Abstract
Many diseases are caused by parasites with complex life cycles that involve several hosts. If parasites cope better with only one of the different types of immune systems of their host species, we might expect a trade-off in parasite performance in the different hosts, that likely influences the evolution of virulence. We tested this hypothesis in a naturally co-evolving host–parasite system consisting of the tapeworm Schistocephalus solidus and its intermediate hosts, a copepod, Macrocyclops albidus, and the three-spined stickleback Gasterosteus aculeatus. We did not find a trade-off between infection success in the two hosts. Rather, tapeworms seem to trade-off adaptation towards different parts of their hosts' immune systems. Worm sibships that performed better in the invertebrate host also seem to be able to evade detection by the fish innate defence systems, i.e. induce lower levels of activation of innate immune components. These worm variants were less harmful for the fish host likely due to reduced costs of an activated innate immune system. These findings substantiate the impact of both hosts' immune systems on parasite performance and virulence.
Keywords: Schistocephalus solidus, virulence, innate immunity, multi-host parasite, host–parasite coevolution, trade-off
1. Introduction
In many theoretical models, virulence is assumed to be a major factor that shapes host–parasite coevolution. Within the field of evolutionary biology, virulence is defined as the reduction in host fitness following parasitic infection (Hansen & Koella 2003). Since parasites exploit their hosts, such reduction can be seen as an unavoidable side effect of growth or multiplication of parasites. Therefore, virulence is closely connected with parasite fitness. Moreover, reduction in host fitness can also result from the activation of the host immune system. Immunity has been shown to be costly, owing to energetic demands and immunopathological effects (Moret & Schmid-Hempel 2000).
The question as to which factors influence the evolution of virulence, especially which factors restrict virulence is of crucial importance to basic research as well as applied issues and has been addressed in many theoretical studies (Bull 1994; Frank 1996; Day 2002). However, these models concentrate on the evolution of virulence in single-host systems (Frank 1996; Galvani 2003), even though many parasites, of medical and veterinary relevance, have complex life cycles that involve several host species (Parker et al. 2003). A recent theoretical study (Gandon 2004) stresses that for understanding the evolution of virulence in complex life cycles, more factors need to be taken into account, especially potential constraints among different parasite traits, both between and within hosts. It is likely that optimal exploitation of one host species leads to a reduced ability to exploit another host. The expected negative correlation in the fitness of a parasite in the different host species could genetically be based on antagonistic pleiotropy, since a gene that enhances fitness in one host could decrease fitness in the other host. In one of the few empirical studies in this field, Davies et al. (2001) found a trade-off in the reproductive success in Schistosoma mansoni across the mammalian and molluscan hosts. Further evidence comes from serial passage experiments (SPEs), where parasites are subsequently cycled through one host species (for more detail see Ebert 1998). In SPEs, virulence normally increases when pathogens are passaged through one host species, but may decrease when intermediate hosts are included again (Ebert 1998).
These studies rarely include the physiological factors that may affect the evolution of parasite virulence such as host immunity. Host immunity can be regarded as a double-edged sword. On the one hand, immunity can restrain parasite growth or multiplication and thereby reduce virulence. On the other hand, immune reactions themselves are costly to produce, not only because they need energy, but also because they may harm the hosts' own tissues (cf. immunopathology) (Sheldon & Verhulst 1996; Moret & Schmid-Hempel 2000; Rolff & Siva-Jothy 2003). Thus, virulence will also depend on the type and degree of host immune responses and their pathological side effects.
In one of the few studies that consider host immunity as a selection pressure that may influence virulence, Mackinnon & Read (2004) recently showed that parasite sibships of Plasmodium chabaudi became more virulent when they evolved in immunized as compared to naive mice. Multi-host life cycles consist of several invertebrate and vertebrate hosts, all with different immune systems. The divergent activation of these immune systems by the parasite may shape and constrain virulence because multi-host parasites may be forced to trade-off between different immune evasive strategies.
In the current paper, we provide a test of this hypothesis using a natural co-evolved host–parasite system. As such, our study contrasts with previous work (Davies et al. 2001; Gower & Webster 2004), in which fitness parameters were measured in parasites adapted to laboratory conditions for many generations. We rather used parasites and hosts that co-occurred in a natural population. Our parasite, the tapeworm Schistocephalus solidus, is confronted with the immune systems of the copepod Macrocyclops albidus, which is a tiny crustacean, the stickleback fish Gasterosteus aculeatus and, as a final host, any species of fish-eating bird. Since the intermediate hosts are the major source for the uptake of resources and are used as a vehicle to reach the next host, an infection with S. solidus leads to serious fitness reduction in both intermediate hosts (Arme & Owen 1967; Wedekind & Milinski 1996; van der Veen & Kurtz 2002; Kurtz & Franz 2003). Selection pressure on defence mechanisms in the host is thus expected to be high and the cestode will therefore be confronted with the problem of coping with competent immune systems in both hosts. We examined whether individuals of S. solidus are genetically constrained in their ability to evade the crustacean and the fish equally well. For this test, we used sibships of tapeworms, as a surrogate of different genotypes, and analysed their performance in both hosts using infectivity and growth as fitness parameters. We expected a trade-off in parasite fitness in the first and the second host, i.e. those sibships that perform well in the copepod might have disadvantages in the fish. Since we suspected performance in the hosts to be mediated through host immune responses, we further analysed parameters that are indicative of the activation status of the immune system of the stickleback host. We also measured the body condition of the fish as an estimate of the damage to the host after infection, to obtain an approximation of the virulence of the parasites.
