Abstract
The deep divergence between the African endemic passerines Picathartidae (rockfowl Picathartes and rockjumpers Chaetops, four species) and the Passerida (ca. 3500 species) suggests an older history of oscines on the African continent than has previously been assumed. In order to determine whether any additional, unexpectedly deep lineages occur in African endemic songbirds, 29 species—including 10 enigmatic focal taxa endemic to southern Africa—were added to a large nuclear sequence dataset gathered from oscine songbirds (Passeri). Phylogenetic analyses of these data resolve many long-standing questions about the affinities of these birds, not all of which were predicted by traditional approaches. The application of a molecular clock indicates that most basal divergences in Passerida occurred in the middle to late Eocene, with divergences between African and Australasian core corvoids occurring somewhat later in the early Miocene. Consistent with inferences for mammals, divergences between Malagasy endemic passerines and their mainland relatives suggests an asynchronous colonization history. This emerging phylogenetic picture reveals that relationships within Old World families are highly informative regarding the early dispersal and radiation of songbirds out of Gondwana. Future analyses will depend on improving resolution of higher-level phylogenetic relationships among these groups, and increasing the density of taxon sampling within them.
Keywords: oscines, Africa, Madagascar, Picathartidae, Old-World warbler
1. Introduction
The application of molecular systematics to the phylogeny of perching birds (Aves: Passeriformes) is providing robust hypotheses of their early history and evolution. Studies using different taxon and character sampling (Barker et al. 2002; Ericson et al. 2002a,b; Ericson & Johansson 2003; Barker et al. 2004) have corroborated a Gondwanan origin for basal branches of suboscines and oscines (Cracraft 2001), and suggest that major revisions to the widely accepted classification of Sibley & Ahlquist (1990) are necessary. The changing topology has already had an effect on reconstructing the evolution of cooperative breeding (Cockburn 2003), and similar insights into other features of life history and behavioural ecology may be anticipated as the passerine tree is resolved. Resolution of the oscine songbird tree promises to provide clues to the phylogenetic and spatial history of its large and often rapid radiation. In this respect, some of the more intriguing results of the molecular systematics analyses are indications that many early divergences involve African endemics. For instance, the emerging molecular phylogeny suggests that the sub-Saharan African rockfowl (Picathartes) and rockjumpers (Chaetops)—placed incertae sedis between Corvida and Passerida by Sibley & Ahlquist (1990)—may be the most basal branch within or sister to the Passerida (Ericson & Johansson 2003; Barker et al. 2004), an extremely speciose clade (approximately 3500 species) of phenotypically diverse oscine passerines.
Although the recent analyses have suggested new, previously unrecognized relationships, as well as increasing signal for some higher-level groups (families and superfamilies), many relationships remain unresolved. While elements of Sibley & Ahlquist's (1990) passeridan superfamilies Muscicapoidea, Sylvioidea and Passeroidea have been corroborated, novel nodes of equivalent rank have emerged, rendering families as well as superfamilies paraphyletic. Thus, wrens, nuthatches and creepers form an assemblage termed the Certhioidea (Cracraft et al. 2004). Another example is Sibley & Ahlquist's (1990) Muscicapoidea, which is suggested to exclude Bombycillidae (Barker et al. 2002; Ericson & Johansson 2003; Cibois & Cracraft 2004; but see Barker et al. 2004) and current studies conflict regarding the relationships among the remaining muscicapid families (Cibois & Cracraft 2004; Voelker & Spellman 2004). Intriguingly, monophyly of both the superfamily Muscicapoidea and the traditional family Monarchidae is violated by recovery of a group of insectivorous ‘flycatching’ taxa that include genera (the African Elminia and Asian Culicicapa) from both of these groups (Pasquet et al. 2002; Barker et al. 2004). The superfamily Passeroidea has received consistent support for the core members plus sunbirds (Nectariniidae) and fairy bluebirds and allies (Irenidae; Ericson & Johansson 2003; Barker et al. 2004), but a new clade not predicted by traditional taxonomy was recovered by Barker et al. (2004), including the southern African sugarbirds (Promerops), spot-throats (Arcanator) and dapple-throats (Modulatrix) as sister to the remaining Passeroidea. The emergence of these new clades, including deeply divergent African endemics, taken in consideration with the basal position of picathartids in Passerida, imply that other African birds traditionally considered ‘relict’ or ‘enigmatic’ (see Clancey 1986) might also provide information about deeper branches in the oscine songbird tree. Here, 29 new passerine taxa, including 10 purportedly ‘paleoendemic’ species selected for special attention, were added to the dataset of Barker et al. (2004).
Ongoing revision of the oscine songbird tree promises to provide not only phylogenetic clues to the reconstruction of its large radiation, but also may yield critical data on its timing. Previous molecular analyses suggested that Passerida arrived in Africa following dispersal across Eurasia in the earliest Miocene, 25 million years ago (Myr; Barker et al. 2002; Ericson, et al. 2002a), while others (Heinsohn & Double 2004) have interpreted the earliest passeridan dispersal to be 15 Myr. Although the early Miocene time frame agrees with the purported mammalian faunal exchange in the early Neogene (less than 27 Myr, Kappelman et al. 2003), recent fossil discoveries in Asia indicate that some biotic interchange between Africa and Asia occurred in the Paleogene (e.g. Marivaux et al. 2002; Seiffert et al. 2003). In any event, the increased taxonomic and genetic sampling of Barker et al. (2004) implied several waves of dispersal out of Australasia, from 45–47 Myr for Picathartidae to 26–29 Myr for crown Corvoidea. These estimates suggest an unexpected role for Africa in the history of oscine songbirds during the Eocene. We evaluate both the relative timing of lineage interchange and diversification, but also begin comparison of the absolute timing of these inferred events to geological and paleoclimatic data.
Within the African biotic region, the temporal assembly and geographical origins of the Malagasy fauna remains an area of particular controversy. Madagascar rifted from continental Africa early in the break-up of Gondwana (approximately 160 Myr; Cracraft 2001), and split from the Indian plate around 84 Myr (Cracraft 2001). However, little of the extant fauna appears to be attributable to these early divergences. For instance, the mammals of Madagascar are the product of asynchronous (but post-Gondwanan) dispersal from African and possibly Asian sources (Jansa et al. 1999; Yoder et al. 2003), and similar conclusions have been reached for much of the herpetofauna (Raxworthy et al. 2002). One major exception appears to be the amphibian fauna, which is much more constrained by oceanic barriers (Bossuyt & Milinkovitch 2001; Biju & Bossuyt 2003). The avifauna of Madagascar is only beginning to be formally analysed: the monophyly of some groups has yet to be established, and the ages of most lineages have not been estimated. Kirchman et al. (2001) reviewed the mitochondrial divergences of endemic (or putatively so) Malagasy radiations, and suggested that most of the sampled groups were old, excepting the novel sylvioid radiation discovered by Cibois et al. (1999, 2001). Given the diversity of genes used in various studies, and lacking explicit tests of rate homogeneity across the diverse lineages (four orders contain putative radiations), they did not specify ages for these lineages, although they argued that the ground rollers (Brachypteraciidae) must be significantly younger than the Eocene. In order to address these questions, we analyse the relationships and inferred divergence times of three Malagasy passerine groups.
