Abstract
Competition between parasites within a host can influence the evolution of parasite virulence and host resistance, but few studies examine the effects of unrelated parasites with conflicting transmission strategies infecting the same host. Vertically transmitted (VT) parasites, transmitted from mother to offspring, are in conflict with virulent, horizontally transmitted (HT) parasites, because healthy hosts are necessary to maximize VT parasite fitness. Resolution of the conflict between these parasites should lead to the evolution of one of two strategies: avoidance, or sabotage of HT parasite virulence by the VT parasite. We investigated two co-infecting parasites in the amphipod host, Gammarus roeseli: VT microsporidia have little effect on host fitness, but acanthocephala modify host behaviour, increasing the probability that the amphipod is predated by the acanthocephalan's definitive host. We found evidence for sabotage: the behavioural manipulation induced by the Acanthocephala Polymorphus minutus was weaker in hosts also infected by the microsporidia Dictyocoela sp. (roeselum) compared to hosts infected by P. minutus alone. Such conflicts may explain a significant portion of the variation generally observed in behavioural measures, and since VT parasites are ubiquitous in invertebrates, often passing undetected, conflict via transmission may be of great importance in the study of host–parasite relationships.
Keywords: Acanthocephala, microsporidia, Gammarus roeseli, conflict, parasite transmission, behavioural manipulation
1. Introduction
There is growing evidence from a wide range of host–parasite systems that mixed infections, involving at least two parasite species or strains, are common in nature (Petnay & Andrews 1998; Read & Taylor 2001). Multiple parasite infections are important determinants of host–parasite interactions (Frank 1996; Read & Taylor 2001), and it is becoming evident that interactions between parasites can influence the evolutionary histories of both hosts and parasites. Competition between parasites within the same host leads to conflicts that could influence the evolution of parasite virulence (van Baalen & Sabelis 1995; Brown et al. 2002), and host resistance (Brown & Grenfell 2001; Read & Taylor 2001; Oliver et al. 2003). For example, direct competition for limited host resources, in theory, can result in increased parasite virulence or competitive exclusion (e.g. Nowak & May 1994; Brown et al. 2002), and this is supported by empirical data (e.g. Read & Taylor 2001; Hughes & Boomsma 2004). However, while many models examine interactions between closely related genotypes, few examine the impact of phylogenetically distant, co-infecting parasites. Indeed, distantly related parasites may use different host resources and be recognized in different ways by host immune systems. The assumptions underlying models of closely related co-infecting parasites may not apply to these cases, as selection may be operating on different levels, and the evolutionary outcomes of competitive interactions may be very different (Friedli & Bacher 2001; Poulin et al. 2003).
However, parasites co-occurring in the same host may have opposite evolutionary interests in the use of their host for their transmission (Thomas et al. 2002). Examples are found in parasites with the same transmission mode, but with different final hosts, e.g. trophically transmitted parasites share the same intermediate host but have different definitive hosts (Cézilly et al. 2000; Lafferty et al. 2000). Another potentially conflicting situation would be co-infection by vertically transmitted (VT) parasites (transmitted from generation to generation, from infected parent to offspring) and horizontally transmitted (HT) parasites (transmitted between unrelated hosts) (Rigaud & Haine 2005). HT parasites are often pathogenic to their host, and this virulence can be beneficial if it is an intrinsic part of parasite fitness (Ebert 1999). In contrast, VT parasites should not be virulent to their host because their fitness is directly dependent on that of the host (Fine 1975). They are transmitted via the cytoplasm of the eggs (Bandi et al. 2001), therefore, their survival and reproduction is intimately linked to survival and reproduction of female hosts. Everything else being equal, they are predicted to have either positive or neutral effects on host fitness, and in particular, on host reproductive success (Smith & Dunn 1991; Ebert & Herre 1996). In theory, a VT parasite has no evolutionary interest in sharing its host with a HT virulent parasite, provided that the selective pressure imposed by the HT parasite on the VT parasite is high enough (Thomas et al. 2002; Rigaud & Haine 2005). This is especially true for VT parasites infecting female hosts, i.e. the transmitting sex (O'Neill et al. 1997). Such conflict should lead to one of two solutions (Thomas et al. 2002): avoidance of the two parasite types within the same host (achieved by parasite-induced resistance in the host, or by one parasite killing the other), or capacity of one parasite to restore damage caused in the host by the other parasite (sabotage of virulence). A recent study showing a positive impact of VT bacteria on aphid host resistance to parasitoids (obligatory killing parasites) (Oliver et al. 2003) supports this prediction.
