Abstract
Insects are the dominant herbivores in tropical forests, with a range of mechanisms for exploiting plant resources. For group-living species, such mechanisms may involve communication. The Neotropical treehopper Calloconophora pinguis (Hemiptera: Membracidae) is a sap-feeding species in which groups of siblings feed on new leaves during the brief period of leaf expansion. Using an experimental approach, a process of cooperative foraging among siblings was documented, in which a few individuals in a group behave as scouts, locating a new feeding site and advertising it using plant-borne vibrational signals. Signalling leads to a period of positive feedback in which newly recruited individuals signal in concert with those already there. The food signalling system of C. pinguis is unique in its use of synchronized group displays and in the tight coordination of receiver responses with collective signals. Examples from a number of taxonomic groups show that vibrational communication can allow group-living insects to solve the challenges of feeding on plants, such as remaining in a foraging group or avoiding predation. While most research has focused on leaf-feeding species, sap-feeding species may remove just as much biomass. This study shows that cooperative vibrational communication underlies the ability of a sap-feeding species to exploit plant resources during a narrow window of availability.
Keywords: cooperation, chorusing, social behaviour, insect–plant interactions
1. Introduction
Insects are the dominant herbivores in tropical forests, and have evolved diverse mechanisms to exploit the changing array of resources created by the dynamics of plant growth, maturation and defence (Coley & Barone 1996). In group-living species, such mechanisms often involve communication. In highly social insects, for example, the efficient allocation of a colony's foraging effort among potential plant resources arises from an ongoing exchange of communication signals among workers (Seeley 1995; Hölldobler & Roces 2000; Wirth et al. 2003). Analogous communication systems have evolved in other plant-feeding insects that live in groups for at least part of their life cycle (Hograefe 1984; Fitzgerald 1995; Costa & Pierce 1997; Costa & Louque 2001; Fitzgerald & Pescador-Rubio 2002; Costa et al. 2004). Although most research on insect herbivory has focused on leaf-chewing species (Coley & Barone 1996), sap-feeding insects may remove an equivalent amount of biomass (Leigh 1999). Furthermore, many fluid-feeding insects feed in groups (e.g. Eickwort 1981; Wood 1984; Stern & Foster 1997), indicating the potential for the kind of recruitment systems seen in leaf- or pollen-feeding species.
The Neotropical treehopper Calloconophora pinguis (Hemiptera: Membracidae) is a sap-feeding insect in which individuals develop to adulthood in sibling groups, and then disperse after reaching adulthood. Groups of immatures feed at the base of young expanding leaves, which provide a high-quality nutritional resource (Coley & Barone 1996; Price & Carr 2000). Because the host plants of C. pinguis are light gap specialists in which leaf expansion occurs rapidly, the location of high-quality feeding sites changes during the insects' one-month development to adulthood. Groups are thus faced with the challenge of exploiting the irregular, ephemeral resource provided by a series of expanding leaves—a challenge that is expected to lead to the evolution of efficient means of discovering new resources (Coley & Barone 1996).
Treehoppers communicate by means of vibrational signals transmitted along the stems and leaves of their host plants (Cocroft & Rodríguez 2005). Vibrational communication occurs on a local scale, of the order of 1–2 m within a single plant (Cokl & Virant-Doberlet 2003). This form of communication is widespread in insect social and ecological interactions, where vibrational signals are far more prevalent than airborne sounds or other forms of mechanical signalling (Cocroft & Rodríguez 2005). Here I show that immature C. pinguis produce vibrational signals during the process of group movement. I then evaluate the hypothesis that signals function to recruit siblings to newly discovered feeding sites, by experimentally testing predictions about the context in which signals are produced, and the effect of signals on the behaviour of individuals searching for a feeding site.
2. Material and methods
(a) Background natural history
The study was conducted in Parque Nacional Soberanía near Gamboa, Panamá, from June 1998 to February 1999 and in January 2000. At that site, C. pinguis feeds on three species in the genus Piper (Piperaceae: Piper auratum, Piper marginatum and Piper reticulatum), and two hosts identified only to family (Bignoniaceae, Araliaceae). The three Piper species are light gap specialists (Grieg 1993; Dyer et al. 1999; Thies & Kalko 2004), and C. pinguis were found on plants in forest gaps or growing along the edges of roads or streams. Within their host plants, C. pinguis feed on stems and petioles just proximal to actively growing apical meristem.