2. Materials and methods
(a) Taxon sampling
Our taxon sampling was focused on passerine species and groups endemic to sub-Saharan Africa, with an emphasis on southern Africa (see table 1 for an overview of their taxonomic status). The species included in this study are listed in Electronic Appendix part A and include representatives of 41 families. In particular, we sampled both genera of Picathartidae (Picathartes and Chaetops), which have been placed incertae sedis by Sibley & Ahlquist (1990) between the oscine parvorders Corvida and Passerida, and which have received weak support as being part of the first branch within the Passerida (Ericson & Johansson 2003; Barker et al. 2004). We have included samples of the enigmatic Herero chat (Namibornis herero), white-tailed shrike or chatshrike (Lanioturdus torquatus), and nicators (Nicator chloris) in order to test their association with the Saxicolini, Malaconotini, and Pycnonotidae, respectively. We have included multiple representatives of the so-called African warblers (Cisticolidae), including the tailorbird (Orthotomus sutorius) which mitochondrial DNA (mtDNA) sequence data place with this group (Cibois et al. 2001), and the cinnamon-breasted warbler (Euryptila subcinnamomea), whose association with the group has not been tested. We have also sampled a wide variety of Old World warbler taxa with uncertain and controversial affinities, including Victorin's warbler (‘Bradypterus’ victorini), the Cape grass-warbler (Sphenoeacus afer), the fairy flycatcher (Stenostira scita), a crombec (Sylvietta), a longbill (Macrosphenus), the hylia (Hylia prasina), the fan-tailed grassbird (Schoenicola brevirostris) and the Damara rockjumper (Achaetops pycnopygius). The genera Sphenoeacus and Achaetops have been suggested to be part of a clade, including the genus Melocichla, owing to similarities in habit and morphological features (e.g. White 1960). By contrast, Olson (1998) determined A. pycnopygius to be so similar as to be congeneric with Chaetops, a taxonomic decision followed by a recent major treatment of African passerines (Fry et al. 2000). We have included data from two members of the endemic Malagasy ‘warbler’ radiation identified by Cibois et al. (1999, 2001); Xanthomixis (Phyllastrephus) zosterops (formerly Pycnonotidae) and Thamnornis chloropetoides (formerly Sylviidae). Finally, we have included data from the spot-throat and dapple-throat (Arcanator and Modulatrix), classified by Sibley and Ahlquist as babblers (tribe Timaliini), and the Cape sugarbird (Promerops cafer), classified with the flowerpeckers and sunbirds (Nectariniidae), all three of which appear to be part of a basal grouping within the Passerida (Barker et al. 2004). To provide a well-sampled framework to test hypotheses of relationship for these taxa, we have included representatives of all but one (Hypocoliidae) oscine passerine family as recognized by Sibley & Monroe (1990), including multiple representatives of most groups, with an emphasis on groups within the Passerida (Electronic Appendix part A). The passerine tree was rooted with sequences from Gallus and Coracias (Barker et al. 2004).
Table 1.
Overview of taxonomic status of focal taxa. (Taxa with sequences new to this study indicated in bold.)
| species | distribution in Africa; habitata | Dowsett & Forbes-Watson (1993) | Monroe & Sibley (1993) | Dickinson (2003) | others |
|---|---|---|---|---|---|
| Achaetops pycnopygius (Rockrunner) | southwest, along Namibian escarpment; grassy hillsides with boulders | Timaliidae incertae sedis | Sylviidae: Acrocephalinae | Timaliidae genus sedis incertae (as Chaetops) | Olson (1989, 1998) placed in Chaetops |
| Sphenoeacus afer (Cape grass-warbler) | southern; tall grasses near fynbos often near water | Sylviidae: Acrocephalinae | White (1960) allied with Achetops and Melocichla in Sphenoeacus | ||
| Bradypterus victorini (Victorin's warbler) | south, along Cape Fold; fynbos | Sylviidae: Acrocephalinae | |||
| Schoenicola brevirostris (Broad-tailed warbler) | sub-Saharan Africa (polytypic); tall grasses near water | Sylviidae: Megalurinae | |||
| Euryptila subcinnamomea (Cinnamon-breasted warbler) | southern; scrub in rocky hills and gorges | Sylviidae: Cisticolinae | Cisticolidae | Cisticolidae | |
| Nicator chloris (Western nicator) | central; forests and woodlands | Malaconotidae incertae sedis | Pycnonotidae | Pynonotidae genus sedis incertae | Olson (1989) placed in Malaconotidae |
| Stenostira scita (Fairy warbler) | southern; summers in Karoo and montane fynbos, winters in thornveld | Monarchidae incertae sedis | Sylviidae: Acrocephalinae | Muscicapidae: Muscicapinae | |
| Promerops cafer (Cape sugarbird) | south, along Cape Fold; flowering Protea stands (sister taxon in eastern grasslands) | Promeropidae | Nectariniidae: Promeropinae | Promeropidae | |
| Namibornis herero (Herero chat) | southwest, along Namibian escarpment; scrub in rocky areas, bases of hills | Turdidae: Saxicolinae | Muscicapidae: Turdinae (Saxicolini) | Muscicapidae: Saxicolinae | |
| Lanioturdus torquatus (White-tailed shrike) | southwest, along Namibian escarpment; thornveld and scrub desert | Malaconotidae (Batis in Platystieridae) | Malaconotidae: Malaconotinae (Batis in Vangini) | Platysteiridae (with Batis) | |
| Chaetops frenatus (Cape rockjumper) | south, along Cape Fold; rocky slopes and scree (sister taxon in eastern grasslands) | Timaliidae incertae sedis | Picathartidae | Timaliidae genus sedis incertae (with Achaetops) | Olson (1989, 1998) made congeneric with A. pycnopygius |
| Picathartes gymnocephalus (White-necked rockfowl) | west; caves and boulders in forest (sister taxon in west Congolia) | Timaliidae incertae sedis | Picathartidae | Picathartidae |
From Clancey (1986), Sinclair et al. (2002), Birds of Southern Africa, and van Perlo (2002), Birds of Western and Central Africa.
(b) Molecular methods
Nucleotide sequences of approximately 3 kb of the single RAG-1 exon, and approximately 1 kb of the single RAG-2 exon, were obtained using primers and procedures previously described (Groth & Barrowclough 1999; Barker et al. 2002, 2004; see Electronic Appendix part B for details) for 108 OTUs, including 41 families of Passeriformes. Phylogenetic analyses included maximum parsimony, maximum likelihood, and Bayesian analysis of the combined dataset (see Electronic Appendix part B).