2. Material and Methods
(a) Study system
To complete its life cycle, the tapeworm S. solidus needs two intermediate hosts, a cyclopoid copepod and the three-spined stickleback G. aculeatus, and one definitive host, which is any species of fish-eating bird. Infestation of intermediate and definitive hosts occurs through ingestion of the parasite or infected intermediate host, respectively. The worms grow only in the two intermediate hosts, but reproduce during a short time span of several days within the bird's gut (Clarke 1954).
(b) Parasites
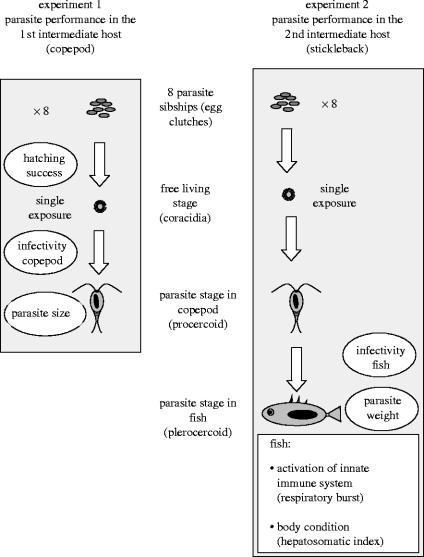
Schistocephalus solidus tapeworms were dissected in February 2003 from sticklebacks, which were caught during autumn 2001 from the brackish ‘Binnenwasser’ near Neustadt, northern Germany, and thereafter kept in large tanks with food ad libitum. The worms (i.e. plerocercoids) were bred in an in vitro system that replaces the bird's gut (Smyth 1946; Wedekind 1997). Worm pairs were matched by body weight to guarantee outcrossing (Lüscher & Milinski 2003). Offspring from each pair will here be referred to as ‘parasite sibship’. A total of eight sibships were used for the experiments (for details on the experimental design, see figure 1). Eggs were stored at 4 °C in the dark. For hatching, eggs were transferred to 20 °C for three weeks and then exposed to light on the day before usage (Dubinina 1966).
Figure 1.
Experimental design. Measures obtained from the tapeworms are shown inside ovals, stickleback traits in a square.
Hatching success was determined three months after exposure to light, to allow all viable larvae to hatch. To this end, the proportion of eggs with an open or removed operculum was determined in 100 eggs per sibship (Swiderski 1994; Schjørring 2004).
(c) Infection of the first intermediate host: copepods
Macrocyclops albidus copepods were kept in culture in the laboratory as described previously (van der Veen & Kurtz 2002). The culture originated from 80 individuals from a small river ‘Kremper Au’ (Neustadt, Germany), which is connected to the Binnenwasser, the source of the parasite population. Thirty days before the start of the experiments, 10 tanks were initiated with 50 adult females each.
Two days before exposure to one tapeworm larva (i.e. coracidium) each, 1152 adult male copepods were filtered from the culture tanks and transferred into individual wells of 24-well plates with 2 ml of water. Thereafter, each copepod was fed ad libitum with five freshly hatched nauplii of Artemia salina three times per week.
Copepods were kept at 20 °C and 16 : 8 light : dark cycle. Ten days post-infection, infection status of each copepod was determined microscopically, without knowledge of parasite sibship (van der Veen & Kurtz 2002).
Two days after checking for parasites, infected copepods were measured (van der Veen 2003) and dissected to obtain the procercoids, which were killed by adding to the water a drop of 20% formalin in phosphate-buffered saline (PBS). For measurement of procercoid size (i.e. area), an image was taken with a video camera and analysed with the image analysis program image J 1.31v (Wayne Rasband, National Institutes of Health, USA). From each procercoid, two pictures were taken to check for repeatability of the measurements, which was 99.6% (calculated using variance components), and the mean of those two measurements was used.
(d) Infection of the second intermediate host: sticklebacks
For infection of sticklebacks, S. solidus infected copepods were obtained as described above, with the exception that, instead of adult males, young copepod stages were used, which are more susceptible (van der Veen & Kurtz 2002). This reduces selection that might occur in the first intermediate host. Such selection could be relevant, since from clutches that perform badly in the copepod host, only the relatively best genotypes might manage to reach the fish host, which could lead to a non-random representation of parasite qualities in the fish host. Infection of the 960 copepods was screened 12 days post-infection.
Stickleback G. aculeatus hosts were bred in the laboratory from adults, derived from the same Binnenwasser population as the parasites. Offspring from four stickleback pairs, all hatched July 2002, were raised in family tanks with 15–25 fish each. During the experiment, 176 fishes were housed in one of two individual compartments of a tank (21×35×25 cm), without any contact (visual or olfactory). The tanks were randomly distributed across the shelves in the aquaria room (18 °C and 16 : 8 light : dark cycle). Fish were fed ad libitum three times per week with frozen chironomids.