Calloconophora pinguis spend much of their lives in groups. Females deposit and guard a single clutch of eggs on a host plant stem, then remain with the developing offspring. Immatures (or nymphs) form a tight, stationary aggregation throughout their approximately one-month development from hatching to adult eclosion. Aggregations may contain up to 80 nymphs (x±s.d.=38.0±22.1, n=24). Pre-reproductive adults remain aggregated for an additional 1–2 weeks before dispersing. Parent females face the aggregation from less than 5 cm away, on the proximal side of the aggregation with respect to the plant stem. However, adult females did not reliably remain with their offspring under laboratory conditions and were not included in the study. Aggregations remain together in the absence of the female, and groups of nymphs without an attending female are encountered in the field (4 out of 28 aggregations in this study).
Family groups move one or more times within their host plant. The initial move occurs after egg hatch: eggs are deposited on mature stems, and newly hatched offspring must move to an appropriate feeding site on new growth. Nymphs in 12 aggregations for which the egg scar was still visible had moved 5–175 cm away from the site where they hatched. Aggregations often move one or more additional times; for five aggregations for which the history of movements was known, each group changed feeding locations at least once, moving 10–175 cm between sites. In four of these cases, the group moved to the petiole of a younger leaf than the one on which it had been feeding; in the other case, both feeding sites were on similar stages of new growth. Laboratory observation of four family groups on a site in which the quality was declining revealed that the group did not move as a unit. Instead, one or a few individuals left the group and explored other parts of the plant.
(b) Context-dependence of signalling
Preliminary observations suggested the hypothesis that nymphs signalled to advertise newly encountered feeding sites. This hypothesis predicts that searching individuals will signal more when they encounter stems with young, expanding leaves than when they encounter stems with mature leaves. I tested this prediction by inducing search behaviour, and placing individuals on host plant stems with and without suitable feeding sites.
I obtained stems of P. marginatum from plants growing at the forest edge. Stems 15–25 cm in length were placed in a florist's water tube, brought into the laboratory and used within 2–4 min. The response variable was the number of signals produced in the 5 min after a nymph was introduced onto the base of a stem. An experimental unit consisted of two nearby stems from the same plant (one with an expanding leaf, one without), plus two nymphs matched for stadium and family. Aggregations from which nymphs were drawn were collected in the field 1–2 days previously, and maintained in the laboratory on cut stems placed in water tubes. Aggregations consisted of nymphs of the same age, alone or attended by one female, and were assumed to represent one clutch of siblings. Removing a nymph from the aggregation and placing it in a plastic container 15 min prior to testing induced searching behaviour.
Vibrational signals were detected with a Knowles BU-1771 accelerometer, attached with wax to the base of the stem, and amplified using a custom-built operational amplifier. The nymphs' behaviour was filmed using a Canon ES 2000 Hi-8 video camera, with signals recorded on the video camera's audio track and on a Marantz PMD 430 cassette recorder. The temperature range for this and the following experiments was 27±1.5 °C.
For statistical purposes, individual nymphs are nested within aggregations. To incorporate aggregation identity into the analysis for this experiment, a two-tailed binomial test was used for each aggregation to calculate the probability of obtaining results as extreme as or more extreme than those observed. An overall p-value across aggregations was then calculated using Fisher's method of combining probabilities (Sokal & Rohlf 1995). Statistical analyses were conducted with SPSS v.10 (SAS Institute, Inc., Cary, NC, USA) unless otherwise noted.
(c) Response to signals: searching behaviour
A second prediction of the hypothesis that nymphs advertise feeding sites is that signals should influence the behaviour of searching individuals. I tested this prediction using vibrational playback of the signals produced at a feeding site. In the first of two experiments, searching nymphs were placed individually on a broad leaf of a potted P. auratum host plant and played a series of recorded signals from an individual in a different aggregation. Noise bursts of the same amplitude, duration and timing were used as a control.
This playback addressed two questions about the influence of signals on search behaviour. First, do natural signals elicit more searching than noise control? The search behaviour of C. pinguis nymphs is intermittent (e.g. Kramer & McLaughlin 2001), consisting of short periods of walking separated by motionless pauses, and the response variable was the number of walking bouts initiated during each stimulus type. The effect of stimulus type on the number of search movements was evaluated using a repeated-measure ANOVA. Second, do nymphs coordinate the timing of walking bouts with that of the playback stimuli? This potential coordination was tested by varying the inter-signal interval during playbacks (as is typical of natural signalling behaviour; see §3). Each playback stimulus was constructed using a recorded signal and four different inter-signal intervals (3, 5, 7 and 9 s) in an unpredictable series chosen using a random numbers table. A series of 12 intervals was generated (e.g. 5 3 3 9 7 5 7 3 5 7 3 7), and this was repeated during the playback. A separate playback stimulus was constructed for each nymph, using a different signal (drawn from recordings of nymphs in the previous experiment) and series of intervals.