We estimated dates of divergences at several nodes based on the assumption that the New Zealand endemic Acanthisitta diverged from all other passeriforms at the same time that New Zealand rifted from Antarctica (Cracraft 2001; Barker et al. 2004), approximately 82 Myr. We applied non-parametric rate smoothing (NPRS; Sanderson 1997) to a maximum likelihood tree and associated branch lengths in order to estimate the age of other nodes (Electronic Appendix part B; see also Barker et al. 2004).
3. Results
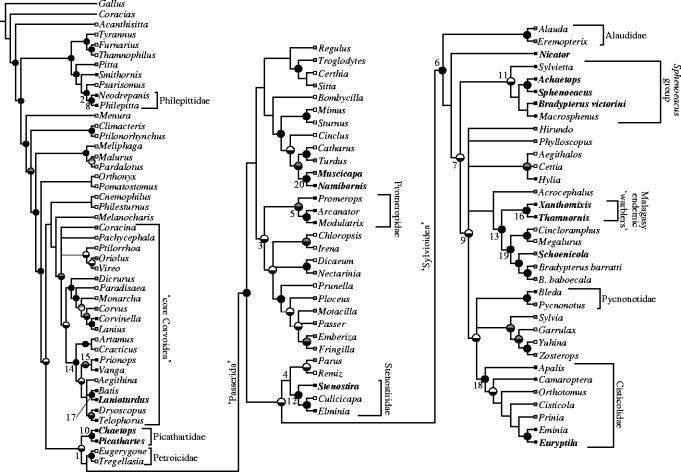
Sequences new to this study are indicated in Electronic Appendix part A along with their associated GenBank accession numbers. The equally weighted parsimony analysis of the RAG-1 and RAG-2 sequences in combination yielded 481 equally parsimonious trees, the consensus of which was well resolved, and which contained a large number of well-supported nodes (figure 1 and table 2). Bayesian analysis of the data yielded estimated nodal posterior probabilities that were completely consistent with the majority rule consensus of most-parsimonious trees (i.e. no node with greater then or equal to 0.95 estimated posterior probability conflicted with the parsimony consensus). The estimates presented are based on eight Metropolis-coupled Markov chain Monte Carlo (MC3) runs of 400 000 generations, with the first 250 000 of each discarded as burn-in. With sampling of every 100th generation, this left 1500 trees per run, for a total of 12 000 sampled trees. The Bayesian analysis differed from the parsimony analysis only in significantly supporting four nodes that were not present in the parsimony consensus, but that were consistent with that consensus. Partitioned Bayesian analysis of RAG-1 and RAG-2 indicated that the two genes are similar, though distinguishable, in evolutionary dynamics (table 2, see Electronic Appendix part B). The estimated nodal posterior probabilities from the partitioned analysis were indistinguishable from those derived from the eight unpartitioned analyses (not shown). A conservative approach was taken to the representation of the support values on the figure, with values over 75% (grey) and 90% (black) for maximum parsimony or over 0.95 estimated Bayesian posterior probability indicated. Estimated divergence dates are shown (table 3) along with minimum and maximum values for selected nodes numbered on the tree figure.
Figure 1.
Relationships among passerines from combined analyses of the nuclear RAG-1 and RAG-2 genes. Presented is the strict consensus of 481 equally parsimonious trees obtained under equally weighted parsimony (see table 2 for summary statistics), with the addition of four nodes not retained in this consensus, but recovered with high estimated Bayesian posterior probability (branches highlighted in grey). Nodal support is indicated by circles, with parsimony bootstrap in the top hemisphere (grey: 75%≥BP<90%; black: BP≥90%) and estimated Bayesian posterior probability in the lower (shaded black if PP≥0.95, otherwise white). Focal taxa in table 1 are highlighted in bold, and numbered nodes correspond to table 3. For illustrative purposes, the distribution of sampled genera is indicated by terminal boxes (white: outside of Africa; grey: distributed both within and outside of Africa; black: endemic to Africa); however, current taxon sampling precludes quantitative biogeographical analyses of the order at this level of resolution.
Table 2.
Data characteristics and estimated evolutionary parameters from phylogenetic analyses. (The number and resolution of trees, tree length (MP); CI (ensemble consistency index), and RI (ensemble retention index) values are derived from equally weighted parsimony analysis of the data. The tree length (BI), and parameters πi, rij, piv, α, and c are derived from simultaneous Bayesian analysis of the partitioned (RAG-1 and RAG-2) and unpartitioned (combined) data (see Electronic Appendix).)
| RAG-1 | RAG-2 | combined | |
|---|---|---|---|
| length of alignment | 2965 | 1152 | 4117 |
| number of variable characters | 1439 | 654 | 2093 |
| number of informative characters | 1084 | 476 | 1560 |
| number of trees (number of nodes) | 7330 (85) | >50 000 (80) | 481 (94) |
| tree length (MP) | 5531 | 2632 | 8215 |
| CI, RI | 0.386, 0.462 | 0.387, 0.525 | 0.384, 0.478 |
| −ln(L) | 51 135.7 (51 116.9–51 157.6) | ←joint estimate | 51 177.1 (51 158.3–51 199.5) |
| tree length (BI) | 2.99 (2.91–3.08) | ←joint estimate | 3.03 (2.92–3.14) |
| πA | 0.33 (0.31–0.34) | 0.32 (0.30–0.34) | 0.33 (0.32–0.34) |
| πC | 0.21 (0.20–0.22) | 0.20 (0.18–0.22) | 0.20 (0.20–0.21) |
| πG | 0.23 (0.22–0.24) | 0.20 (0.18–0.21) | 0.22 (0.21–0.22) |
| πT | 0.24 (0.23–0.25) | 0.29 (0.27–0.30) | 0.26 (0.25–0.263) |
| rAC | 1.69 (1.45–1.99) | 0.80 (0.61–1.01) | 1.29 (1.13–1.43) |
| rAG | 5.95 (5.28–6.67) | 5.18 (4.17–6.15) | 5.50 (4.98–6.01) |
| rAT | 0.81 (0.69–0.93) | 0.48 (0.38–0.60) | 0.64 (0.56–0.71) |
| rCG | 1.71 (1.45–1.98) | 1.58 (1.23–1.95) | 1.66 (1.48–1.89) |
| rCT | 11.53 (10.18–13.13) | 5.69 (4.67–6.75) | 8.91 (7.98–9.69) |
| piv | 0.39 (0.36–0.41) | 0.29 (0.24–0.33) | 0.36 (0.34–0.38) |
| α | 1.20 (1.06–1.35) | 0.90 (0.75–1.06) | 1.05 (0.95–1.16) |
| c | 0.92 (0.89–0.95) | 1.21 (1.14–1.30) | NA |
Table 3.
