Abstract
Octenyl succinic anhydride (OSA) modified early Indica rice starch was prepared in aqueous slurry systems using response surface methodology. The paste properties of the OSA starch were also investigated. Results indicated that the suitable parameters for the preparation of OSA starch from early Indica rice starch were as follows: reaction period 4 h, reaction temperature 33.4 °C, pH of reaction system 8.4, concentration of starch slurry 36.8% (in proportion to water, w/w), amount of OSA 3% (in proportion to starch, w/w). The degree of substitution was 0.0188 and the reaction efficiency was 81.0%. The results of paste properties showed that with increased OSA modification, the starch derivatives had higher paste clarity, decreased retrogradation and better freeze-thaw stability.
Keywords: Early Indica rice, OSA starch, Response surface methodology, Paste properties
INTRODUCTION
Early Indica rice is widely planted in southern China, where it is particularly predominant as an early-season crop. Its inferior eating and cooking qualities account for its lower price in the national market (Zhong et al., 2005). Therefore, the modification of early Indica rice starch is important to widen the usage of Indica rice in China (Song et al., 2006). Octenyl succinic anhydride (OSA) modified starch was patented by Caldwell and Wurzburg (1953). In the United States, FDA approved OSA starch for food use in 1972. The maximum level of OSA treatment allowed is 3% (degree of substitution (DS)≈0.02). However, in China the investigation and application of OSA starch are scarce.
OSA modification made the starch an effective emulsifier due to the addition of dual functional hydrophilic and hydrophobic groups (Tesch et al., 2002). OSA starch has been used in food products such as sauces, puddings, and baby foods for more than 30 years. Recently, OSA starch was reported to have special nutritional values (Heacock et al., 2004). Heacock et al.(2004) found that esterification of starch with OSA might impair the binding of α-amylase, thus decreasing the extent of starch digestion. Their study indicated that OSA starch appeared to be a resistant starch, which could be used as a functional fiber for the treatment of certain diseases.
Although OSA starches have been investigated regarding their preparative conditions (Jeon et al., 1999), pasting and digestive properties (Viswanathan, 1999; Bao et al., 2003; Shih and Daigle, 2003; Park et al., 2004), relatively little work has been done on the preparation conditions and paste properties of OSA starch from early Indica rice starch. In this study, OSA starches were prepared from early Indica rice starch in aqueous slurry systems. The major factors affecting the esterification, including reaction temperature, pH of the reaction system, and starch concentration were evaluated by means of response surface methodology. The paste properties of OSA starches with different DS were also investigated. It is hoped that the results may give the optimum operating conditions to obtain OSA starch with a high degree of substitution. In addition, they provide valuable information on the paste properties of OSA modified early Indica rice starch, which might be useful for food industry.
MATERIALS AND METHODS
Materials
The early Indica rice starch used for this study was Jiazao312 (The approximate composition of Jiazao312 was as follows: amylose content 11.9%±0.2%, crude protein content 0.19%±0.02%, crude fat content 0.23%±0.03%, moisture content 9.00%±0.16%). High purity OSA was purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). The other chemicals used in the study were of analytical grade.
Preparation of OSA starch
Early Indica rice starch (30 g, dry weight) was suspended in distilled water with agitation. The pH of the suspension was adjusted throughout the reaction with a pH meter by adding 3% NaOH solution. OSA (3%, in proportion to starch, w/w) was added slowly in 2 h. The reaction was continued for the required time. After reaction, the pH was adjusted to 6.5 with 3% HCl solution, the mixture was centrifuged, washed two times with distilled water and two times with 70% aqueous alcohol, and the solid oven-dried at 40 °C for 24 h, then passed through a 180-mesh nylon sieve (90 μm opening).
