Abstract
Goals
Previous investigations have shown that women undergoing chemotherapy for breast cancer experience both disturbed sleep and fatigue. However, most of the previous research examined women either during or after chemotherapy. This study examined sleep, fatigue, and circadian rhythms in women with breast cancer before the start of chemotherapy.
Patients and methods
Eighty five women with Stages I–IIIA breast cancer who were scheduled to begin adjuvant or neo-adjuvant anthracycline-based chemotherapy participated. Each had sleep/wake activity recorded with actigraphy for 72 consecutive hours and filled out questionnaires on sleep, fatigue, depression, and functional outcome.
Main results
On average, the women slept for about 6 h a night and napped for over an hour during the day. Sleep was reported to be disturbed and fatigue levels were high. Circadian rhythms were robust, but women who were more phase-delayed reported more daily dysfunction (p<0.01).
Conclusions
The data from the current study suggest that the women with breast cancer likely experience both disturbed sleep and fatigue before the beginning of chemotherapy. Although their circadian rhythms are robust, breast cancer patients with more delayed rhythms experience more daily dysfunction secondary to fatigue. These data suggest that strategies to improve disturbed sleep and to phase-advance circadian rhythms prior to initiation of chemotherapy may be beneficial in improving daily function in breast cancer patients.
Keywords: Fatigue, Sleep, Circadian rhythms, Quality of life, Breast cancer
Abbreviations : CES-D: Center for Epidemiological Studies-Depression scale, CI: Confidence Intervals, FACT-B: Functional Assessment of Cancer Therapy-Breast, FOSQ: Functional Outcome of Sleep Questionnaire, MFSI-SF: Multidimensional Fatigue Symptom Inventory-Short Form, PSQI: Pittsburgh Sleep Quality Index
Introduction
One of the most frequent and disturbing complaints of patients with cancer is fatigue [37, 40, 41, 45]. There is no universally accepted definition of fatigue, but what one descriptor often included is sleepiness during the day accompanied by poor sleep at night [23, 45]. In addition to fatigue, women with breast cancer also often complain of difficulty in sleeping. Ancoli-Israel et al. [3], in a review of sleep and fatigue in cancer, suggested that fatigue is caused by multiple factors including physiological factors such as pain or anemia, psychological factors such as depression or anxiety, socio-cultural factors and chronobiological factors such as sleep and circadian rhythms.
Circadian rhythms are biological cycles that repeat every 24 h. Examples of circadian rhythms include core body temperature, hormone secretion (such as melatonin or cortisol), and the sleep/wake cycle. Circadian rhythms determine if someone is an “owl” (has a delayed sleep phase, which means they like to stay up late and sleep late) or a “lark” (has an advanced sleep phase, which means they like to go to bed early and wake up early). Although these rhythms are generally entrained to periodic environmental events, they can be modified overtime by changes in the sleep/wake cycle and exposure to bright light.
Early studies examined subjective sleep reports in cancer patients without objectively recording sleep and found that the patients with cancer had significantly more complaints of difficulty in sleeping than controls [8, 28]. Whereas some studies supported the findings that women with breast cancer experience sleep problems [7, 29, 39], other studies have suggested that the sleep disturbance might be secondary to the pain and psychological distress experienced by these patients [24, 25, 42]. However, sleep disturbances were reported even when pain and anxiety were low, suggesting that the sleep problem may be independent of these psychological/physiological factors [16].
A better approach for examining sleep is through objective recordings. Two objective methodologies have been used: wrist actigraphy, which measures sleep/wake activity patterns and circadian rhythms over multiple days [2]; and polysomnography, which is the gold standard for measuring sleep stages and sleep disturbances for one night. Studies using actigraphy have shown that in patients with cancer, sleep was fragmented, sleep efficiency (the amount of sleep given the amount of time in bed) was low, patients were more restless at night during treatment, and circadian activity rhythms showed little variation between night and day (i.e., were not robust and were desynchronized), with equal amounts of activity during the day and night instead of high activity during the day and low activity at night [6, 32, 33]. One study used polysomnography to study breast cancer patients and found that they had lower sleep efficiency, took longer to fall asleep, and spent more time awake during the night than the normal sleepers. However, these studies collected data during or after chemotherapy and not before the start of treatment. For a complete review of sleep studies in cancer, the reader is referred to Ancoli-Israel et al. [3].
A similar problem exists with studies of fatigue. Although many cancer studies have examined fatigue during and after chemotherapy, only a small number evaluated fatigue before chemotherapy. These studies found that younger age, female gender, presence of metastases, sleep problems, mood disturbance, lower cognitive function, and poorer performance were all associated with more severe pretreatment fatigue among cancer patients [16, 26, 34, 43].
Studies examining the relationship between sleep and fatigue have primarily found that, during chemotherapy, the two are associated. Anderson et al. [4] and Broeckel et al. [11] found that reports of poor sleep quality were significantly correlated with reports of fatigue during treatment. Andrykowski et al. [5] showed that women with breast cancer reported more fatigue and less vitality than women with benign breast problems. Miaskowski and Lee [32], in a study of patients with bone metastasis, found that these patients reported only moderate fatigue but significant sleep disturbance. Several investigators found that fatigue was also related to depression, anxiety, and/or pain in patients undergoing chemotherapy [36, 43] and after the end of chemotherapy [9, 43]. Three studies examined the relationship between fatigue and reports of sleep disruption before treatment [16, 26, 34], but no studies examined fatigue and objectively recorded sleep before treatment. Therefore, although these studies all demonstrated relationships between fatigue and sleep disturbance during and after treatment for cancer, little is known about levels of fatigue or objective measures of sleep, and the relationship between them, before the start of treatment.