Inferred dates of oscine divergences. (Shown are the average, minimum, and maximum values from relaxed molecular clock analysis of 100 bootstrap replicates of the complete RAG-1 and RAG-2 matrix (see §2). Numbered nodes are labelled on figure 1.)
| node | description | mean | minimum | maximum |
|---|---|---|---|---|
| 1 | Picathartidae/Passerida and Petroicidae | 44.5 | 40.7 | 51.9 |
| 2 | Philepittidae/Psarisomus | 42.0 | 33.3 | 50.1 |
| 3 | Promeropids/Passeroids | 38.1 | 33.3 | 43.4 |
| 4 | Stenostirids/Parids | 37.5 | 32.1 | 42.1 |
| 5 | Promerops/Arcanator and Modulatrix | 33.4 | 28.3 | 39.5 |
| 6 | Alaudids/other Sylvioids | 33.8 | 29.2 | 37.7 |
| 7 | Sylvietta et al./other Sylvioids | 32.3 | 27.7 | 37.3 |
| 8 | Philepitta/Neodrepanis | 31.8 | 22.2 | 39.1 |
| 9 | Megalurines/other Sylvioids | 31.3 | 27.1 | 37.3 |
| 10 | Picathartes/Chaetops | 31.2 | 23.2 | 36.9 |
| 11 | Sylvietta/other Spheanoeacus clade | 30.1 | 25.6 | 35.4 |
| 12 | Stenostira/Culicicapa and Elminia | 29.8 | 25.4 | 34.8 |
| 13 | Malagasy/other Megalurines | 25.2 | 21.4 | 31.7 |
| 14 | ‘Vangidae’ et al./Artamidae | 23.1 | 20.5 | 29.8 |
| 15 | Vanga/Prionops | 19.7 | 16.8 | 27.0 |
| 16 | Thamnornis/Phyllastrephus | 19.2 | 14.9 | 24.4 |
| 17 | Lanioturdus/Batis | 14.3 | 11.0 | 22.9 |
| 18 | Apalis/other Cisticolidae | 17.2 | 12.5 | 22.2 |
| 19 | Schoenicola and Bradypterus/ Megalurus and Cincloramphus | 14.7 | 11.3 | 21.7 |
| 20 | Namibornis/Muscicapa | 7.1 | 3.4 | 12.6 |
The relationships among higher taxa inferred here are largely congruent with those reported previously (Barker et al. 2002, 2004), given the differences in taxon and character sampling. Namely, we recover Acanthisitta as sister to all other passerines, and two major groups corresponding to the traditionally recognized suboscines and oscines. Further, within the oscine passerines, we recover a basal grade of Australasian groups, with Menura sister to all other oscines (figure 1, see also Electronic Appendix part B), rendering Sibley & Ahlquist's (1990) parvorder ‘Corvida’ a paraphyletic grade. Most corvoid lineages are comprised in a single, large, well supported group that has been termed the ‘core Corvoidea’ (Barker et al. 2002, 2004). A large group roughly corresponding to Sibley & Ahlquist's parvorder Passerida finds strong support, as do three groups roughly corresponding to their superfamilies Muscicapoidea, Passeroidea and Sylvioidea (figure 1, see also Electronic Appendix part B). As previously, this ‘Passerida’ clade forms an effective trichotomy with two other lineages, the Australasian Petroicidae, and the African Picathartidae, with optimal trees favouring a sister group relationship between the Passerida and the Petroicidae.
In addition to this broad overview, we elaborate on the relationships of the focal enigmatic African taxa added in this study. Within the ‘core Corvoidea’ (figure 1), L. torquatus received strong support as the sister to Batis, but this lineage was not further resolved within a clade of corvidans that include Asian ioras and African bushshrikes. In the Passerida, the Herero chat (N. herero) appears to be more closely related to the saxicolines than to the turdines, although the composition of each of those groups is in flux (compare Cibois & Cracraft 2004; Voelker & Spellman 2004). Nicators (N. chloris) were not supported as bush shrikes (Malaconotidae) or bulbuls (Pycnonotidae), instead appearing in a basal grade with Alaudidae (larks) and many of Sibley & Ahlquist's (1990) Sylvioidea, which name we use for this group for brevity's sake. Given the basal position of Nicator, we suggest that the three species of nicators comprise a new family.
Three different clades with ‘African warblers’ are well resolved in this monophyletic assemblage of Sylvioidea. The first includes the crombecs and a disparate array of southern African endemics, including the Damara rockjumper (A. pycnopygius), Cape grass-warbler (S. afer) and Victorin's warbler (Bradypterus/Cryptillas victorini); this novel clade is relatively basal within the Sylvioidea, sister to more than 1000 species. This clade probably also includes the African endemic Melocichla, which is grouped with Sphenoeacus and Sylvietta in Sibley & Ahlquist's (1990, pp. 798, 866) DNA hybridization study, and which some traditional taxonomists allied with Sphenoeacus (Dickinson 2003). The second large group in the monophyletic assemblage of Sylvioidea includes the tailorbird (O. sutorius, placed in acrocephaline warblers by Sibley & Ahlquist (1990)) and the Kopje warbler (E. subcinnamomea) in the Cisticolidae. The third clade includes the megalurine warblers, the broad-tailed warbler, and the endemic Malagasy radiation (Cibois 1999).
Two additional clades are noteworthy in that they were not predicted by traditional classifications. The first includes Promerops, Arcanator and Modulatrix, all endemic to sub-Saharan Africa; we propose that the latter two taxa either be included in Promeropidae or be recognized with their own family name. This new clade is sister to an unresolved group that includes other Old World nectarivores (Nectariniidae, Irenidae and Dicaeidae) and core passeridans. Surprisingly, the African endemic fairy flycatcher (S. scita) is strongly supported as part of the previously recognized ‘sylvioid flycatcher’ clade, comprising the genera Elminia and Culicicapa. We suggest that these taxa, with Stenostira (Viellot 1818) having seniority, be recognized as the family Stenostiridae. The possible affinity of the African endemic Myioparus to Stenostira, suggested by Urban et al. (1997, p. 498), remains to be tested; other African or Asian species might conceivably fall into this novel clade. The taxonomic status of all these new higher-level groups will depend on further sampling within Passerida.