Determination of the degree of substitution
Degree of substitution (DS) is the average number of hydroxyl groups substituted per glucose unit. The DS of OSA starch was determined using titration method (Kweon et al., 2001). OSA starch (5 g, dry weight) was accurately weighed and dispersed in 25 ml of 2.5 mol/L HCl-isopropyl alcohol solution by stirring for 30 min. A total of 100 ml of 90% isopropyl alcohol solution (in proportion to water, v/v) was added, stirred for an additional 10 min. The suspension was filtered through a glass filter and the residue was washed with 90% isopropyl alcohol solution until no Cl− was detected (using 0.1 mol/L AgNO3 solution). The starch was re-dispersed in 300 ml distilled water, and then the dispersion was cooked in a boiling water-bath for 20 min. The starch solution was titrated with 0.1 mol/L standard NaOH solution, using phenolphthalein as an indicator. A blank was simultaneously titrated with native starch as a control. The DS was calculated by the following equation:
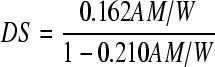 , ,
|
where A is the titration volume of NaOH solution (ml); M is the molarity of NaOH solution; W is the dry weight (g) of the OSA starch.
The reaction efficiency (RE) was calculated as follows:
 . .
|
The theoretical DS was calculated assuming that all of the added anhydride reacted with starch to form the ester derivative.
Experimental design and statistical analysis
According to previous studies, the most important parameters for the OSA modification were pH, temperature and starch concentration (Song et al., 2006). In this study, response surface methodology was used to evaluate the effect of reaction temperature, pH of reaction system and starch concentration on DS (Table 1). The central composite design scheme consisted of 20 treatments, whose central point was replicated six times for the calculation of the experimental error.
Table 1.
Level and coding of each factor
| Levels | Temperature (°C) (x1) | pH (x2) | Starch concentration (%) (x3) |
| −1.682 | 26.59 | 6.82 | 26.59 |
| −1 | 30.00 | 7.50 | 30.00 |
| 0 | 35.00 | 8.50 | 35.00 |
| 1 | 40.00 | 9.50 | 40.00 |
| 1.682 | 43.41 | 10.18 | 43.41 |
Statistical analysis was performed with SAS 8.0 software. The variables were coded according to the following equation (Montagomery, 1991):
| xi=(Xi−X0)/Δxi, | (1) |
where xi is the coded value of an independent variable, Xi is the real value of an independent variable, X 0 is the real value of an independent variable at the center point, and Δxi is the step change value. The model proposed for the response (Y) was:
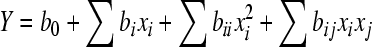 , ,
|
(2) |
where b 0 is the value of the fixed response at the central point of the experiment, which is the point (0, 0); bi, bii and bij are the linear, quadratic and cross product coefficients, respectively.
Light transmittance (T%) of starch paste
T% of starch paste was determined according to the method of Craig et al.(1989). Starch (0.5 g, dry weight) was suspended in distilled water (50 ml) in screwcap tubes in a boiling water bath for 20 min. The tubes were thoroughly shaken every 5 min. After cooling to room temperature, the percent transmittance (T%) at 650 nm was determined against a water blank in a UV-2102PC spectrophotometer (Unico Instruments Co. Ltd., Shanghai, China).
Retrogradation of starch paste
Retrogradation studies were performed according to He et al.(2004) with modifications. Twenty-five milliliters of 1% starch paste was placed in a graduated flask. Retrogradation was determined as the volume of free water (ml) after storage at room temperature (25 °C) for 6, 24, 48, 480 and 720 h.
Freeze-thaw stability
Freeze-thaw stability was determined according to the previous methods (Zheng and Sosulski, 1998; Bhandari and Singhal, 2002; van Hung and Morita, 2005) with slight modification. Starch (3.0 g, dry weight) was suspended in distilled water (100 ml) in screwcap tubes in a boiling water bath for 20 min. After cooling, the paste was divided into four equal parts and then transferred to centrifuge tubes, separately. The samples were kept refrigerated at −20 °C for 24 h, and then thawed at room temperature for 6 h. One of the tubes was taken out every time and centrifuged at 3000 r/min (1700×g) for 20 min in L-550 centrifuge (Xiang-Yi Centrifuge Instrument Co. Ltd., Changsha, China). The water layer was decanted and the residual paste was weighed. The percentage of water separated after each freeze-thaw cycle was measured and expressed as in the following equation:
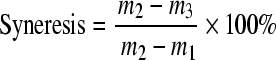 , ,
|
where m 1 is the weight of centrifuge tubes (g); m 2 is the weight of centrifuge tubes and starch paste (g); m 3 is the weight of centrifuge tubes and starch paste after centrifuging (g).