To fully understand the extent of the sleep problems in women undergoing chemotherapy, one needs to first know what the baseline levels of fatigue, sleep, and circadian rhythms are before the start of treatment. Only by knowing prechemotherapy levels will we be able to understand the best treatment approaches and whether specific treatments for the fatigue and sleep should begin even before the start of chemotherapy.
In an attempt to elucidate whether there is a relationship between fatigue, sleep, and circadian rhythms before the start of treatment, we studied women with newly diagnosed breast cancer, recorded their sleep and activity rhythms with actigraphy, and administered questionnaires on sleep, fatigue, functional outcome, and depression after diagnosis but before chemotherapy and during chemotherapy. We describe here the data on fatigue, sleep, and circadian rhythms in the period after diagnosis but before the start of chemotherapy. The hypothesis was that fatigue and sleep disturbances would be present even before chemotherapy, suggesting that these complaints are not just a function of treatment.
Methods
Subjects
Data are presented from 85 women with a mean age of 51.2 years (SD 10.0 years, range 34–79). Of those participating, 74% were Caucasian, 67% were married, 76% had at least some college, and 81% reported an annual income of more than $30,000. All women had been diagnosed with stage 1 (33%), stage II (41%), or stage IIIA (26%) breast cancer and were scheduled to begin neoadjuvant (chemotherapy before surgery) or adjuvant (chemotherapy after surgery) anthracycline-based chemotherapy. Breast cancer disease staging was performed by the referring medical oncologist typically utilizing the American Joint Committee on Cancer Staging Manual 5th Edition. Neoadjuvant patients (N=13) received anthracycline-based chemotherapy after a biopsy to confirm invasive disease and after clinical staging. Adjuvant patients (N=72) received anthracycline-based chemotherapy after clinical staging and definitive surgical treatment with either lumpectomy or mastectomy and axillary staging with sentinel node biopsy or axillary lymph node dissection according to institutional standards. Of the 72 adjuvant patients, 36 had a lumpectomy, 32 had a mastectomy, 3 had a double mastectomy, and 1 is unknown. None of the women had radiation therapy before the chemotherapy.
Potential participants were recruited from the University of California San Diego (UCSD) Cancer Center and from oncologists in the San Diego, CA, and Yakima, WA, areas. Although the study was posted on the UCSD Clinical Trials website and although brochures were placed in areas around the county where potential subjects might see them, this yielded no eligible subjects. Because of the strict inclusion criteria and because of the short window of opportunity between diagnosis and the start of chemotherapy, every woman included in the study was referred to us by her oncologist.
Exclusion criteria included pregnancy, undergoing bone marrow transplants, metastatic or IIIB (including inflammatory) breast cancer, confounding underlying medical illnesses, and significant preexisting anemia or other physical or psychological impairments which would have limited participation. Of the 128 women who requested information about the study, 29 were not consented (19 were not interested in participating and 10 were not eligible). The remaining 99 were consented, but 7 turned out to be ineligible due to different chemotherapy regimens and 6 dropped out (mostly due to feelings of being overwhelmed). As some women are still in the midst of the study, data are presented on the 85 that have completed the protocol.
Apparatus
An Actillume (Ambulatory Monitoring Inc., Ardsley, NY, USA) was used to record sleep/wake behavior and to measure circadian rhythms. The Actillume is a small device, approximately 1×3×6 cm, worn on the wrist. It contains a piezoelectric linear accelerometer (sensitive to 0.003 g and above), a log-linear photometric transducer (sensitive from <0.01 to >100,000 lx), a microprocessor, 32K RAM memory, and associated circuitry. The orientation and sensitivity of the accelerometer are optimized for highly effective sleep–wake inference from wrist activity, which has been previously validated [1, 2, 17].
Action 3 (Ambulatory Monitoring Inc.) was used to analyze the actigraphy data. The output supplied information about percent sleep during the day and night, percent wake during the day and night, number of awakenings per night, length of awakenings at night, and duration of naps.
Procedure
The study was approved by the University of California Committee on Protection of Human Subjects and by the Rebecca and John Moores UCSD Cancer Center’s Protocol Review and Monitoring Committee. After consent forms were signed, medical records were abstracted for medical history and current medication use. Standard baseline blood tests, including complete blood counts, and chemistry profiles were obtained as part of the patient’s breast cancer management.
Women were studied after diagnosis but before the start of chemotherapy with data collection beginning a mean of 7.3 days (SD 5.9, range 1–28 days) before the start of treatment. Each woman wore the actigraph recorder for three consecutive 24-h periods (i.e., 72 h) beginning at 9:00 a.m. on day 1, and each woman completed an accompanying sleep log. In addition, the Pittsburgh Sleep Quality Index (PSQI) [12, 13], the Multidimensional Fatigue Symptom Inventory–Short Form (MFSI-SF) [41], the Functional Outcome of Sleep Questionnaire (FOSQ) [44], the Functional Assessment of Cancer Therapy–Breast (FACT-B) [15], and the Center for Epidemiological Studies–Depression scale (CES-D) [35] were completed within the 72-h period.
The PSQI [12, 13] is a 19-item questionnaire which rates patients’ reports of their sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleep medication, and daytime dysfunction. The total PSQI scores can range from 0–21 with high scores reflecting poor sleep quality. A score above 5 is generally considered poor sleep.
The MFSI-SF [41] is a 30-item fatigue questionnaire with five subscales of fatigue: General, Emotional, Physical, Mental, and Vigor. The sum of General, Physical, Emotional, and Mental subscale scores minus the Vigor subscale score generates a total fatigue score. The range of possible scores for each subscale is 0 to 24, and the range for the total fatigue score is −24 to 96, with higher scores indicating more severe fatigue, except for the Vigor sub-scale, where larger score indicates less fatigue (more Vigor). In a study of adults with no cancer, the mean total MFSI-SF score was 0.85 [41].
