Abstract
Dutch elm disease is caused by the fungal pathogen Ophiostoma novo-ulmi which is transmitted by the native elm bark beetle, Hylurgopinus rufipes. We have found that four semiochemicals (the monoterpene (−)-β-pinene and the sesquiterpenes (−)-α-cubebene, (+)-spiroaxa-5,7-diene and (+)-δ-cadinene) from diseased American elms, Ulmus americana, synergistically attract H. rufipes, and that sesquiterpene emission is upregulated in elm trees inoculated with O. novo-ulmi. The fungus thus manipulates host trees to enhance their apparency to foraging beetles, a strategy that increases the probability of transportation of the pathogen to new hosts.
Keywords: Dutch elm disease, Ulmus americana, fungal pathogen, Ophiostoma novo-ulmi, Hylurgopinus rufipes, semiochemicals
1. Introduction
Non-motile parasites that complete one or more stages of their life cycle in intermediate or definitive hosts can manipulate these hosts to optimize transportation to new hosts (Poulin 2002). For example, protozoan parasites, Toxoplasma gondii, cause their intermediate rat hosts to approach and be eaten by cats, the definitive host (Berdoy et al. 2000). Likewise, the fungal pathogen Ophiostoma ulmi kills elm trees (Hubbes 1999; Brasier 2001) and then requires transportation to new elms (Agrios 1988). Since its introduction into the United States in the 1930s, it has ravaged forest and urban American elms across the northeastern United States and Canada. With the appearance in the 1960s of the more virulent strain Ophiostoma novo-ulmi, elms have been severely decimated across all geographical locations. In North America, O. novo-ulmi relies on the smaller European elm bark beetle, Scolytus multistriatus, or the native elm bark beetle, Hylurgopinus rufipes, to be transported to new host elms (Millar et al. 1986; Agrios 1988; Hubbes 1999). In the prairie regions of North America, H. rufipes can withstand cold winter temperatures (Agrios 1988), and is the primary vector of Dutch elm disease.
Plants are known to synthesize and emit semiochemicals in response to invading or damaging organisms (Turlings et al. 1990) in order to recruit natural enemies of those organisms. Tobacco (Nicotiana tabacum), cotton (Gossypium hirsutum) and maize (Zea maize) plants each produce distinct semiochemical blends in response to damage by caterpillars of two closely related herbivore species. The specialist parasitic wasp Cardiochiles nigriceps exploits these differences to distinguish infestation by its host Heliocoverpa virescens from that by the non-host Heliocoverpa zea (DeMoraes et al. 1998). Volicitin, N-(17-hydroxylinolenoyl)-l-glutamine, in the oral secretion of beet armyworms (Spodoptera exigua) triggers the release of plant semiochemicals which attract natural enemies of the caterpillar (Alborn et al. 1997; Paré et al. 1998). Insects that feed by sucking plant sap also induce changes in plants' semiochemicals to attract parasitic wasps (Guerrieri et al. 1993; Du et al. 1996; Powell et al. 1998). cis-Jasmone has been found to attract an insect predator and parasitoid of aphids (Birkett et al. 2000); it may even serve as a phyto-pheromone in plant–plant communications (Powell & Pickett 2003). Trees under attack by bark beetles that carry symbiotic fungi respond by forming necrotic lesions around the infection, and by increasing the concentration of allelochemicals with fungistatic properties within the lesions (Raffa 1988). In all these cases, the plants' response helps alleviate the impact of the damage caused by insects or fungi. Here, we show that the fungal plant pathogen O. novo-ulmi induces change in the elm's semiochemical blend that is detrimental to the tree in that it attracts insect vectors that kill the host and carry the pathogen to new hosts.
2. Material and Methods
(a) Collection of semiochemicals from elm wood
Trunk sections of American elm wood were cut and ground into fine sawdust which was weighed and placed in a Pyrex glass aeration chamber (15.5 cm inner diameter (i.d.)×20 cm). For 96 h, a water-driven aspirator drew purified air at 1 l min−1 through the chamber and a downstream Pyrex glass column (140×5 mm i.d.) filled with Porapak Q (50–80 mesh, Waters, Milford, MA, USA). Volatiles were eluted from Porapak Q with 2 ml of freshly distilled pentane.