RESULTS AND DISCUSSION
Model analysis
The DS and RE were determined for all the 20 treatments and are given in Table 2. The DS ranged from 0.0124 to 0.0188 and the RE from 53.4% to 81.0%. With 3% OSA concentration, the highest values of DS and RE were obtained at the following reaction conditions: reaction period 4 h, reaction temperature 35 °C, pH of reaction system 8.5, concentration of starch slurry 35%. Table 2 also indicated RE was positively correlated with DS, and therefore the regression analysis was only done for DS as the response variable.
Table 2.
DS and RE for the OSA modified rice starches
| Experiment | Variables |
Responses |
|||
| x1 | x2 | x3 | DS | RE (%) | |
| 1 | −1 | −1 | −1 | 0.0164 | 70.7 |
| 2 | −1 | −1 | 1 | 0.0179 | 77.2 |
| 3 | −1 | 1 | −1 | 0.0161 | 69.4 |
| 4 | −1 | 1 | 1 | 0.0158 | 68.1 |
| 5 | 1 | −1 | −1 | 0.0149 | 64.2 |
| 6 | 1 | −1 | 1 | 0.0155 | 66.8 |
| 7 | 1 | 1 | −1 | 0.0150 | 64.6 |
| 8 | 1 | 1 | 1 | 0.0141 | 60.8 |
| 9 | −1.682 | 0 | 0 | 0.0167 | 72.0 |
| 10 | 1.682 | 0 | 0 | 0.0157 | 67.7 |
| 11 | 0 | −1.682 | 0 | 0.0143 | 61.6 |
| 12 | 0 | 1.682 | 0 | 0.0124 | 53.4 |
| 13 | 0 | 0 | −1.682 | 0.0154 | 66.4 |
| 14 | 0 | 0 | 1.682 | 0.0182 | 78.4 |
| 15 | 0 | 0 | 0 | 0.0188 | 81.0 |
| 16 | 0 | 0 | 0 | 0.0182 | 78.4 |
| 17 | 0 | 0 | 0 | 0.0183 | 78.9 |
| 18 | 0 | 0 | 0 | 0.0185 | 79.7 |
| 19 | 0 | 0 | 0 | 0.0187 | 80.6 |
| 20 | 0 | 0 | 0 | 0.0185 | 79.7 |
Determination of the optimum operating conditions
Analysis of variance indicated that reaction temperature, pH and starch concentration had significant influence at 95% level on the OSA modification of early Indica rice starch. x 1 2, x 2 2 and x 3 2 also had significant influence on the esterification. But the effects of cross products were not significant.
The application of response surface methodology yielded a regression equation of empirical relationship between the DS and the variables in coded units.
| DS=0.018486−0.000614x1−0.000505x2+0.000411x3−0.000719x12+0.000137x1x2−0.000187x1x3−0.001726x22−0.000412x2x3−0.000507x32, | (3) |
where DS is the response and x 1, x 2 and x 3 are coded values of temperature, pH and starch concentration, respectively. R 2 was calculated to be 0.9440 (Table 3). This implies that the sample variation of 94.40% could be attributed to the independent variables and that the model did not explain only 5.6% of the total variations. The model’s F-value of 18.742 and the P-value of 0.0000 imply that the model is statistically significant at 99% confidence level.
Table 3.
