Abstract
The potential for trichromacy in mammals, thought to be unique to primates, was recently discovered in two Australian marsupials. Whether the presence of three cone types, sensitive to short- (SWS), medium- (MWS) and long- (LWS) wavelengths, occurs across all marsupials remains unknown. Here, we have investigated the presence, distribution and spectral sensitivity of cone types in two further species, the quokka (Setonix brachyurus) and quenda (Isoodon obesulus). Immunohistochemistry revealed that SWS cones in the quokka are concentrated in dorso-temporal retina, while in the quenda, two peaks were identified in naso-ventral and dorso-temporal retina. In both species, MWS/LWS cone spatial distributions matched those of retinal ganglion cells. Microspectrophotometry (MSP) confirmed that MWS and LWS cones are spectrally distinct, with mean wavelengths of maximum absorbance at 502 and 538 nm in the quokka, and at 509 and 551 nm, in the quenda. Although small SWS cone outer segments precluded MSP measurements, molecular analysis identified substitutions at key sites, accounting for a spectral shift from ultraviolet in the quenda to violet in the quokka.
The presence of three cone types, along with previous findings in the fat-tailed dunnart and honey possum, suggests that three spectrally distinct cone types are a feature spanning the marsupials.
Keywords: cone topography, spectral sensitivity, marsupial, opsin
1. Introduction
Until recently, dichromacy was thought to be the most prevalent system of mammalian colour vision, based on two cone pigments sensitive to short (SWS) and medium-to-long (MWS/LWS) wavelengths (Jacobs 1993; Szél et al. 1996; Ahnelt & Kolb 2000). The only exception was thought to be in primates where a third cone pigment had arisen by duplication of the MWS/LWS gene (Bowmaker 1991; Dulai et al. 1994; Yokoyama 2000; Ebrey & Koutalos 2001). The genetic division gave rise to two spectrally distinct cone types with sensitivities at approximately 530 nm (MWS/LWS green) and 560 nm (MWS/LWS red). Among the mammals, the trichromatic colour vision of primates was thought to be unique (Mollon 1991; Yokoyama 2000; Regan et al. 2001).
However, the discovery of three spectrally distinct cone types (SWS, MWS and LWS) in two Australian marsupials, the fat-tailed dunnart (Sminthopsis crassicaudata) and honey possum (Tarsipes rostratus), has provided evidence for the potential of trichromacy in mammals other than primates (Arrese et al. 2002).
To date, it is unknown whether other marsupials also possess the basis for a trichromatic colour system. A behavioural study (Hemmi 1999) and a molecular analysis (Deeb et al. 2003) indicated dichromacy in the tammar wallaby, however, microspectrophotometric (MSP) data have not been obtained for this species. In addition, the presence, distribution and spectral characteristics of different cone types in marsupials remain poorly understood. The present study investigates spectral sensitivity in a small wallaby, the quokka (Setonix brachyurus) and a bandicoot, the quenda (Isoodon obesulus). These emblematic Western Australian marsupials are representative of the two major taxonomic divisions of marsupials, the diprotodonts and polyprotodonts, respectively.
Here, we provide evidence for the presence of three spectrally distinct cone types in both species, and describe the topography of the two immunohistochemically defined cone populations, SWS and M/LWS. As SWS cone outer segments in both species were unusually small for mammalian cones, we determined their opsin sequence to investigate and compare spectral tuning mechanisms.
2. Materials and methods
(a) Animals
Quokkas (n=6) were obtained from a breeding colony established at the University of Western Australia (Animal Service Division, Shenton Park). Quendas (n=6) were collected in the Harry Waring Marsupial Reserve using cage traps under licence from the Department of Conservation and Land Management. Owing to the threatened status of the species (2000 IUCN Red List of Threatened Species), access to material was limited. Animals were terminally anaesthetized with Saffan (alphaxalone and alphadolone acetate, 0.1 ml per 10 g body weight, intraperitoneally). For MSP measurements, animals were dark adapted overnight before euthanasia. The study was approved by the Animal Ethics and Experimentation Committee of the University of Western Australia.