4. Discussion
(a) Inferred dates of divergence
We estimate the date of divergence of Picathartidae from Petroicidae and Passerida at 44.5 Myr (table 3), slightly later than the 47 Myr derived from the different taxon sample in Barker et al. (2004). Somewhat later in the Eocene, divergence between Promerops and the cosmopolitan passeroids is estimated at 38.1 Myr, and between the fairy-flycatcher clade (‘Stenostiridae’) and Holarctic/African parids at 37.5 Myr. The inferred presence of several lineages in Africa from the middle Eocene imply an extensive role for that continent in passeridan history. Many clades wholly endemic to Africa, as well as others more widely distributed, appear to include divergences in the Oligocene, for example, between Picathartes and Chaetops at 31.2 Myr, and between Sylvietta and its ‘Sphenoeacus’ clade sisters at 30.1 Myr. The basal divergences among Old World warblers range from 32.3 Myr between the Sphenoeacus clade and remaining warblers to 31.3 Myr between megalurine and other warblers. Although further work is required to identify the pattern of cladogenesis in the passeridan tree, these results indicate that much of current Old World warbler diversity may be attributable to radiation in and subsequent dispersal out of Africa.
Certain focal enigmatic taxa did not appear to be particularly ‘old’, as indicated by, for example, the estimated age of the divergence between Lanioturdus and Batis at 14.3 Myr, or that between Namibornis and Muscicapa at 7.1 Myr, although these estimates are certainly older than the Plio-Pleistocene time-frame traditionally associated with African avian diversification. Similarly, the date of divergence for the cinnamon-breasted warbler (Euryptila) is 6.1 Myr (±1.0 s.d.), and the most basal split within the Cisticolidae sampled—between Apalis and the other genera—appears to be relatively young, in the middle Miocene (17.2 Myr ±2.0 s.d.).
Our data provide the first quantitative assessment of divergence times for all of the endemic passerine radiations in Madagascar, which suggests that as for mammals, dispersal of these lineages into Madagascar was asynchronous. The oldest of these is the suboscine Philepittidae (2 genera, 4 species), which we estimate dispersed into Madagascar between 42.0 and 31.8 Myr, based on divergences from the broadbill Psarisomus and divergences within the family (table 3). These dates clearly postdate Gondwanan rifting, but are consistent with putative landbridge (or near-landbridge) connections between Madagascar and mainland Africa during the Late Eocene/Early Oligocene (McCall 1997; Yoder et al. 2003). A second major group of Malagasy birds dated by our data are the endemic Vangidae (10 genera, 15 species). Monophyly of this group of birds is controversial (Schulenberg 1995, unpublished Ph.D. dissertation; Fjeldså et al. 1999). Although a recent molecular study (Yamagishi et al. 2001) supported monophyly of the group, it included only one species (Laniarius ferrugineus), which might credibly test its monophyly, as available data suggest its closest relatives lie among members of the Prionopinae and Malaconotinae (figure 1; Sibley & Ahlquist 1990). Regardless of the status of the group as a whole, our data indicate divergence of one Malagasy lineage (Vanga) from its closest mainland relative (Prionops) approximately 19.7 Myr, placing an upper limit on dispersal of the Vangidae (or a subset of the family pertaining to Vanga) in the Early Miocene. Finally, based on their divergence from other megalurine warblers, and divergences within the group, we estimate dispersal of the endemic sylvioid clade of Cibois et al. (1999, 2001; represented here by Thamnornis and ‘Phyllastrephus’ equal to Xanthomixis) 25.2–19.2 Myr, a range that overlaps the maximum value for the Vangidae. Both of these latter ranges also overlap the inferred age of Malagasy carnivores (Yoder et al. 2003), but no geological scenario has been forwarded that might explain this congruence. Additional divergence dates for other lineages of Malagasy birds, which vary considerably in their extant diversity, will offer tests of alternative models (i.e. constant rates diversification versus ecological release and adaptive radiation) for their diversification.
(b) Biogeographic implications
Our effort to clarify the enigmatic status of key taxa with molecular data has provided several novel hypotheses of relationship among oscine songbirds and suggested new biogoegraphic themes for research on passerine evolution. The unanticipated extent to which African endemics span the passeridan tree suggests a complicated role for Africa in the history of these oscine passerines. Interestingly, there is no readily available paleogeographic context for the estimated age of dispersal of Picathartidae (or the common ancestor of Picathartidae and Passerida) into Africa (44.5 Myr). The middle Eocene dispersal precedes the mammalian faunal exchange between Afro-Arabia and Eurasia (less than 27 Myr; Kappelman et al. 2003), but postdates the applicable domain of the Kerguelen plateau and Indian continental drift hypothesis derived from systematic data for non-passeridans (Phasianidae, Dinesen et al. 1994; Struthionidae, Cooper et al. 2001; Tyranni, Fjeldså et al. 2003). Thus, the paleogeographic scenario involving island chains associated with drifting India seems too old to apply to many of the Eocene divergences suggested by our data. However, recent discoveries of fossil mammals are beginning to reveal Eocene interchanges between Africa and Asia (Seiffert et al. 2003; Tsubamoto et al. 2003); at present, therefore, an emerging consensus of evidence for mammals and birds suggest a historical scenario—Eocene faunal exchange—not yet grounded in paleogeography. Two paleogeographic scenarios may provide reasonable hypotheses for early episodes of dispersal of songbirds into Africa, that is, via the Indian plate (e.g. Wan et al. 2004) or via small landmasses at the leading edge of the Australian plate (Hall 1998), both effective after 65 Myr; these will have to be examined in the light of future analyses.
Within Africa, our data suggest future avenues for exploring the geographical context of speciation. The deep node subtending the Picathartidae in our analysis supports the idea that lowland forest areas serve as a ‘museum’ of ancient diversity (Fjeldså 1994; Fjeldså & Lovett 1997). However, the existence of an apparently ancient montane clade, including Promerops, Modulatrix and Arcanator, underscores the spatio-temporal heterogeneity of montane areas. This suggests that African montane regions not only have an important role in recent diversification (Roy 1997; Roy et al. 1997), but also in harbouring ancient diversity. The existence of this clade suggests, on the one hand, that other southern and Eastern Arc relationships may be found, and on the other, that additional montane endemics may be informative about phylogenetic pattern. Thus, sampling other highland endemics such as the bush blackcap (Lioptilus), the grey-chested thrush-babbler (Kakamega; J. Fjeldså personal communication), and possibly the Abyssinian catbird (Parophasma) should also be considered in the near future.
(c) Systematic implications
The topology of Barker et al. (2004) considered along with these results make the challenge for resolving the deeper nodes in the oscine songbird tree clear; in addition, recent molecular studies of more distal taxa show that many traditional tribes and genera are not monophyletic (e.g. Beresford 2003; Voelker & Spellman 2004). Yet, in order to understand the assemblage of the African avifauna, monophyletic groups and their sister taxa must be delineated. Future studies designed to extract biogeographical signals should be designed carefully, since different higher-level groups will, by virtue of their affinity to other faunas, contain different kinds of signal. Thus, although it may be tempting to clarify area relationships with a large sample of African birds, these results indicate that excluding Australasian or New World exemplars from such a study may be ill-advised. In order to determine whether or not Old World warblers, or crown Sylvoidea, is ancestrally an African group, both the sister relationship of the clade (to larks or nicators) as well as the ancestral area of that sister clade will have to be known.