The amphipod Gammarus roeseli is an intermediate host for the acanthocephalans Pomphorynchus laevis (Bauer et al. 2000), whose definitive hosts are fish, and Polymorphus minutus (Bauer et al. 2005), whose definitive hosts are freshwater birds (Hynes & Nicholas 1963). Acanthocephalan parasites have life-cycles involving different hosts at different life-stages. They are known to modify the behaviour of their intermediate hosts in order to increase the probability of predation of the intermediate host by the definitive host (Bethel & Holmes 1977), which can be seen as an extreme case of virulence (Lafferty 1999; Poulin & Combes 1999). Polymorphus parasites, and particularly P. minutus in G. roeseli (Bauer et al. 2005), modify the geotaxis of their intermediate hosts, a change that increases the probability of predation by freshwater birds (Hynes & Nicholas 1963). On the other hand, Pomphorynchus parasites modify the phototaxis of their intermediate host G. pulex, but do not have a significant effect on the behaviour of G. roeseli (Bauer et al. 2000). The behavioural manipulations expressed by each parasite genus seem to be quite specialized, since Polymorphus minutus does not affect phototaxis and Pomphorhynchus laevis does not affect geotaxis in Gammarus pulex (Cézilly et al. 2000). However, it is not known if this specificity applies to G. roeseli. P. minutus is also known to totally castrate its host Gammarus pulex, where infection results in ovary degeneration in all infected females (Bollache et al. 2002), but this effect has not been investigated in G. roeseli. By increasing predation probability and inducing castration, P. minutus is clearly detrimental to its host. P. laevis has a negative effect on the fecundity of G. pulex (Bollache et al. 2002), but this has not been investigated in G. roeseli.
Gammarus roeseli is also infected by three species of VT microsporidia, which usually co-occur in the same populations (Haine et al. 2004). These microsporidia transmit vertically from mother to offspring through transovarial transmission, and appear to have very little, if any, horizontal transmission (Haine et al. 2004). Thus, since the microsporidia are present in the host from the egg stage, they always precede the potential acanthocephalan infection. Clearly, for microsporidia that rely on vertical transmission from generation to generation, co-infection of its host by Polymorphus minutus is detrimental, while the consequences of co-infection with Pomphorhynchus laevis are questionable because this acanthocephalan does not appear to alter host behaviour (Bauer et al. 2000). One could, nevertheless, propose that this absence of behavioural change could be due to the presence of microsporidia inducing a sabotage on behavioural modification (Rigaud & Haine 2005).
In this study, we looked for negative associations between microsporidia and acanthocephala to test for avoidance of acanthocephala by microsporidia. We also assessed whether microsporidia sabotaged acanthocephala by interfering with castration or behavioural changes in amphipods. We predicted that avoidance and/or sabotage by microsporidia would be stronger when G. roeseli is infected by the manipulating parasite (Polymorphus minutus) compared to the non-manipulative parasite (Pomphorhychus laevis).
2. Material and Methods
(a) Behaviour experiments
Gammarus roeseli were collected randomly (Hynes 1954) from two sites in Côte d'Or, France, in a geographic area of ca 10 km2: Chour (river Ausson) and Maillys (river Tille).
Virulence of P. minutus and P. laevis was measured as the parasite's ability to modify host geotactic and phototactic behaviours, respectively (Poulin & Combes 1999). To test for specialization of behavioural manipulation (as for G. pulex, Cézilly et al. 2000), we also tested whether P. minutus modifies host phototaxis and P. laevis modifies geotaxis. The protocols were as described previously (Perrot-Minnot 2004; Bauer et al. 2005). To standardize the conditions of behavioural experiments, the animals were maintained for 24 h in the lab prior to experiments, in dechlorinated aerated tap water, in a room maintained at 15 °C±1 °C. To test geotaxis, animals were individually placed in a graduated 500 ml measuring cylinder. The top of the water was covered with a small net that animals could cling onto (increased clinging is a component of the parasitic-induced behavioural changes) (Bauer et al. 2005; Helluy & Holmes 1990). To avoid an effect of direct light, the measuring cylinder was placed on a black plastic sheet and covered by a similar sheet. The measuring cylinder was vertically graduated with 5 equal size sections in the water column. The position of the animal determined its geotaxis score: from 1 at the bottom of the water column, to 5 at the top. After 5 min of acclimatization in this apparatus, geotaxis scores were taken every 30 s during 5 min. Thus, the minimum score was 10 (positive geotaxis) and maximum 50 (negative geotaxis) for a single individual. To test phototaxis, a tube 23 cm long by 3 cm diameter was used, of which one half was completely covered in black and the other half was transparent. The tube was placed horizontally on a white surface, filled with water and the animal was introduced into the tube through a hole in the middle. After 5 min of acclimatization in this apparatus, phototaxis scores (0=dark, 1=light) were taken every 30 s during 5 min. Thus, the minimum score was 0 (photophobic) and maximum 10 (photophilic) for a single individual. Behavioural experiments were always made in the afternoon, and water in cylinders or tubes was changed after each measurement.