(b) Immunohistochemistry
(i) Tissue preparation
Eyes were removed and immersed in 4% paraformaldehyde in 0.1 M phosphate buffer saline (PBS) at pH 7.4 for 60 min. A dorsal cut was placed in the cornea for orientation. Wholemounts were prepared by dissecting the retina from the eye cup after removal of the lens and vitreous. The sclera and pigment epithelium were removed and the retina flattened, photoreceptor layer uppermost, on a gelatinized slide. The wholemounted retina was viewed in a light microscope and an outline, with salient features, was drawn to allow identification of sample areas.
(ii) Immunolabelling
Affinity purified rabbit antisera, JH455 (raised against human ‘blue-cone’ opsin) and JH492 (raised against human green- and red-cone opsin) were kindly supplied by Dr J. Nathans (Johns Hopkins University School of Medicine, Baltimore, MD, USA). Immunolabelling was performed on wholemounts, as previously described (Arrese et al. 2003).
(iii) Topography
Immunolabelled cones were counted using transmitted light microscopy at 1000× with a 100× oil immersion objective. Single cones were counted separately from double cones (considered one cone) but data were combined for density estimates. For each wholemount, 5% of retinal area was sampled at regular intervals using an eyepiece graticule (Beazley & Dunlop 1983). Sampling was increased in regions where cone density was high. Shrinkage owing to processing (less than 10%) was taken into account when constructing cell density maps.
(iv) Microspectrophotometry
Following enucleation, eyes were immersed in PBS and retinal tissue was dissected for examination by microspectrophotometry, following procedures described by Shand et al. (2002). Briefly, dissections were conducted in darkness under infrared (IR) illumination and visualized using a microscope fitted with a Find-R-Scope (IR Viewer, Electrophysics, Fairfield, NJ, USA). Retinal preparations were mounted in a solution of PBS containing 10% dextran (Sigma 250k RMM), on a rectangular 50 mm×22 mm No. 1 cover-slip, covered with a smaller cover-slip (19 mm×19 mm), and sealed with nail varnish to prevent dehydration.
(v) Measurements of absorbance spectra
A single-beam wavelength scanning microspectrophotometer was used to measure the spectral absorbance of the outer segments of individual photoreceptors and of the associated oil droplets of cones. The equipment has been described previously by Partridge et al. (1992), with recent modifications improving the optics and, hence, the transmission delivery of short wavelengths to the specimen (Shand et al. 2002). Sample and baseline scans were made from cellular and tissue-free samples, respectively. Measurements were made by placing the outer segment in the path of the measuring beam (2×2 μm2) and scanning over the wavelength range 350–750 nm. All cone inner segments possessed an oil droplet. For each outer segment or oil droplet, one ‘pre-bleach’ sample scan was combined with two separate baseline scans from an adjacent area, and the two absorbance spectra obtained were averaged. Following the pre-bleach scans, outer segments were bleached for 2 min with white light from the monochromator, and an identical number of sample and baseline scans were made to confirm that the molecules were photolabile (i.e. indicated a visual pigment).
(vi) Analysis
Baseline and sample data were converted to absorbance values at 1 nm intervals. Upward and downward scans were averaged together by fitting a weighed three-point running average to the absorbance data (Hart et al. 2000). Absorbance spectra were normalized to the peak and long-wavelength offset absorbances were obtained by fitting a variable-point unweighted running average. Following the method of MacNichol (1986), a regression line was fitted to the long wavelength limb of the normalized absorbance spectrum between 30 and 70% of the normalized maximum absorbance. The regression equation was used to predict the wavelength of maximum sensitivity (λmax) and fit the visual pigment template following the methods of Govardovskii et al. (2000). Acceptable pre-bleach spectra showing a clear alpha peak and that were fitted well by the A1 template were averaged together and reanalysed (Levine & MacNichol 1985).
(vii) Molecular biology
Total RNA was extracted from freshly dissected retinae using the EpiCentre MasterPure RNA Purification Kit. Purification of mRNA was performed using Qiagen Oligotex Spin Columns. Single stranded cDNA was synthesized using an oligo-d(T) anchor primer and Invitrogen Superscript III RT Polymerase.
For both species, primers SWS1F63 and SWS1R905 (table 1) were used to generate a 843 bp fragment. The 5′ sequence was completed with primers by SWS1F1 and SWS1R209 and the 3′ sequence with primers SWS1F777 and 3′ adapter primer. PCR products were visualized by agarose gel electrophoresis and cloned into a Promega pGEM T-Easy plasmid. Positive colonies were sequenced using T7 and SP6 primers. Sequencing was carried out on both strands using Big Dye Terminator Version 3.1 and an ABI 3730 sequencer.