The future analysis of other Old World enigmatic taxa might also resolve some of the basal passeridan nodes, such as the current polytomy between the rock-fowl, rockjumpers and Australo-Papuan robins. Olson (1979) has suggested a relationship between Picathartes and the southeast Asian ‘rail-babbler’ Eupetes. Inclusion of this enigmatic taxon in the phylogenetic analysis of passerines should be considered a priority. More broadly, increased sampling of all passerine groups distributed in Asia and southeast Asia should clarify the history of forest-mediated interchange with Africa. In particular, sampling of endemic Asian sylvioids will be informative with regard to the possible African origins of this group. Additionally, determining the relationships between endemic African groups and the Malagasy vangas, Oriental aegithinids (ioras), and the Australo-Paupuan boatbills (Dicrurinae: Monarchini; Monroe & Sibley 1993; Machaerirhynchidae; Dickinson 2003) should help clarify the history of corvoid dispersal.
5. Conclusion
The paleogeological picture of the break-up of Gondwana enjoys ongoing clarification (e.g. Marks & Tikku 2001; Gelabert et al. 2002), even with respect to the African continental margin (Hofmann et al. 1997; Muelenkamp & Sissingh 2003). In addition to the possible role of drifting India (Cracraft 2001), the presence of Palaeocene dispersal routes west of drifting India, west of Madagascar, and east of Antarctica should be explored (in association with confidence limits around dates estimated both by molecular systematists and paleogeographers). The general problem might be mitigated if extinct lineages can be incorporated into this assessment, since the observation that the only sampled (extant) sister taxa to Passerida are narrowly distributed on the remnant of western Gondwana does not necessarily require that the common ancestor of Picathartidae and Passerida was so distributed. Thus, there are two aspects to reconstructing early passeridan history that should be explored: the available paleogeographic scenarios and alternative models of phylogenetic relationships (perhaps incorporating extinction probabilities).
Fortunately, many more geographical signals for the biotic assemblage of the African passeridan fauna await clarification from well-sampled analyses of relationships at and below the family level. The proximity and ultimate contact of Africa to Eurasia, starting from approximately 40 Myr with the Iberian plate in the northwest, across the Mediterranean and the closing Tethys, from 35 to 30 Myr, to the meeting of the Arabian and Eurasian plates approximately 20 Myr, provides a complicated set of alternative routes of dispersal between Africa and Eurasia, and it is in this context that resolution of the sister relationships of Promeropidae, ‘Stenostiridae’ and the Spheonoeacus clade might be particularly informative. It seems probable that dispersal between Africa and Eurasia was first possible in the northwest in the late Eocene (notwithstanding the extensive submersion of parts of Europe at that time), followed by more eastern Arabian routes, similar to those inferred for the late Miocene (Voelker 1999) and presently in use by migrating species and genera distributed in Africa, Eurasia, or both (e.g. Griswold & Baker 2002; Drovetski et al. 2004). The contribution of multiple studies for different groups of birds will be critical for detecting patterns from congruence.
The near future of passeridan systematics also promises to add new dimensions to other evolutionary hypotheses, such as independent origins of passerine flycatching (e.g. Monarchini, Muscicapini and Stenostiridae) and modes of passeridan speciation (Cockburn 2003; Ricklefs 2003). Having a resolved passeriform tree also provides an opportunity to explore the description and distribution of morphological characters, some of which have been rendered homoplasious by the emerging topology (e.g. the ‘corvoid’ humerus; Bock 1962). In terms of historical biogeography, the challenge for avian systematic biogeographers in reconstructing the African passeridan faunal assemblage is to detect Paleogene episodes of dispersal, and clarify the Neogene continuum of long-distance dispersal, species dispersion and isolating effects of migratory processes, and relate those to abiotic factors current or historical, as well as to patterns discerned from other groups.
Acknowledgments
PB was supported by a post-doctoral bursary from the University of Cape Town Research Foundation. FKB was supported in part by the Frank M. Chapman Memorial Fund at the American Museum of Natural History. TMC and PGR's research was supported by grants from the South African National Research Foundation and the University of Cape Town Research Committee. R. Bowie and C. Cohen generously provided assistance with sample collection. In addition to specimens provided by the Percy FitzPatrick Institute for African Ornithology (PFI), additional specimens were generously provided by the Burke Museum of Natural History and Culture (S. Edwards, S. Birks), the Field Museum (FMNH; S. Hackett, D. Willard), the Zoological Museum of the University of Copenhagen (ZMUC; J. Fjeldså, J.B. Kristensen) and the American Museum of Natural History (AMNH; P. Sweet). Analyses were performed using resources of the Computational Genetics Laboratory, University of Minnesota Supercomputing Institute. This research is part of the South African Department of Science and Technology's Centre of Excellence Programme led by the Percy FitzPatrick Institute. This paper is a contribution from the Monell Molecular Laboratory and the Cullman Research Facility in the Department of Ornithology, American Museum of Natural History, and has received generous support from the Lewis B. and Dorothy Cullman Program for Molecular Systematics Studies, a joint initiative of The New York Botanical Garden and The American Museum of Natural History.
Footnotes
As this paper exceeds the maximum length normally permitted, the authors have agreed to contribute to production costs.
Present address: The City College of New York, Convent Avenue at 138th Street, New York, NY 10031, USA.