The FOSQ [44] is designed to measure functional status in situations that produce sleepiness. There are five sub-scales: Vigilance, Intimacy and Sexual Relationships, General Productivity, Activity Level, and Social Outcome. The scales are added together for a weighted total score. A score below 18 is considered functional impairment, with lower scores indicating more dysfunction.
The FACT-B measures the effect of having cancer on functional status [10, 15, 46]. Four domains are measured: Physical Well-Being, Social/Family Well-Being, Emotional Well-Being, and Functional Well-Being. Normative data in women with breast cancer suggest that a score below 112 indicates functional impairment, with lower scores indicating more dysfunction. Two additional scales, Additional Concerns and Trial Outcome (the sum of the Physical and Functional Well-Being scales plus the Additional Concerns) are also computed.
The CES-D scale [35] is a 20-item scale of depressive symptoms. Inasmuch as the CES-D reflects cognitive and affective symptoms rather than somatic symptoms of depression, it is highly recommended for use with patients with medical problems. The range of scores on the CES-D is 0–60, with higher scores representing more symptoms of depression. The mean score for patients with a diagnosis of clinical depression is 39 [35].
Data analyses and statistical plan
The objectives of this study were to determine how much fatigue, poor sleep, depressed mood, poor functional outcome, and desynchronized rhythms women experience after the diagnosis of breast cancer but before the start of chemotherapy as well as to examine the relationships between fatigue, mood, functional outcome, sleep (subjective reports and objective findings), and circadian rhythms before the start of chemotherapy. Our prospective statistical analysis plan was to compute means and standard deviations and Spearman rank correlations. As this was both a descriptive and exploratory study, multiple statistical comparisons were computed between the different variables. To balance experiment-wise Type I and Type II error rates, results of all statistical tests are reported without a Bonferroni or other correction for multiplicity. Statistical analyses were conducted using SAS version 8.02 [38]. All statistical tests were two-sided with significance levels of 0.05 rigidly adhered to.
After the actigraphy data were automatically scored with Action 3 for sleep/wake for each minute of recording, the complete sleep/wake record for each 3-day record was analyzed by a custom SAS program that identified the start and stop time of each sleep and wake epoch and computed the duration of each epoch. The summary statistics for wake and sleep durations were computed separately for the in-bed and out-of-bed recording times, which were obtained from the patients’ diaries (time to sleep and final awakening time).
Circadian rhythms were analyzed by fitting each subject’s actigraphy data to a 5-parameter extended cosine model [30]. Briefly, the model measures the “goodness of fit” of the rhythm (how robust and consistent from day to day, i.e., synchronized, the rhythm is), the acrophase (time of day of the peak of the rhythm), the time of day when the women switched from low activity to high activity (i.e, increases from below the mean of the rhythm to above the mean called low–high), and the time of day when they switched from high activity to low activity (i.e., decreases from above the mean to below the mean of the rhythm called high–low).
There were no significant differences between women scheduled to receive adjuvant or neoadjuvant therapy in any of the subscale or total scores of the MFSI-SF, PSQI, FOSQ, or CES-D scores or on any of the objective sleep variable measures; therefore, data are reported for all women as one group.
Results
Descriptive variables
Objective sleep
Based on 3 days and nights of actigraphy, the women were asleep for an average of 6.0 h per night and napped for an average of 1.1 h per day. Table 1 lists the nighttime and daytime sleep variables. A sample wrist actigraph recording from one patient is shown in Fig. 1.
Table 1.
Sleep characteristics during the night and day (n=73)
| Mean | CI | Range | |
|---|---|---|---|
| Night | |||
| Total sleep time (h/night) | 6.0 | 5.6–6.4 | 1.2–9.6 |
| Percent sleep at night | 76 | 73–79 | 26–97 |
| Wake after sleep onset (h/night) | 1.8 | 1.6–2.0 | 0.24–7.1 |
| Percent wake at night | 24 | 21–27 | 2.9–74 |
| Number of sleep episodes/night | 59 | 51–67 | 9–203 |
| Number of wake episodes/night | 60 | 52–68 | 9–204 |
| Day | |||
| Total nap time (h/day) | 1.1 | 0.9–1.3 | 0.02–5.1 |
| Percent sleep during the day | 9 | 6.4–11.6 | 0.3–84 |
| Total wake time (h/day) | 12.2 | 11.6–12.8 | 4.0–15.8 |
| Percent wake during the day | 92 | 90–94 | 60–99.7 |
| Mean duration of naps (min) | 7.5 | 6.6–8.4 | 5–18.6 |
Fig. 1.
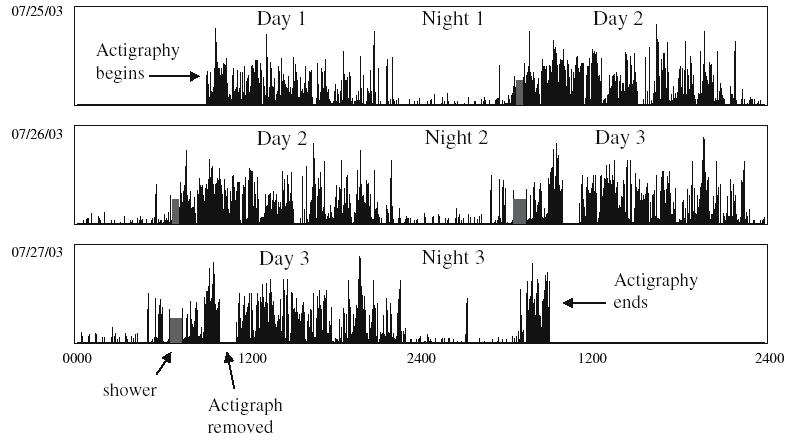
Three days of sleep/wake activity. Note that this is a double plot such that the second 24-h period (day 2) on the first line is repeated as the first 24-h period on the second line, etc
Subjective sleep
On average, the women reported disturbed sleep with a mean total PSQI score of 7.0 (CI 6.2–7.8, range 0–16). The sleep disturbance subscale and the quality of sleep subscale had the highest (most disturbed) scores. Means and confidence intervals (CIs) for the sub-scales are reported in Table 2.