The nymph's behaviour was filmed with the camcorder while recording the playback signal via an accelerometer attached to the base of the leaf. The video signals were then digitized and the timing of each bout of walking was measured to the nearest full frame (1/30 s) using Final Cut Pro v.1.2.5 (Apple Computer, Inc., Cupertino, CA, USA) on a Macintosh G4 computer. The timing of the playback signals was measured from the audio track.
Stimuli were played from the stem just below the base of the leaf, using an electromagnet held approximately 3 mm away from a small magnet glued to the stem (e.g. Michelsen et al. 1982). Signals were played from a Macintosh PowerBook 2400 laptop computer with a Pioneer A-305 amplifier. Filtering properties of the plant stem were estimated by playing a broadband noise stimulus through the magnet and recording it with an accelerometer approximately 5 cm away on the leaf. The filter characteristics of the stem were then used to create a compensating digital filter using Matlab v.5.0 (Mathworks, Inc., Natick, MA, USA), so that the frequency spectrum of the stimulus matched that of the original recording to within ±1.5 dB (see Cocroft 1996; Cocroft & Rodríguez 2005). Signal amplitude was adjusted to that of a signalling nymph by setting the amplitude of each signal to be equal to that of the original recording.
A randomization test (Manly 1991) was conducted in Matlab v.5.2.1 to evaluate the relationship between the timing of an individual's bouts of walking and that of the playback signals. First, a cross-correlation score was obtained between a vector representing the start times of the first 20 playback signals (time increment: 0.25 s), and a vector representing the start times of the walking bouts that occurred during the same period. This score was compared with a distribution of scores obtained by randomizing the order of the intervals between walking bouts and cross-correlating the new vector with the playback signal vector (1000 iterations). An overall p-value was obtained by using Fisher's method of combining p-values first within an aggregation, then across all aggregations (Sokal & Rohlf 1995).
(d) Response to signals: orientation
If vibrational signals function in recruitment to a feeding site, then responding individuals should not only alter their searching behaviour, but also approach the source of the signals. This prediction was tested using a Y-maze. Woody stems of P. reticulatum with a symmetrical Y-shaped branch were cut and brought into the laboratory with the stem base in a water tube. The stems contained no appropriate feeding sites, and all leaf material was removed except for the leaf base at the tip of each side. Eight different stems were used for 1–2 days each. The stem bases below the Y were 15–20 cm long, as were the side branches, for a total path length from stem base to playback magnet of 30–40 cm. Stems were positioned vertically using a clamp just above the point where the stem base entered the water tube.
To test for orientation to the playback signals, each nymph was placed at the base of the stem, then played a series of vibrational signals from a sibling at a rate of one every 5 s for 5 min. Each individual was tested first with no signals. It was then tested with the signal played from one side of the Y, and again with the signal played from the opposite side, with the side order of the playbacks alternated between nymphs. There was a 20 min interval between successive treatments. To provide the same visual cues on both sides of the Y, an identical electromagnet/magnet pair was positioned on each side. The signal's frequency and amplitude characteristics were adjusted as above.
An experimental unit consisted of one unique playback signal and one nymph. Two response variables were examined, based on video recordings. The 5 min trial period was divided into three periods: time spent on the base of the Y, time spent on the left branch and time spent on the right branch (and thus there are two degrees of freedom). The first response variable was the arcsine-transformed proportion of time spent searching on the left side during each treatment (arbitrarily chosen; the prediction was that more time would be spent on the left when the playback was from the left, and less when the playback was from the right). The influence of stimulus location was evaluated using a repeated-measures ANOVA (Littell et al. 1996) with one within-subjects fixed factor (playback type: none, on left or on right) and one between-subjects random factor (aggregation of origin). The second response variable was the nymph's initial choice of which side of the Y to ascend. The influence of stimulus location on initial turn direction was evaluated using logistic regression (Allison 1999) in SAS v.6.12 (SAS Institute, Inc.); the dependent variable was turn direction (left=1, right=0), and the predictor variables were stimulus location (absent, left or right) and aggregation.
(e) Coordination among signallers
Field observations suggested that newly arrived individuals contribute to a growing chorus by signalling in unison with those already there. This apparent coordination among signallers was evaluated by introducing a second individual onto a stem with a growing shoot on which the first individual was already signalling. The signalling behaviour of both individuals was recorded with the camcorder, with the signals on the audio track (signalling is accompanied by a slight raising of the abdomen, allowing identification of the signaller from the video recording). The coordination of signalling among pairs was evaluated using a randomization test similar to that described above for the coordination of walking bouts with signals. In this case, an initial cross-correlation score was calculated for vectors representing the starting times of the signals of both individuals, using a time increment of 0.1 s (results of this test were not sensitive to the particular time increment used). This score was compared with a distribution of 1000 scores obtained by randomizing the order of inter-signal intervals of the second signaller. Signalling behaviour was analysed for eight pairs, with one pair drawn from each of eight aggregations.