Two days before exposure to infected copepods, stickleback weight (to the nearest 0.1 mg) and size (from the snout to the base of the tail, to the nearest millimetre) were determined and a condition factor (cf) (Frischknecht 1993) calculated as cf=100×W/Lb (fish weight W in g, fish length L in cm and the exponent from the linear regression analysis b=2.495 of log-transformed values of W and L). Fish were transferred into small tanks with 2 l water and starved to enhance consumption of copepods. On the day of exposure, each fish was given one copepod that was infected with one parasite larva. Individuals of the different fish families were randomly assigned to the eight different parasite sibships, and the combination of fish family and parasite sibship was balanced. Two days post-infection, the fish were returned to their larger tanks.
One-week post-infection half of the fish were dissected and the body cavity screened for tapeworms by rinsing tissues in PBS. At this stage, the worms are still translucent and very small. The parasites were fixed and measured as described for the procercoids. However, repeatability of this measure turned out to be low, due to the flexible shape of the small plerocercoids. We thus did not consider this measure for further analyses. Remaining fish were killed five weeks post-infection. At this time, the tapeworms are much bigger and their weight could be determined to the nearest 0.1 mg. We also determined again fish length, weight and condition. Moreover, liver weight was determined (to the nearest 0.1 mg) and a hepatosomatic index (IH) calculated as 100×liver weight/fish weight. IH is a correlate of medium term energy reserves, thus a good measure of fish metabolic body condition (Chellappa et al. 1995).
(e) Immunity of sticklebacks
We analysed the activity of the innate immune system of the sticklebacks, using leucocytes from the head kidney, which is the major immune organ of bony fish (Zapata et al. 1996). Unfortunately, we did not possess a method to assess the adaptive immune system at the time of the experiment.
Leucocytes were processed as described in Kurtz et al. (2004) with the following modifications: during washing of the cells, centrifugation was performed at 550g for 10 min at 4 °C. Differential cell counts were obtained on a Becton Dickinson FACSCalibur flow cytometer using the cellquest pro 4.02 software for acquisition and analysis. All samples were supplemented with propidium iodide (2 mg l−1, Sigma Aldrich) to detect dead cells. Forward- and sidescatter values (FSC/SSC characteristics) of at least 10 000 cells were acquired in linear mode; fluorescence intensities at wavelengths of 530 and 585 nm were acquired at log-scale. Cellular debris with low FSC characteristics and dead cells (propidium iodide-positive) were excluded from further evaluation. Different cellular subsets were identified according to their characteristic FSC/SSC profiles cf. Scharsack et al. (2004), and denoted as lymphocytes (low FSC/low SSC) and granulocytes (high FSC/high SSC). For the adjustment of cell numbers for the subsequent in vitro respiratory burst assay, absolute cell counts were determined with the standard cell dilution assay (Pechhold et al. 1994) in a modified form, by addition of 2×105 green fluorescent particles (4 μm, Polyscience, USA) to each tube, as a standard for counting (Scharsack et al. 2004).
As a functional estimate of innate immune activity, we quantified the respiratory burst reaction. During the respiratory burst, reactive oxygen intermediates are generated to kill pathogens. We analysed the respiratory burst (relative luminescence units (RLU)) associated with phagocytosis of zymosan particles in vitro in a lucigenin-enhanced chemiluminescence assay (Scott & Klesius 1981). For this assay, the cell density was adjusted to 1.25×106 live cells per ml, corresponding to 2×105 cells per assay (Kurtz et al. 2004). Unfortunately, we did not obtain enough cells for the immune assays from all fish so that data from 153 individuals went into the final analyses.
(f) Heritability and covariance between parasite traits
For the estimates of heritability and genetic correlation between traits, an analysis of full-sib families (parasite sibships) was performed. Such designs only give an upper estimate of heritability, because dominance variance and common environment effects cannot be excluded (Falconer & Mackay 1996; Roff 1997). In our system, the latter is assumed to be negligible because once inside their host, siblings do not encounter a common environment any more. To estimate the heritability for the continuous and threshold traits, we performed an ANOVA (analysis of variance) and calculated the standard errors for unequal family size as described in Roff (1997).
When assessing the covariance between traits in full-sib family correlations, the ‘family’ component of variance contains additive, non-additive and interaction genetic covariance plus maternal covariance, which cannot be separated (Via 1984; Falconer & Mackay 1996; Roff 1997). Thus, all correlations computed here are genetic correlations in a broad sense, which can serve only as a first step towards a more precise estimation.
Some traits are measured in different hosts (environments). Therefore, the analysis cannot be performed on the same individual. We applied an approach, first suggested by Via (1984), where the genetic correlation can be estimated by using the Pearson product–moment correlation between family means (also suggested by Roff (1997)). However, we preferred to calculate a more conservative test, the Spearman rank correlation, to account for the relatively small sample size of eight sibships, and the potential non-normality of the data.
(g) Data analysis
We checked for differences in infectivity of the parasites in the two intermediate hosts with log-linear models (all effect variables mentioned were included in one model for each host). To analyse which factors influenced parasite size, an ANOVA contained parasite size in the copepod as response and parasite sibship and copepod size as effect variables. For parasite size in the fish an ANOVA was calculated with worm weight as the response and parasite sibship, fish family, fish gender and fish weight before infection as effect variables.