Table 2.
Pittsburgh Sleep Quality Index (n=75)
| Variablea | Possible range | Mean | CI | Range |
|---|---|---|---|---|
| Subjective sleep quality | 0–3 | 1.2 | 1.0–1.4 | 0–3 |
| Sleep latency | 0–3 | 0.96 | 0.94–0.98 | 0–3 |
| Sleep duration | 0–3 | 0.75 | 0.61–0.89 | 0–3 |
| Habitual sleep efficiency | 0–3 | 0.87 | 0.65–1.09 | 0–3 |
| Sleep disturbances | 0–3 | 1.4 | 1.3–1.5 | 0–3 |
| Use of sleep medications | 0–3 | 0.96 | 0.66–1.26 | 0–3 |
| Daytime dysfunction | 0–3 | 0.77 | 0.62–0.92 | 0–2 |
| Totalb | 0–21 | 7.0 | 6.2–7.8 | 0–16 |
Subscales: subjective sleep quality is the self-evaluated global satisfaction of sleep quality; sleep latency, self-reported time it takes to fall asleep after lights out; duration, self-reported actual sleep time during night; habitual sleep efficiency, number of hours reported in sleeping divided by the number of hours reported in bed multiplied by 100; sleep disturbances, usual reasons that interfere nighttime sleep; sleep medications, the frequency of use of medicine to help sleep; and daytime dysfunction, the influence of sleepiness on daytime performance
A total score above 5 is generally considered poor sleep
Fatigue
On average, the women reported a mean total fatigue score on the MFSI-SF of 6.7 (CI 2.3–11.1, range −23–68). Scores for the subscales are presented in Table 3.
Table 3.
Multidimensional fatigue symptom inventory (n=76)
| Variablea | Possible range | Mean | CI | Range |
|---|---|---|---|---|
| Emotion | 0–24 | 5.3 | 4.2–5.4 | 0–19 |
| General | 0–24 | 5.9 | 4.6–7.2 | 0–24 |
| Mental | 0–24 | 3.7 | 2.9–4.5 | 0–17 |
| Physical | 0–24 | 2.8 | 2.2–3.4 | 0–16 |
| Vigor | 0–24 | 11.1 | 9.8–12.4 | 0–24 |
| Total scoreb | −24–96 | 6.7 | 2.3–11.1 | −23–68 |
Subscales: emotion is the feelings of emotional distress and discomfort; General, feelings of general fatigue; Mental, feeling of decreased mental ability; Physical, feeling of bodily discomfort and pain; and Vigor, feelings of alertness and vigor
Higher score indicates more fatigue, except in the Vigor scale where a higher score indicates more vigor
Functional outcome
On average, the women reported a mean total FACT-B score of 107.1 (CI 103.1–111.1, range 51–134) and a mean FOSQ total score of 18.2 (CI 17.7–18.7, range 11.5–20).
Depression
On average, there was very little depression among the women. The mean CES-D total score was 11.4 (CI 9.3–13.1, range 0–39), and the median was 12; 18% of the women had scores above 16, 8% had scores above 22, and none had scores above 39 (which would suggest clinical depression).
Circadian rhythms
All the patients who complied with the actigraphy instructions (n=65) had strong, synchronized circadian rhythms (mean F=668, CI 571–765, range 137–1946). The time of the peak of the activity rhythm, i.e., the mean acrophase, was 2:40 p.m. (CI 2:22–2:58 p.m., range 11:43 a.m.–7:41 p.m.). Analyses showed that for almost all the women, the pattern of activity could be described as a periodic alternation between low activity and high activity, with rapid transitions. The mean low–high time (i.e., time when women “got going” in the morning) was 7:13 a.m. (CI 6:54–7:32 a.m., range 4:54–11:36 a.m.). The mean high–low value (i.e., the time when women “settled down” in the evening) was 10:20 p.m. (CI 9:55–10:45 p.m., range 4:50 p.m.–3:45 a.m.).
Correlations
Objective measure of sleep with subjective measures of sleep, fatigue, and functional status
There were no significant correlations between the objective sleep variables at night or during the day with fatigue (MFSI-SF) or with functional outcome (FOSQ or FACT-B).
At night, objective total sleep time significantly correlated with the PSQI sleep duration subscale score (r=0.32, p=0.013), such that women who slept more at night reported fewer hours of sleep. During the day, total nap time (r=0.35, p=0.0058) and percent time spent napping (r=0.33, p=0.010) were significantly correlated with the PSQI sleep duration subscale score, such that women who spent more time napping during the day reported fewer hours of sleep at night. Total wake time was associated with total PSQI score (r=−0.31, p=0.013) and with the sleep disturbance (r=−0.29, p=0.017), sleep medication use (r=−0.28, p=0.030), and daytime dysfunction subscale scores (r=−0.27, p=0.035), such that women who sleep more at night reported better overall sleep quality, less sleep disturbance, less medication use, and daytime dysfunction. The number of daytime naps was significantly correlated with the sleep onset score (r=0.27, p=0.038) and with the sleep duration score (r=−0.45, p=0.0003), such that women who took more naps reported more difficulty in falling asleep and more hours of sleep at night.