(f) Variation in vibrational signals
If nymphs do advertise feeding sites, it is possible that variation in signals may correlate with variation in feeding site quality (Seeley et al. 1990). In this study that correlation was not evaluated. Instead, as a first step, signal variation was quantified within and between individuals to identify signal features with high coefficients of variation and thus the potential to convey information about differences among feeding sites. Signals were measured using Canary v.1.2.4 (Cornell University Laboratory of Ornithology, Ithaca, NY, USA) for a sample of individuals recorded during the course of the ‘context-dependence of signalling’ experiment. Signals (see figure 1a) consist of a set of harmonics with a fundamental of approximately 100 Hz and the majority of the energy below 1 kHz. Inter-signal interval, signal length and frequency of the third harmonic were quantified, as measured from audiospectrograms (the third harmonic was chosen arbitrarily to provide a measure of frequency variation among signals). A second, much shorter signal type was occasionally produced, but was not included here.
Figure 1.
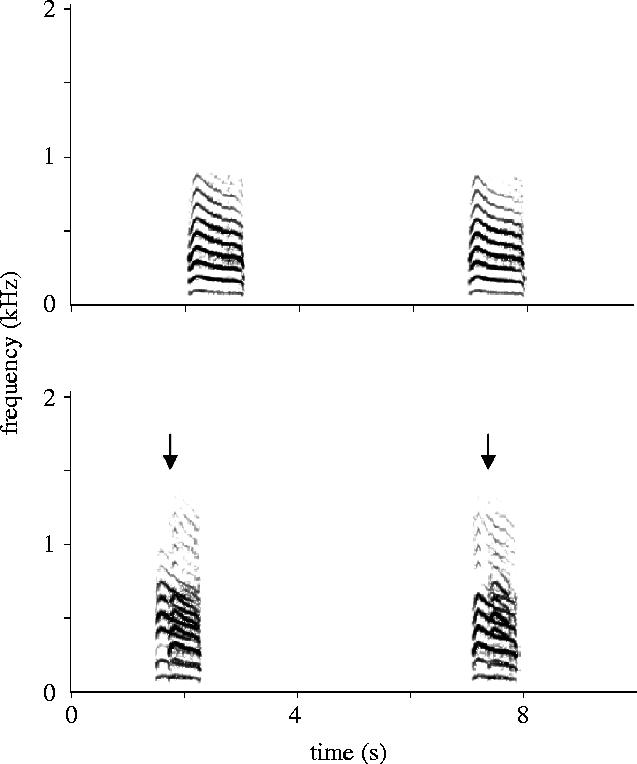
Spectrograms of vibrational signals of C. pinguis nymphs. (a) One individual signalling after discovery of a new shoot; (b) two individuals signalling together, with arrows indicating the start of a second individual's signals.
3. Results
(a) Context-dependence of signalling
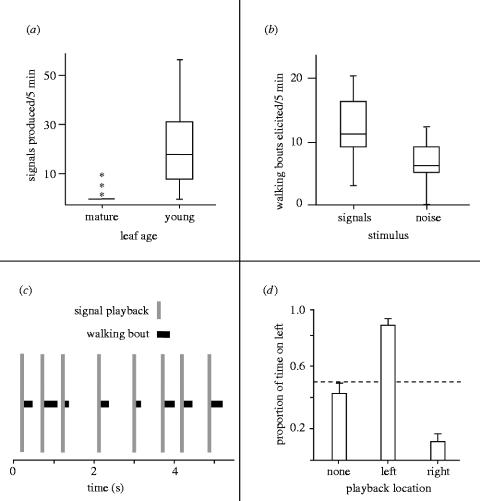
Nymphs produced over 14 times more signals on stems with new growth than on stems without new growth (χ142=34.55, p<0.01; n=37 pairs of nymphs in seven aggregations; figure 2a). Overall, 93% of signals were produced on stems with new growth. Signalling began once the nymph encountered the new leaf and inserted its feeding stylets.
Figure 2.
Signalling behaviour and its influence on receivers in C. pinguis nymphs. (a) Boxplot showing signalling as a function of the presence or absence of a suitable feeding site, with extreme values indicated by asterisks; (b) the number of walking bouts elicited by playback of 20 stimuli; (c) the coordination of walking with signals, in an example from a playback experiment. Grey vertical bars show timing of playback signals, solid horizontal bars show timing of walking bouts by the responding individual; (d) influence of signal playback location on searching behaviour of nymphs on a branching stem.