Table 1.
Sequences of oligonucleotide primers.
| primer no. | sequence (5′ to 3′) |
|---|---|
| SWS1F63 | SCCYCAGTACCACMTYGC |
| SWS1R905 | GCAGTAGATGATGGGRTTGTA |
| SWS1F1 | ATGTCAGGGGAYGAGGAGTT |
| SWS1R209 | ATGTAGTTGAGTGGCTGGCG |
| SWS1F777 | CTATGTGCCCTATGCTGCC |
| 3′ adapter primer | GACTCGAGTCGACATCGATA |
3. Results
(a) Immunohistochemistry
The presence of unlabelled cones in retinae incubated with JH455 or JH492 confirmed the specificity of the antisera. The two cone types were strongly immunopositive to their respective antisera. In both species, the outer segments of SWS cones were considerably smaller than those of M/LWS cones (figure 1a,c,f,h). As reported for the fat-tailed dunnart and honey possum (Arrese et al. 2003), SWS cones were far outnumbered by M/LWS cones. All cone populations appeared to contain an oil droplet.
Figure 1.
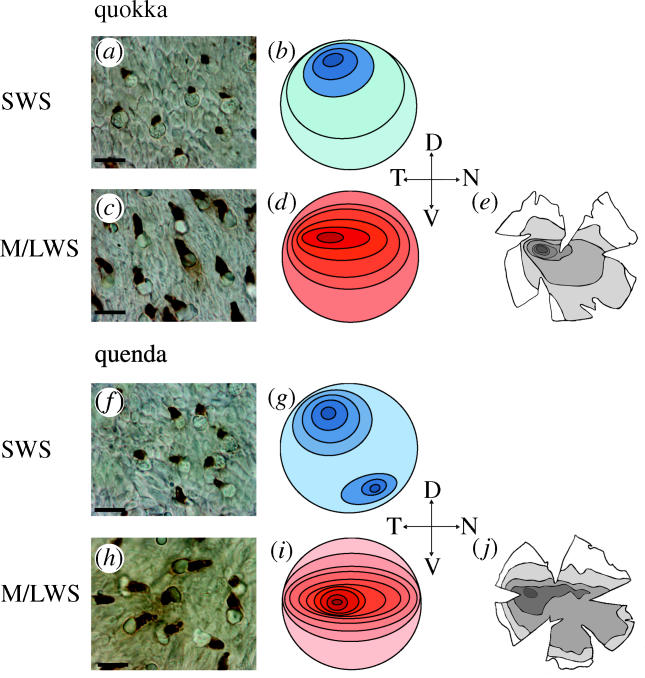
Immunolabelling of quokka (a,c) and quenda (f,h) retinae. Photomicrographs of wholemounts treated with JH455 (a,f) and JH492 (c,h). Scale bar, 8 μm. Contour maps for each cone type in quokka (b,d) and quenda (g,i): darker shades indicate highest densities, lighter shades, lowest densities. Retinal ganglion cell topographies in quokka (e; modified from Beazley & Dunlop 1983) and quenda (j; modified from Tancred 1981), showing distributions similar to M/LWS cones.
In the quokka, SWS cone density was highest in the dorso-temporal retina (2900 mm−2, s.d. ±128, figure 1b), with the lowest density located in far-ventral periphery (2100 mm−2, s.d. ±89, figure 1b). Densities elsewhere were uniform (between 2400 and 2500 mm−2, s.d. ±113, figure 1b). In the quenda, the topography of the SWS cones revealed two regions of peak density located in dorso-temporal (2300 mm−2, s.d. ±121, figure 1g) and naso-ventral retina (2200 mm−2, s.d. ±117, figure 1g). SWS cone densities decreased concentrically with the lowest concentration of SWS found in far-nasal and -temporal retina (1500 mm−2, s.d. ±63, figure 1g).