(b) Measurements of host reproduction and parasite identification
After behavioural experiments, each host individual was measured (height of the fourth coxal plate; Bollache et al. 2002), and the infection status of each individual was investigated. Presence of acanthocephalan parasites was assessed by dissection, and morphological identification of the species was according to Perrot-Minnot (2004) and Crompton & Nickol (1985). For females, the eggs in the brood pouch were counted and the ovaries were checked for degeneration (Bollache et al. 2002). Gonads of each individual were collected and stored in 100% ethanol at −20 °C.
Microsporidia presence and identity was determined using a PCR-RFLP protocol (Haine et al. 2004). Briefly, DNA was extracted from the gonads, with CTAB buffer followed by a phenol–chloroform purification. A fragment of the microsporidian 16S ribosomal gene was amplified with the specific primers V1f and 530r (Haine et al. 2004). The PCR product was then digested by three restriction enzymes (BglII, VspI and Bst1107I), following the manufacturer's instructions (MBI Fermentas), allowing us to identify which microsporidia species was infecting each individual G. roeseli (the three enzymes specifically digest PCR products from the following parasites: Nosema granulosis, Dictyocoela muelleri and Dictyocoela sp. (roeselum), respectively (Haine et al. 2004).
(c) Statistical analyses
Most of the comparisons were made according to host infection status, i.e. (i) individuals not infected by microsporidia or acanthocephalans; (ii) individuals infected by microsporidia only, (iii) individuals infected by acanthocephalans only; (iv) individuals infected by both microsporidia and acanthocephalans. Frequency data were analysed either with Pearson χ2-test or Fisher exact test (two tailed), where possible. Continuous data were analysed with parametric statistics when they met normality and homogeneity of variances criteria (after Box–Cox power transformation if necessary), or using non-parametric statistics otherwise (Siegel & Castellan 1988). Statistical analyses were performed using JMP software version 5 (SAS Institute, Cary, NC, USA).
3. Results
Of the two species of Acanthocephala, P. minutus was found at Chour, while P. laevis was found at Maillys. Only one species of microsporidia, Dictyocoela sp. (roeselum), was found at Chour, while all three microsporidia species were found at Maillys. Dictyocoela sp. (roeselum) was predominant at Maillys (Haine et al. 2004). We, therefore, excluded the rare individuals (<5%) infected by both N. granulosis and D. muelleri from the analyses at Maillys.
At Maillys, the prevalence of P. laevis in G. roeseli was 12.32% (n=276), with no significant difference according to host sex (17.65% in the 85 males and 9.95% in the 191 females; Fisher exact test, two tailed, p=0.08). We found 80 female hosts infected by the VT microsporidia Dictyocoela sp. (roeselum), while only eight males were infected. The difference in microsporidia prevalence between the sexes was highly significant (Fisher exact test, two tailed, p<0.0001), and, given that females are the transmitting sex for VT microsporidia, the following analyses were made in females only.
There was no significant difference in acanthocephalan prevalence between microsporidia-infected and uninfected hosts (Fisher exact test, p=0.34). Thus, there was no effect of the VT parasite infection on the distribution of the HT parasite. All females sampled had functional ovaries. Female egg number was strongly influenced by the size of the female, and by the infection by P. laevis in interaction with host size (table 1, figure 1). In fact, the relationship between female size and fecundity was significant only in uninfected females (x=−59.89+24.39 y; r2=0.52; p<0.0001) but not in females infected by P. laevis (r2=0.03; p=0.17) (figure 1). The microsporidian infection status had no significant effect on egg number, either alone or in interaction with acanthocephalan infection (table1). Thus, the microsporidia infection did not restore fecundity loss in females also infected by the HT parasite. Experiments on host phototaxis, made with 143 females homogenous for size according to their infection status (ANOVA: F3,139=2.00, p=0.12) (37 uninfected, 28 infected by P. laevis only, 41 infected by Dictyocoela sp. (roeselum) only, 37 bi-infected), revealed no significant effect of either the infection by the acanthocephalan P. laevis or the microsporidia Dictyocoela sp. (roeselum), either alone or in association (Kruskal–Wallis: H=5.85; 3 d.f.; p=0.12). Phototaxis score was slightly negatively correlated with female size (Spearman rs=−0.17, p=0.04). A comparison of host geotaxis, measured on a subset of 53 females (12 uninfected, 15 infected by P. laevis, 13 infected by Dictyocoela sp., 13 bi-infected), revealed no significant difference according to infection status (Kruskal–Wallis: H=3.10; 3 d.f.; p=0.38). These geotaxis scores were not correlated with female size (Spearman rs=0.03, p=0.80).
