Abstract
Tropical lowland areas have often been seen as the centres of terrestrial species proliferation, but recent evidence suggests that young species may be more frequent in montane areas. Several montane speciation modes have been proposed, but their relative frequencies and predominant evolutionary sequence remain unclear because so few biogeographic and phylogenetic studies have tested such questions. I use morphological data to generate a phylogenetic hypothesis for all 11 species of the riodinid butterfly genus Ithomiola (Riodininae: Mesosemiini: Napaeina). These species are shown here to be all strictly geographically and elevationally allo- or parapatrically distributed with respect to their closest relatives in lowland and montane regions throughout the Neotropics. The overwhelming pattern in Ithomiola is of repeated upward parapatric speciation across an elevational gradient, and the genus appears to provide the clearest example to date of vertical montane speciation. All of the young derived species are montane and all of the old basal species are confined to the lowlands, supporting the hypothesis of montane regions largely as ‘species pumps’ and lowland regions as ‘museums’. Possible reasons for the post-speciation maintenance of parapatric ranges in Ithomiola are discussed.
Keywords: biogeography, Ithomiola, montane, parapatric speciation, Riodinidae, vertical speciation
1. Introduction
Tropical montane forests remain one of the last frontiers in an effort to inventory and conserve the world's terrestrial biodiversity (Churchill et al. 1995), but only recently has their full potential role in generating that diversity begun to be investigated (Roy et al. 1997). The study of geographic speciation patterns in the tropics has traditionally focused on the lowlands, the area often viewed as the main centre of historical species proliferation, and numerous mechanisms, such as the refuge hypothesis (Haffer 1969; Whitmore & Prance 1987), have been proposed to explain the patterns of species distributions we see today (Haffer 1997). However, recent evidence from Afrotropical and Neotropical birds that young species predominantly occur in montane areas, and old species in lowland areas (Fjeldså 1994), suggests that the tropical montane regions of the world may more frequently be acting as the ‘species pumps’, with the lowlands functioning in part as ‘museums’, where dispersed older species accumulate (Fjeldså 1994; Fjeldså & Lovett 1997; Roy et al. 1997). Clearly, being able to distinguish between speciation centres and areas of species accumulation has considerable implications for several fields, particularly conservation (Fjeldså 1994).
To gain a better understanding of current species richness patterns, it is necessary to go beyond the simple examination of species distributions (Vuilleumier & Monasterio 1986), and harness the explanatory power of phylogenies (Barraclough & Vogler 2000). With a robust phylogenetic hypothesis for a diverse monophyletic taxon containing a mixture of lowland and montane species, coupled with detailed geographic and elevational range data, it is possible to test and assess the relative importance of the five main proposed montane speciation modes. A tropical montane fauna can evolve through upward or downward speciation across an elevational gradient, horizontal speciation within or between mountain chains or colonization from a higher (subtropical to temperate) latitude (Chapman 1917; Willmott et al. 2001). As collating the necessary taxonomic and biogeographic data is difficult, few studies have explicitly tested these hypotheses, and almost all of those that have used molecular data on plants (Knox & Palmer 1995), birds (Bates & Zink 1994; Arctander & Fjeldså 1994; Roy 1997; García-Moreno et al. 1998) or other vertebrates (Patton & Smith 1992).
In this paper, I use morphological data to generate a phylogenetic hypothesis for the Neotropical riodinid butterfly genus Ithomiola, one of six genera in the subtribe Napaeina (figure 1a), nested in the Mesosemiini, the most basal tribe in the speciose Riodininae (Hall 2003). I have then used the phylogeny to reconstruct the geographic and elevational radiation of the group, test the prevalence of the above montane speciation modes and discuss the maintenance of parapatric ranges. This genus of 11 species has recently been revised by myself (Hall in press), critically ensuring that the units of speciation being used in this analysis are taxonomically sound and irreducible (Riddle & Hafner 1999). Three species are confined to Central America and the western Andes, five to the eastern Andes, two largely to the Amazon, and one to southeastern South America. Three are confined to the lowlands, six to montane habitats and two share both life zones. Importantly, prior to detailed study, most species seemed to be allo- or parapatrically distributed, indicating minimal post-speciation dispersal might have occurred in the genus to obscure speciation patterns (Lynch 1989; Chesser & Zink 1994).
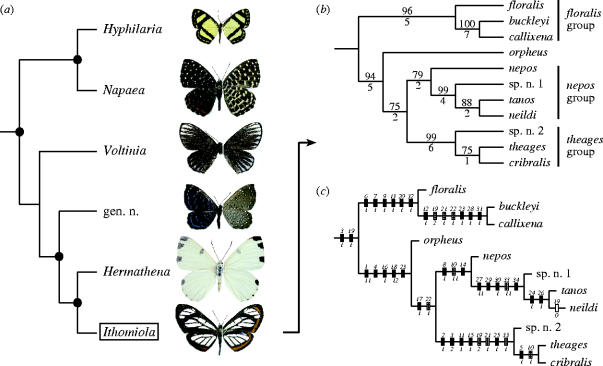
Figure 1.
(a) Generic-level phylogenetic hypothesis for the Napaeina (Riodinidae: Riodininae: Mesosemiini) (Hall 2003, in press), with males of type species shown to the right (Hyphilaria nicia, Napaea eucharila, Voltinia radiata, ‘Cremna’ alector, Hermathena candidata and Ithomiola floralis floralis; dorsal surface at left, ventral surface at right); nodes with a reported bootstrap value of 70 or higher and/or a decay index value of two or higher are indicated with a black circle. (b) Single most parsimonious cladogram resulting from the exhaustive analysis of 34 morphological characters for all 11 species of Ithomiola (see tables 1 and 2); branch support is given as bootstrap values higher than 50 above branches and decay index values below branches. (c) Cladogram from (b), illustrating the distribution of character states, with black bars indicating unique apomorphies, striped bars homoplasious apomorphies, and white bars reversals.
