Abstract
The Labridae is one of the most structurally and functionally diversified fish families on coral and rocky reefs around the world, providing a compelling system for examination of evolutionary patterns of functional change. Labrid fishes have evolved a diverse array of skull forms for feeding on prey ranging from molluscs, crustaceans, plankton, detritus, algae, coral and other fishes. The species richness and diversity of feeding ecology in the Labridae make this group a marine analogue to the cichlid fishes. Despite the importance of labrids to coastal reef ecology, we lack evolutionary analysis of feeding biomechanics among labrids. Here, we combine a molecular phylogeny of the Labridae with the biomechanics of skull function to reveal a broad pattern of repeated convergence in labrid feeding systems. Mechanically fast jaw systems have evolved independently at least 14 times from ancestors with forceful jaws. A repeated phylogenetic pattern of functional divergence in local regions of the labrid tree produces an emergent family-wide pattern of global convergence in jaw function. Divergence of close relatives, convergence among higher clades and several unusual ‘breakthroughs’ in skull function characterize the evolution of functional complexity in one of the most diverse groups of reef fishes.
Keywords: convergence, feeding, skull, Labridae, phylogenetics
1. Introduction
A central goal of biology is to understand the evolution of diversity in form, function and behaviour in large species groups, which provide our most cogent examples of evolutionary change (Schluter 2000a,b; Autumn et al. 2002). Integrating studies of phylogeny, ecology and physiology of vertebrate clades has advanced our understanding of the structural, functional and ecological radiation of such disparate lineages as fishes (Basolo 1990; Westneat 1995; Stoddard 1999), amphibians (Ryan 1998), lizards (Harmon et al. 2003), birds (Burns et al. 2002; Lovette et al. 2002) and bats (Ruedi & Mayer 2001). The family Labridae (the wrasses), with about 600 species, is the 11th largest vertebrate family and second largest marine fish family (Parenti & Randall 2000), and is among the most morphologically and ecologically diversified families of fishes on coral and rocky reefs. The group is globally distributed in tropical and temperate habitats (Bellwood & Wainwright 2002), and represents an oceanic counterpart to the well-known cichlid fishes (Verheyen et al. 2003) to whom they may be closely related (Stiassny & Jensen 1987; Streelman & Karl 1997). Labrids play a critical role in reef ecology as they occupy virtually all major feeding guilds on reefs, feeding on gastropods, bivalves, crustaceans, fishes, coral mucous, zooplankton, ectoparasites, detritus and algae (Randall 1967; Westneat 1994; Wainwright & Bellwood 2002). Each feeding mode in the family has specific mechanical requirements for generating bite force or jaw speed for prey capture that influence the dynamics of skull function. In some cases the diversity of skull form is extreme even among close relatives that have diverged along axes of skull function and dietary specialization (Westneat 1995). These colourful, widespread reef fishes thus provide an excellent system for examining the evolution of skull diversity and mechanical design. To explore this diversity and investigate patterns of divergence and convergence, we investigated the evolutionary history of skull function in the labrid fishes.
Recent analyses have demonstrated that the evolution of form, function and behaviour in diverse organisms is best understood by combining phylogenetic information on a group with mechanistic studies of organismal function to yield a picture of historical change in key traits that may be otherwise unattainable (Lauder & Liem 1989; Westneat 1995; Autumn et al. 2002; Harmon et al. 2003). The phylogenetic relationships of several subfamilies of labrid fishes have been clarified (Westneat 1993; Bellwood 1994; Gomon 1997; Hanel et al. 2002; Streelman et al. 2003; Clements et al. 2004; Barber & Bellwood 2005) but a higher-level phylogeny for the family has only recently emerged (Westneat & Alfaro in press). Here, we use a higher-level molecular phylogeny of the Labridae (Westneat & Alfaro in press) for evolutionary analysis of feeding biomechanics. The mechanisms of skull kinesis, suction generation and force transmission during feeding in fishes have been the subject of intensive research in recent years, with the Labridae and their relatives playing a central role as test subjects in this field (Westneat 1994, 2003; Hulsey & Wainwright 2002; Wainwright et al. 2004). Biomechanical models of skull function provide a means of assessing critical features of fish feeding systems in a large species sample suitable for evolutionary analysis. Using phylogenetics and biomechanics, we ask two questions: (i) how have diverse feeding designs been assembled during the evolution of labrids and (ii) how often have particular solutions to biomechanical feeding challenges evolved? The answers to these questions reveal that functional diversity is assembled by largely independent evolution of mechanical traits in a complex feeding system and that repeated patterns of divergence among close relatives yield a higher-level pattern of repeated biomechanical convergence.