Supplementary Material
References
- Barker F.K, Barrowclough G.F, Groth J.G. A phylogenetic hypothesis for passerine birds: taxonomic and biogeographic implications of an analysis of nuclear DNA sequence data. Proc. R. Soc. B. 2002;269:295–308. doi: 10.1098/rspb.2001.1883. (doi:10.1098/rspb.2001.1883.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker F.K, Cibois A, Schikler P, Feinstein J, Cracraft J. Phylogeny and diversification of the largest avian radiation. Proc. Natl Acad. Sci. USA. 2004;101:11 040–11 045. doi: 10.1073/pnas.0401892101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beresford P. Molecular systematics of Alethe, Sheppardia and other African forest robins (Aves: Muscicapae) Ostrich. 2003;74:58–73. [Google Scholar]
- Biju S.D, Bossuyt F. New frog family from India reveals an ancient biogeographic link with the Seychelles. Nature. 2003;425:711–714. doi: 10.1038/nature02019. [DOI] [PubMed] [Google Scholar]
- Bock W.J. The pneumatic fossa of the humerus in the Passeres. Auk. 1962;79:425–443. [Google Scholar]
- Bossuyt F, Milinkovitch M.C. Amphibians as indicators of Early Tertiary “out-of-India” dispersal of vertebrates. Science. 2001;292:93–95. doi: 10.1126/science.1058875. [DOI] [PubMed] [Google Scholar]
- Cibois A, Cracraft J. Assessing the passerine “Tapestry”: phylogenetic relationships of the Muscicapoidea inferred from nuclear DNA sequences. Mol. Phylogenet. Evol. 2004;32:264–273. doi: 10.1016/j.ympev.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Cibois A, Pasquet E, Shulenberg T.S. Molecular systematics of the Malagasy babblers (Passeriformes: Timaliidae) and warblers (Passeriforms: Sylviidae), based on cytochrome b and 16S rRNA sequences. Mol. Phylogenet. Evol. 1999;13:581–595. doi: 10.1006/mpev.1999.0684. [DOI] [PubMed] [Google Scholar]
- Cibois A, Slikas B, Schulenberg T.S, Pasquet E. An endemic radiation of Malagasy songbirds is revealed by mitochondrial DNA sequence data. Evolution. 2001;55:1198–1206. doi: 10.1111/j.0014-3820.2001.tb00639.x. [DOI] [PubMed] [Google Scholar]
- Clancey P.A. Endemicity in the southern African avifauna. Durban Mus. Novit. 1986;13:245–283. [Google Scholar]
- Cockburn A. Cooperative breeding in oscine passerines: does sociality inhibit speciation? Proc. R. Soc. B. 2003;270:2207–2214. doi: 10.1098/rspb.2003.2503. (doi:10.1098/rspb.2003.2503.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper A, Lalueza-Fox C, Anderson S, Rambaut A, Austin J, Ward R. Complete mitochondrial genome sequences of two extinct moas clarify ratite evolution. Nature. 2001;409:704–707. doi: 10.1038/35055536. [DOI] [PubMed] [Google Scholar]
- Cracraft J. Avian evolution, Gondwana biogeography and the Cretaceous–Tertiary mass extinction event. Proc. R. Soc. B. 2001;268:459–469. doi: 10.1098/rspb.2000.1368. (doi:10.1098/rspb.2000.1368.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cracraft J, et al. Phylogenetic relationships among modern birds (Neornithes): towards an avian tree of life. In: Cracraft J, Donoghue M.J, editors. Assembling the tree of life. Oxford University Press; New York: 2004. pp. 468–489. [Google Scholar]
- Dickinson E.C, editor. The Howard & Moore complete checklist of the birds of the world. 3rd edn. Princeton University Press; 2003. [Google Scholar]
- Dinesen L, Lehmberg T, Svendsen J.O, Hansen L.A, Fjeldså J. A new genus and species of perdicine bird (Phasianidae, Perdicini) from Tanzania: a relict form with Indo-Malayan affinities. Ibis. 1994;136:3–11. [Google Scholar]
- Dowsett R.J, Forbes-Watson A.D. Tuaraco Press; Liege, Belgium: 1993. Checklist of birds of the Afrotropical and Malagasy Regions. [Google Scholar]
- Drovetski S.V, Zink R.M, Fadeev I.V, Nesterov E.V, Koblik E.A, Red'kin Y.A, Rohwer S. Mitochondrial phylogeny of Locustella and related genera. J. Avian Biol. 2004;35:105–110. [Google Scholar]
- Ericson P.G.P, Johansson U.S. Phylogeny of Passerida (Aves: Passeriformes) based on nuclear and mitochondrial sequence data. Mol. Phylogenet. Evol. 2003;29:126–138. doi: 10.1016/s1055-7903(03)00067-8. [DOI] [PubMed] [Google Scholar]
- Ericson P.G.P, Christidis L, Cooper A, Irestedt M, Jackson J, Johansson U.S, Norman J.A. A Gondwanan origin of passerine birds supported by DNA sequences of the endemic New Zealand wrens. Proc. R. Soc. B. 2002;269:235–241. doi: 10.1098/rspb.2001.1877. (doi:10.1098/rspb.2001.1877.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson P.G.P, Christidis L, Irestedt M, Norman J.A. Systematic affinities of the lyrebirds (Passeriformes: Menura), with a novel classification of the major groups of passerine birds. Mol. Phylogenet. Evol. 2002;25:53–62. doi: 10.1016/s1055-7903(02)00215-4. [DOI] [PubMed] [Google Scholar]
- Fjeldså J. Geographical patterns for relict and young species of birds in Africa and South America and implications for conservation priorities. Biodivers. Conserv. 1994;3:207–226. [Google Scholar]
- Fjeldså J, Lovett J.C. Geographical patterns or old and young species in African forest biota: the significance of specific montane areas as evolutionary centres. Biodivers. Conserv. 1997;6:325–346. [Google Scholar]
- Fjeldså J, Goodman S.M, Schulenberg T.S, Slikas B. Molecular evidence for relationships of Malagasy birds. In: Adams N.J, Slotow R.H, editors. Proc. 22nd Int. Ornithological Congr. Birdlife South Africa; Johannesburg: 1999. pp. 3084–3094. [Google Scholar]
- Fjeldså J, Zuccon D, Irestedt M, Johansson U.S, Ericson P.G.P. Sapayoa aenigma: a New World representative of ‘Old World suboscines’. Proc. R. Soc. B. (Suppl.S2) 2003;270:S238–S241. doi: 10.1098/rsbl.2003.0075. (doi:10.1098/rsbl.2003.0075.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry C.H, Keith S, Urban E.K, editors. The birds of Africa volume VI. Academic Press; New York: 2000. [Google Scholar]
- Gelabert B, Sàbart F, Rodriguez-Perea A. A new proposal for the late Cenozoic geodynamic evolution of the western Mediterranean. Terra Nova. 2002;14:93–100. [Google Scholar]
- Griswold C.K, Baker A.J. Time to the most recent common ancestor and divergence times of populations of common chaffinches (Fringilla coelebs) in Europe and North Africa: insights into Pleistocene refugia and current levels of migration. Evolution. 2002;56:143–153. doi: 10.1111/j.0014-3820.2002.tb00856.x. [DOI] [PubMed] [Google Scholar]