2. Material and Methods
(a) Phylogenetic analysis
The phylogenetic analysis was based on morphological characters derived from the adult body, wings, and male and female genitalia. With only three Ithomiola species reared to date (Hall in press), no characters of the immature stages could meaningfully be included. Phylogenetically uninformative autapomorphies were excluded. All the characters were equally weighted and unordered. The maximum parsimony analysis was performed using an exhaustive search in PAUP 4.0b4a (Swofford 2000). Branch support was estimated by performing 1000 bootstrap replicates in PAUP (Felsenstein 1985) and by calculating decay indices (Bremer 1988) using Autodecay 4.0 (Eriksson 1998) in combination with PAUP.
The analysis included all 11 species treated by Hall (in press) in Ithomiola. Heretofore, the name Ithomiola has been applied exclusively to the three members of the monophyletic floralis group (Callaghan & Lamas 2004), but adding nepos and relatives (formerly treated in Napaea) creates a more meaningful evolutionary unit (Hall 2003, in press). Hermathena is the sister genus to Ithomiola (Hall 2003, in press), but the wing patterns of its species are so highly apomorphic (figure 1a) that it would rarely have been possible to determine homology and polarity in wing pattern characters. Therefore, the sole member of the sister genus (currently without a name) to the Hermathena + Ithomiola clade (Hall in press), alector, is used as the outgroup taxon.
(b) Field and museum research
Ithomiola specimens were examined in 16 European and American collections to complete the taxonomic revision of the genus (full list in Hall in press) and to record geographic and elevational range data. The majority of the precise elevational data required for this study, however, were recorded during 16 months of field work over a 14-year period throughout Ecuador, which harbours nine of the 11 known Ithomiola species. During the course of this Ecuadorian research, two new Ithomiola species, I. buckleyi and I. neildi, were discovered and described (Hall & Willmott 1998), and a third, I. sp. n. 1, was recognized as being distinct from I. tanos (Hall in press).
3. Results of phylogenetic analysis
Thirty-four characters were identified (table 1) from the adult body (5), wing shape and venation (18), and male (8) and female (3) genitalia (data matrix in table 2). I found relatively little codable genital variation, especially in females, and those genital characters that I was able to code only helped in resolving the most distal nodes. Therefore, the phylogenetic hypothesis presented here is based predominantly on external characters of the wings and body. The exhaustive search generated a single most parsimonious cladogram with a length of 43 steps, a consistency index of 0.86 and a retention index of 0.92. This cladogram is shown in figure 1b and c, with the distribution of characters and their states mapped onto the latter.
Table 1.
The 34 characters of adult morphology used in the phylogenetic analysis.
| body | ||
| 1. four white spots on dorsum of thorax | (0) absent; (1) present. CI=1; RI=1 | |
| 2. a tuft of long androconial setae attached to inner distal tip of a shortened tibia of male hindleg and held in a pouch along inner edge of a lengthened first tarsal segment | (0) present; (1) absent. CI=1; RI=1. The presence of long androconial setae, or hairpencils, on the male hindleg of certain Napaeina species was only reported recently (Hall & Harvey 2002b). They occur in eight species of Ithomiola, all three Hermathena species, Hyphilaria thasus and ‘Cremna’ alector (Hall 2003, in press). Such leg hairpencils are not known elsewhere in the Papilionoidea, or true butterflies | |
| 3. a tibial spur on male hindleg | (0) absent; (1) small; (2) large. CI=1; RI=1. Note that the presence of a large instead of a small tibial spur is probably correlated with the absence of a hairpencil socket in the same distal region of the tibia | |
| 4. pale rings around abdomen | (0) absent; (1) present. CI=1; RI=1 | |
| 5. blue scaling at tip only of male abdomen | (0) absent; (1) present. CI=1; RI=1 | |
| wing venation and pattern | ||
| 6. forewing discal cell | (0) less than half length of forewing; (1) more than half length of forewing. CI=1; RI=1 | |
| 7. markings in forewing discal cell | (0) narrow vertical bars; (1) enlarged and laterally elongated spots. CI=1; RI=1 | |
| 8. rufous-brown tinge to ground colour of dorsal forewing | (0) absent; (1) present. CI=1; RI=1 | |
| 9. hyaline wing markings | (0) absent; (1) present. CI=1; RI=1 | |
| 10. contrasting purple colouration within otherwise white basal spots of dorsal forewing in male | (0) absent; (1) present. CI=0.5; RI=0.8 | |
| 11. pale markings immediately distal to forewing discal cell | (0) absent or a narrow bar (entire or medially divided); (1) enlarged into a big round spot. CI=1; RI=1 | |
| 12. continuous pale blue scaling along anal margin of dorsal forewing | (0) absent; (1) present. CI=1; RI=1 | |
| 13. pale postdiscal marking in forewing cell Cu1 | (0) round or square; (1) elongate and rectangular. CI=1; RI=1 | |
| 14. pale postdiscal marking in forewing cell M3 | (0) approximately same size as that in cell Cu1 (no less than about half of its size); (1) considerably smaller than that in cell Cu1 (only a tiny fraction of its size). CI=1; RI=1 | |