M/LWS cone topography of the quokka revealed a horizontal streak with a peak density in mid-dorso-temporal retina (27 000 mm−2, s.d. ±1100, figure 1d) decreasing to values of 18 000 mm−2 (s.d. ±650) in ventral periphery (figure 1d). M/LWS cone distribution was similar to that of retinal ganglion cells, which form an area centralis in mid-temporal retina embedded in a visual streak (figure 1e; Beazley & Dunlop 1983). In the quenda, M/LWS density decreased concentrically from a horizontal streak in mid-temporal retina (21 000 mm−2, s.d. ±760, figure 1i), to the dorsal and ventral periphery (12 000 mm−2, s.d. ±530, in figure 1i). Except for a more centrally located peak density, M/LWS cone distribution resembled that of retinal ganglion cells (figure 1j; Tancred 1981).
In both species, as in the fat-tailed dunnart and honey possum (Arrese et al. 2003), consecutive incubations of a same wholemount with the two antisera revealed the presence of a small minority of unlabelled cones (figure 2).
Figure 2.
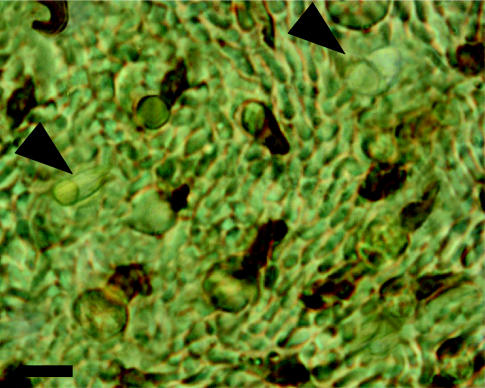
Wholemounted retina of quenda incubated with JH455 and JH492 revealing the presence of unlabelled outer segments (arrow head). Scale bar, 10 μm.
(i) Microspectrophotometry
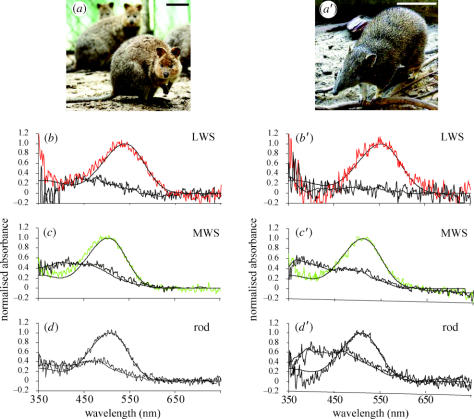
The mean wavelengths of maximum absorbance (λmax) of the cone visual pigments were 502 nm (n=17, s.d.=5.4) and 538 nm (n=12, s.d.=9.8) in the quokka, and 509 nm (n=10, s.d.=6.9) and 551 nm (n=5, s.d.=10.3) in the quenda, representing sensitivity to MWS and LWS, respectively (figure 3). The MWS- and LWS-visual pigments were found in either single or double cones, but not as MWS/LWS pairings. A minority of cones with small outer segments (presumed SWS cones) were measured, but they did not provide useable spectra. The λmax of the rod visual pigment was determined at 505 nm (n=12, s.d.=5.7) in the quokka and 508 nm (n=9, s.d.=5.1) in the quenda (figure 3d,d′). Oil droplets were found to be transparent in both species, as indicated by their negligible absorbance (<0.002) between 350 and 750 nm.
Figure 3.
The diprotodont quokka (a) and polyprotodont quenda (a′). Normalized mean averaged absorbance spectra of visual pigments in the quokka (b–d) and quenda (b′–d′), showing pre-bleach spectra (upper traces) with best-fitted visual pigment templates (solid lines) and post-bleach spectra (lower traces) with running averages (solid lines). Scale bar, 10 cm.
(ii) Molecular biology
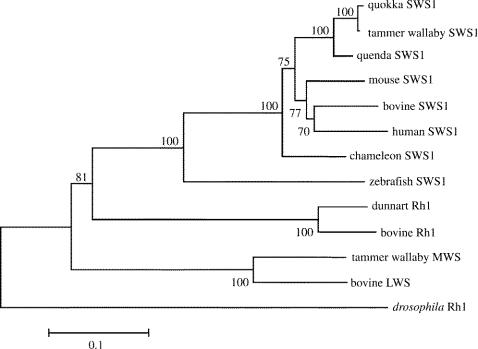
Coding sequences of the SWS1 opsin for the quokka and quenda, derived from retinal cDNA, have been deposited in GenBank (accession numbers AY726545 and AY726544, respectively). A phylogenetic tree was generated by neighbour-joining (Saitou & Nei 1987) from amino acid sequence data of the two marsupial opsins together with other mammalian and non-mammalian opsins (figure 4). The cladding of the quokka and quenda SWS1 with the SWS1 sequences of placental mammals demonstrates that the genes are orthologous.