Table 1.
Analysis of covariance testing the effects on the female fecundity (number of eggs) of the infection by microsporidia Dictyocoela sp. and the infection by Acanthocephala P. laevis, with the female size as covariate. Both size and egg number data were Box–Cox transformed prior to analysis to meet normality assumptions.
| source | d.f. | sum of squares | F-ratio | p |
|---|---|---|---|---|
| size | 1 | 1492.71 | 19.58 | <0.0001 |
| microsporidia | 1 | 21.21 | 0.28 | 0.60 |
| Acanthocephala | 1 | 81.98 | 1.08 | 0.30 |
| size×microsporidia | 1 | 7.61 | 0.10 | 0.75 |
| size×Acanthocephala | 1 | 421.61 | 5.53 | 0.02 |
| microsporidia× Acanthocephala | 1 | 119.00 | 1.56 | 0.22 |
| error | 59 | 4497.05 |
Whole model: F6,65=6.14; p<0.0001.
Figure 1.
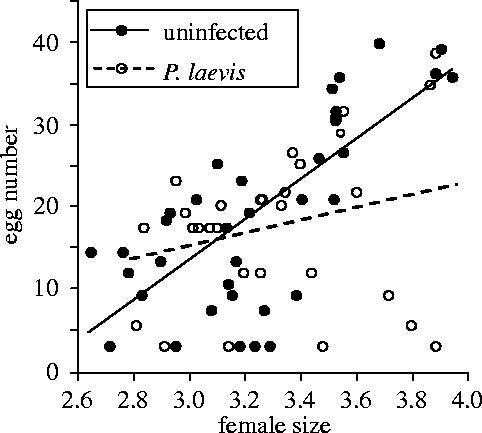
Relationship between G. roeseli female size and fecundity, according to P. laevis infection status.
At Chour, the prevalence of P. minutus in G. roeseli was 17.20% (n=157), with no difference according to host sex (10.42% in the 48 males and 20.18% in the 109 females; Fisher's exact test, two tailed, p=0.17). We found 38 female hosts infected by the VT microsporidia Dictyocoela sp. (roeselum), and no infected males (comparison between sexes: Fisher exact test, two tailed, p<0.0001). Again, the following analyses were made in females only.
There was no significant difference in acanthocephalan infection between microsporidia-infected and uninfected hosts (Fisher exact test, p=0.30). Thus, there was no effect of the microsporidia parasite infection on the distribution of the acanthocephalan parasite. We found 41 females with non-functional ovaries (i.e. castrated), all of them infected by P. minutus. However, the acanthocephalan castration was not total, since 15/56 (26.8%) of infected females possessed functional ovaries. The microsporidian infection status did not influence the degree of castration among females infected by P. minutus (Fisher exact test, p=0.75). Thus, the microsporidia infection did not restore fertility in females also infected by P. minutus. All behavioural experiments were made on 105 females, that did not differ significantly in size according to their infection status (ANOVA: F3,101=1.86; p=0.14). A comparison of host phototaxis revealed no significant effect according to infection status (Kruskal–Wallis: H=6.28; 3 d.f.; p=0.10). Phototaxis score was not correlated with female size (rs=−0.002, p=0.98). Our experiments on host geotaxis confirmed the results of Bauer et al. (2005) in that uninfected G. roeseli generally stay at the bottom of the water column while those infected by acanthocephala were more often found at the top (figure 2). Infection by microsporidia alone only slightly affected host geotaxis compared to uninfected hosts. Individuals infected by both VT and HT parasites had a geotaxis score significantly lower than those infected by acanthocephala only (p<0.0005), and comparable to uninfected and microsporidia-infected individuals (figure 2). The presence of microsporidia resulted in a decrease in the behavioural change induced by Acanthocephala and can be seen as a sabotage of host manipulation. Geotaxis score was not correlated with female size (rs=−0.165, p=0.10).
Figure 2.