| 15. a pale postdiscal spot toward base of dorsal forewing cell M1 in male | (0) present; (1) absent. CI=1; RI=1 | |
| 16. costal four spots of forewing post-discal band | (0) in an approximate straight line; (1) variably jagged. CI=1; RI=1 | |
| 17. prominent black rays proximal to anal and costal series of postdiscal spots on ventral forewing | (0) absent; (1) present. CI=1; RI=1 | |
| 18. white submarginal spots along entire margin of dorsal forewing | (0) absent; (1) present. CI=1; RI=1 | |
| 19. pale area on dorsal hindwing | (0) absent; (1) does extend to or near distal margin; (2) does not extend to or near distal margin. CI=0.5; RI=0.6 | |
| 20. black venal stripes crossing pale area on dorsal hindwing | (0) absent; (1) present. CI=1; RI=1. As ‘Cremna’ alector and I. neildi have no pale area on the dorsal hindwing, they are coded with a ‘?’ | |
| 21. a distal chalk-blue patch on dorsal hindwing | (0) absent; (1) present. CI=0.5; RI=0.75 | |
| 22. bluish-white scaling across base of ventral hindwing in male | (0) absent; (1) present. CI=0.5; RI=0.5 | |
| 23. hindwing fringe | (0) brown; (1) brown with prominent white in apex only; (2) checkered black and white. CI=1; RI=1 | |
| male genitalia | ||
| 24. smoothly upturned, ‘sickle’-shaped lower posterior valve process | (0) absent; (1) present. CI=1; RI=1 | |
| 25. lower posterior valve process projecting from centre of posterior valve margin, with dorsal margin of process intruding into valve centre | (0) absent; (1) present. CI=1; RI=1 | |
| 26. inner margin of lower posterior valve process | (0) smooth; (1) angular and connected to a small point before upper posterior process. CI=1; RI=1 | |
| 27. upper posterior valve process extending posteriorly at least half distance of a lower posterior valve process | (0) absent; (1) present. CI=1; RI=1 | |
| 28. an abruptly downturned posterior tip to aedeagus | (0) absent; (1) present. CI=1; RI=1 | |
| 29. a paired row of parallel cornuti along lateral margins of everted vesica | (0) absent; (1) present. CI=1; RI=1 | |
| 30. a large dorsal patch of tiny spines at base of everted vesica | (0) absent; (1) present. CI=1; RI=1 | |
| 31. a dorsal section at base of everted vesica with each hardened wavy line ending in a small anteriorly directed sclerotized spine | (0) absent; (1) present. CI=1; RI=1 | |
| female genitalia | ||
| As I have not been able to examine the female genitalia of I. buckleyi and I. tanos, these species receive a ‘?’ for all three of the following characters | ||
| 32. signa | (0) approximately symmetrically placed; (1) asymmetrically placed. CI=1; RI=1 | |
| 33. signa | (0) gradually tapered and spine-like; (1) laterally flattened and blade-like. CI=0.5; RI=0.75 | |
| 34. a long and straight, sclerotized, posterior section to ductus bursae | (0) absent; (1) present. CI=1; RI=1 | |
Table 2.
Data matrix used in the phylogenetic analysis.
| Taxon | 1 | 1 | 2 | 2 | 3 | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5 | 0 | 5 | 0 | 5 | 0 | |||||||||||||||||||||||||||||
| alector | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ? | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| floralis | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| buckleyi | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | ? | ? | ? |
| callixena | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 |
| orpheus | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| nepos | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| sp. n. 1 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 |
| tanos | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 2 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | ? | ? | ? |
| neildi | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 1 | 0 | ? | 0 | 1 | 2 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 |
| sp. n. 2 | 1 | 1 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 2 | 0 | 1 | 1 | 2 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| theages | 1 | 1 | 2 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 2 | 0 | 1 | 1 | 2 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| cribralis | 1 | 1 | 2 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 2 | 0 | 1 | 1 | 2 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
Ithomiola consists of three well supported monophyletic groups (indicated in figure 1b), plus I. orpheus, related as follows: floralis group + (orpheus + (nepos group + theages group)). Each clade has a bootstrap value of 75 or higher and a decay index value of two or higher, as do all but one of the other nodes, indicating that this phylogenetic hypothesis is sufficiently robust to form the foundation for further evolutionary and biogeographical analysis.
4. Discussion
(a) Montane speciation
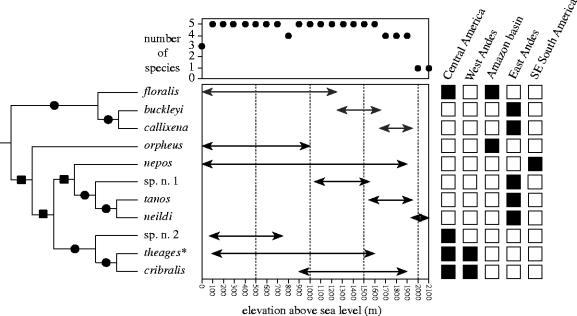
Every Ithomiola species was found to be allo- or parapatrically distributed with respect to its closest relative (figure 2). Ithomiola orpheus and I. nepos are geographically allopatric (18% of Ithomiola species), as are the nepos and theages groups, and the remaining Ithomiola species are all elevationally parapatric (82%). In other words, allopatric speciation seems to be indicated for the three potentially oldest cladogram nodes, and parapatric speciation for all the younger nodes.
Figure 2.
