 10.1016/0022-3956(94)90033-7. [DOI] [PubMed] [Google Scholar]