Subjective measures of sleep and fatigue
There were some significant relationships between reported sleep and fatigue with women reporting poor sleep and who were also reporting fatigue. The correlation between total PSQI and total MFSI-SF was 0.46 (p<0.0001). In general, more reported disturbances on the PSQI subscales of subjective sleep quality, sleep disturbance, use of sleep medication, and daytime dysfunction were significantly correlated with more reported fatigue on all the fatigue subscales (rs ranged from 0.22 to 0.71; the exception was the lack of correlation between use of sleep medication and the MFSI-SF Emotion subscale). The PSQI subscales of sleep latency, sleep duration, and habitual sleep efficiency were not significantly correlated with any of the fatigue subscales.
Subjective measures of sleep and functional outcome
There were significant relationships between reported sleep and functional outcome secondary to cancer, with women reporting poor sleep who were also reporting that cancer affected their ability to function. Total FACT-B was significantly correlated with the total PSQI score (rs = −0.45, p<0.0001). The FACT-B was also significantly correlated with all subscales of the PSQI except sleep duration, with correlations ranging from −0.28 to −0.56 (all p<0.01).
Reports of poor sleep were also significantly related to functional outcome secondary to sleepiness. Total FOSQ was significantly correlated with total PSQI (rs = −0.22, p<0.05), with subscale correlations ranging from −0.33 to −0.55 (all p<0.01).
Fatigue and functional outcome
Correlations between fatigue (MFSI) and FOSQ showed that all the total and subscale scores of MFSI were correlated with the total and subscales of FOSQ (r=0.22–0.80, p=0.074 to <0.0001). In addition, correlations between MFSI and FACT-B showed that all the total and subscale scores of MFSI were also significantly correlated with those of all the total and subscales of FACT-B (r=0.29–0.65, p=0.019 to <0.0001), except for the Social/Family Well-Being subscale. In all cases, more severe fatigue and less vigor were correlated with worse functional outcomes.
Depression and subjective measures of sleep and fatigue and functional outcome
Although none of the women scored in the range of clinical depression on the CES-D, the CES-D total score was significantly correlated with almost all of the other subjective ratings, with more symptoms of depression associated with poorer sleep, more fatigue, and decreased functional outcome (PSQI total rs=0.47, p<0.0001; MFSI-SF total rs=0.76, p<0.0001; FACT-B total rs=−0.75, p<0.0001; FOSQ total rs=−0.41, p<0.001).
Because of these significant correlations with CES-D, stepwise regression and partial correlations were done to examine whether the relationship between subjective sleep and FACT-B might be dependent upon their relationships to depression. The results showed that poor functional outcome was related to poor sleep after controlling for depressed mood (p<0.001).
Circadian rhythms and subjective measures of sleep, fatigue, and functional outcome
Correlations were computed between the rhythm summary statistics and subjective ratings of fatigue (MFSI-SF), functional outcome (FACT-B and FOSQ), subjective reports of sleep (PSQI), and depression (CES-D). There were no significant correlations between any of the rhythm variables and fatigue (MFSI-SF), sleep (PSQI), depression (CES-D), or functional outcome of sleep (FOSQ).
Functional impairment secondary to cancer (FACT-B) was correlated with circadian rhythm variables. The results suggest that those with more functional impairment were more phase delayed as indicated by “get going” later in the morning (Functional Well-Being r=−0.26, p<0.05; Physical Well-Being r=−0.28, p<0.05), “settling down” later in the evening (i.e., switched from low to high activity later in the day and from high activity to low activity later in the evening) (Physical Well-Being r=−0.33, p<0.02; Trial Outcome Index r=−0.25, p<0.05), and having later acrophases (i.e., later peak of activity) (total FACT-B r=−0.25, p<0.05; Functional Well-Being r=−0.31, p<0.01; Physical Well-Being r=−0.36, p<0.01; Trial Outcome Index r=−0.30, p<0.05).
Discussion
Studies have shown that sleep is disturbed and fatigue levels are high during chemotherapy. The results of this study of sleep, fatigue, and circadian rhythms after the diagnosis of breast cancer but before the start of chemotherapy suggest that sleep is already disturbed and women already report fatigue before chemotherapy begins.
On average, the women were only asleep 75% of the night and spent over an hour a day napping (although not in one continuous nap). The subjective reports of sleep confirm the objective findings. Women reported poor sleep, complaining particularly about the quality of sleep and the number of sleep disturbances. The total score on the sleep questionnaire was 7.0, with normative data suggesting any score higher than 5 as being indicative of poor sleep. Although both objective and subjective reports of sleep independently indicated poor sleep, the correlations between the two measures suggested that more hours spent sleeping at night and more time spent napping during the day was associated with reports of sleeping fewer hours at night and more daytime dysfunction. However, objective and subjective sleep measures are rarely related, as patients will often overestimate how long they sleep and underestimate the time it takes them to fall asleep [14]. In addition, the time frame for the objective and subjective measures was different, inasmuch as the PSQI asks about sleep over the previous 7 days.
Although the MFSI does not report cutoff scores for defining fatigue, a study by Stein et al. [41] reported that in adults with no cancer, the mean total MFSI score was 0.85. Inasmuch as the women in this study reported levels close to 7, this suggested that women with breast cancer were already experiencing some fatigue before the start of chemotherapy. Although in most studies fatigue was examined as a side effect of chemotherapy [19, 27], several studies did report findings similar to this study on pre-treatment fatigue. Cimprich [16] used the Symptom Distress Scale [31] to measure distress symptoms during the pretreatment period of 74 women who were newly diagnosed with breast cancer and found that 90% reported some mood disturbance, 65% reported some loss of concentration, and 77% reported fatigue defined as tiredness. All three of these types of distress are included in the definition of fatigue in this study. Jacobsen et al. [26] reported that fatigue, measured with three different fatigue scales, was more severe in a pretreatment group of 54 breast cancer patients than in a comparison group of 54 non-cancer controls.