Analysis of variance (ANOVA) for the model
| Regression | DF | R2 | F-values | P-values |
| Linear | 3 | 0.1685 | 10.036 | 0.0023 |
| Quadratic | 3 | 0.7479 | 44.542 | 0.0000 |
| Cross product | 3 | 0.0277 | 1.647 | 0.2405 |
| Total model | 9 | 0.9440 | 18.742 | 0.0000 |
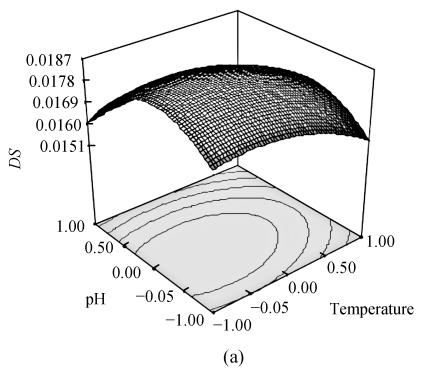
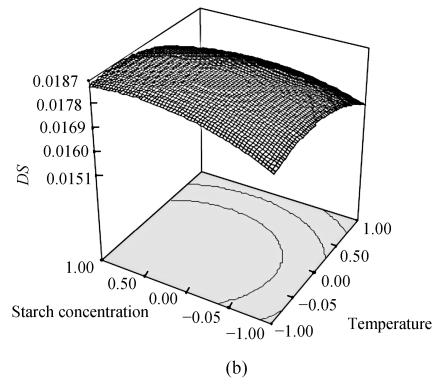
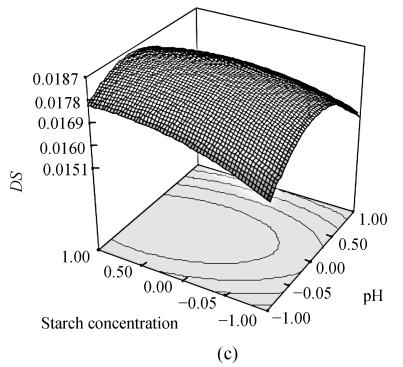
To optimize OSA modification, we obtained the response surface curves by the statistically significant model (Fig.1). The plots showed that the DS reached its maximum value (DS=0.0188) at a combination of coded level −0.314 (x 1), −0.142 (x 2) and 0.357 (x 3). To examine the possible reaction conditions of reaction temperature 33.4 °C, pH 8.4, starch concentration 36.8%, DS under such conditions was examined. The data showed that the DS was 0.0188±0.0003 and RE was 81.0%±1.1%. The above results indicate that the model is adequate under these conditions and is useful for the production of OSA starch.
Fig. 1.
Response surface plot for the DS as a function of temperature and pH (a); temperature and starch concentration (b) and pH and starch concentration (c) by keeping the other factor constant
Light transmittance (T%) of starch paste
The T% values of the native and OSA starch pastes are shown in Table 4. OSA starch pastes showed a considerably higher T% than the native starch. Within the range of our experiments, the higher the DS, the more the transmittance increased. Bhandari and Singhal (2002) also reported increased paste clarity for the succinylated derivatives of corn and amaranth starches. The increase in the T% of the OSA starches might be attributed to the introduction of carboxyl group, which retained the water molecules to form hydrogen bonds in the starch granules, thereby, increasing the clarity of starch pastes. Moreover, the chemical substitution of the hydroxyl group by succinyl moiety caused the inhibition of ordered structure of the starch paste, thus retarding retrogradation and resulting in a more fluid paste with improved clarity (Craig et al., 1989; Bhandari and Singhal, 2002). Improved paste clarity is a useful property in the manufacture of some foods like jellies, sausages and fruit pastes, which require transparency (Jyothi et al., 2005).
Table 4.
Paste clarity of native and OSA modified rice starches with different DS
| DS | T%a |
| 0 | 12.2±0.1 |
| 0.0124 | 19.1±1.0 |
| 0.0141 | 25.6±0.6 |
| 0.0164 | 30.5±0.6 |
| 0.0179 | 34.9±0.4 |
| 0.0188 | 35.2±0.3 |
Mean of 3 determinations±standard deviation
Retrogradation of starch paste
Retrogradation is a general term for the behavior of recrystallization of gelatinized starches on cooling and storage, and is accompanied by gel hardening and the leakage of water from the starch gel (Ishiguro et al., 2000). Retrogradation is an important factor for starch used as a food ingredient in processing and preservation, because the quality of the food’s texture and physical properties deteriorate due to retrogradation as time passes.
After OSA modification, the retrogradation of starch paste from early Indica rice decreased significantly (Table 5). Moreover, with increase in the DS of OSA starches, the water separated from the starch paste decreased. The bulky succinyl groups in the modified starch could prevent the formation of an ordered structure of the starch paste, thus retarding retrogradation (Lawal, 2004).
Table 5.