2. Material and methods
(a) Phylogenetic reconstruction
A phylogeny of the Labridae (see Westneat & Alfaro in press) was generated by sequencing four gene fragments from 98 fish species (84 labrid fishes and 14 outgroups). Taxa were chosen from all major labrid clades and most major ocean regions where labrid fishes exist. In most cases we used a single species to represent a genus, although in large or morphologically diverse genera several species are often represented. We used standard techniques for DNA extraction (Puregene DNA isolation kit—Gentra Systems, Minneapolis, MN), polymerase chain reaction (PCR; primers available from M. Westneat, PCR performed using an MJ Research PTC-200 Peltier Thermal Cycler—MJ Research Incorporated, Watertown, MA) and sequencing (ABI PRISM 3100 Genetic Analyser—Applied Biosystems, Foster City, CA). From the mitochondrial genome we sequenced portions of 12S rRNA (1000 bp) and 16S rRNA (585 bp), which were aligned by using a secondary structure model. From the nuclear genome, we sequenced part of the protein-coding genes RAG2 (846 bp) and Tmo4C4 (541 bp). GenBank accession numbers are provided in Electronic Appendix 1.
Parsimony, maximum-likelihood and Bayesian methods were used during searches for phylogenetic topologies that best fit the data (complete analysis in Westneat & Alfaro in press). We explored both individual partitions and total evidence phylogenetic trees using the computer program Paup* v.4.0b10 (Swofford 2000). Heuristic searches to find the most parsimonious tree(s) were performed using tree bisection–reconnection branch swapping, 1000 random sequence addition replicates and non-parametric bootstrapping to measure support of clades. A likelihood analysis using the GTR+Γ+I model and heuristic search with 10 random sequence additions was used to find the optimal tree. To calculate posterior probabilities of clades, we used MrBayes v.3.0 (Huelsenbeck & Ronquist 2001) to run a 10 million generation Markov chain. The four gene partitions were assigned separate (unlinked) parameters for an HKY+Γ+I model of sequence evolution. A majority rule consensus tree calculated from 19 000 post burn-in trees was constructed and used to determine the posterior probabilities of clades. All methods arrived at a similar topology that is used here for evolutionary analysis of functional characters.
(b) Biomechanical modelling and character analysis
We analysed the skull morphology of labrid fishes using preserved dissected fishes or specimens cleared and double stained for cartilage and bone (Taylor & Van Dyke 1985). Characters were defined according to recent biomechanical models and morphometrics of the jaws. We used two biomechanical models as the foundation for morphometrics of the labrid lower jaw (Westneat 1994, 2003; Wainwright & Richard 1995) and the anterior jaws linkage for jaw protrusion (Westneat 1994). Computer models are available from M. Westneat for calculating the biomechanics of lower jaw levers or the kinematics of cranial four-bar linkage design. Morphometric data were collected from each of the taxa represented in the phylogeny, using either direct distance measures (with calipers) or coordinate data taken from digital images of dissected specimens. Morphometric data were analysed using lever and linkage models to calculate mechanical advantage (force transmission) and kinematic transmission (KT) variables. Three biomechanical variables were calculated: (i) the jaw-opening mechanical advantage is the ratio of jaw-opening inlever to jaw outlever; (ii) the jaw-closing mechanical advantage is the ratio of the jaw-closing inlever of the A2 division of the jaw adductor muscle to the jaw outlever; and (iii) maxillary KT is maxillary rotation output per degree of lower jaw rotation input during jaw opening. Characters 1 and 2 are estimates of relative force (and their inverses scale with speed), whereas character 3 is a speed metric whose inverse is proportional to force transmission (Westneat 1994).
Phylogenetic histories of biomechanical characters were analysed in two ways: as continuous quantitative characters and as discrete characters. Continuous characters were optimized on the phylogeny using squared-change parsimony, whereas discrete characters were generated using gap coding and optimized on the tree topology using Farris optimization (both implemented in MacClade v.4.0). Character homoplasy (independent origin and reversal) was assessed by calculating the character consistency index. Tests of character correlation were performed using independent contrasts, a phylogenetically adjusted regression technique implemented using the software CAIC (Purvis & Rambaut 1995).