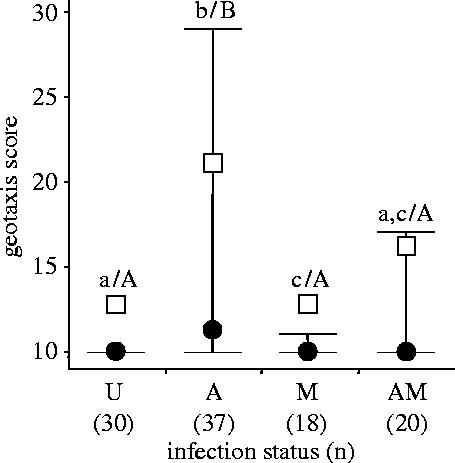
Geotaxis score in G. roeseli females, according to their infection status. U, uninfected; A, infected by the Acanthocephala P. minutus only; M, infected by microsporidia Dictyocoela sp. only; AM, infected by both acanthocephala and microsporidia. Dots are medians, squares are means, and bars are interquartiles. Kruskal–Wallis test: H=11.99; 3 d.f.; p=0.007. Bars with the same letters are not significantly different, after Siegel & Castellan post hoc test (small letters: α=0.05; capital letters: α=0.0005).
4. Discussion
This study presents contrasting results on the relationships between VT microsporidia and HT Acanthocephala. The distribution of both acanthocephalan species was not influenced by VT microsporidia infection. Others studies revealed either avoidance or resistance of VT parasites versus detrimental parasites (Omacini et al. 2001; Oliver et al. 2003). In our case, absence of these phenomena could be because the various infection groups are not affected by the same demographic processes, but the similar size of females according to their infection status suggests they belong to the same cohort. Absence of avoidance could also be because HT parasites do not impose a high enough selective pressure for the VT parasites to evolve an avoidance strategy. Acanthocephala prevalence may not be high enough to give a favourable costs versus benefits ratio for the microsporidia. The effects of acanthocephala infection on host reproduction were not influenced by microsporidian infection. We, nevertheless, found that the behavioural manipulation induced by P. minutus in hosts also co-infected by the microsporidia Dictyocoela sp. (roeselum) was weaker compared to hosts infected by P. minutus only. Such a difference was not found in populations infected by P. laevis.
In fact, while P. laevis infection results in lower fecundity in large G. roeseli females, it does not manipulate host behaviour (either phototaxis or geotaxis). The selective pressure imposed by P. laevis on the microsporidia can be considered to be low. In contrast, both castration (decreasing fertility) and geotaxis manipulation (increased probability of mortality via predation; Hynes & Nicholas 1963) induced by P. minutus are harmful for both the host and VT microsporidia (note that, as observed in G. pulex (Cézilly et al. 2000), P. minutus does not affect phototaxis behaviour of G. roeseli). Restoration of one of these two components of host fitness would, therefore, increase the chances of VT parasite maintenance in the population. This is all the more critical because Dictyocoela sp. (roeselum) vertical transmission rate is relatively low (around 55% on average, Haine et al. (2004)), and vertical transmission rate is a critical parameter for the maintenance of a VT parasite in a host population (the number of infected daughters produced by an infected mother should be higher than the number of uninfected daughters produced by an uninfected mother; Hatcher & Dunn 1995). Consequently, any decrease in host fitness would be harmful for the parasite. Thus, the microsporidian sabotage of acanthocephalan behavioural alteration might be important for the maintenance of the former parasite in the host population: it increases the probability of reproduction of the 25% remaining fertile female hosts, and increases microsporidia transmission. We do not know whether the castration is a permanent or dynamic phenomenon (i.e. if the 25% fertile females infected by the Acanthocephala are females that have not yet been castrated, or are females that have recovered their fertility), or if it occurs before or after behavioural manipulation. However, if females recover fertility after a phase of castration, the VT parasite-induced sabotage of the HT parasite's behavioural effect is even more efficient for fitness of both the host and the microsporidia.
An additional, but untested, resolution of the conflict between VT and HT parasites may involve the timing of host reproduction. For example, G. roeseli females infected by microsporidia reproduce earlier in the reproductive season relative to uninfected females (Haine et al. 2004). Haine et al. (2004) suggested that VT microsporidia may share the host's interest in investing in reproduction before infection and subsequent damage by an HT parasite. Induction of early host reproduction, followed by sabotage of the HT parasite's virulent effects (castration, increased probability of predation) may, in combination, be important for the maintenance of a VT parasite in a host population.