Retrogradation of native and OSA modified rice starches with different DS
| DS | Separated water (ml)a |
||||
| 6 h | 24 h | 48 h | 480 h | 720 h | |
| 0 | 2.5±0.2 | 9.0±0.2 | 11.5±0.3 | 15.0±0.2 | 16.0±0.1 |
| 0.0124 | Nil | Nil | Nil | 2.0±0.1 | 3.0±0.2 |
| 0.0141 | Nil | Nil | Nil | 1.0±0.1 | 2.0±0.2 |
| 0.0164 | Nil | Nil | Nil | 0.3±0.1 | 1.5±0.2 |
| 0.0179 | Nil | Nil | Nil | 0.2±0.0 | 1.5±0.1 |
| 0.0188 | Nil | Nil | Nil | 0.2±0.0 | 0.8±0.1 |
Mean of 3 determinations±standard deviation
Freeze-thaw stability
When pasted starch is employed as a thickening agent in frozen foods, the accelerated retrogradation at low temperature may produce undesirable physical changes including gel formation and syneresis. The effect of freezing/thawing on the OSA starch pastes is shown in Table 6. OSA starches at all levels of DS had lower degree of syneresis than the native starch. Moreover, the freeze-thaw stability increased along with the increase in DS within the range of 0 to 0.0188. During the fourth freeze-thaw cycle, the native rice starch gels had a spongy-like texture and exuded 54.4% water, while the gels from OSA starch (DS=0.0188) were soft and elastic, and discharged only 4.2% water. Consequently, OSA modified early Indica rice starch could be used to stabilize starch gels in low temperature conditions that favor retrogradation.
Table 6.
Freeze-thaw stability of native and OSA modified rice starches with different DS
| DS | Syneresis (%)a |
|||
| Cycle 1 | Cycle 2 | Cycle 3 | Cycle 4 | |
| 0 | 37.9±0.5 | 44.6±0.4 | 49.0±0.2 | 54.4±0.4 |
| 0.0124 | 11.5±0.3 | 25.2±0.5 | 31.5±0.3 | 35.2±0.3 |
| 0.0141 | 5.2±0.3 | 7.2±0.3 | 12.3±0.1 | 16.1±0.4 |
| 0.0164 | Nil | 1.8±0.2 | 6.4±0.2 | 10.7±0.1 |
| 0.0179 | Nil | Nil | 4.1±0.2 | 7.3±0.3 |
| 0.0188 | Nil | Nil | 2.7±0.1 | 4.2±0.2 |
Mean of 3 determinations±standard deviation
CONCLUSION
OSA modified early Indica rice starch was prepared in aqueous slurry systems. The optimum conditions were selected as follows: reaction period 4 h, reaction temperature 33.4 °C, pH of reaction system 8.4, concentration of starch slurry 36.8% (in proportion to water, w/w), amount of OSA 3% (in proportion to starch, w/w). Under these conditions, the degree of substitution (DS) was 0.0188 and the reaction efficiency (RE) was 81.0%. Predicted values using the model were shown to correspond well with the experimental results. The study on the paste properties showed that with increased OSA modification, the starch derivatives had higher paste clarity, decreased retrogradation and better freeze-thaw stability. For the application of OSA starch in food industry, we can make use of the information for reference.