Patterns of geographic and elevational diversity in Ithomiola. The symbols at each cladogram node indicate whether the species in the two clades are geographically allopatric (squares) or elevationally parapatric (circles). The asterisk beside Ithomiola theages signifies that its elevational range varies geographically (see figure 4).
The five ways in which montane biotas can evolve that were outlined in the introduction produce differing phylogeographic predictions. Based on the phylogenetic and biogeographic data analysed here (figure 2), only two of these modes seem to have been involved in Ithomiola diversification. If the phylogenetic hypothesis is correct, the three most derived nepos group species in the eastern Andes were derived not from a lowland Amazonian ancestor, but from a southeast South American ancestor like I. nepos (I. nepos itself ranges from 0–1900 m in southeastern Brazil, southeastern Argentina and Paraguay). The current parapatric distributions of I. orpheus and I. sp. n. 1 are therefore misleading, and an I. nepos-like ancestor and its descendant(s) appear in the past to have moved from the slightly cooler southern environment north into the Andes along an altitudinal corridor of similar climate, and upon secondarily encountering an I. orpheus-like species become confined to elevations above 1000 m. This scenario essentially constitutes colonization from a higher latitude.
When recurrent patterns of vertical stratification across mountain slopes are identified, the elevationally neighbouring species are often assumed to be closely related, and each vertical group thought to be a monophyletic clade. However, the phenomenon is often the result of more recent horizontal speciation (Adams 1985), which creates a paraphyletic group. Horizontal speciation, either within or between montane regions, does not appear to have contributed to Ithomiola diversification. Explicit phylogeographic studies on altitudinally stratified sigmodontine mice in the Peruvian Andes (Patton & Smith 1992) and tapaculos (birds) in the Ecuadorian Andes (Arctander & Fjeldså 1994) showed that horizontally, not vertically, adjacent populations and species in the same elevational zone were sister entities. Strict serial elevational stratification in butterflies is uncommon, and has previously been well documented only in the nymphalid subfamily Satyrinae (Pronophilina). As in the above vertebrate examples, although vertical speciation must surely have occurred at some earlier point during the evolutionary history of the group, pronophiline sister species typically appear to occupy the same elevational band in neighbouring regions of an extended mountainous range, or in a disjunct cordillera (Adams 1985; Viloria 1998; Pyrcz & Wojtusiak 2002). Phylogenies are needed, however, to explicitly test these observations and hypotheses. Such horizontal speciation is believed to be a product of dispersal subsequent to historical climate induced elevational fluctuations in vegetation zones, which may have ‘migrated’ up and down the Andes by as much as 1500 m (Van der Hammen 1974).
The overwhelming pattern discernible in Ithomiola is of vertical speciation, presumably as parapatric speciation across an elevational gradient (Endler 1977), one elevational band at a time, following or concomitant with orogenic cycles of uplift. Either way, modelling shows that the narrow geographic and elevational ranges typical of montane Ithomiola species, especially when coupled with small population sizes, low dispersal rates and the presence of an environmental gradient, would have accelerated the speciation process (Gavrilets et al. 2000; Doebell & Dieckmann 2003). From the few published montane phylogeographic studies, there are examples of both downward speciation, generally from open highland areas or elfin forest down into middle elevation montane forest, in East African senecio plants (Knox & Palmer 1995), Andean chat-tyrants (birds) (García-Moreno et al. 1998) and Hypanartia nymphalid butterflies (Willmott et al. 2001), and upward speciation from the lowlands into montane forest, in east African greenbuls (birds) (Roy 1997) and Andean flycatchers (birds) (Bates & Zink 1994). The utility of most of these studies, however, is compromised by combinations of insufficiently well resolved phylogenies, incomplete or imprecise elevational range data, and conclusions based on more vague species group rather than species level patterns and speciation events tied more to specific microhabitat adaptations than to elevation per se. Only the flycatcher study (Bates & Zink 1994) presents a fully resolved phylogeny that points to a clear species level pattern, but even here the elevational ranges partially overlap, the example is of only a single potential triple serial replacement, and the outgroup is uncertain. Ithomiola apparently represents one of the clearest examples to date of vertical montane speciation, in large part because the terminal clades appear to be relatively young, and post-speciation dispersal has been minimal. The detailed pattern of elevational distributions and speciation is still there to be seen. Although uplift of the southern Andes was perhaps completed prior to the Tertiary, the northern Andes are not believed to have achieved heights above 1000 m until the Mid-Pliocene (Simpson 1979; Gregory-Wodzicki 2000). The Andean members of the floralis and nepos groups are thus probably no older than 4 or 5 million years.