(b) Analyses of volatiles
Aliquots of Porapak volatile extracts were analysed by coupled gas chromatographic–electroantennographic detection (GC–EAD) (Arn et al. 1975; Gries et al. 2002). An H. rufipes antenna was removed and the base inserted into the tip of a glass capillary filled with a saline solution (Staddon & Everton 1980). The club of the antenna was pierced with a sharply pointed open tip of a second capillary also filled with saline. Volatiles that elicited responses from male or female antennae were analysed by GC–mass spectrometry (MS), employing a Varian Saturn 2000 Ion Trap GC–MS fitted with a DB-5 column (30 m×0.32 mm i.d.; J&W Scientific, Folsom, CA, USA).
High-performance liquid chromatography (HPLC) of samples employed a Waters LC626 and a Waters 486 variable wavelength UV/visible detector set to 210 nm, hp chemstation software (Rev.A.07.01), and a reverse-phase Nova-Pak C18 column (60 Å, 4 μm, 3.9×300 mm).
(c) Acquisition of candidate semiochemicals
(−)-β-Pinene (1) and (−)-α-cubebene (2) were purchased (Fluka Chemika-Biochemika, Buchs, Switzerland CH-9470; Sigma-Aldrich, Oakville, Ontario L6H 2J8). (+)-Spiroaxa-5,7-diene (3) was formed as a minor product by palladium-catalysed rearrangement of 2 during hydrogenation. Reduced palladium (5% on barium sulphate, 200 mg) was added to a solution of 2 (30 mg) in 10 ml pentane. While stirring, hydrogen was bubbled through the suspension. We monitored the reaction by GC analysis of aliquots and terminated it after 3–6 min when the yield of 3 reached its maximum (approx. 3%). Compound 3 was isolated from the mixture by HPLC on a reverse-phase column (see above) eluted with acetonitrile (1 ml min−1). Elution with 88% aq. acetonitrile afforded (+)-δ-cadinene (4) which was also produced by heating a solution of 2 (20 mg) in 1,4-dioxane (1 ml) in the presence of 0.2 ml 0.1 M HCl (50 °C, 2–4 h) and extraction with pentane (Ohta et al. 1968).
To determine the molecular structure and absolute configuration of 3, the dextro- and levorotatory enantiomers were synthesized by TiO2/SO42−-catalysed rearrangement (Polovinka et al. 2000) of (−)-ent-aromadendrene (a sesquiterpene derived from bicyclogermacrene which was isolated from the liverwort Mylia taylorii; von Reuβ et al. 2004) and (+)-aromadendrene, respectively, and analysed by GC on an octakis-(2,6-di-O-methyl-3-O-pentyl)-β-cyclodextrin column which separated them with complete baseline resolution.
(d) Laboratory bioassay experiments
Response of H. rufipes to aliquots of Porapak Q extract of diseased elm wood volatiles was tested in a Y-tube olfactometer (Delury et al. 1999) at 22–26 °C and 40–44% relative humidity. The olfactometer was enclosed on three sides with white poster board, and illuminated by two overhead light tubes (fluorescent GE Plant and Aquarium F40PL/AQ Wide Spectrum and Sylvania Daylight Deluxe F40DX 40W). Treatment and control odour sources were micro-pipetted onto Whatman No. 2 filter paper (13.5 mm diameter) assigned near the orifice of side arms. For each replicate, a new male or female beetle, a clean Y-tube, and new filter papers were used, with test stimuli assigned randomly to one of the side arms. Air was drawn through the olfactometer at a rate of 1 l min−1 with a water-driven aspirator. Thirty seconds after placement of stimuli, a beetle was released into the entrance of the olfactometer. Beetles walking up-wind that reached within 5 min a filter paper emanating host-derived odour or pentane as the control stimulus were classed as responders, and included in statistical analyses.