Footnotes
Project (NO. 2003C12009) supported by the Science and Technology Ministry of Zhejiang Province, China
References
- 1.Bao JS, Xing J, Phillips DL, Corke H. Physical properties of octenyl succinic anhydride modified rice, wheat, and potato starches. J Agric Food Chem. 2003;51(8):2283–2287. doi: 10.1021/jf020371u. [DOI] [PubMed] [Google Scholar]
- 2.Bhandari PN, Singhal RS. Effect of succinylation on the corn and amaranth starch pastes. Carbohyd Polym. 2002;48(3):233–240. doi: 10.1016/S0144-8617(01)00310-1. [DOI] [Google Scholar]
- 3.Caldwell CG, Wurzburg OB. U.S. Pat. 2661349. Polysaccharides Derivative of Substituted Dicarboxylic Acids. 1953
- 4.Craig SAS, Maningat CC, Seib PA, Hoseney RC. Starch paste clarity. Cereal Chem. 1989;66(3):173–182. [Google Scholar]
- 5.He CB, Pan LJ, Jiang ST, Chen L, Li L. Influence of phosphorylation on retrogradation of phosphate monoesters of sweet potato starch. Journal of the Chinese Cereals and Oils Association. 2004;19(1):84–88. (in Chinese) [Google Scholar]
- 6.Heacock PM, Hertzler SR, Wolf B. The glycemic, insulinemic, and breath hydrogen responses in humans to a food starch esterified by 1-octenyl succinic anhydride. Nutr Res. 2004;24(8):581–592. doi: 10.1016/j.nutres.2003.10.015. [DOI] [Google Scholar]
- 7.Ishiguro K, Noda T, Kitahara K, Yamakawa O. Retrogradation of sweetpotato starch. Starch/Stärke. 2000;52(1):13–17. doi: 10.1002/(SICI)1521-379X(200001)52:1<13::AID-STAR13>3.0.CO;2-E. [DOI] [Google Scholar]
- 8.Jeon YS, Viswanathan A, Gross RA. Studies of starch esterification: reactions with alkenylsuccinates in aqueous slurry systems. Starch/Stärke. 1999;51(2-3):90–93. doi: 10.1002/(SICI)1521-379X(199903)51:2<90::AID-STAR90>3.3.CO;2-D. [DOI] [Google Scholar]
- 9.Jyothi AN, Rajasekharan KN, Moorthy SN, Sreekumar J. Synthesis and characterization of low DS succinate derivatives of cassava (Manihot esculenta crantz) starch. Starch/Stärke. 2005;57(7):319–324. doi: 10.1002/star.200400374. [DOI] [Google Scholar]
- 10.Kweon DK, Choi JK, Kim EK, Lim ST. Adsorption of divalent metal ions by succinylated and oxidized corn starches. Carbohyd Polym. 2001;46(2):171–177. doi: 10.1016/S0144-8617(00)00300-3. [DOI] [Google Scholar]
- 11.Lawal OS. Succinyl and acetyl starch derivatives of a hybrid maize: physicochemical characteristics and retrogradation properties monitored by differential scanning calorimetry. Carbohyd Res. 2004;339(16):2673–2682. doi: 10.1016/j.carres.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 12.Montagomery DC. Design and Analysis of Experiments. 3rd Ed. NY: Wiley; 1991. [Google Scholar]
- 13.Park S, Chung MG, Yoo B. Effect of octenylsuccinylation on rheological properties of corn starch pastes. Starch/Stärke. 2004;56(9):399–406. doi: 10.1002/star.200300274. [DOI] [Google Scholar]
- 14.Shih FF, Daigle KW. Gelatinization and pasting properties of rice starch modified with 2-octen-1-ylsuccinic anhydride. Nahrung/Food. 2003;47(1):64–67. doi: 10.1002/food.200390015. [DOI] [PubMed] [Google Scholar]
- 15.Song XY, He GQ, Ruan H, Chen QH. Preparation and properties of octenyl succinic anhydride modified early Indica rice starch. Starch/Stärke. 2006;58(2):109–117. doi: 10.1002/star.200500444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tesch S, Gerhards C, Schubert H. Stabilization of emulsions by OSA starches. J Food Eng. 2002;54(2):167–174. doi: 10.1016/S0260-8774(01)00206-0. [DOI] [Google Scholar]
- 17.van Hung P, Morita N. Physicochemical properties of hydroxypropylated and cross-linked starches from A-type and B-type wheat starch granules. Carbohyd Polym. 2005;59(2):239–246. doi: 10.1016/j.carbpol.2004.09.016. [DOI] [Google Scholar]
- 18.Viswanathan A. Effect of degree of substitution of octenyl succinate starch on enzymatic degradation. Journal of Polymers and the Environment. 1999;7(4):185–190. doi: 10.1023/A:1022878631495. [DOI] [Google Scholar]
- 19.Zheng GH, Sosulski FW. Determination of water separation from cooked starch and flour pastes after refrigeration and freeze-thaw. J Food Sci. 1998;63(1):134–139. doi: 10.1111/j.1365-2621.1998.tb15693.x. [DOI] [Google Scholar]
- 20.Zhong LJ, Cheng FM, Wen X, Sun ZX, Zhang GP. The deterioration of eating and cooking quality caused by high temperature during grain filling in early-season Indica rice cultivars. J Agron Crop Sci. 2005;191(3):218–225. [Google Scholar]