We have no data explaining how this sabotage may occur, but, even as speculation, we can propose that microsporidia have a disruptive effect on the neuromediator manipulation by acanthocephalans (Helluy & Holmes 1977). Vertically transmitted microsporidia are known to alter gammarid host physiology, by disrupting the production of circulating sex hormones (Rodgers-Gray et al. 2004), and it is, therefore, possible that they can alter other aspects of host physiology. Another possibility could be that microsporidia-infected animals suffer an energetic depletion that results in reduced activity, and therefore, a lower probability that bi-infected animals would swim upwards. This, however, is improbable, since Haine et al. (2004) failed to find any cost associated with microsporidia infection on other host life-history traits (e.g. reproduction). Whatever the mechanism, sabotage of acanthocephalan harmful effects is likely to be costly for the microsporidia. For example, Dictyocoela sp. (roeselum) does not interfere with P. laevis, even though infection by this acanthocephalan results in reduced host fecundity. One must assume that the costs of sabotage on microsporidia are balanced by the benefits of interfering and in the case of P. minutus, reduced fecundity combined with increased probability of predation exert a high enough selective pressure that sabotage of the behavioural manipulation is selected for.
In the context of parasite-induced behavioural manipulation, such sabotage may explain some of the variance generally observed in behavioural changes associated with infection by manipulating parasites (Thomas et al. 2002), while the rest of the variation (i.e. the remaining variation in the behaviour of hosts infected by acanthocephala only, figure 2), is likely to be a result of genetic variability in hosts, parasites, or both. In a more general way, and if future studies reveal similar effects of VT parasites on HT pathogenic parasites, conflict via transmission induced by co-infections may be of great importance. Since infection by VT microbial parasites are often asymptomatic (they have neutral, or at least, very few pathogenic effects on their hosts) or induce unusual phenotypes unrelated to host health, most of them are presently undetected and, due to advances in molecular diagnosis of microparasites, more and more studies reveal new infections (e.g. Terry et al. 2004; Zchori-Fein & Perlman 2004). Therefore, the ubiquity of some VT microbial infections (e.g. Wolbachia bacteria in invertebrates (Stouthamer et al. 1999) could be important for host protection, and, in this respect, our results strengthen those of Oliver et al. (2003).
Acknowledgments
This work was funded by an ATIP grant ‘Dynamique de la Biodiversité’ from the CNRS to T. R., and a CNRS postdoctoral position for E. H. We thank Kevin Hume and Emilie Brondani for field and laboratory assistance. We also thank Yannick Moret, Frédéric Thomas and Frank Cézilly for advice on the content of an earlier version of the manuscript, and two anonymous referees for their comments.
Footnotes
Current address: Department of Animal and Plant Sciences, University of Sheffield, Western Bank, Sheffield S10 2TN, UK.
References
- Bandi C, Dunn A.M, Hurst G.D.D, Rigaud T. Hereditary symbiosis, sex specific virulence and reproductive parasitism. Trends Parasitol. 2001;17:88–94. doi: 10.1016/s1471-4922(00)01812-2. 10.1016/S1471-4922(00)01812-2 [DOI] [PubMed] [Google Scholar]
- Bauer A, Trouvé S, Grégoire A, Bollache L, Cézilly F. Differential influence of Pomphorhynchus laevis (Acanthocephala) on the behaviour of native and invader gammarid species. Int. J. Parasitol. 2000;30:1453–1457. doi: 10.1016/s0020-7519(00)00138-7. 10.1016/S0020-7519(00)00138-7 [DOI] [PubMed] [Google Scholar]
- Bauer, A., Haine, E. R., Perrot-Minnot, M.-J. & Rigaud, T. 2005 The acanthocephalan parasite Polymorphus minutus alters the geotactic and clinging behaviours of two sympatric amphipods hosts: the native Gammarus pulex and the invasive Gammarus roeseli J. Zool in press.