Only data from infected animals were considered in subsequent analyses. Immune and fitness data of two fish had to be excluded: their livers were extremely swollen and yellow and they also produced extreme values in the respiratory burst reaction and hepatosomatic index. This jointly suggested that besides S. solidus they suffered from another illness. In general, all interactions were insignificant and thus removed from the models, except for the analysis of the parasite size in the fish where we did not include interactions due to saturation of the model. All test statistics refer to two-tailed tests. We considered effects significant at a level of p<0.05. Analyses were performed with the jmp v. 5.0.1.2 (SAS) software for Macintosh.
3. Results
(a) Heritability and covariance between parasite traits
Three heritability estimates obtained from full-sib analysis were significantly larger than zero (table 1). Heritability of infection success and size of the parasites in the copepod host was 0.65 and 0.29, respectively, which indicates a considerable additive genetic component to the phenotypic variance. Genetic (full-sib) correlations between traits within and across hosts are shown in table 2 and addressed in more detail below.
Table 1.
Broad sense heritability estimates (h2±s.e.) for parasite traits in the two intermediate hosts.
| trait | full-sib h2 | n | N | |
|---|---|---|---|---|
| hatching | 0.00±0.00 | 800 | 8 | |
| copepod (procercoid) | infectivity | 0.65±0.01 | 1072 | 8 |
| size | 0.29±0.01 | 332 | 8 | |
| fish (plerocercoid) | infectivity | −0.09±0.01 | 170 | 8 |
| weight | −0.35±0.04 | 21 | 7 | |
| respiratory burst | 0.17±0.04 | 27 | 8 | |
| hepatosomatic index | −0.28±0.02 | 29 | 8 |
Sample size (n) and number of sibships (N) are given. Estimates that are significantly larger than zero are indicated in bold type.
Table 2.
Genetic correlations for traits within and among hosts for full-sib families (parasite sibships).
| hatching | infectivity in copepod | size in copepod | infectivity in fish | weight in fish | RLU in fish | hepatosomatic index | |
|---|---|---|---|---|---|---|---|
| hatching | 1 | 0.04 [0%] | 0.11 [1%] | −0.86* [74%] | 0.59* [35%] | −0.05 [0%] | −0.10 [1%] |
| infectivity in copepod | 1 | 0.74* [54%] | 0.20 [4%] | 0.18 [3%] | −0.95* [90%] | 0.81* [66%] | |
| size in copepod | 1 | 0.04 [0%] | 0.39 [15%] | −0.71* [51%] | 0.43 [18%] | ||
| infectivity in fish | 1 | −0.79* [62%] | −0.12 [1%] | 0.12 [1%] | |||
| weight in fish | 1 | 0 [0%] | −0.14 [2%] | ||||
| respiratory burst in fish | 1 | −0.88* [78%] | |||||
| hepatosomatic index | 1 |
r2 from the family mean correlation is shown in square brackets as an estimate of the proportion of genetic variance attributable to effects of the same or linked genetic factors. *p<0.05.
(b) Fitness of tapeworms in their first intermediate host, the copepod
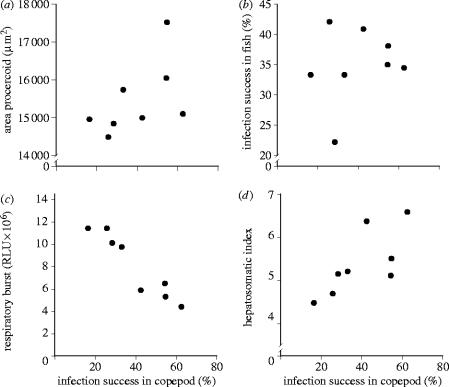
In total, 1072 male copepods survived the experiment and were included in the analysis. Mortality of copepods (0.8%) did not depend on the parasite sibship they had been exposed to (Likelihood Ratio (LR) χ72=3.098, p=0.88). On average, 39.83% of the copepods were infected. The infection rate varied significantly among parasite sibships (LR χ72=110.61, p<0.0001). Body size of the tapeworm larvae in the copepod (i.e. procercoid size) was significantly influenced by parasite sibship and by copepod size (effect of sibship: F7,323=8.20, p<0.0001 and copepod size: F1,323=8.52, p=0.0038). Worms that originated from sibships with a higher infection success also reached a larger size in the copepod: rs=0.74, N=8, p=0.0366 (figure 2a).
Figure 2.
Relation between infectivity (%) of sibships of the tapeworm S. solidus in its first intermediate host (the copepod M. albidus) and (a) worm size 12 d.p.i. in the copepod, (b) infectivity in the second intermediate host (the stickleback fish G. aculeatus), (c) stickleback innate immune response (respiratory burst 7 d.p.i.) and (d) stickleback body condition (hepatosomatic index 7 d.p.i.). Each data point shows the mean of a parasite sibship, i.e. 22–54 individuals in the copepod host and 2–6 individuals in the fish host, respectively.