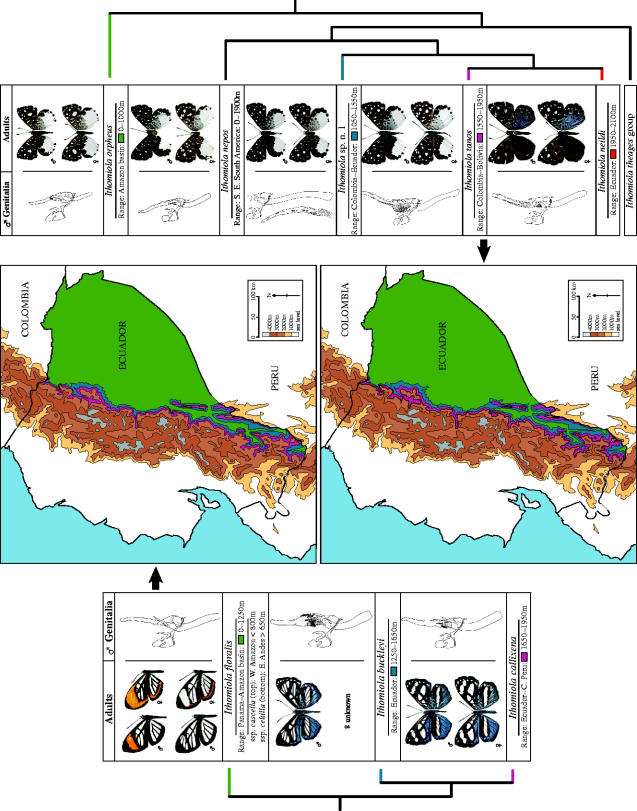
The cladogram and elevational data in figure 2 provide good evidence to suggest that both the floralis and nepos groups diversified primarily through upward speciation (see figure 3). In the floralis group, there seem to have been two upward speciation events. The proto-Ithomiola ancestor to the floralis group is presumed to have been a lowland species because the three highly derived Hermathena species range from 500–2100 m, and the next most basal taxa, ‘Cremna’ alector and Voltinia (figure 1a), all occupy the lowlands (Hall in press). The sister species I. buckleyi (1250–1650 m) and I. callixena (1650–1950 m) therefore certainly seem to have evolved from a lowland ancestor similar to I. floralis (0–1250 m). Although it is not possible to determine the original elevational range of the ancestor of I. buckleyi and I. callixena, the most parsimonious scenario is colonization of middle elevations adjacent to the range of the lowland ancestor, followed by subsequent speciation into upper elevations, producing I. callixena. Using similar reasoning, there appears to have been three upward speciation events in the nepos group. The cladogram indicates that I. sp. n. 1 (1050–1550 m), I. tanos (1550–1950 m) and I. neildi (1950–2100 m) evolved from a lowland ancestor similar to I. nepos and I. orpheus, and that the sister species pair of I. tanos and I. neildi most plausibly evolved from a mid elevation ancestor. Again, although the original elevations of ancestral species are indeterminate, it also seems most parsimonious to conclude that I. neildi evolved through upward speciation. The evolution of the theages group appears to have been a more convoluted process, but in broad outline I hypothesize that after an I. orpheus-like ancestor dispersed from the Amazon basin into and throughout much of Central America, there was an initial vicariant speciation event centred around the Panama Canal Zone, followed ultimately by the evolution of the upland I. cribralis from an I. theages-like ancestor by upward speciation (see figure 4).
Figure 3.
Elevational parapatry in the I. floralis group (left and top) and the nepos group +I. orpheus (right and bottom). Thumbnails in phylogenetic sequence of adults (dorsal surface at left, ventral surface at right) and their male genitalia (vertically positioned in lateral view) allow identification and illustrate most characters in the phylogenetic analysis. Female genitalia provide many fewer characters for either purpose and, although omitted here, are illustrated by Hall (in press). The known geographic and elevational range of each species is indicated. To better visualize the parapatric ranges of these species, the colour-coded elevational bands for each species (except I. nepos) are marked on maps of Ecuador, the only country from which they have almost all been recorded. Their geographic ranges within Ecuador have been extrapolated for effect, but all species are known from more-or-less throughout the zones indicated except I. buckleyi, which has been recorded to date from only the southern half of the country (see range maps in Hall in press).
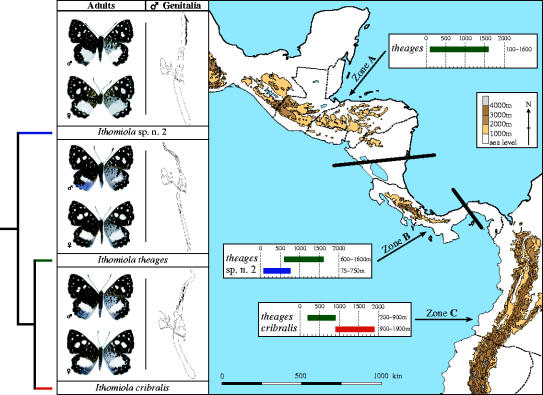
Figure 4.
Elevational parapatry and geographic variation in elevational range in the I. theages group. Thumbnails of adults (dorsal surface at left, ventral surface at right) and their male genitalia (vertically positioned in lateral view) allow identification and illustrate most characters in the phylogenetic analysis. The map of Central America and adjoining areas encompasses the known geographic ranges of the three species (see range maps in Hall in press), and the two black bars demarcate three zones, A to C, where different combinations of species are macrosympatric. When I. theages is the only species present (zone A), its elevational range is wide, covering lowland and montane habitats, but where it broadly co-occurs with the lowland specialist I. sp. n. 2 (zone B) it is confined to montane areas, and where it broadly co-occurs with the montane specialist I. cribralis (zone C) it is confined to lowland areas.
To summarize, in each of the three species groups, successively more derived species occupy successively higher elevational bands, with a sister species pair occupying the highest elevational bands. These data seem to support the hypothesis that relatively young species are predominantly evolving in montane areas and old species are mostly confined to the lowlands (Fjeldså 1994; Fjeldså & Lovett 1997). However, further corroboration is needed. A more nuanced and unifying theory to account for all aspects of tropical montane biotic diversification can only come through the synthesis of further case studies such as this one, so for example the frequencies and predominant evolutionary sequence of the different speciation modes can be determined. Ithomiola gives an apparently rare insight into the process of vertical speciation, as mostly anecdotal evidence suggests that this phase is generally succeeded by horizontal dispersal and diversification that obscures it.