Figure 4.
Phylogenetic tree of marsupial SWS1 and other opsins generated by the neighbour-joining method (Saitou & Nei 1987) with 1000 bootstrap replications. The calibration bar shows substitutions per site. Amino acid sequences were aligned by Clustal X (Higgins et al. 1996).
Sequence comparison between the two species reveals a total of 17 amino acid differences, including site 86, which is critical in determining ultraviolet (UV) or violet sensitivity. In the quokka, Tyr86 implies that a violet sensitive (VS) SWS1 pigment is present, whereas Phe86 in the quenda indicates a UVS pigment. Tyr86 is present in ungulate (Cowing et al. 2002; Fasick et al. 2002) and tammar wallaby (Deeb et al. 2003) VS pigments, whereas Phe86 is retained in mouse and rat UVS pigments (Yokoyama 2000). In addition, the two SWS1 sequences differed at site 114, with Gly in the quokka and Ala in the quenda.
4. Discussion
Our results provide evidence for the presence of three spectrally distinct cone photoreceptor types in the quokka and quenda. The three cone types, SWS, MWS and LWS, forming the basis for trichromacy, were also reported in the fat-tailed dunnart and honey possum (Arrese et al. 2002). The findings are consistent in the four phylogenetically distant species, indicating that the presence of three spectrally distinct cone types is a common feature of marsupials.
Cone topographies suggesting regional specializations related to visual tasks (Hemmi & Grünert 1999; Ahnelt & Kolb 2000; Peichl et al. 2000; Arrese et al. 2003) varied between the quokka and quenda.
(a) Cone topographies
Cone topographies in the two marsupials reveal three distinct regions of distribution, with species-specific differences. In both species, the combination of a high SWS cone distribution with a moderate to high M/LWS cone density in dorsal retina, would optimize spectral discrimination in the inferior field of view, as reported for the tammar wallaby (Hemmi & Grünert 1999) and fat-tailed dunnart (Arrese et al. 2003). In the mid-temporal retina, the high density of M/LWS cones coincides with the ganglion cell area centralis embedded in a visual streak (quokka; Beazley & Dunlop 1983, quenda; Tancred 1981). Visual acuity would be enhanced in these regions of highest densities of M/LWS cones and ganglion cells. In the ventral retina of the quokka, the high convergence of M/LWS cones to a low density of ganglion cells would enhance contrast sensitivity in the superior field of view (Hemmi & Grünert 1999). By contrast, spectral discrimination in the superior field of view of the quenda would be optimized by the higher density in naso-ventral retina of SWS cones and their combination with a moderate M/LWS cone distribution in naso-ventral retina. Spectral discrimination and visual acuity in the inferior and frontal fields would enhance food detection and acquisition in both species. However, different capabilities in the superior fields of the quokka and quenda may be related to their different habitats. Although contrast sensitivity is compatible with the detection of airborne predators in the open habitat of the quokka, enhanced spectral discrimination may further increase predator visibility against the background of Banksia woodland foliage.
(b) Spectral sensitivities
Measurements in the quokka and quenda provide evidence for the presence of MWS cones, which have been lost from placental mammals. In both species, MWS cones possess similar spectral sensitivities and photochemical properties to those of rods (figure 3b,c,e,f), with a post-bleach build-up of photoproduct at wavelengths below 450 nm, as reported for the fat-tailed dunnart and honey possum (Arrese et al. 2002) and birds (Bowmaker et al. 1997; Das et al. 1999; Hart 2001). The λmax of MWS cones is identical in the polyprotodont quenda and fat-tailed dunnart (509 nm) and similar in the diprotodont quokka (502 nm) and honey possum (505 nm; Arrese et al. 2002). In contrast, the LWS cones absorb at longer wavelengths, with a wider range of λmax values at 535 nm (fat-tailed dunnart), 538 nm (quokka), 551 nm (quenda) and 557 nm (honey possum), respectively. The λmax of the rod visual pigment in the quokka (505 nm) and quenda (508 nm) is characteristic of mammalian rods.