(b) Response to signals: searching behaviour
Natural signals were more effective than noise bursts in eliciting walking behaviour (repeated-measures ANOVA: F1,5=12.4, p<0.05; figure 2b; no effect of aggregation from which nymph was drawn: F5,10=0.96, p=0.48; n=16 nymphs in six aggregations).
Searching individuals responded to playback by initiating a short burst of walking (x±s.d.=5.2±2.1 s, n=16), then stopping and remaining motionless until after the next signal. The timing of initiation of walking bouts (1.07±0.51 s after the beginning of the playback signal) was significantly correlated with the timing of signals (Fisher's method of combining p-values: χ122=55.26, p<0.01; figure 2c).
(c) Response to signals: orientation
Nymphs approached the source of the signals on a Y-shaped stem (F2,12=49.33, p<0.001; figure 2d; no effect of aggregation from which an individual was drawn: F6,19=0.74, p=0.62; n=26 nymphs in seven aggregations). Of 26 nymphs tested, 24 (92%) spent more time searching on the side of the Y from which signals were played. Individuals walked upward along the stem, typically coming to a temporary stop just at or before the branching point (88% of trials). An individual's initial choice of which stem to ascend was influenced by the playback location, according to the logistic regression (χ22=10.86, p<0.01), but not by the aggregation from which it was drawn (χ62=6.74, p=0.35; n=26 nymphs in seven aggregations). Individuals made a ‘correct’ initial choice in about 73% of the playback trials. When individuals ascended the side opposite to that from which the signals were played, they often stopped a few centimetres above the branching point and then reversed direction and ascended the side from which the signals were played.
The behaviour of searching nymphs appeared to be influenced by the location of signals alone, rather than by chemical cues associated with prior individuals on the stem. There was no evidence that the initial direction an individual took when first placed on the stem (with no signal played back) was influenced by the last turn made by the previous individual on the stem, or by the amount of time the previous individual spent on each side (n=18, logistic regression, for both tests p>0.5). Furthermore, there was a significant influence of playback stimulus location (as in figure 2d), even when only the first individual on each stem was included in the analysis (n=eight individuals, repeated-measures ANOVA: F2=10.5, p<0.01).
(d) Coordination among signallers
Individuals placed onto a stem with another individual already signalling from a new shoot began walking in coordination with the signals. Once they arrived at the feeding site, they signalled in concert with the first individual. The timing of signals from the second individual was significantly correlated with that of the first individual (Fisher's method of combining p-values: χ162=75.64, p<0.01; also see figure 1b and the Electronic Appendix). Signals of the second individual were given 284±234 ms (n=8 pairs) after the beginning of the signals of the first individual, and overlapped those signals. However, signals of the second individual were also shorter, such that both signals typically ended at approximately the same time (see figure 1b). When the two individuals changed the order of signalling (n=3 pairs), their relative signal lengths changed accordingly. Additional field (n=4) and laboratory (n=5) observations revealed that the number of signallers continued to increase as more individuals located the advertised feeding site, and that signalling continued to be synchronized. Signalling continued for several minutes after the last individual had joined the group.
(e) Variation in vibrational signals
Inter-signal interval and signal length had relatively high within- and among-individual coefficients of variation (table 1). In contrast, signal frequency had a relatively low coefficient of variation.
Table 1.
Variation in the vibrational signals produced by individual C. pinguis nymphs at a newly discovered feeding site. (CV, coefficient of variation. n=18 individuals, 10 signals per individual.)
| signal trait | x ± s.d | CV (within-individual, %) | CV (among-individual, %) |
|---|---|---|---|
| length (ms) | 682±127 | 14 | 22 |
| interval (s−1) | 6.9±1.1 | 34 | 24 |
| frequency (Hz) | 300±17 | 1.4 | 5.4 |
4. Discussion
Herbivory in tropical forests is especially intense on young leaves, which are high in nitrogen and water content, and anti-herbivore defence for many species involves rapid leaf expansion and maturation (Coley & Barone 1996). The ephemeral nature of this valuable resource may select for efficient resource-finding mechanisms on the part of herbivores. In the treehopper C. pinguis, group-living immatures feed on a series of young leaves, and communication with plant-borne vibrational signals underlies their ability to locate new resources. During the transition from one feeding site to another, searching individuals signal when they have discovered a new feeding site. A positive feedback process then occurs, in which newly recruited individuals signal in concert with those already there, and the process ends after all of the group members have been recruited to the new site.