3. Results and discussion
(a) Phylogeny of the Labridae
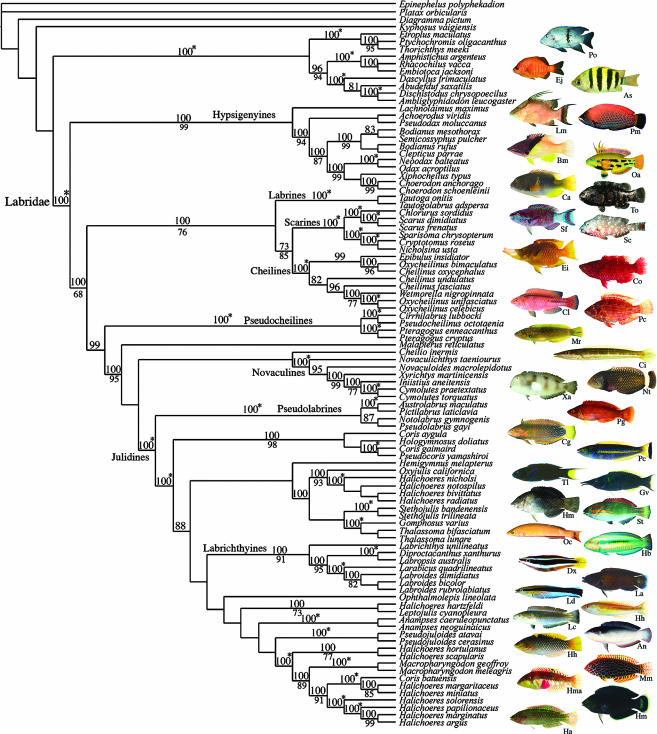
A well-resolved phylogeny (figure 1, from Westneat & Alfaro in press) was generated for 84 labrid taxa from all major clades in the family. Similar results from each tree-building method and high support for many nodes in the tree allow confidence in the broad structure of the tree. This phylogeny is an important step towards resolution of a long-standing problem in ichthyology (Kaufman & Liem 1982; Westneat 1993) and presents an outstanding opportunity to frame evolutionary questions about a key group of reef fishes. Highlights of the tree include confirmation that both the parrotfish (scarines) and rock whitings (represented by Odax) are nested deeply within labrid clades rather than standing apart as separate families, and strong support for the monophyly of the family Labridae (including scarines and odacines). Evidence was not sufficient to test the monophyly of the suborder Labroidei. Strong support was found for monophyly of many major tribal groupings of the family, including the Hypsigenyini, Labrini, Novaculini and Labrichthyinae. However, the cheilines are allied with the scarines in this topology rather than being closely related to the pseudocheilines as in previous hypotheses (Westneat 1993), and relationships within and between genera of the julidine lineage suggest that many currently recognized genera may not be natural groups.
Figure 1.
Phylogeny of the family Labridae (from Westneat & Alfaro in press), based on mitochondrial and nuclear DNA sequences, with images of representative members of each major clade. Shown is the Bayesian MAP (maximum a posterior probability) topology. Numbers above branches are posterior probabilities from a consensus tree of all post burn-in topologies visited by the Markov chain and numbers below branches are bootstrap support values from parsimony analyses. The symbol 100* indicates that the clade is recovered at 100% under both methods. This phylogeny supports monophyly of the family and provides clear higher-level support for the branching pattern among most labrid groups. Photographs are identified by initials of genus and species nearby in the tree. Photograph credits are given in Electronic Appendix, part C.