(c) Molecular biology
The majority of amino acid differences in SWS1 sequences of the quokka and quenda are conservative changes that would not be expected to affect the spectral characteristics of the pigment (Yokoyama 2000; Cowing et al. 2002; Fasick et al. 2002; Hunt et al. 2004). Among the key tuning sites at which substitutions generate a spectral shift in mammalian SWS1 pigments (Yokoyama 2000; Cowing et al. 2002; Fasick et al. 2002), the amino acid at site 86 was found to differ between the two marsupial opsins (table 2). Phe86 is thought to be present in the ancestral, ultraviolet sensitive SWS1 pigment (Yokoyama & Shi 2000; Hunt et al. 2001; Shi et al. 2001). As demonstrated by Cowing et al. (2002) and Fasick et al. (2002), a single Phe/Tyr substitution at site 86 alone is responsible for the generation of the VS pigment in bovine from the ancestral UVS pigment. Having retained Phe at site 86, the SWS1 of the quenda is UVS, as found in other mammalian and non-mammalian UVS pigments (Hunt et al. 2001, 2004). In the quokka, a substitution of Phe86 by Tyr indicates a long-wave shift, generating a VS pigment, as found in the related tammar wallaby (Deeb et al. 2003). Although further long-wave shifting may result from the substitution of Ala114 by Gly in the quokka (table 2), the single Phe86Tyr substitution would account for the majority of the spectral shift between the two opsins (Yokoyama 2000). Because UVS pigments are found in other members of the order Diprotodontia (e.g. honey possum, Arrese et al. 2002), the evolution of VS pigments by a Phe86Tyr substitution may have occurred at the base of the Macropodidae lineage. A study of other diprotodonts is needed to confirm this inference.
Table 2.
Variation at five key amino acid sites of vertebrate SWS1 pigments.
| species | λmax (nm) | 46 | 52 | 86 | 114 | 118 |
|---|---|---|---|---|---|---|
| bovine | 438 | Phe | Thr | Tyr | Ala | Cys |
| human | 424 | Thr | Phe | Leu | Gly | Thr |
| tammar wallaby | 424 | Phe | Thr | Tyr | Gly | Ser |
| quokka | (VS) | Phe | Thr | Tyr | Gly | Ser |
| quenda | (UVS) | Phe | Thr | Phe | Ala | Ser |
| mouse | 358 | Phe | Thr | Phe | Ala | Ser |
| chameleon | 358 | Phe | Thr | Phe | Ala | Ser |
| zebrafish | 362 | Phe | Thr | Phe | Ala | Ser |
In conclusion, our results add to the evidence presented previously for SWS, MWS and LWS cones in Australian marsupials, with SWS cones showing the greatest degree of variation in topography and spectral sensitivity between species. The presence of three spectrally distinct cone types in four phylogenetically distant species indicates that the potential for trichromacy is a common feature among the Australian marsupials. Although no evidence was provided by molecular analysis for the presence of an MWS visual pigment in the tammar wallaby, it is probable that, to date, MSP affords the most reliable approach to determine the presence of a third cone type in marsupials. Consequently, an MSP investigation would be critical to verify the potential for trichromacy in the tammar wallaby.
Acknowledgments
This research was supported by a grant from the Australian Research Council to C.A. (35322000). N.H. was supported by a National Health and Medical Research Council (NHMRC) grant to David Vaney; J.S. by the NHMRC programme grant no. 993219. We thank Dr Nicole Thomas for assistance with MSP and Dr Julian Partridge for helpful discussions. Dr Alexander Larcombe kindly provided the photograph of the quenda.
Footnotes
As this paper exceeds the maximum length normally permitted, the authors have agreed to contribute to production costs.