Food signalling in C. pinguis is cooperative, in that there is a benefit to the group—the location of a new feeding site—that requires collective action (Mesterton-Gibbons & Dugatkin 1992). What are the costs and benefits of signalling? The energetic costs of signalling (and the mechanism of signal production) are unknown. However, there are probably predation risks of producing signals that can be detected by vibration-sensitive predators (Cocroft & Rodríguez 2005). The inclusive fitness benefits of signalling may be substantial, depending on within-brood relatedness, the extent to which advertising a feeding site reduces search costs for siblings and the relationship between group size and individual fitness. The influence of grouping on predation risk has not been assessed for C. pinguis, but individual benefits from dilution effects will vary with group size, position within the group and the effect of grouping on the probability of predator encounters (Hamilton 1971; Mooring & Hart 1992; Krause 1994; Cocroft 2002). In C. pinguis, maternal defence against predators may also be important (e.g. Wood 1978), and such defence will probably be more effective when offspring are clumped (Windsor 1987). Other possible benefits to insect herbivores from groups range from enhanced thermoregulation to more efficient use of food resources (Fitzgerald 1995; Costa & Pierce 1997; Cocroft 2001). Signalling by individuals that have arrived at a new feeding site is thus likely to have a range of fitness benefits. Indirect evidence suggests that signalling may also recruit individuals from a different aggregation. During playbacks in this study, nymphs responded to signals of both siblings and non-siblings. In the field, 40% of females (21/52) with eggs or nymphs occurred together on the same host plant with one or more other aggregations, and three aggregations contained two broods, as evidenced by the presence of two attending females and distinct size classes of nymphs (R. B. Cocroft, personal observations, this study). Whether recruitment of individuals from a second aggregation represents a benefit or a cost is unclear.
There may be less overlap of interests between siblings at the exploratory stage of a move. Because a solitary individual moving onto new areas of the host plant is likely to be at a relatively high risk from spiders or other predators attracted to moving prey, there may be a higher fitness pay-off of remaining at the old site while other individuals search. What then determines which individuals behave as ‘scouts’? The present study provides two insights into this question. First, the experiments above show that virtually any individual can be induced to search and signal by removing it from a group and placing it on a stem with new growth. Second, in four groups filmed in the pre-dispersal period (R. B. Cocroft, personal observations, this study), there was a characteristic pattern of behaviour that suggests an assessment process. Individuals began periodically rocking or ‘walking in place’, bringing them into contact with adjacent individuals, some of which responded with a similar behaviour. This episodic rocking behaviour gradually spread to more and more individuals, after which a few individuals eventually left the group and walked down the stem towards the rest of the plant. Strikingly similar patterns of tactile stimulation occur in other nomadically foraging insects prior to a move by the group (Fitzgerald & Pescador-Rubio 2002; Fitzgerald et al. 2004). Determination of which individuals leave the group to discover new sites is thus more likely to depend on variation in nutritional state, position in the group, or other factors that influence response probability than on variation in the ability to signal when a new site is discovered.
The use of synchronized acoustical displays to advertise a food resource is a striking feature of communication in C. pinguis, with no clear parallels even among the highly social insects (Kirchner 1997). Since the interval between group displays is variable, synchrony must be maintained on a cycle-by-cycle basis, rather than through adjustment to a common rate, as occurs, for example, in the synchronous visual signalling of some fireflies (Greenfield 2002). Mirroring this synchrony are the responses of receivers, which approach in short bursts during the intervals between signals. This alternation of signals and responses probably facilitates signal detection and localization. The principal vibration receptors in insects are in the legs (Kalmring 1985), and walking reduces the number of legs in contact with the substrate. Furthermore, movement along a plant stem, especially by multiple individuals, generates vibrational noise (R. B. Cocroft, unpublished data) that may interfere with the detection of signals. Receivers will thus experience a higher signal-to-noise ratio if signals and movement are alternated, rather than if they occur simultaneously.
Vibrational communication underlies the social behaviour of other species of herbivorous insects with group-living immatures, with functions that vary among species with different foraging ecologies. For nomadically foraging species, signalling can be important during the formation of foraging groups (Cocroft 2001; Greenfield 2002), in ‘activation’ of existing groups (Costa et al. 2004), and in recruiting other group members to feeding sites (Hograefe 1984). By contrast, in species that complete their development to adulthood on a single stem, vibrational communication may have an anti-predator rather than a foraging function. In some treehoppers, females remain with stationary broods of offspring (Wood 1983), and sibling groups produce synchronized vibrational signals to elicit maternal defence against predators (Cocroft 1996, 2002).
Given that substrate-borne vibrational communication is the predominant form of mechanical signalling in insects (Cocroft & Rodríguez 2005), and that short-range vibrational signals are easily overlooked, many more such communication systems probably await discovery. The above examples show that vibrational communication can allow group-living insects to solve a range of challenges faced by social herbivores, from remaining in a foraging group to avoiding predation. This study describes a cooperative communication system underlying the ability of a sap-feeding species to make use of a series of ephemeral plant resources. For group-living species, vibrational communication may be a widespread and important feature of insect–plant interactions.