One interpretation of the increased fatigue would be that the women were depressed after learning they have cancer, and the depression manifested itself as fatigue. Increased fatigue was associated with increased symptoms of depression in this study; however, none of the women were clinically depressed. The relationship between depression and fatigue needs to be more fully explored. A second interpretation would be that the daytime fatigue is secondary to poor sleep, as there was a significant relationship between increased fatigue and reports of poor sleep. However, there were no significant correlations between the reports of fatigue and objective sleep parameters. It might be worthwhile to examine if improving women’s perception of their sleep would improve feelings of fatigue.
Women also reported that both their poor sleep and fatigue contributed to decreased functional outcome in the week prior to chemotherapy. Ganz and colleagues [20–22] have shown that quality-of-life and functional outcome are decreased during and after treatment, but our data suggest a decrement in these parameters before treatment. Significant correlations were observed between reports of poor sleep and the FACT-B instrument, which measures the impact of acknowledging a cancer diagnosis on daily functioning. Reports of poor sleep were also significantly correlated with every subscale of the FOSQ, a scale which measures daytime consequences of sleep problems.
It was surprising that objective measures of sleep did not correlate with fatigue or with any of the functional outcome measures. However, the functional tests are all global measures, whereas the objective sleep measures were specific to the day/nights recorded. Objective measures of sleep were clearly suggestive of disrupted and insufficient sleep. The women were napping during the day, which was in part likely due to disrupted sleep at night. The objective daytime napping was correlated with less satisfaction with sleep, and less satisfaction with sleep was associated with worse functional outcome. Although not conclusive, these results suggest that future trials should examine whether treating the fatigue and poor sleep before chemotherapy begins might both alleviate these symptoms and improve quality of life during chemotherapy.
The hypothesis that women with desynchronized rhythms would experience more fatigue could not be tested, as all the women had strong, synchronized rhythms, with activity alternating between high and low levels. On average, the women “got going” at about 7:13 in the morning and “settled down” at about 10:20 in the evening, with the peak of activity occurring at 2:40 in the afternoon. There was no relationship between the circadian rhythm variables and subjective sleep variables, fatigue ratings, or daytime consequences of disturbed sleep. However, the functional outcome secondary to cancer was related to several circadian rhythm features. Those breast cancer patients with worse functional outcome had activity rhythms that peaked later in the day and switched from high activity to low activity (became less active) later in the afternoon as well as from low activity to high activity (became more active) later in the morning, suggesting that the women were more phase delayed (their biological clock was delayed compared to the environment). Future research to examine whether phase advancing these women would also improve their functional outcome is warranted.
One limitation of this study is the lack of a control group. An interesting replication would include a comparison with a group of women with breast cancer who did not plan on having chemotherapy or a group who were age-matched and did not have breast cancer. A potential second limitation was, as mentioned above, that this was both a descriptive and exploratory study; therefore, multiple statistical comparisons were computed. Results of all statistical tests were reported in an attempt to balance experiment-wise Type I and Type II error rates. However, no Bonferroni or other correction for multiplicity was performed.
A third limitation of this study was that information about the length of time from surgery to the start of chemotherapy was not available for all women and therefore could not be included as a covariate. It is possible that those still recovering from surgery experienced more fatigue and poorer sleep. Nevertheless, the conclusions remain the same regardless of the reason for the fatigue and poor sleep. Clinicians may want to consider beginning treatment of these complaints before the start of chemotherapy.
In summary, this was the first descriptive study to examine sleep and fatigue before the start of chemotherapy in women with breast cancer. Previous investigations have shown that women with breast cancer undergoing chemotherapy experience both disturbed sleep and increased fatigue [18, 26, 37, 40, 41, 45]. The data from the current study showed that the women experienced both disturbed sleep and fatigue before they began chemotherapy. This does not suggest that chemotherapy does not contribute to fatigue, but rather that being fatigued before chemotherapy even begins might make fatigue during chemotherapy worse. Although their circadian rhythms were in general robust, those breast cancer patients with more delayed circadian rhythms experienced more daily dysfunction. We are currently exploring how these factors change once chemotherapy begins. These data suggest that strategies to improve disturbed sleep and to phase-advance circadian rhythms prior to initiation of chemotherapy may be beneficial in improving daily function in breast cancer patients.
Acknowledgments
Supported by NCI CA85264, NIA AG08415, NCI R25 CA 65745, the UCSD General Clinical Research Center (MO1-RR00827), the Rebecca and John Moores UCSD Cancer Center (NCI P30 CA-23100), the Department of Veterans Affairs VISN-22 Mental Illness Research, Education and Clinical Center (MIRECC), and the Research Service of the Veterans Affairs San Diego Healthcare System.