References
- Ahnelt K.P, Kolb H. The mammalian photoreceptor mosaic-adaptive design. Prog. Retin. Eye Res. 2000;19:711–777. doi: 10.1016/s1350-9462(00)00012-4. [DOI] [PubMed] [Google Scholar]
- Arrese C.A, Hart N.S, Thomas N, Beazley L.D, Shand J. Trichromacy in Australian marsupials. Curr. Biol. 2002;12:657–660. doi: 10.1016/s0960-9822(02)00772-8. [DOI] [PubMed] [Google Scholar]
- Arrese C.A, Rodger J, Beazley L.D, Shand J. Topographies of retinal cone photoreceptors in two Australian marsupials. Vis. Neurosci. 2003;20:303–311. doi: 10.1017/s0952523803203096. [DOI] [PubMed] [Google Scholar]
- Beazley L.D, Dunlop S.A. The evolution of the area centralis and visual streak in the marsupial Setonix brachyurus. J. Comp. Neurol. 1983;216:211–231. doi: 10.1002/cne.902160208. [DOI] [PubMed] [Google Scholar]
- Bowmaker J.K. Evolution of photoreceptors and visual pigments. In: Cronly-Dillon J.R, Gregory R.L, editors. In Evolution of the eye and visual pigments. CRC Press; Boca Raton, FL: 1991. pp. 63–81. [Google Scholar]
- Bowmaker J.K, Heath L.A, Wilkie S.E, Hunt D.M. Visual pigments and oil droplets from six classes of photoreceptor in the retinas of birds. Vision Res. 1997;37:2183–2194. doi: 10.1016/s0042-6989(97)00026-6. [DOI] [PubMed] [Google Scholar]
- Cowing J.A, Poopalasundaram S, Wilkie S.E, Robinson P.R, Bowmaker J.K, Hunt D.M. The molecular mechanism for the spectral shifts between vertebrate ultraviolet- and violet-sensitive cone visual pigments. Biochem. J. 2002;367:129–135. doi: 10.1042/BJ20020483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das D, Wilkie S.E, Hunt D.M, Bowmaker J.K. Visual pigments and oil droplets in the retina of a passerine bird, the canary Serinus canaria: microspectrophotometry and opsin sequences. Vision Res. 1999;39:2801–2815. doi: 10.1016/s0042-6989(99)00023-1. [DOI] [PubMed] [Google Scholar]
- Deeb, S., Wakefield, M. J., Tada, T., Marotte, L., Yokoyama, S. & Marshall Graves J. A. 2003 The cone visual pigments of an Australian marsupial, the tammar wallaby (Macropus eugenii): sequence, spectral tuning and evolution. Mol. Biol. Evol.20, 1642–1649. [DOI] [PubMed]
- Dulai K.S, Bowmaker J.K, Mollon J.D, Hunt D.M. Sequence divergence, polymorphism and evolution of the middle-wave and long-wave visual pigment genes of Great apes and Old World monkeys. Vision Res. 1994;34:2483–2491. doi: 10.1016/0042-6989(94)90233-x. [DOI] [PubMed] [Google Scholar]
- Ebrey T, Koutalos Y. Vertebrate photoreceptors. Prog. Retin. Eye Res. 2001;20:49–94. doi: 10.1016/s1350-9462(00)00014-8. [DOI] [PubMed] [Google Scholar]
- Fasick J.I, Applebury M.L, Oprian D.D. Spectral tuning in the mammalian short-wavelength sensitive cone pigments. Biochemistry. 2002;41:6860–6865. doi: 10.1021/bi0200413. [DOI] [PubMed] [Google Scholar]
- Govardovskii V.I, Fyhrquist N, Reuter T, Kuzmin D.G, Donner K. In search of the visual pigment template. Vis. Neurosci. 2000;17:509–528. doi: 10.1017/s0952523800174036. [DOI] [PubMed] [Google Scholar]
- Hart N.S. The visual ecology of avian photoreceptors. Prog. Retin. Eye Res. 2000;20(5):675–703. doi: 10.1016/s1350-9462(01)00009-x. [DOI] [PubMed] [Google Scholar]
- Hart N.S, Partridge J.C, Bennett A.T.D, Cuthill I.C. Visual pigments, cone oil droplets and ocular media in four species of estrildid finch. J. Comp. Physiol. A. 2000;186:681–694. doi: 10.1007/s003590000121. [DOI] [PubMed] [Google Scholar]
- Hemmi J.M. Behavioural colour vision in an Australian marsupial, the tammar wallaby (Macropus euigenii) J. Comp. Physiol. A. 1999;185:509–515. doi: 10.1007/s003590050411. [DOI] [PubMed] [Google Scholar]