Acknowledgments
I thank the staff at the Smithsonian Tropical Research Institute, especially Maria Leone for assistance in obtaining research permits, A. Aiello and D. Windsor for plant identifications and S. Rand and M. Jennions for the loan of field vehicles. I thank J. Christy, A. Herre, M. Jennions, S. Rand, B. Wcislo and D. Windsor for stimulating discussions, C. Dietrich for membracid identification, and M. Ellerseick for statistical advice. I also thank K. Konrad for video analysis and C. P. Lin, K. Ramaswamy, R. L. Rodríguez, L. E. Sullivan and three anonymous reviewers for comments on the manuscript. This research was conducted under permits INRENARE 6-97 and ANAM 13-99.
Footnotes
Present address: Division of Biological Sciences, University of Missouri-Columbia, Columbia, MO 65211, USA.
Supplementary Material
References
- Allison P.D. SAS Institute; Cary, NC: 1999. Logistic regression using the SAS system. [Google Scholar]
- Cocroft R.B. Insect vibrational defence signals. Nature. 1996;382:679–680. [Google Scholar]
- Cocroft R.B. Vibrational communication and the ecology of group-living, herbivorous insects. Am. Zool. 2001;41:1215–1221. [Google Scholar]
- Cocroft R.B. Maternal defense as a limited resource: unequal predation risk in broods of a subsocial insect. Behav. Ecol. 2002;13:125–133. [Google Scholar]
- Cocroft R.B, Rodríguez R.L. The behavioral ecology of insect vibrational communication. BioScience. 2005;55:323–334. [Google Scholar]
- Cokl A, Virant-Doberlet M. Communication with substrate-borne signals in small plant-dwelling insects. Annu. Rev. Entomol. 2003;48:29–50. doi: 10.1146/annurev.ento.48.091801.112605. [DOI] [PubMed] [Google Scholar]
- Coley P.D, Barone J.A. Herbivory and plant defenses in tropical forests. Annu. Rev. Ecol. Syst. 1996;27:305–335. [Google Scholar]
- Costa J.T, Louque W. Group foraging and trail following behavior of the red-headed pine sawfly Neodiprion lecontei (Fitch) (Hymenoptera: Symphyta: Diprionidae) Ann. Entomol. Soc. Am. 2001;94:480–489. [Google Scholar]
- Costa J.T, Pierce N.E. Social evolution in the Lepidoptera: ecological context and communication in larval societies. In: Choe J.C, Crespi B.J, editors. The evolution of social behavior in insects and arachnids. Cambridge University Press; Cambridge, UK: 1997. pp. 407–442. [Google Scholar]
- Costa J.T, Fitzgerald T.D, Pescador-Rubio A, Mays J, Janzen D.H. Group foraging behavior of the larvae of the Neotropical processionary weevil Phelypera distigma (Boheman) (Coleoptera: Curculionidae: Hyperinae) Ethology. 2004;110:515–530. [Google Scholar]
- Dyer L.A, Letourneau D.K, Williams W, Dodson C. A commensalism between Piper marginatum Jacq. (Piperaceae) and a coccinellid beetle at Barro Colorado Island, Panama. J. Trop. Ecol. 1999;15:841–846. [Google Scholar]
- Eickwort G.C. Presocial insects. In: Hermann H.R, editor. Social insects. vol. 2. Academic Press; New York: 1981. pp. 199–280. [Google Scholar]
- Fitzgerald T.D. Cornell University Press; Ithaca, NY: 1995. The tent caterpillars. [Google Scholar]
- Fitzgerald T.D, Pescador-Rubio A. The role of tactile and chemical stimuli in the formation and maintenance of the processions of the social caterpillar Hylesia lineata (Lepidoptera: Saturniidae) J. Insect Behav. 2002;15:659–674. [Google Scholar]
- Fitzgerald T.D, Pescador-Rubio A, Turna M.T, Costa J.T. Trail marking and processionary behavior of the larvae of the weevil Phelypera distigma (Coleoptera: Curculionidae) J. Insect Behav. 2004;17:627–646. [Google Scholar]
- Greenfield M.D. Oxford University Press; 2002. Signalers and receivers: mechanisms and evolution of arthropod communication. [Google Scholar]
- Grieg N. Regeneration mode in Neotropical Piper: habitat and species comparisons. Ecology. 1993;74:2125–2135. [Google Scholar]
- Hamilton W.D. Geometry for the selfish herd. J. Theor. Biol. 1971;31:295–311. doi: 10.1016/0022-5193(71)90189-5. [DOI] [PubMed] [Google Scholar]