(e) Field trapping experiments
We suspended adhesive cardboard traps (45×67 cm) (Phero Tech, Inc., Delta, BC, V4G 1E9, Canada) between poles at a height of approximately 2 m and spacings of 20–25 m in randomized complete blocks separated by 2–5 km. Trap baits consisted of a piece of dental cotton roll (10×15 mm) (Richmond Dental, Charlotte, NC 28234, USA) that was impregnated with (−)-α-cubebene (2), (+)-spiroaxa-5,7-diene (3) and (+)-δ-cadinene (4), and affixed to a 400-μl polyethylene microcentrifuge tube containing a 5-μl capillary tube filled with (−)-β-pinene (1) (Sigma-Aldrich Canada Ltd, Oakville, Ont. L6H 2J8). Release rates of 1, 2, 3 and 4 were, respectively, 25, 2, 2 and 184 μg/24 h, approximating the ratio found in diseased elm wood (figure 1). We recorded the number of H. rufipes captured in traps 24 h after trap placement, replaced lures and traps, and re-randomized their location within blocks.
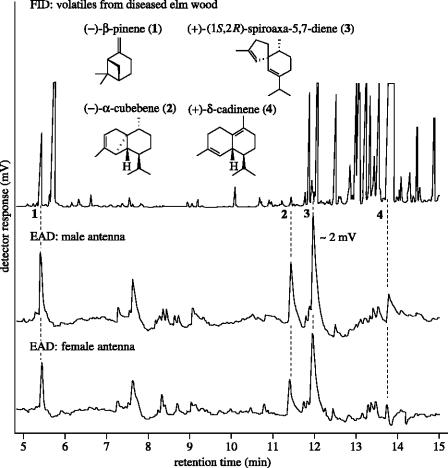
Figure 1.
Gas chromatograms of volatiles (desorbed from Porapak Q) emanating from ground elm wood infected with the fungal pathogen Ophiostoma novo-ulmi. Hewlett Packard 5890A gas chromatograph with DB-5 column (30 m×0.32 mm i.d.; J&W Scientific, Folsom, CA 95630) with flame ionization (FID) or electroantennographic detector (EAD: male or female Hylurgopinus rufipes antenna); splitless injection; temperature program: 50 °C (2 min), then 10 °C min−1 to 280 °C.
(f) Inoculation of elm saplings with Ophiostoma novo-ulmi
To determine whether O. novo-ulmi emits these four semiochemicals or induces host trees to emit them, we conducted the following inoculation experiment. We flooded culture plates of O. novo-ulmi grown on potato dextrose agar with distilled water and diluted the suspended conidia to 6×107 ml−1. Using a sterile probe, we then punched 10 holes similar in diameter to those bored by H. rufipes into the stem and twig crotches of potted 2 m tall healthy elm saplings (n=3) maintained in a quarantine greenhouse. Into each hole, we pipetted 10 μl of O. novo-ulmi spore suspension. Control saplings (n=3) with the same number and distribution of holes received equivalent volumes of distilled water, and additional control saplings (n=3) received no treatment. After 12 weeks, when treatment saplings exhibited disease symptoms, we ground wood tissue from all nine saplings into separate samples of fine sawdust, weighed them, and collected volatiles on Porapak Q (see §2a).
3. Results and discussion
Our data show that the fungal plant pathogen O. novo-ulmi induces change in the elm's semiochemical blend that is detrimental to the tree in that it attracts insect vectors that kill the host and carry the pathogen to new hosts. To identify these semiochemicals, we adsorbed the volatiles from finely ground diseased American elm trunk wood on Porapak Q and eluted them with pentane. Subsequent bioassays showed strong attraction of male and female H. rufipes to the eluate. We analysed aliquots of the Porapak Q extract by GC–EAD (Arn et al. 1975; Gries et al. 2002), using H. rufipes antennae as detectors. Four compounds elicited consistent and significant antennal responses (figure 1). Comparative analyses by GC–MS of volatiles from diseased elm wood and of authentic standards revealed that these four compounds were (−)-β-pinene (1), (−)-α-cubebene (2), (+)-spiroaxa-5,7-diene (3) and (+)-δ-cadinene (4). The absolute configuration of (−)-β-pinene was determined by GC analysis on a chiral column. The presence of 1, 2 and 4 in volatiles from diseased elm trees has been previously reported (Millar et al. 1986). (+)-Spiroaxa-5,7-diene (3), discovered as a semiochemical for the first time in this study, was present in only trace quantities but elicited the strongest response from H. rufipes antennae (figure 1). Stronger antennal responses to synthetic (+)-(1S,2R)-spiroaxa-5,7-diene than to its antipode (data not shown) support the absolute configuration assignment of 3.