- Hemmi J.M, Grünert U. Distribution of photoreceptor types in the retina of a marsupial, the tammar wallaby (Macropus eugenii) Vis. Neurosci. 1999;16:291–302. doi: 10.1017/s0952523899162102. [DOI] [PubMed] [Google Scholar]
- Hunt D.M, Wilkie S.E, Bowmaker J.K, Poopalasundaram S. Vision in the ultraviolet. Cell. Mol. Life Sci. 2001;58:1583–1598. doi: 10.1007/PL00000798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt D.M, Cowing J.A, Wilkie S.E, Parry J, Poopalasundaram S, Bowmaker J.K. Divergent mechanisms for the tuning of shortwave sensitive visual pigments in vertebrates. Photochem. Photobiol. Sci. 2004;3:713–720. doi: 10.1039/b314693f. [DOI] [PubMed] [Google Scholar]
- Jacobs G.H. The distribution and nature of colour vision among the mammals. Biol. Rev. Camb. Phil. Soc. 1993;68:413–471. doi: 10.1111/j.1469-185x.1993.tb00738.x. [DOI] [PubMed] [Google Scholar]
- Levine J.S, MacNichol E.F. Microspectrophotometry of primate photoreceptors: art, artifact and analysis. In: Fein A, Levine J.S, editors. In The visual system. Liss; New York: 1985. pp. 73–87. [Google Scholar]
- MacNichol E.F. A unifying presentation of photopigment spectra. Vision Res. 1986;26(9):1543–1556. doi: 10.1016/0042-6989(86)90174-4. [DOI] [PubMed] [Google Scholar]
- Mollon J. The uses and evolutionary origins of primate colour vision. In: Cronly-Dillon J.R, Gregory R.L, editors. In Evolution of the eye and visual pigments. CRC Press; Boca Raton, FL: 1991. pp. 306–319. [Google Scholar]
- Partridge J.C, Speare P, Shand J, Muntz W.R.A, Williams D.McB. Microspectrophotometric determinations of rod visual pigments in some adult and larval Australian amphibians. Vis. Neurosci. 1992;9:137–142. doi: 10.1017/s0952523800009597. [DOI] [PubMed] [Google Scholar]
- Peichl L, Künzle H, Vogel P. Photoreceptor types and distributions in the retinae of insectivores. Vis. Neurosci. 2000;17:937–948. doi: 10.1017/s0952523800176138. [DOI] [PubMed] [Google Scholar]
- Regan B.C, Julliot C, Simmen B, Viénot F, Charles-Dominique P, Mollon J.D. Fruit, foliage and the evolution of primate colour vision. Phil. Trans. R. Soc. B. 2001;356:229–283. doi: 10.1098/rstb.2000.0773. (doi:10.1098/rstb.2000.0773) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitou N, Nei M. The neighbour-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Shand J, Hart N.S, Thomas N, Partridge J.C. Developmental changes in the cone visual pigments of black bream Acanthopagrus butcher. J. Exp. Biol. 2002;205:3361–3367. doi: 10.1242/jeb.205.23.3661. [DOI] [PubMed] [Google Scholar]
- Shi Y, Radlwimmer F.B, Yokoyama S. Molecular genetics and the evolution of ultraviolet vision in vertebrates. Proc. Natl Acad. Sci. USA. 2001;98(20):11731–11736. doi: 10.1073/pnas.201257398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szél A, Röhlich P, Caffe A.R, van Veen T. Distribution of cone photoreceptors in the mammalian retina. Microsc. Res. Tech. 1996;35:445–462. doi: 10.1002/(SICI)1097-0029(19961215)35:6<445::AID-JEMT4>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Tancred E. The distribution and sizes of ganglion cells in the retina of five Australian marsupials. J. Comp. Neurol. 1981;196:585–603. doi: 10.1002/cne.901960406. [DOI] [PubMed] [Google Scholar]
- Yokoyama S. Molecular evolution of vertebrate visual pigment. Prog. Retin. Eye Res. 2000;19:385–419. doi: 10.1016/s1350-9462(00)00002-1. [DOI] [PubMed] [Google Scholar]
- Yokoyama S, Shi Y. Genetics and evolution of ultraviolet vision in vertebrates. FEBS Lett. 2000;486(2):167–172. doi: 10.1016/s0014-5793(00)02269-9. [DOI] [PubMed] [Google Scholar]