- Bethel W.M, Holmes J.C. Increased vulnerability of amphipods to predation owing to altered behaviour induced by larval acanthocephalans. Can. J. Zool. 1977;55:110–116. doi: 10.1139/z77-013. [DOI] [PubMed] [Google Scholar]
- Bollache L, Rigaud T, Cézilly F. Effects of two acanthocephalan parasites on the fecundity and pairing status of female Gammarus pulex (Crustacea: Amphipoda) J. Invertebr. Pathol. 2002;79:102–110. doi: 10.1016/s0022-2011(02)00027-7. 10.1016/S0022-2011(02)00027-7 [DOI] [PubMed] [Google Scholar]
- Brown S.P, Grenfell B.T. An unlikely partnership: parasites, concomitant immunity and host defence. Proc. R. Soc. B. 2001;268:2543–2549. doi: 10.1098/rspb.2001.1821. 10.1098/rspb.2001.1821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S.P, Hochberg M.E, Grenfell B.T. Does multiple infection select for raised virulence? Trends Microbiol. 2002;10:401–405. doi: 10.1016/s0966-842x(02)02413-7. 10.1016/S0966-842X(02)02413-7 [DOI] [PubMed] [Google Scholar]
- Cézilly F, Grégoire A, Bertin A. Conflict between co-occurring manipulative parasites? An experimental study of the joint influence of two acanthocephalan parasites on the behaviour of Gammarus pulex. Parasitology. 2000;120:625–630. doi: 10.1017/s0031182099005910. 10.1017/S0031182099005910 [DOI] [PubMed] [Google Scholar]
- Crompton D.W.T, Nickol B.B. Cambridge University Press; Cambridge: 1985. Biology of the Acanthocephala. [Google Scholar]
- Ebert D. The evolution and expression of virulence. In: Stearns S.C, editor. Evolution in health and disease. Oxford University Press; Oxford: 1999. pp. 161–172. [Google Scholar]
- Ebert D, Herre E.A. The evolution of parasitic diseases. Parasitol. Today. 1996;12:96–101. doi: 10.1016/0169-4758(96)80668-5. 10.1016/0169-4758(96)80668-5 [DOI] [PubMed] [Google Scholar]
- Fine P.E.M. Vectors and vertical transmission: an epidemiologic perspective. Ann. N Y Acad. Sci. 1975;266:173–194. doi: 10.1111/j.1749-6632.1975.tb35099.x. [DOI] [PubMed] [Google Scholar]
- Frank S.A. Host-symbiont conflict over the mixing of symbiotic lineages. Proc. R. Soc. B. 1996;263:339–344. doi: 10.1098/rspb.1996.0052. [DOI] [PubMed] [Google Scholar]
- Friedli J, Bacher S. Mutualistic interaction between a shoot-base boring weevil and a rust fungus, two parasites of the weed creeping thistle. Oecologia. 2001;129:571–576. doi: 10.1007/s004420100763. 10.1007/s004420100763 [DOI] [PubMed] [Google Scholar]
- Haine E.R, Brondani E, Hume K.D, Perrot-Minnot M.-J, Gaillard M, Rigaud T. Coexistence of three microsporidia parasites in populations of the freshwater amphipod Gammarus roeseli: evidence for vertical transmission and positive effect on reproduction. Int. J. Parasitol. 2004;34:1137–1146. doi: 10.1016/j.ijpara.2004.06.006. 10.1016/j.ijpara.2004.06.006 [DOI] [PubMed] [Google Scholar]
- Hatcher M.J, Dunn A.M. Evolutionary consequences of sex ratio distortion by cytoplasmically inherited feminizing factors. Phil. Trans. R. Soc. B. 1995;348:445–456. [Google Scholar]
- Helluy S, Holmes J.C. Serotonin, octopamine, and the clinging behavior induced by the parasite Polymorphus paradoxus (Acanthocephala) in Gammarus lacustris (Crustacea) Can. J. Zool. 1990;68:1214–1220. [Google Scholar]
- Hughes W.O.H, Boomsma J.J. Let your enemy do the work: within-host interactions between two fungal parasites of leaf-cutting ants. Proc. R. Soc. B. 2004;271:S104–S106. doi: 10.1098/rsbl.2003.0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes H.B.N. The ecology of Gammarus duebeni lilljeborg and its occurrence in fresh water in western Britain. J. Anim. Ecol. 1954;23:38–84. [Google Scholar]
- Hynes H.B.N, Nicholas W.L. The importance of the acanthocephalan Polymorphus minutus as a parasite of domestic ducks in the United Kingdom. J. Helminthol. 1963;37:185–198. doi: 10.1017/s0022149x00003771. [DOI] [PubMed] [Google Scholar]
- Lafferty K.D. The evolution of trophic transmission. Parasitol. Today. 1999;15:111–115. doi: 10.1016/s0169-4758(99)01397-6. 10.1016/S0169-4758(99)01397-6 [DOI] [PubMed] [Google Scholar]
- Lafferty K.D, Thomas F, Poulin R. Evolution of host phenotype manipulation by parasites and its consequences. In: Poulin R, Morand S, Skorping A, editors. Evolutionary biology of host parasite relationships: theory meets reality. Elsevier; Amsterdam: 2000. pp. 117–127. [Google Scholar]