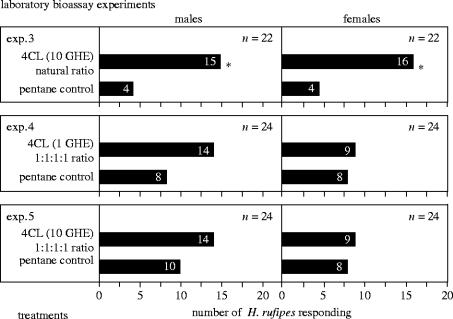
The attractiveness of synthetic 1, 2, 3 and 4 was tested in field trapping experiments in elm forests near Regina, Saskatchewan, Canada. Traps baited with the 4-component blend in an approximately natural ratio captured significantly more male and female H. rufipes than unbaited control traps (figure 2, experiment 1), whereas blends lacking any one of the four compounds were as unattractive as unbaited traps (figure 2, experiment 2). In laboratory bioassay experiments, a synthetic blend containing equal amounts of all four components did not elicit a behavioural response from the beetles (figure 3). Thus, the attractiveness of the semiochemical blend requires both the presence of all four components and their release at a natural ratio.
Figure 2.
Results of field experiments 1 (2–5 June 2004; 12 replicates) and 2 (17–20 August 2004; 24 replicates), comparing captures of H. rufipes on adhesive traps baited with a blend of (−)-β-pinene, (−)-α-cubebene, (+)-spiroaxa-5,7-diene and (+)-δ-cadinene in release rates of, respectively, 25, 2, 2 and 184 μg per 24 h, or baited with lures lacking one of the four components (experiment 2). Location: near Regina, Saskatchewan, Canada. Bars with different letters are significantly different (ANOVA, Zar 1999, followed by Tukey–Kramer HSD comparison of means; jmp statistical software, p<0.05).
Figure 3.
Number of male and female Hylurgopinus rufipes responding in Y-tube olfactometers to a 4-component lure (4CL) of synthetic (−)-β-pinene, (−)-α-cubebene, (+)-spiroaxa-5,7-diene and (+)-δ-cadinene at a natural ratio (20 : 1.5 : 1.25 : 175; determined in volatile blend of diseased elm (figure 1)) of components (experiment 3), or at a non-natural ratio (1 : 1 : 1 : 1) of components (experiments 4, 5). One GHE=1 g-h equivalent=amount of semiochemicals released from 1 g of ground diseased elm wood during 1 h of volatile acquisition. Number of insects responding to each stimulus given in bars, number of insects tested given in parenthesis. For each experiment an asterisk (*) indicates a significant preference for a particular treatment; χ2 test with Yates correction for continuity, treatment versus control; *p<0.05.
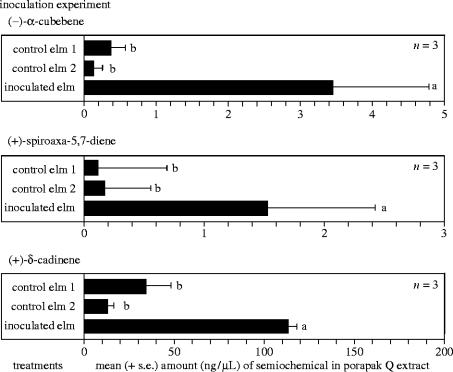
To determine whether O. novo-ulmi emits these four semiochemicals or induces host trees to emit them, we inoculated healthy elm saplings with O. novo-ulmi and analysed by quantitative GC–MS the volatiles collected on Porapak Q (as above). Spiroaxa-5,7-diene, α-cubebene and δ-cadinene were found to be significantly more abundant in pathogen-inoculated than in healthy elm saplings (figure 4), while the concentration of β-pinene, which is typically released in response to severe mechanical injury (Trapp & Croteau 2001), was not affected. None of the four semiochemicals was present in volatiles emitted by O. novo-ulmi grown on potato dextrose, indicating that the pathogen does not produce them. Whether other factors causing ill health in elms, such as different pathogens or herbicide poisoning, may induce similar changes in the trees' semiochemical blend has not yet been determined.