(c) Fitness of tapeworms in their second intermediate host, the stickleback
Of the 176 fish that had been exposed to infected copepods, 14 (7.9%) died before the end of the experiment and two fish were excluded from parts of the analyses because they suffered from another illness (see §2 for details). Mortality of fish was independent of the parasite sibship (LR χ72=5.83, p=0.56). In total, 33.95% of the fish were infected. The likelihood of infection was not significantly explained by any of the following factors included into the model: parasite sibship (LR χ72=2.37, p=0.94), fish family (LR χ32=6.72, p=0.08), fish gender (LR χ12=0.30, p=0.59), and fish condition factor before infection (LR χ12=0.76, p=0.38). Size of parasite (N=21) was not significantly influenced by any of the effect variables in the model: parasite sibship (F6,9=1.31, p=0.34), fish family (F3,9=2.23, p=0.15), fish gender (F1,9=3.02, p=0.17) or weight pre-infection (F1,9=0.42, p=0.54). In contrast to the first intermediate host, where a positive correlation between the infectivity and the mean procercoid size of the tapeworm sibships had been found, we observed a negative correlation between infectivity and mean tapeworm (i.e. plerocercoid) size in the fish host (rs=−0.79, N=7, p=0.0362; table 2).
(d) Trade-offs in fitness between the different developmental stages of the parasite
Parasite sibships varied in hatching success from egg clutches (LR χ72=43.31, p<0.0002). We may thus expect that sibships, which did not hatch well, might compensate for this disadvantage later during their life, i.e. by being better in infecting the first or second intermediate host. We found such a relationship with regard to infection of the fish host (rs=−0.86, N=8, p=0.0061), but not in the copepod host (rs=0.04, N=8, p=0.93; table 2).
We expected to find a trade-off between the infection success of parasite genotypes in the copepod and in the fish host. However, among worm sibships there was no negative correlation between these traits (rs=0.20, N=8, p=0.63) (figure 2b). Another factor that could have influenced infectivity in the fish is the size of the procercoid; bigger parasites could potentially have an advantage when infecting a fish. We could not show that in this experiment: rs=0.04, N=8, p=0.93. Furthermore, there was no significant relation between mean procercoid size (in the copepod) and mean plerocercoid weight (in the fish) among parasite sibships (rs=0.39, N=7, p=0.38; table 2).
(e) Parasite virulence and host immunity
Tapeworm sibships varied in the intensity of activation of their fish hosts' innate immune response, as estimated from the respiratory burst reaction. Since previous work has shown that the impact of S. solidus on the fish immune system could be assessed best with the respiratory burst reaction (Kurtz et al. 2004), we concentrated on results from this assay in further analyses. This measure correlated with another measure for the activity of the innate immune system, the G : L ratio (F1,151=22.97, p<0.0001, r=0.36), i.e. the proportion of granulocytes in relation to lymphocytes. Moreover, the use of the G : L ratio as an innate immune measure instead of the respiratory burst would lead to qualitatively similar conclusions, although effects are more pronounced with respiratory burst as measure.
Sibships better at infecting the copepods also elicited a weaker innate immune reaction (respiratory burst) 7 days post-infection (p.i.) in the stickleback (rs=−0.95, N=8, p=0.0003) (figure 2c). Moreover, fish infected with these genotypes were in better condition as estimated from the hepatosomatic index (rs=0.81, N=8, p=0.0149) (figure 2d). However, 35 days p.i., the correlation between infectivity in the copepod and fish immune stimulation disappeared (rs=0.21, N=7, p=0.64), while the correlation with the hepatosomatic index remained as a trend (rs=0.75, N=7, p=0.0522). This was not due to a positive correlation between plerocercoid weight and hepatosomatic index (rs=−0.143, N=7, p=0.76), showing that fish were not in worse condition in consequence of infection with parasites that may exploit them more.
4. Discussion
Sibships of the tapeworm S. solidus varied considerably in fitness parameters in the first and the second intermediate host, copepods and sticklebacks. Analysis of heritability indicates that such variation was partly genetic, especially for traits measured in the copepod. It should be noted, however, that a full-sib design does not control for common environmental, dominance and maternal effects. For parasite traits in the fish intermediate host, most heritability estimates were not significantly greater than zero. This might be caused by the lower sample sizes of parasites in the fish, but could also be a consequence of strong phenotypic variation in parasite traits that is caused by the fish host. This interpretation is corroborated by previous studies showing that phenotypic and genetic factors of the stickleback influence growth of S. solidus (Kurtz et al. 2004; Barber 2005).
Contrary to our initial expectation, we did not find that parasite sibships, which performed weakly in the copepod host, were superior in the fish host. This absence of a trade-off, combined with the indication that parasite performance is partly based on genetic variation, raises the question as to what might maintain apparently inferior genotypes in the population. One possible explanation is the above-mentioned lack of heritability of traits measured in the fish host. Another possibility is that trade-offs might occur with parasite performance in the definitive, bird host, rather than the second intermediate host. This interpretation is especially intriguing since previous studies found trade-offs in fitness between intermediate and definitive hosts (Davies et al. 2001; Gower & Webster 2004). However, we consider this interpretation unlikely in our particular system, where it is fairly safe to assume that the definitive host is rather unimportant for parasite fitness. First, S. solidus appears to cause little harm to the bird (Clarke 1954; Tierney & Crompton 1992), which leads to the assumption that there is no relevant selection against S. solidus in the definitive host. Second, and most importantly, the worms do not grow any more in the bird (Clarke 1954). They seem to entirely rely on the resources obtained from the intermediate hosts for the short phase of reproduction in the bird's gut (Tierney & Crompton 1992; Schärer & Wedekind 1999). This implies that worm size achieved in the intermediate hosts determines establishment and egg production in the definitive host (Tierney & Crompton 1992). Therefore, the relevance of the two intermediate hosts for parasite fitness in this system is comparable to the intermediate and definitive hosts in other systems.