References
- 1.Ancoli-Israel S, Clopton P, Klauber MR, Fell R, Mason WJ. Use of wrist activity for monitoring sleep/wake in demented nursing home patients. Sleep. 1997;20:24–27. doi: 10.1093/sleep/20.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ancoli-Israel S, Cole R, Alessi CA, Chambers M, Moorcroft WH, Pollak C. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26:342–392. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- 3.Ancoli-Israel S, Moore P, Jones V. The relationship between fatigue and sleep in cancer patients: a review. Eur J Cancer Care. 2001;10:245–255. doi: 10.1046/j.1365-2354.2001.00263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson KO, Getto CJ, Mendoza TR, Palmer SN, Wang XS, Reyes-Gibby CC, Cleeland C. Fatigue and sleep disturbance in patients with cancer, patients with clinical depression, and community-dwelling adults. J Pain Symptom Manage. 2003;25:307–318. doi: 10.1016/s0885-3924(02)00682-6. [DOI] [PubMed] [Google Scholar]
- 5.Andrykowski MA, Curran SL, Lightner R. Off-treatment fatigue in breast cancer survivors: a controlled comparison. J Behav Med. 1998;21:1–18. doi: 10.1023/a:1018700303959. [DOI] [PubMed] [Google Scholar]
- 6.Berger AM. Patterns of fatigue and activity and rest during adjuvant breast cancer chemotherapy. Oncol Nurs Forum. 1998;25:51–62. [PubMed] [Google Scholar]
- 7.Berglund G, Bolund C, Fornander T, et al. Late effects of adjuvant chemotherapy and postoperative radiotherapy on quality of life among breast cancer patients. Cancer. 1991;27:1075–1081. doi: 10.1016/0277-5379(91)90295-o. [DOI] [PubMed] [Google Scholar]
- 8.Beszterczey A, Lipowski ZJ. Insomnia in cancer patients. Can Med Assoc J. 1977;116:355. [PMC free article] [PubMed] [Google Scholar]
- 9.Bower JE, Ganz PA, Desmond KA, Rowland JH, Meyerowitz BE, Belin TR. Fatigue in breast cancer survivors: occurrence, correlates, and impact on quality of life. J Clin Oncol. 2000;18:743–753. doi: 10.1200/JCO.2000.18.4.743. [DOI] [PubMed] [Google Scholar]
- 10.Brady MJ, Cella DF, Mo F, Bonomi AE, Tulsky DS, Lloyd SR, Deasy S, Cobleigh M, Shiomoto G. Reliability and validity of the functional assessment of cancer therapy–B (FACT-B) quality of life instrument. J Clin Oncol. 1997;15:974–986. doi: 10.1200/JCO.1997.15.3.974. [DOI] [PubMed] [Google Scholar]
- 11.Broeckel J, Jacobsen PB, Horton J, Balducci L, Lyman GH. Characteristics and correlates of fatigue after adjuvant chemotherapy for breast cancer. J Clin Oncol. 1998;16:1689–1696. doi: 10.1200/JCO.1998.16.5.1689. [DOI] [PubMed] [Google Scholar]
- 12.Buysse DJ, Reynolds CFI, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 13.Buysse DJ, Reynolds CRI, Monk TH, Hoch CC, Yeager AL, Kupfer DJ. Quantification of subjective sleep quality in healthy elderly men and women using the Pittsburgh Sleep Quality Index (PSQI) Sleep. 1991;14 (4):331–338. [PubMed] [Google Scholar]
- 14.Carskadon MA, Dement WC, Mitler MM, Guilleminault C, Zarcone VP, Spiegel R. Self-reports versus sleep laboratory findings in 122 drug-free subjects with complaints of chronic insomnia. Am J Psychiatr. 1976;133:1382–1388. doi: 10.1176/ajp.133.12.1382. [DOI] [PubMed] [Google Scholar]
- 15.Cella DF. Quality of life outcomes: measurement and validation. Oncology. 1996;10:233–246. [PubMed] [Google Scholar]
- 16.Cimprich B. Pretreatment symptom distress in women newly diagnosed with breast cancer. Cancer Nurs. 1999;22:185–194. doi: 10.1097/00002820-199906000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Cole RJ, Kripke DF, Gruen W, Mullaney DJ, Gillin JC. Automatic sleep/wake identification from wrist activity. Sleep. 1992;15:461–469. doi: 10.1093/sleep/15.5.461. [DOI] [PubMed] [Google Scholar]
- 18.de Jong N, Candel MJ, Schouten HC, Abu-Saad HH, Courtens AM. Prevalence and course of fatigue in breast cancer patients receiving adjuvant chemotherapy. Ann Oncol. 2004;15:896–905. doi: 10.1093/annonc/mdh229. [DOI] [PubMed] [Google Scholar]
- 19.de Jong N, Courtens AM, Abu-Saad HH, Schouten HC. Fatigue in patients with breast cancer receiving adjuvant chemotherapy: a review of the literature. Cancer Nurs. 2002;25:283–297. doi: 10.1097/00002820-200208000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Ganz P, Coscarelli A, Fred C, et al. Breast cancer survivors: psychosocial concerns and quality of life. Breast Cancer Res Treat. 1996;38:183–199. doi: 10.1007/BF01806673. [DOI] [PubMed] [Google Scholar]
- 21.Ganz PA, Day R, Ware JE, Redmond C, Fisher B. Base-line quality-of-life assessment in the National Surgical Adjuvant Breast and Bowel Project Breast-Cancer Prevention Trial. J Natl Cancer Inst. 1995;87:1372–1382. doi: 10.1093/jnci/87.18.1372. [DOI] [PubMed] [Google Scholar]
- 22.Ganz PA, Kwan L, Stanton AL, Krupnick JL, Rowland JH, Meyerowitz BE, Bower JE, Belin TR. Quality of life at the end of primary treatment of breast cancer: first results from the moving beyond cancer randomized trial. J Natl Cancer Inst. 2004;96:376–387. doi: 10.1093/jnci/djh060. [DOI] [PubMed] [Google Scholar]