Figure 4.
Results of an inoculation experiment, comparing quantities of α-cubebene, spiroaxa-5,7-diene and δ-cadinene in Porapak Q volatile extracts of wood tissue from potted elm saplings (n=3) 12 weeks after inoculation with an aqueous spore suspension of O. novo-ulmi (inoculated elm; n=3), distilled water (control elm 1; n=3) or left untreated (control elm 2; n=3). β-Pinene occurred in amounts too low for analysis. Statistics: see figure 2 caption.
Our data provide strong evidence that H. rufipes uses a blend of four elm-derived semiochemicals to find a susceptible host. All four occur at low quantities in healthy elms. By an as yet unknown mechanism, O. novo-ulmi then upregulates the production of these semiochemicals, thus enhancing the apparency of host trees to foraging beetles, and increasing the probability of transportation of the pathogen to new hosts.
Acknowledgments
We thank Steve Hyde, Glen Chernick, Ryan Johnston, Gary McLeod and Ashley Ulrich for field assistance, John Borden, Wittko Francke and Eberhard Kiehlmann for review of the manuscript, and Grigori Khaskin for discussions. The research was supported by (a) Saskatchewan Environment, (b) an Industrial Scholarship to G.M. from the Natural Sciences and Engineering Research Council of Canada with Phero Tech, Inc. as the industrial sponsor, (c) a Dr John Yorston Memorial Scholarship to G.M. and (d) Simon Fraser University. Insect and plant materials were kept in SFU's Global Forest quarantine facility.
Footnotes
Dedicated to Professor Dr Wilfried A. König in memoriam.
References
- Agrios G.N. Plant Pathology. 3rd edn. Academic Press; San Diego, CA: 1988. [Google Scholar]
- Alborn H.T, Turlings T.C.J, Jones T.H, Stenhagen G, Loughrin J.H, Tumlinson J.H. An elicitor of plant volatiles from beet armyworm oral secretion. Science. 1997;276:945–949. 10.1126/science.276.5314.945 [Google Scholar]
- Arn H, Städler E, Rauscher S. The electroantennographic detector—a selective and sensitive tool in the gas chromatographic analysis of insect pheromones. Z. Naturforsch. [C] 1975;30:1137–1141. [Google Scholar]
- Berdoy M, Webster J.P, Macdonald D.W. Fatal attraction in rats infected with Toxoplasma gondii. Proc. R. Soc. B. 2000;267:1591–1594. doi: 10.1098/rspb.2000.1182. 10.1098/rspb.2000.1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkett M.A, et al. New roles for cis-jasmone as an insect semiochemical in plant defense. Proc. Natl Acad. Sci. USA. 2000;97:9329–9334. doi: 10.1073/pnas.160241697. 10.1073/pnas.160241697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasier C. Rapid evolution of introduced plant pathogens via interspecific hybridization. Bioscience. 2001;51:123–133. [Google Scholar]
- Delury N.C, Gries R, Gries G, Judd G.J.R, Khaskin G. Moth scales-derived kairomones used by egg-larval parasitoid Ascogaster quadridentata to locate eggs of its host, Cydia pomonella. J. Chem. Ecol. 1999;25:2419–2431. 10.1023/A:1020861821919 [Google Scholar]
- DeMoraes C.M, Lewis W.J, Paré P.W, Alborn H.T, Tumlinson J.H. Herbivore-infested plants selectively attract parasitoids. Nature. 1998;393:570–573. 10.1038/31219 [Google Scholar]
- Du Y.-J, Poppy G.M, Powell W. Relative importance of semiochemicals from first and second trophic level in host foraging behavior of Aphidius ervi. J. Chem. Ecol. 1996;22:1591–1605. doi: 10.1007/BF02272400. [DOI] [PubMed] [Google Scholar]