The arguably most important reason for the absence of a trade-off in infectivity between the two intermediate hosts is revealed by a closer look at the interaction of the tapeworms with their hosts' immune systems. We here propose that worm sibships that are well adapted to components of the innate immune system will benefit in both host species. This proposition is not unrealistic, since invertebrate and vertebrate innate immunological receptors recognize similar pathogen-associated molecular patterns, such as peptidoglycans (Janeway et al. 1999; Salzet 2001; Janeway & Medzhitov 2002; Guan et al. 2004). Worms that remain undetected by the copepod immune system may thus represent worms that are well adapted to evade innate immune systems in general. However, such worms might have a disadvantage when interacting with fish adaptive immunity that will normally displace innate immunity later during an infection. This hypothesis is supported by our observations that (i) tapeworm sibships, which are highly infective to the copepod, elicit a weaker innate immune response, as estimated from the respiratory burst reaction, at an early stage of fish infection (7 days p.i.); (ii) this relation disappeared at a later stage of the infection (35 days p.i.); (iii) there was a negative correlation between the infectivity of a sibship (that seems dominated by innate defence) and its mean plerocercoid size 35 days p.i. (that might be influenced by adaptive immunity). Unfortunately, we do not have any direct measures of adaptive immunity in the current experiment. However, we have reason to believe that adaptive immunity influences worm size in the stickleback, since size (but not the likelihood of infection per se) has previously been shown to depend on stickleback MHC genetics, which are central to the adaptive immune system (Kurtz et al. 2004). Taken together, these results indicate that parasites with complex life cycles could face a dilemma when adapting to different components of their hosts' immune systems. Since each individual parasite has a limited pool of resources to invest, it might have to allocate its resources strategically (e.g. into diverse immune evasive strategies).
Does this have any consequences for the virulence of the different parasite genotypes? We found that worm sibships that are highly infective to copepods were also in fish with better body condition, as estimated from the hepatosomatic index. In sticklebacks, this measure of energy reserves is a meaningful estimate of the negative consequences of tapeworm infection (Kurtz et al. 2004). The severe effects on fish condition may be a direct, immunopathological consequence of the activated innate immune system, as has been found in other systems (see Lochmiller & Deerenberg 2000). Importantly, the harmful effects of a parasite on its host might be a consequence of deficient adaptation of the parasite to parts of its host's immune system rather than a direct effect resulting from resource drain to the parasite. This seems to be the case in our system, since the more harmful parasites for the stickleback did not grow bigger. Thus, parasites may not necessarily benefit from such harmful effects, i.e. virulence and fitness of a parasite might be uncoupled. What could then maintain the more virulent genotypes in the population? One possibility, yet unstudied here, is transmission to the definitive host, which might be enhanced in fish that suffer more from the infection (Lester 1970; Giles 1983).
What are the population consequences of the above findings? Under natural situations, most sticklebacks will be infected with worm sibships that are highly infective to the copepod. At the same time, these genotypes are also relatively benign for the fish. As a side effect, infection of the invertebrate host thus represents a ‘filter’ that reduces infection of the subsequent vertebrate host with the most harmful parasites. These findings might also give an explanation for observations from SPEs, where the exclusion of a first, intermediate host from a parasite's life cycle, through artificial transfer of parasites among individuals of the second host alone, leads to increased virulence in that host (Ebert 1998; Mackinnon & Read 2004).
In conclusion, if different genotypes of parasites in multi-host life cycles are adapted to cope either with parts of the invertebrate or the vertebrate immune systems, this could explain performance and virulence of parasites in this and potentially other multi-host parasites.
Acknowledgments
We are grateful for the help of W. Derner, A. Hasselmeyer, R. Leipnitz, L. Janke, and G. Augustin during the experiment. We thank everybody in our department for catching and dissecting infected sticklebacks. We thank all people of our department, especially M. Kalbe, M. Milinski and I. van der Veen for helpful discussions and I. van der Veen and G. Rauch for statistical advice. M. Milinski, I. van der Veen and H. Schulenburg made valuable comments on earlier versions of this manuscript.
Footnotes
Present address: Ecology and Evolution, ETH Zürich, ETH Zentrum CHN J12.1, 8092 Zürich, Switzerland.