- 23.Glaus A. Fatigue in patients with cancer: analysis and assessment. Recent Results Cancer Res. 1998;145:1–172. [PubMed] [Google Scholar]
- 24.Holland JC, Plumb M. A comparative study of depressive symptoms in patients with advanced cancer. Proc Am Assoc Cancer Res. 1977;18:201. [Google Scholar]
- 25.Hu D, Silberfarb PM. Management of sleep problems in cancer patients. Oncology. 1991;5:23–27. [PubMed] [Google Scholar]
- 26.Jacobsen PB, Hann DM, Azzarello LM, Horton J, Balducci L, Lyman GH. Fatigue in women receiving adjuvant chemotherapy for breast cancer: characteristics, course, and correlates. J Pain Symptom Manage. 1999;18:233–242. doi: 10.1016/s0885-3924(99)00082-2. [DOI] [PubMed] [Google Scholar]
- 27.Jacobsen PB, Stein K. Is fatigue a long-term side effect of breast cancer treatment? Cancer Control. 1999;6:256–263. doi: 10.1177/107327489900600304. [DOI] [PubMed] [Google Scholar]
- 28.Kaye J, Kaye K, Madow L. Sleep patterns in patients with cancer and patients with cardiac disease. J Psychol. 1983;114:107–113. doi: 10.1080/00223980.1983.9915403. [DOI] [PubMed] [Google Scholar]
- 29.Knobf MT. Physical and psychological distress associated with adjuvant chemotherapy in women with breast cancer. J Clin Oncol. 1986;4:678–684. doi: 10.1200/JCO.1986.4.5.678. [DOI] [PubMed] [Google Scholar]
- 30.Martin J, Marler MR, Shochat T, Ancoli-Israel S. Circadian rhythms of agitation in institutionalized patients with Alzheimer’s disease. Chronobiol Int. 2000;17:405–418. doi: 10.1081/cbi-100101054. [DOI] [PubMed] [Google Scholar]
- 31.McCorkle R, Young K. Development of a symptom distress scale. Cancer Nurs. 1978;1:373–378. [PubMed] [Google Scholar]
- 32.Miaskowski C, Lee KA. Pain, fatigue and sleep disturbances in oncology outpatients receiving radiation therapy for bone metastasis: a pilot study. J Pain Symptom Manage. 1999;17:320–332. doi: 10.1016/s0885-3924(99)00008-1. [DOI] [PubMed] [Google Scholar]
- 33.Mormont MC, De Prins J, Levi F. Assessment of activity rhythms by wrist actigraphy: preliminary results in 30 patients with colorectal cancer. Biol Rhythm Res. 1995;6:423. [Google Scholar]
- 34.Pater JL, Zee B, Palmer M, Johnston D, Osoba D. Fatigue in patients with cancer: results with National Cancer Institute of Canada Clinical Trials Group studies employing the EORTC QLQ-C30. Support Care Cancer. 1997;5:410–413. doi: 10.1007/s005200050100. [DOI] [PubMed] [Google Scholar]
- 35.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 36.Redeker NS, Lev EL, Ruggiero J. Insomnia, fatigue, anxiety, depression, and quality of life of cancer patients undergoing chemotherapy. Sch Inq Nurs Pract. 2000;14:275–290. [PubMed] [Google Scholar]
- 37.Richardson A. Fatigue in cancer patients: a review of the literature. Eur J Cancer Care. 1995;4:20–32. doi: 10.1111/j.1365-2354.1995.tb00049.x. [DOI] [PubMed] [Google Scholar]
- 38.SAS Institute Inc. (1999) SAS/STAT User’s Guide, Version 8. 1–3884
- 39.Silberfarb PM, Hauri PJ, Oxman TE, Schnurr PP. Assessment of sleep in patients with lung cancer and breast cancer. J Clin Oncol. 1993;11:997–1004. doi: 10.1200/JCO.1993.11.5.997. [DOI] [PubMed] [Google Scholar]
- 40.Smets EMA, Garssen B, Cull A, de Haes JC. Application of the multidimensional fatigue inventory in cancer patients receiving radiotherapy. Br J Cancer. 1996;73:241–245. doi: 10.1038/bjc.1996.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stein KD, Martin SC, Hann DM, Jacobsen PB. A multidimensional measure of fatigue for use with cancer patients. Cancer Pract. 1998;6:143–152. doi: 10.1046/j.1523-5394.1998.006003143.x. [DOI] [PubMed] [Google Scholar]
- 42.Strang P, Qvarner H. Cancer-related pain and its influence on quality of life. Anticancer Res. 1990;10:109–112. [PubMed] [Google Scholar]
- 43.Visser MRM, Smets EMA. Fatigue, depression and quality of life in cancer patients: how are they related? Support Care Cancer. 1998;6:101–108. doi: 10.1007/s005200050142. [DOI] [PubMed] [Google Scholar]
- 44.Weaver TE, Laizner AM, Evans LK, Maislin G, Chugh DK, Lyon K, Smith PL, Schwartz AR, Redline S, Pack AI, Dinges DF. An instrument to measure functional status outcome for disorders of excessive sleepiness. Sleep. 1999;20:835–843. [PubMed] [Google Scholar]
- 45.Winningham ML, Nail LM, Burke MB, Brophy L, Cimprich B, Jones LS, Pickard-Holley S, Rhodes V, Pierre B, Beck S, Glass EC, Mock VL, Mooney KH, Piper B. Fatigue and the cancer experience; the state of the knowledge. Oncol Nurs Forum. 1994;21:23–36. [PubMed] [Google Scholar]
- 46.Yellen BY, Cella DF, Webster K, Blendowski C, Kaplan E, Sarokhan B. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manage. 1997;13:63–74. doi: 10.1016/s0885-3924(96)00274-6. [DOI] [PubMed] [Google Scholar]