(b) Maintenance of parapatric ranges
No two closely related Ithomiola species are sympatric, despite obvious morphological differentiation, and this begs an explanation. The elevational species replacements in Ithomiola are so precise that there are no modern data indicating closely related species overlap even slightly, and I have seen no evidence of interspecific hybridization in the genus. It has long been recognized that the location of a species boundary might be affected by the nearby presence of a related species (Darwin 1869), but the mechanisms maintaining the boundary between such apparently fully differentiated parapatric species are still debated today (Bull 1991). Traditionally, ecotonal change between one vegetation type or zone and another and competitive exclusion have been the most commonly cited mechanisms (Terborgh 1971; Key 1982; Haffer 1986; Bull 1991). For example, based on field studies in the Peruvian Andes, in which different transects were sampled where ecotonal changes occurred at different elevations and closely related congeners were variably absent, Terborgh concluded that about one-sixth of the distributional limits of Andean birds could be attributed to ecotones and two-thirds to direct and diffuse competitive exclusion (Terborgh 1971, 1985; Terborgh & Weske 1975).
In Ithomiola, ecotones seem unlikely to be significantly contributing to the maintenance of parapatric boundaries. Along the same elevational transect in the eastern Andes of Ecuador, the transition zones from one species to another in the floralis and nepos groups are similar but not identical (1250 m versus 1000 m and 1650 m versus 1550 m, respectively). If ecotonal changes were largely responsible for the positioning of these transitions, then one would expect them to be at nearly identical elevations. Further evidence comes from the theages group.
The three theages group species have heretofore been treated as a single species (Callaghan & Lamas 2004), but are consistently morphologically distinct and occupy differing geographic and elevational ranges (Hall in press). Ithomiola theages is widespread from southern Mexico (Veracruz) to western Ecuador, I. sp. n. 2 is apparently confined to southern Nicaragua, Costa Rica and western Panama (west of the Canal Zone), and I. cribralis ranges from eastern Panama (east of the Canal Zone) through western Colombia (west of the Cordillera Occidental) to western Ecuador (Hall in press). When I. theages is the only species present, it ranges widely across lowland and montane habitats from near sea level to at least 1600 m, but in zones where it broadly co-occurs with a close relative it has become confined to either montane areas only above 600 m (by I. sp. n. 2) or lowland areas only below 900 m (by I. cribralis) (see figure 4). This phenomenon, of a species being able to expand its elevational range in the absence of its most closely related congeners, has rarely been documented in such detail in insects, but less complex scenarios have been recorded for two closely related pairs of Lymanopoda and Corades satyrine butterflies in the Venezuelan Andes (Pyrcz & Wojtusiak 2002).
This revealing clue from the theages group would seem to indicate that interspecific competition is playing a critical role in maintaining Ithomiola species boundaries. The related species certainly seem to have very similar ecological requirements. However, based on anecdotal field observations, neither the larval food plants (Napaeina caterpillars are generally polyphagous feeders on their abundant bromeliad and orchid foodplants (Hall in press)), adult food sources, nor streamside male perching sites ever appear to be in short supply. It is thus not clear at present what resources might need to be competed for, and how one species might be excluded from the range of another, a situation that has also been noted in the case of elevationally parapatric satyrines (Pyrcz & Wojtusiak 2002). Traditionally, in the absence of environmental factors, competition alone was generally assumed to be responsible for the maintenance of parapatric boundaries (e.g. Hairston 1951), but more recently other ecological processes, including interactions with predators and parasites, have been suggested as additional or alternative explanations (Bull 1991).
Although speculative at this juncture, one such possible explanation in the case of Ithomiola might be something similar to the ‘satyr effect’ (Ribeiro & Spielman 1986), a form of reproductive interference. We know nothing about the mate recognition system in Ithomiola. Males typically establish perching leks along streamsides, where they defend small territories waiting for passing females (Hall in press), but it is unclear how mates are chosen and, for example, whether or how they use their leg hairpencils in courtship. In other riodinid genera, such as Theope (Hall 1999), as many as 15 species can be found perching on a single lowland hilltop, where each species will often have a unique perching niche in time and space. It is possible that such premating isolating mechanisms are not yet fully developed in Ithomiola, and attempts at interspecific pairings may constantly be taking place near boundary zones. Given the considerable differences in the configuration of the male genitalia in most parapatric Ithomiola species, especially in the positioning and arrangement of cornuti on the everted aedeagal vesica, it seems likely such pairings would lead to considerable, perhaps sometimes irreparable, damage to copulatory structures. The female genitalia, in particular, would be susceptible to tears in the often membranous ductus bursae, and the reproductive life of any individual so afflicted would be finished. The maintenance of parapatric distributions through reproductive interference and other mechanisms has been shown to be enhanced by low dispersal rates (Ribeiro & Spielman 1986; Hewitt 1990), and riodinids are known to be relatively low vagility organisms (Hall & Harvey 2002a; Hall et al. 2004). Presumably rarity, a factor certainly applicable to most Ithomiola species, has the same effect.
Acknowledgements
I thank all the curators who gave me access to collections and loaned specimens (full list in Hall in press); K. Willmott for the photograph of female I. tanos, topographic map data, preliminary digital manipulation of most of the adult images and the contribution of elevational data from Ecuador; D. Harvey for doing many of the male genitalia dissections; D. Janzen, K. Willmott and several anonymous reviewers for critical comments on the manuscript; the Museo Ecuatoriano de Ciencias Naturales and the Ministerio del Ambiente, in Quito, for Ecuadorian research permits; and the National Geographic Society (#5751-96) and the National Science Foundation (BS&I #0103746) for financial support (full list in Hall in press).