References
- Arme C, Owen R.W. Infections of the three-spined stickleback, Gasterosteus aculeatus L., with the plerocercoid larvae of Schistocephalus solidus (Müller, 1776), with special reference to pathological effects. Parasitology. 1967;57:301–314. doi: 10.1017/s0031182000072103. [DOI] [PubMed] [Google Scholar]
- Barber I. Parasites grow larger in faster growing fish hosts. Int. J. Parasitol. 2005;35:137–143. doi: 10.1016/j.ijpara.2004.11.010. 10.1016/j.ijpara.2004.11.010 [DOI] [PubMed] [Google Scholar]
- Bull J.J. Perspective—virulence. Evolution. 1994;48:1423–1437. doi: 10.1111/j.1558-5646.1994.tb02185.x. [DOI] [PubMed] [Google Scholar]
- Chellappa S, Huntingford F.A, Strang R.H.C, Thomson R.Y. Condition factor and hepatosomatic index as estimates of energy status in male three-spined stickleback. J. Fish Biol. 1995;47:775–787. [Google Scholar]
- Clarke A.S. Studies on the life cycle of the pseudophyllidean cestode Schistocephalus solidus. Proc. Zool. Soc. Lond. 1954;124:257–304. [Google Scholar]
- Davies C.M, Webster J.P, Woolhouse M.E.J. Trade-offs in the evolution of virulence in an indirectly transmitted macroparasite. Proc. R. Soc. B. 2001;268:251–257. doi: 10.1098/rspb.2000.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day T. The evolution of virulence in vector-borne and directly transmitted parasites. Theor. Popul. Biol. 2002;62:199–213. doi: 10.1006/tpbi.2002.1595. 10.1006/tpbi.2002.1595 [DOI] [PubMed] [Google Scholar]
- Dubinina M.N. Nauka; Moscow, Leningrad: 1966. Tapeworms (Cestoda, Ligulidae) of the Fauna of the USSR—Remnetsy (Cestoda, Ligulidae) Fauny SSSR. [Google Scholar]
- Ebert D. Evolution—experimental evolution of parasites. Science. 1998;282:1432–1435. doi: 10.1126/science.282.5393.1432. 10.1126/science.282.5393.1432 [DOI] [PubMed] [Google Scholar]
- Falconer D.S, Mackay T.F.C. 4th edn. Longman; London: 1996. Introduction to quantitative genetics. [Google Scholar]
- Frank S.A. Models of parasite virulence. Q. Rev. Biol. 1996;71:37–78. doi: 10.1086/419267. [DOI] [PubMed] [Google Scholar]
- Frischknecht M. The breeding coloration of male 3-spined sticklebacks (Gasterosteus aculeatus) as an indicator of energy investment in vigor. Evol. Ecol. 1993;7:439–450. 10.1007/BF01237640 [Google Scholar]
- Galvani A.P. Epidemiology meets evolutionary ecology. Trends Ecol. Evol. 2003;18:132–139. 10.1016/S0169-5347(02)00050-2 [Google Scholar]
- Gandon S. Evolution of multihost parasites. Evolution. 2004;58:455–469. [PubMed] [Google Scholar]
- Giles N. Behavioural effects of the parasite Schistocephalus solidus (Cestoda) on an intermediate host, the three-spined stickleback Gasterosteus aculeatus L. Anim. Behav. 1983;31:1192–1194. [Google Scholar]
- Gower C, Webster J. Fitness of indirectly transmitted pathogens: restraint and constraint. Evolution. 2004;58:1178–1184. doi: 10.1111/j.0014-3820.2004.tb01698.x. [DOI] [PubMed] [Google Scholar]
- Guan R, Roychowdhury A, Ember B, Kumar S, Boons G.-J, Mariuzza R.A. Structural basis for peptidoglycan binding by peptidoglycan recognition proteins. Proc. Natl Acad. Sci. USA. 2004;101:17 168–17 173. doi: 10.1073/pnas.0407856101. 10.1073/pnas.0407856101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen M.H.H, Koella J.C. Evolution of tolerance: the genetic basis of a host's resistance against parasite manipulation. Oikos. 2003;102:309–317. 10.1034/j.1600-0706.2003.12537.x [Google Scholar]
- Janeway C.A, Medzhitov R. Innate immune recognition. Annu. Rev. Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. 10.1146/annurev.immunol.20.083001.084359 [DOI] [PubMed] [Google Scholar]
- Janeway C.A, Travers P, Walport M, Capra J.D. Current Biology Publications; London: 1999. Immunobiology: the immune system in health and disease. [Google Scholar]
- Kurtz J, Franz K. Evidence for memory in invertebrate immunity. Nature. 2003;425:37–38. doi: 10.1038/425037a. 10.1038/425037a [DOI] [PubMed] [Google Scholar]
- Kurtz J, Kalbe M, Aeschlimann P.B, Häberli M.A, Wegner K.M, Reusch T.B.H, Milinski M. Major histocompatibility complex diversity influences parasite resistance and innate immunity in sticklebacks. Proc. R. Soc. B. 2004;271:197–204. doi: 10.1098/rspb.2003.2567. 10.1098/rspb.2003.2567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester R.J.G. The influence of Schistocephalus plerocercoids on the respiration of Gasterosteus and a possible resulting effect on the behavior of the fish. Can. J. Zool. 1970;49:361–366. doi: 10.1139/z71-052. [DOI] [PubMed] [Google Scholar]
- Lochmiller R.L, Deerenberg C. Trade-offs in evolutionary immunology: just what is the cost of immunity? Oikos. 2000;88:87–98. 10.1034/j.1600-0706.2000.880110.x [Google Scholar]