- Groth J.G, Barrowclough G.F. Basal divergences in birds and the phylogenetic utility of the nuclear RAG-1 gene. Mol. Phylogenet. Evol. 1999;12:115–123. doi: 10.1006/mpev.1998.0603. [DOI] [PubMed] [Google Scholar]
- Hall R. The plate tectonics of Cenozoic SE Asia and the distribution of land and sea. In: Hall R, Holloway J.D, editors. Biogeography and geological evolution of SE Asia. Backhuys Publishers; Leiden: 1998. pp. 99–131. [Google Scholar]
- Heinsohn R, Double M.C. Cooperate or speciate: new theory for the distribution of passerine birds. Trends Evol. Ecol. 2004;19:55–57. doi: 10.1016/j.tree.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Hoffman C, Courtillot V, Feraud G, Rochette P, Yirgu G, Ketefo E, Pik R. Timing of the Ethiopian flood basalt event and implications for plume birth and global change. Nature. 1997;389:838–841. [Google Scholar]
- Jansa S.A, Goodman S.M, Tucker P.M. Molecular phylogeny and biogeography of Madagascar's native rodents (Muridae: Nesomyinae): a test of the single origin hypothesis. Cladistics. 1999;15:253–270. doi: 10.1111/j.1096-0031.1999.tb00267.x. [DOI] [PubMed] [Google Scholar]
- Kappelman J, et al. Oligocene mammals from Ethiopia and faunal exchange between Afro-Arabia and Eurasia. Nature. 2003;426:549–552. doi: 10.1038/nature02102. [DOI] [PubMed] [Google Scholar]
- Kirchman J.J, Hackett S.J, Goodman S.M, Bates J.M. Phylogeny and systematics of ground rollers (Brachypteraciidae) of Madagascar. Auk. 2001;118:849–863. [Google Scholar]
- McCall R.A. Implications of recent geological investigations of the Mozambique Channel for the mammalian colonization of Madagascar. Proc. R. Soc. B. 1997;264:663–665. doi: 10.1098/rspb.1997.0094. (doi:10.1098/rspb.1997.0094.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marivaux L, Vianey-Liaud M, Welcomme J.L, Jaeger J.J. The role of Asia in the origin and diversification of hystricognathous rodents. Zool. Scr. 2002;31:225–239. [Google Scholar]
- Marks K.M, Tikku A.A. Cretaceous reconstructions of East Antartica, Africa and Madagascar. Earth Planet. Sci. Lett. 2001;186:479–495. [Google Scholar]
- Monroe B.L, Sibley C.G. Yale University Press; New Haven: 1993. A world checklist of birds. [Google Scholar]
- Muelenkamp J.E, Sissingh W. Tertiary paleogeography and tectonostratigraphic evolution of the Northern and Southern Peri-Tethys platforms and the intermediate domains of the African–Eurasian convergent plate bounday zone. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2003;196:209–228. [Google Scholar]
- Olson S.L. Picathartes—another West African forest relict with probable Asian affinities. Bull. Br. Orn. Club. 1979;99:112–113. [Google Scholar]
- Olson S.L. Preliminary systematic notes on some Old World passerines. Riv. Ital. Orn. 1989;59:183–185. [Google Scholar]
- Olson S.L. Notes on the systematics of the Rockrunner Achetops (Passeriformes Timaliidae) and its presumed relatives. Bull. Br. Orn. Club. 1998;118:47–52. [Google Scholar]
- Pasquet E, Cibois A, Ballon F, Érard C. What are African monarchs (Aves, Passeriformes)? A phylogenetic analysis of mitochondrial genes. CR Biol. 2002;325:1–12. doi: 10.1016/s1631-0691(02)01409-9. [DOI] [PubMed] [Google Scholar]
- Raxworthy C.J, Forstner M.R.J, Nussbaum R.A. Chameleon radiation by oceanic dispersal. Nature. 2002;415:784–787. doi: 10.1038/415784a. [DOI] [PubMed] [Google Scholar]
- Ricklefs R.E. Global diversification rates of passerine birds. Proc. R. Soc. B. 2003;270:2285–2291. doi: 10.1098/rspb.2003.2489. (doi:10.1098/rspb.2003.2489.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy M.S. Recent diversification in African greenbuls (Pynonotidae: Andropadus) supports a montane speciation model. Proc. R. Soc. B. 1997;264:1337–1344. (doi:10.1098/rspb.1997.0185.) [Google Scholar]
- Roy M.S, Cardoso da Silva J.M, Arctander P, García-Moreno J, Fjeldså J. The speciation of South American and African birds in montane regions. In: Mindell D.P, editor. Avian molecular evolution and systematics. Academic Press; San Diego: 1997. pp. 325–343. [Google Scholar]
- Sanderson M.J. A nonparametric approach to estimating divergence times in the absence of rate constancy. Mol. Biol. Evol. 1997;14:1218–1231. [Google Scholar]
- Seiffert E.R, Simons E.L, Attia Y. Fossil evidence for an ancient divergence of lorises and galagos. Nature. 2003;427:421–424. doi: 10.1038/nature01489. [DOI] [PubMed] [Google Scholar]
- Sibley C.G, Ahlquist J.E. Yale University Press; New Haven: 1990. Phylogeny and classification of birds: a study in molecular evolution. [Google Scholar]
- Sibley C.G, Monroe B.L., Jr. Yale University Press; New Haven: 1990. Distribution and taxonomy of the birds of the world. [Google Scholar]
- Sinclair I, Hockey P, Tarboton W. Princeton University Press; 2002. Birds of southern Africa. [Google Scholar]
- Tsubamoto T, Takai M, Egi N, Shigehara N. Mammalian faunal change in Eocene Asia and the Pondaung mammal fauna of Myanmar. Primate Res. 2003;19:43–64. [Google Scholar]
- Urban E.K, Fry C.H, Keith S, editors. The birds of Africa volume V. Academic Press; New York: 1997. [Google Scholar]
- van Perlo B. Princeton University Press; 2002. Birds of western and central Africa. [Google Scholar]
- Voelker G. Dispersal, vicariance, and clocks: historical biogeography and speciation in a cosmopolitan passerine genus (Anthus: Motacillidae) Evolution. 1999;53:1536–1552. doi: 10.1111/j.1558-5646.1999.tb05417.x. [DOI] [PubMed] [Google Scholar]
- Voelker G, Spellman G.M. Nuclear and mitochondrial DNA evidence of polyphyly in the avian superfamily Muscicapoidea. Mol. Phylogenet. Evol. 2004;30:386–394. doi: 10.1016/s1055-7903(03)00191-x. [DOI] [PubMed] [Google Scholar]
- Wan, Xia Qiao, Jansa L.F, Sarti M. Cretaceous and Paleogene boundary strata in southern Tibet and their implication for the India–Eurasia collision. Lethaia. 2004;35:131–146. [Google Scholar]
- White C.M.N. Notes on some African warblers. Bull. Br. Orn. Club. 1960;80:18–21. [Google Scholar]
- Yamagishi S, Honda M, Eguchi K, Thorstrom R. Extreme endemic radiation of the Malagasy vangas (Aves: Passeriformes) J. Mol. Evol. 2001;53:39–46. doi: 10.1007/s002390010190. [DOI] [PubMed] [Google Scholar]
- Yoder A.D, Burns M.M, Zehr S, Delefosse T, Veron G, Goodman S.M, Flynn J. Single origin of Malagasy Carnivora from an African ancestor. Nature. 2003;421:734–737. doi: 10.1038/nature01303. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.