References
- Adams M.J. Speciation in the pronophiline butterflies (Satyridae) of the northern Andes. J. Res. Lepid. 1985;(Suppl. 1):33–49. [Google Scholar]
- Arctander P, Fjeldså J. Andean tapaculos of the genus Scytatopus (Aves, Rhinocryptidae): a study of speciation using DNA sequence data. In: Loeschcke V, Tomiuk J, Jain S.K, editors. Conservation genetics. Birkhäuser Verlag; Basel: 1994. pp. 205–225. [DOI] [PubMed] [Google Scholar]
- Barraclough T.G, Vogler A.P. Detecting the geographical pattern of speciation from species-level phylogenies. Am. Nat. 2000;155:419–434. doi: 10.1086/303332. 10.1086/303332 [DOI] [PubMed] [Google Scholar]
- Bates J.M, Zink R.M. Evolution into the Andes: molecular evidence for species relationships in the genus Leptopogon. The Auk. 1994;111:507–515. [Google Scholar]
- Bremer K. The limits of amino acid sequence data in angiosperm phylogenetic reconstruction. Evolution. 1988;42:795–803. doi: 10.1111/j.1558-5646.1988.tb02497.x. [DOI] [PubMed] [Google Scholar]
- Bull C.M. Ecology of parapatric distributions. Annu. Rev. Ecol. Syst. 1991;22:19–36. 10.1146/annurev.es.22.110191.000315 [Google Scholar]
- Callaghan C.J, Lamas G. Riodinidae. In: Lamas G, editor. Checklist: part 4A. Hesperioidea–Papilionoidea. Scientific Publishers; Gainesville, FL: 2004. pp. 141–170. [Google Scholar]
- Chapman F.M. The distribution of bird life in Colombia. Bull. Am. Mus. Nat. Hist. 1917;31:1–169. [Google Scholar]
- Chesser R.T, Zink R.M. Modes of speciation in birds: a test of Lynch's method. Evolution. 1994;48:490–497. doi: 10.1111/j.1558-5646.1994.tb01326.x. [DOI] [PubMed] [Google Scholar]
- Churchill S.P, Balslev H, Forero E, Luteyn J.L, editors. Biodiversity and conservation of neotropical montane forests. New York Botanical Garden; New York: 1995. [Google Scholar]
- Darwin C. Murray; London: 1869. On the origin of species by means of natural selection. [Google Scholar]
- Doebell M, Dieckmann U. Speciation along environmental gradients. Nature. 2003;421:259–264. doi: 10.1038/nature01274. 10.1038/nature01274 [DOI] [PubMed] [Google Scholar]
- Endler J.A. Princeton University Press; Princeton, NJ: 1977. Geographic variation, speciation and clines. [PubMed] [Google Scholar]
- Eriksson, T. 1998 Autodecay, v. 4.0 Distributed by author, Department of Botany, Stockholm University, Stockholm.
- Felsenstein J.F. Confidence limits on phylogenies: an approach using bootstrap. Evolution. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- Fjeldså J. Geographical patterns of relict and young species of birds in Africa and South America and implications for conservation priorities. Biodivers. Conserv. 1994;3:107–126. [Google Scholar]
- Fjeldså J, Lovett J.C. Geographical patterns of old and young species in African forest biota: the significance of specific montane areas as evolutionary centres. Biodivers. Conserv. 1997;6:325–346. [Google Scholar]
- García-Moreno J, Arctander P, Fjeldså J. Pre-pleistocene differentiation among chat-tyrants. Condor. 1998;100:629–640. [Google Scholar]
- Gavrilets S, Li H, Vose M. Patterns of parapatric speciation. Evolution. 2000;54:1126–1134. doi: 10.1111/j.0014-3820.2000.tb00548.x. [DOI] [PubMed] [Google Scholar]
- Gregory-Wodzicki K.M. Uplift history of the central and northern Andes: a review. Geol. Soc. Am. Bull. 2000;112:1091–1105. 10.1130/0016-7606(2000)112%3C1091:UHOTCA%3E2.3.CO;2 [Google Scholar]
- Haffer J. Speciation in Amazonian forest birds. Science. 1969;165:131–137. doi: 10.1126/science.165.3889.131. [DOI] [PubMed] [Google Scholar]
- Haffer J. Superspecies and species limits in vertebrates. Zeit. Zool. Syst. Evol. 1986;24:169–190. [Google Scholar]
- Haffer J. Alternative models of vertebrate speciation in Amazonia: an overview. Biodivers. Conserv. 1997;6:451–476. 10.1023/A:1018320925954 [Google Scholar]
- Hairston N.G. Interspecies competition and its probable influence upon the vertical distribution of Appalachian salamanders of the genus Plethodon. Ecology. 1951;32:266–274. [Google Scholar]
- Hall J.P.W. Scientific Publishers; Gainesville, FL: 1999. A revision of the genus Theope: its systematics and biology (Lepidoptera: Riodinidae: Nymphidiini) [Google Scholar]
- Hall J.P.W. Phylogenetic reassessment of the five forewing radial-veined tribes of the Riodininae (Lepidoptera: Riodinidae) Syst. Entomol. 2003;28:23–37. 10.1046/j.1365-3113.2003.00196.x [Google Scholar]
- Hall, J. P. W. In press. A phylogenetic revision of the Napaeina (Lepidoptera: Riodinidae: Mesosemiini). Washington, DC: Entomological Society of Washington.