- Lüscher A, Milinski M. Simultaneous hermaphrodites reproducing in pairs self-fertilize some of their eggs: an experimental test of predictions of mixed-mating and Hermaphrodite's Dilemma theory. J. Evol. Biol. 2003;16:1030–1037. doi: 10.1046/j.1420-9101.2003.00552.x. 10.1046/j.1420-9101.2003.00552.x [DOI] [PubMed] [Google Scholar]
- Mackinnon M.J, Read A.F. Immunity promotes virulence evolution in a malaria model. Plos Biol. 2004;2:1286–1292. doi: 10.1371/journal.pbio.0020230. 10.1371/journal.pbio.0020230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moret Y, Schmid-Hempel P. Survival for immunity: the price of immune system activation for bumblebee workers. Science. 2000;290:1166–1168. doi: 10.1126/science.290.5494.1166. 10.1126/science.290.5494.1166 [DOI] [PubMed] [Google Scholar]
- Parker G.A, Chubb J.C, Ball M.A, Roberts G.N. Evolution of complex life cycles in helminth parasites. Nature. 2003;425:480–484. doi: 10.1038/nature02012. 10.1038/nature02012 [DOI] [PubMed] [Google Scholar]
- Pechhold K, Pohl T, Kabelitz D. Rapid quantification of lymphocyte subsets in heterogeneous cell-populations by flow-cytometry. Cytometry. 1994;16:152–159. doi: 10.1002/cyto.990160209. 10.1002/cyto.990160209 [DOI] [PubMed] [Google Scholar]
- Roff D.A. 1st edn. Chapman & Hall; New York: 1997. Evolutionary quantitative genetics. [Google Scholar]
- Rolff J, Siva-Jothy M.T. Invertebrate ecological immunology. Science. 2003;301:472–475. doi: 10.1126/science.1080623. 10.1126/science.1080623 [DOI] [PubMed] [Google Scholar]
- Salzet M. Vertebrate innate immunity resembles a mosaic of invertebrate immune responses. Trends Immunol. 2001;22:285–288. doi: 10.1016/s1471-4906(01)01895-6. 10.1016/S1471-4906(01)01895-6 [DOI] [PubMed] [Google Scholar]
- Schärer L, Wedekind C. Lifetime reproductive output in a hermaphroditic cestode when reproducing alone or in pairs. Evol. Ecol. 1999;13:381–394. 10.1023/A:1006789110502 [Google Scholar]
- Scharsack J, Kalbe M, Derner R, Kurtz J, Milinski M. Modulation of granulocyte responses in three-spined sticklebacks Gasterosteus aculeatus infected with the tapeworm Schistocephalus solidus. Dis. Aquat. Org. 2004;59:141–150. doi: 10.3354/dao059141. [DOI] [PubMed] [Google Scholar]
- Schjørring S. Delayed selfing in relation to the availability of a mating partner in the cestode Schistocephalus solidus. Evolution. 2004;58:2591–2596. doi: 10.1111/j.0014-3820.2004.tb00887.x. [DOI] [PubMed] [Google Scholar]
- Scott A.L, Klesius P.H. Chemiluminescence: a novel analysis of phagocytosis in fish. Dev. Biol. Stand. 1981;49:243–254. [Google Scholar]
- Sheldon B.C, Verhulst S. Ecological immunology: costly parasite defences and trade-offs in evolutionary ecology. Trends Ecol. Evol. 1996;11:317–321. doi: 10.1016/0169-5347(96)10039-2. 10.1016/0169-5347(96)10039-2 [DOI] [PubMed] [Google Scholar]
- Smyth J.D. Studies on tapeworm physiology. 1. The cultivation of Schistocephalus solidus in vitro. J. Exp. Biol. 1946;23:47–70. doi: 10.1242/jeb.23.1.47. [DOI] [PubMed] [Google Scholar]
- Swiderski Z. Origin, differentiation and ultrastructure of egg envelopes surrounding the coracidia of Bothriocephalus clavibothrium (Cestoda, Pseudophyllidae) Acta Parasitol. 1994;39:73–81. [Google Scholar]
- Tierney J.F, Crompton D.W.T. Infectivity of plerocercoids of Schistocephalus solidus (Cestoda: Ligulidae) and fecundity of the adults in an experimental definitive host, Gallus gallus. J. Parasitol. 1992;78:1049–1054. [PubMed] [Google Scholar]
- van der Veen I.T. Is body size or activity of copepods related to ingestion of parasite larvae? Parasitology. 2003;126:173–178. doi: 10.1017/s0031182002002652. 10.1017/S0031182002002652 [DOI] [PubMed] [Google Scholar]
- van der Veen I.T, Kurtz J. To avoid or eliminate: cestode infections in copepods. Parasitology. 2002;124:465–474. doi: 10.1017/s0031182001001275. 10.1017/S0031182001001275 [DOI] [PubMed] [Google Scholar]
- Via S. The quantitative genetics of polyphagy in an insect herbivore. II. Genetic correlations in larval performance within and among host plants. Evolution. 1984;38:896–905. doi: 10.1111/j.1558-5646.1984.tb00360.x. [DOI] [PubMed] [Google Scholar]
- Wedekind C. The infectivity, growth, and virulence of the cestode Schistocephalus solidus in its first intermediate host, the copepod Macrocyclops albidus. Parasitology. 1997;115:317–324. doi: 10.1017/s0031182097001406. 10.1017/S0031182097001406 [DOI] [PubMed] [Google Scholar]
- Wedekind C, Milinski M. Do three-spined sticklebacks avoid consuming copepods, the first intermediate host of Schistocephalus solidus? An experimental analysis of behavioural resistance. Parasitology. 1996;112:371–383. [Google Scholar]
- Zapata A.G, Chibá A, Varas A. Cells and tissues of the immune system of fish. In: Nakanishi G.I.T, editor. The fish immune system: organism, pathogen, and environment. Academic Press; San Diego: 1996. pp. 1–62. [Google Scholar]