- Nowak M.A, May R.M. Superinfection and the evolution of parasite virulence. Proc. R. Soc. B. 1994;255:81–89. doi: 10.1098/rspb.1994.0012. [DOI] [PubMed] [Google Scholar]
- Oliver K.M, Russell J.A, Moran N.A, Hunter M.S. Facultative bacterial symbionts in aphids confer resistance to parasitic wasps. Proc. Natl Acad. Sci. USA. 2003;100:1803–1807. doi: 10.1073/pnas.0335320100. 10.1073/pnas.0335320100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omacini M, Chaneton E.J, Ghersa C.M, Mueller C.B. Symbiotic fungal endophytes control insect host–parasite interaction webs. Nature. 2001;409:78–81. doi: 10.1038/35051070. 10.1038/35051070 [DOI] [PubMed] [Google Scholar]
- O'Neill S.L, Hoffmann A.A, Werren J.H. Oxford University Press; Oxford: 1997. Influential passengers. [Google Scholar]
- Perrot-Minnot M.J. Larval morphology, genetic divergence, and contrasting levels of host manipulation between forms of Pomphorhynchus laevis (Acanthocephala) Int. J. Parasitol. 2004;34:45–54. doi: 10.1016/j.ijpara.2003.10.005. 10.1016/j.ijpara.2003.10.005 [DOI] [PubMed] [Google Scholar]
- Petnay T.N, Andrews R.H. Multiparasite communities in animals and humans: frequency, structure and pathogenic significance. Int. J. Parasitol. 1998;28:377–393. doi: 10.1016/s0020-7519(97)00189-6. 10.1016/S0020-7519(97)00189-6 [DOI] [PubMed] [Google Scholar]
- Poulin R, Combes C. The concept of virulence: interpretations and implications. Parasitol. Today. 1999;15:474–475. doi: 10.1016/s0169-4758(99)01554-9. 10.1016/S0169-4758(99)01554-9 [DOI] [PubMed] [Google Scholar]
- Poulin R, Nichol K, Latham A.D.A. Host sharing and host manipulation by larval helminths in shore crabs: cooperation or conflict? Int. J. Parasitol. 2003;33:425–433. doi: 10.1016/s0020-7519(03)00002-x. 10.1016/S0020-7519(03)00002-X [DOI] [PubMed] [Google Scholar]
- Read A.F, Taylor L.H. The ecology of genetically diverse infections. Science. 2001;292:1099–1102. doi: 10.1126/science.1059410. 10.1126/science.1059410 [DOI] [PubMed] [Google Scholar]
- Rigaud T, Haine E.R. Conflict between co-occurring parasites as a confounding factor in manipulation studies? Behav. Proc. 2005;68:259–262. doi: 10.1016/j.beproc.2004.09.005. 10.1016/j.beproc.2004.09.005 [DOI] [PubMed] [Google Scholar]
- Rodgers-Gray T.P, Smith J.E, Ashcroft A.E, Isaac R.E, Dunn A.M. Mechanisms of parasite-induced sex reversal in Gammarus duebeni. Int. J. Parasitol. 2004;34:747–753. doi: 10.1016/j.ijpara.2004.01.005. 10.1016/j.ijpara.2004.01.005 [DOI] [PubMed] [Google Scholar]
- Siegel S, Castellan N.J. Nonparametric statistics for the behavioural sciences. 2nd edn. McGraw-Hill; New York: 1988. [Google Scholar]
- Smith J.E, Dunn A.M. Transovarial transmission. Parasitol. Today. 1991;7:146–148. doi: 10.1016/0169-4758(91)90283-t. 10.1016/0169-4758(91)90283-T [DOI] [PubMed] [Google Scholar]
- Stouthamer R, Breeuwer J.A.J, Hurst G.D.D. Wolbachia pipentis: microbial manipulator of arthropod reproduction. Annu. Rev. Microbiol. 1999;53:71–102. doi: 10.1146/annurev.micro.53.1.71. 10.1146/annurev.micro.53.1.71 [DOI] [PubMed] [Google Scholar]
- Terry R.S, Smith J.E, Sharpe R.G, Rigaud T, Littlewood D.T.J, Ironside J.E, Rollinson D, Bouchon D, MacNeil C, Dick J.T.A, et al. Widespread vertical transmission and associated host sex ratio distortion within the eukaryotic phylum Microspora. Proc. R. Soc. B. 2004;271:1783–1789. doi: 10.1098/rspb.2004.2793. 10.1098/rspb.2004.2793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas F, Fauchier J, Lafferty K.D. Conflict of interest between a nematode and a trematode in an amphipod host: test of the ‘sabotage’ hypothesis. Behav. Ecol. Sociobiol. 2002;51:296–301. 10.1007/s00265-001-0442-2 [Google Scholar]
- van Baalen M, Sabelis M.W. The dynamics of multiple infection and the evolution of virulence. Am. Nat. 1995;146:881–910. 10.1086/285830 [Google Scholar]
- Zchori-Fein E, Perlman S.J. Distribution of the bacterial symbiont Cardinium in arthropods. Mol. Ecol. 2004;13:2009–2016. doi: 10.1111/j.1365-294X.2004.02203.x. 10.1111/j.1365-294X.2004.02203.x [DOI] [PubMed] [Google Scholar]