- Hall J.P.W, Harvey D.J. The phylogeography of Amazonia revisited: new evidence from riodinid butterflies. Evolution. 2002a;56:1489–1497. doi: 10.1111/j.0014-3820.2002.tb01460.x. [DOI] [PubMed] [Google Scholar]
- Hall J.P.W, Harvey D.J. A survey of androconial organs in the Riodinidae (Lepidoptera) Zool. J. Linn. Soc. 2002b;136:171–197. 10.1046/j.1096-3642.2002.00003.x [Google Scholar]
- Hall J.P.W, Willmott K.R. Four new riodinid species from eastern Ecuador (Lepidoptera: Riodinidae) Lambillionea. 1998;98:325–334. [Google Scholar]
- Hall J.P.W, Robbins R.K, Harvey D.J. Extinction and biogeography in the Caribbean: new evidence from a fossil riodinid butterfly in Dominican amber. Proc. R. Soc. B. 2004;271:797–801. doi: 10.1098/rspb.2004.2691. 10.1098/rspb.2004.2691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt G.M. Divergence and speciation as viewed from an insect hybrid zone. Can. J. Zool. 1990;68:1701–1715. [Google Scholar]
- Key K.H.L. Species, parapatry, and the morabine grasshoppers. Syst. Zool. 1982;30:425–458. [Google Scholar]
- Knox E.B, Palmer J.D. Chloroplast DNA variation and the recent radiation of the giant senecios (Asteraceae) on the tall mountains of eastern Africa. Proc. Natl Acad. Sci. USA. 1995;92:10 349–10 353. doi: 10.1073/pnas.92.22.10349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch J.D. The gauge of speciation: on the frequencies of modes of speciation. In: Otte D, Endler J.A, editors. Speciation and its consequences. Sinauer Associates; Sunderland, MA: 1989. pp. 527–553. [Google Scholar]
- Patton J.L, Smith M.F. MtDNA phylogeny of Andean mice: a test of diversification across ecological gradients. Evolution. 1992;46:174–183. doi: 10.1111/j.1558-5646.1992.tb01992.x. [DOI] [PubMed] [Google Scholar]
- Pyrcz T.W, Wojtusiak J. The vertical distribution of pronophiline butterflies (Nymphalidae, Satyrinae) along an elevational transect in Monte Zerpe (Cordillera de Mérida, Venezuela) with remarks on their diversity and parapatric distribution. Global Ecol. Biogeogr. 2002;11:211–221. 10.1046/j.1466-822X.2002.00285.x [Google Scholar]
- Ribeiro J.M.C, Spielman A. The satyr effect: a model predicting parapatry and species extinction. Am. Nat. 1986;128:513–528. 10.1086/284584 [Google Scholar]
- Riddle B.R, Hafner D.J. Species as units of analysis in ecology and biogeography: time to take the blinders off. Global Ecol. Biogeogr. 1999;8:433–441. 10.1046/j.1365-2699.1999.00170.x [Google Scholar]
- Roy M.S. Recent diversification in African greenbuls (Pycnonotidae: Andropadus) supports a montane speciation model. Proc. R. Soc. B. 1997;264:1337–1344. 10.1098/rspb.1997.0185 [Google Scholar]
- Roy M.S, da Silva J.M.C, Arctander P, García-Moreno J, Fjeldså J. The speciation of South American and African birds in montane regions. In: Mindell D, editor. Avian molecular evolution and systematics. Academic Press; San Diego, CA: 1997. pp. 325–343. [Google Scholar]
- Simpson B.B. Quaternary biogeography of the high montane regions of South America. In: Duellman W.E, editor. The South American herpetofauna: its origins, evolution and dispersal. 1979. pp. 157–188. Monographs of the Museum of Natural History, University of Kansas, no.7, pp. 157–188. [Google Scholar]
- Swofford D.L. v. 4.0b4a. Sinauer Associates; Sunderland, MA: 2000. PAUP*: phylogenetic analysis using parsimony (*and other methods) [Google Scholar]
- Terborgh J. Distribution on environmental gradients: theory and a preliminary interpretation of distributional patterns in the avifauna of the Cordillera Vilcabamba, Peru. Ecology. 1971;52:23–40. [Google Scholar]
- Terborgh J. The role of ecotones in the distribution of Andean birds. Ecology. 1985;66:1237–1246. [Google Scholar]
- Terborgh J, Weske J.S. The role of competition in the distribution of Andean birds. Ecology. 1975;56:562–576. [Google Scholar]
- Van der Hammen T. The pleistocene changes of vegetation and climate in tropical South America. J. Biogeogr. 1974;1:3–26. [Google Scholar]
- Viloria, A. L. 1998 Studies on the systematics and biogeography of some montane satyrid butterflies (Lepidoptera). Ph.D. Dissertation, King's College, London.
- Vuilleumier F, Monasterio M, editors. High altitude tropical biogeography. Oxford University Press; Oxford: 1986. [Google Scholar]
- Whitmore T.C, Prance G.T, editors. Biogeography and quaternary history in tropical America. Oxford monographs in biogeography, no. 3. Oxford Science Publications; Oxford: 1987. [Google Scholar]
- Willmott K.R, Hall J.P.W, Lamas G. Systematics of Hypanartia (Lepidoptera: Nymphalidae: Nymphalinae), with a test for speciation mechanisms in the Andes. Syst. Entomol. 2001;26:369–399. 10.1046/j.1365-3113.2001.00157.x [Google Scholar]