- Hograefe T. Substrat-stridulation bei den koloniebildenden blattwespenlarven von Hemichroa crocea (Geoff.) (Hymenoptera: Tenthredinidae) Zool. Anz. Jena. 1984;213:234–241. [Google Scholar]
- Hölldobler B, Roces F. The behavioral ecology of stridulatory communication in leafcutting ants. In: Dugatkin L, editor. Model systems in behavioral ecology: integrating empirical, theoretical and conceptual approaches. Princeton University Press; 2000. pp. 92–109. [Google Scholar]
- Kalmring K. Vibrational communication in insects (reception and integration of vibratory information) In: Kalmring K, Elsner N, editors. Acoustic and vibrational communication in insects. Paul Parey; Berlin: 1985. pp. 127–134. [Google Scholar]
- Kirchner W.H. Acoustical communication in social insects. In: Lehrer M, editor. Orientation and communication in arthropods. Birkhäuser; Basel, Switzerland: 1997. pp. 273–300. [Google Scholar]
- Kramer D.L, McLaughlin R.L. The behavioral ecology of intermittent locomotion. Am. Zool. 2001;41:137–153. [Google Scholar]
- Krause J. Differential fitness returns in relation to spatial position in groups. Biol. Rev. 1994;69:187–206. doi: 10.1111/j.1469-185x.1994.tb01505.x. [DOI] [PubMed] [Google Scholar]
- Leigh E.G. Oxford University Press; New York: 1999. Tropical forest ecology: a view from Barro Colorado Island. [Google Scholar]
- Littell R.C, Milliken G.A, Stroup W.W, Wolfinger R.D. SAS Institute; Cary, NC: 1996. SAS system for mixed models. [Google Scholar]
- Manly B.F.J. Chapman & Hall; London: 1991. Randomization and Monte Carlo methods in biology. [Google Scholar]
- Mesterton-Gibbons M, Dugatkin L.A. Cooperation among unrelated individuals: evolutionary factors. Q. Rev. Biol. 1992;67:267–281. [Google Scholar]
- Michelsen A, Fink F, Gogala M, Traue D. Plants as transmission channels for insect vibrational songs. Behav. Ecol. Sociobiol. 1982;11:269–281. [Google Scholar]
- Mooring M.S, Hart B.L. Animal grouping for protection from parasites: selfish herd and encounter-dilution effects. Behaviour. 1992;123:173–193. [Google Scholar]
- Price P.W, Carr T.G. Comparative ecology of membracids and tenthredinids in a macroevolutionary context. Evol. Ecol. Res. 2000;2:645–655. [Google Scholar]
- Seeley T.D. Harvard University Press; London: 1995. The wisdom of the hive: the social physiology of honey bee colonies. [Google Scholar]
- Seeley T.D, Camazine S, Sneys J. Collective decision-making in honey bees: how colonies choose among nectar sources. Behav. Ecol. Sociobiol. 1990;28:277–290. [Google Scholar]
- Sokal R.R, Rohlf F.J. 3rd edn. Freeman; New York: 1995. Biometry. [Google Scholar]
- Stern D.L, Foster W.A. The evolution of sociality in aphids: a clone's-eye view. In: Choe J.C, Crespi B.J, editors. The evolution of social behavior in insects and arachnids. Cambridge University Press; Cambridge, UK: 1997. pp. 150–165. [Google Scholar]
- Thies W, Kalko E.K.V. Phenology of Neotropical pepper plants (Piperaceae) and their association with their main dispersers, two short-tailed fruit bats, Carollia perspicillata and C. castanea (Phyllostomidae) Oikos. 2004;104:362–376. [Google Scholar]
- Windsor D.M. Natural history of a subsocial tortoise beetle, Acromis sparsa Boheman (Chrysomelidae, Cassidinae) in Panama. Psyche. 1987;94:127–150. [Google Scholar]
- Wirth R, Herz H, Ryel R.J, Beyschlag W, Hölldobler B, Molnar Z.D. Herbivory of leaf-cutting ants: a case study on Atta colombica in the tropical rainforest of Panama. Ecol. Stud. 2003;164:1–230. [Google Scholar]
- Wood T.K. Parental care in Guayaquila compressa Walker (Homoptera: Membracidae) Psyche. 1978;85:135–145. [Google Scholar]
- Wood T.K. Brooding and aggregating behavior of the treehopper, Umbonia crassicornis. Nat. Geogr. Res. 1983;15:753–758. [Google Scholar]
- Wood T.K. Life history patterns of tropical membracids (Homoptera: Membracidae) Sociobiology. 1984;8:299–344. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.