- Gries R, Khaskin G, Gries G, Bennett R.G, King G.G.S, Morewood P, Slessor K.N, Morewood W.D. (Z,Z)-4,7-Tridecadien-(S)-2-yl acetate: sex pheromone of Douglas-fir cone gall midge, Contarinia oregonensis. J. Chem. Ecol. 2002;28:2201–2212. doi: 10.1023/a:1021005517389. [DOI] [PubMed] [Google Scholar]
- Guerrieri E, Pennacchio F, Trembay E. Flight behaviour of the aphid parasitoid Aphidius ervi (Hymentopera: Braconidae) in response to plant and host volatiles. Eur. J. Entomol. 1993;90:415–421. [Google Scholar]
- Hubbes M. The American elm and Dutch elm disease. Forest. Chron. 1999;75:265–273. [Google Scholar]
- Millar J.G, Zhao C, Lanier G.N, O'Callaghan D.P, Griggs M, West J.R, Silverstein R.M. Components of moribund American elm trees as attractants to elm bark beetles, Hylurgopinus rufipes and Scolytus multistriatus. J. Chem. Ecol. 1986;12:583–608. doi: 10.1007/BF01012095. 10.1007/BF01012095 [DOI] [PubMed] [Google Scholar]
- Ohta Y, Ohara K, Hirose Y. Acid-catalyzed isomerization of α-cubebene, α-copaene and α-ylangene. Tetrahedron Lett. 1968;39:4181–4184. 10.1016/S0040-4039(00)75403-7 [Google Scholar]
- Paré P.W, Alborn H.T, Tumlinson J.H. Concerted biosynthesis of an insect elicitor of plant volatiles. Proc. Natl Acad. Sci. USA. 1998;95:13 971–13 975. doi: 10.1073/pnas.95.23.13971. 10.1073/pnas.95.23.13971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polovinka M.P, Shal'ko A.A, Korchagina D.V, Gatilov Y.V, Shcherbukhin V.V, Zenkovets G.A, Barkash V.A. Rearrangements of some sesquiterpenes of the aromadendrane and guaiane series in acidic media. Russ. J. Org. Chem. 2000;36:49–61. [Google Scholar]
- Poulin R. Parasite manipulation of host behaviour. In: Lewis E.E, Campbell J.F, Sukhdeo M.V.K, editors. The behavioural ecology of parasites. CAB International; Wallingford, UK: 2002. pp. 243–257. [Google Scholar]
- Powell W, Pickett J.A. Manipulation of parasitoids for aphid pest management: progress and prospects. Pest Manag. Sci. 2003;59:149–155. doi: 10.1002/ps.550. 10.1002/ps.550 [DOI] [PubMed] [Google Scholar]
- Powell W, Pennacchio F, Poppy G.M, Tremblay E. Strategies involved in the location of hosts by the parasitoid Aphidius ervi Haliday (Hymenoptera: Braconidae: Aphidiinae) Biol. Control. 1998;11:104–112. 10.1006/bcon.1997.0584 [Google Scholar]
- Raffa K.F. Strategies and mechanisms of host colonization by bark beetles. In: Berryman A.A, editor. Dynamics of forest insect populations. Plenum Press; New York: 1988. pp. 505–530. [Google Scholar]
- Staddon B.W, Everton I.J. Haemolymph of milkweed bug Oncopectus fasciatus (Heteroptera: Lygaeidae): inorganic constituents and amino acids. Comp. Biochem. Physiol. 1980;65:371–374. [Google Scholar]
- Trapp S, Croteau R. Defensive resin biosynthesis in conifers. Annu. Rev. Plant Phys. 2001;52:689–724. doi: 10.1146/annurev.arplant.52.1.689. 10.1146/annurev.arplant.52.1.689 [DOI] [PubMed] [Google Scholar]
- Turlings T.C.J, Tumlinson J.H, Lewis W.J. Exploitation of herbivore-induced plant odors by host-seeking parasitic wasps. Science. 1990;250:1251–1253. doi: 10.1126/science.250.4985.1251. [DOI] [PubMed] [Google Scholar]
- von Reuβ S.H, Wu C.L, Muhle H, König W.A. Sesquiterpene constituents from the essential oils of the liverworts Mylia taylorii and Mylia nuda. Phytochemistry. 2004;65:2277–2291. doi: 10.1016/j.phytochem.2004.04.039. 10.1016/j.phytochem.2004.04.039 [DOI] [PubMed] [Google Scholar]
- Zar J.H. Biostatistical analysis. Prentice-Hall; Upper Saddle River, NJ: 1999. [Google Scholar]