(b) Morphology and biomechanics
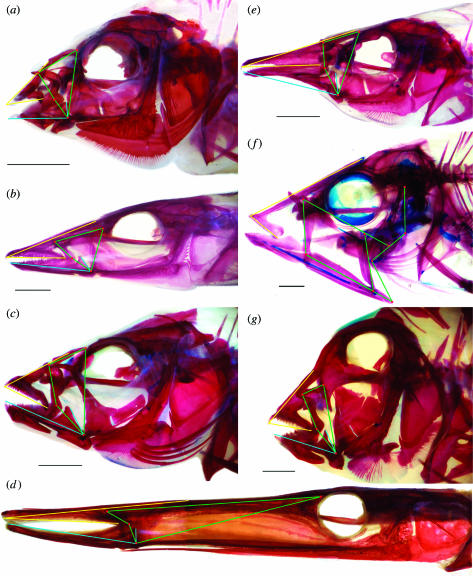
The skulls of labrid fishes are complex mechanical systems with many movable elements and a diversity of anatomical designs (figure 2). Morphological diversity in the wrasses includes deep heads with strong jaws (figure 2c,g), often with unusual teeth (figure 2a), slender heads with fast jaws and long snouts (figure 2b,e), and skulls that are simply bizarre, including the extremely elongate head of Siphonognathus argyrophanes (figure 2d) and the sling-jaw mechanism of Epibulus insidiator (figure 2f). Engineering models were developed to analyse the biomechanics of feeding in labrids as a system of levers and linkages that transmits the forces of contracting muscles to the jaws. The first model is that of the mandibular lever (figure 3), a simple lever model for opening and closing mechanics (Westneat 2003). Wrasse mandibles range from heavy jaws with large teeth to long slender jaws specialized for speed (figure 3a–g). Lever models of labrid jaws estimate force transmission during jaw opening and closing using the principle of mechanical advantage from engineering theory (figure 3h). Jaw-closing mechanical advantage is a critical factor in bite force transmission, and is highly variable, ranging from 0.14 to 0.68 (see Electronic Appendix, part B). This range of variation is nearly as broad as that of all ray-finned fishes, which have closing advantages ranging from 0.05 to 0.70 (Westneat 2004). At one extreme, labrids such as parrotfish and tuskfish possess robust jaws and jaw-closing muscles that provide a high mechanical advantage. Parrotfish bite chunks out of the reef and tuskfish are capable of biting legs off crabs and even lifting rocks. Their jaws emphasize force transmission. At the other extreme, many labrids have long slender jaws with small teeth and protrusible mouths. These fishes have a low mechanical advantage, rendering them less forceful but capable of increased jaw speed for picking plankton or capturing evasive prey such as amphipods or fishes. Many taxa are intermediates on the force–velocity continuum, perhaps as a means of employing a ‘jack-of-all-trades’ strategy. Lower jaw levers provide a primary axis of functional variation along which labrid fishes have evolved to exploit a wide range of prey that demand alternative force and velocity capabilities.
Figure 2.
Diversity of the skull and feeding mechanism in the Labridae, illustrated with skulls cleared and double-stained for bone (red) and cartilage (blue). Green lines indicate the four-bar linkage of the jaws, blue the lower jaw-closing lever and yellow the sliding upper jaw. (a) Anampses neoguinaicus, (b) Cheilio inermis, (c) Cheilinus trilobatus, (d) Siphonognathus argyrophanes, (e) Gomphosus varius ( f) Epibulus insidiator, (g) Xyrichtys pavo. Scale bars, 5 mm.
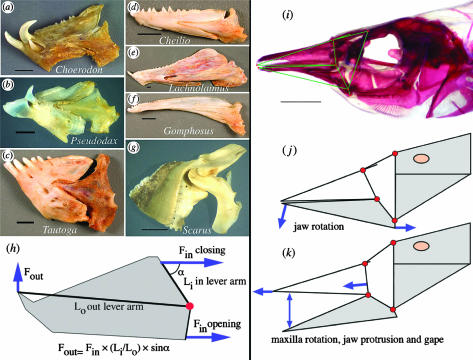
Figure 3.
Diversity of jaw morphology and the function of jaw levers and linkages in the Labridae. Jaws (a–c) are powerful with a high mechanical advantage (Li/Lo) for jaw closing. Jaws (d–f) are elongate and have a lower force (but higher velocity) transmission. These lever mechanisms are proposed to be homologous and are coded as either continuous or gap-coded states. Jaw (g) is a parrotfish with a novel intramandibular joint, making this jaw mechanism a different character state from the simple levers. (h) Lever model of jaw opening and closing force, in which muscles rotate the jaw and transmit force to the jaw tip. (i) Cranial morphology of Gomphosus varius, demonstrating the jaw's four-bar linkage (j, k) by which the upper jaw is protruded. The lower jaw rotates downward, forcing the maxilla and premaxilla anteriorly to cause jaw protrusion. Scale bars, 5 mm.
In addition to the jaw lever, a linkage system in the jaws (figure 3i–k) transmits lower jaw rotation to the maxilla and the sliding upper jaw, the premaxilla (Westneat 1994). This is a four-bar linkage, an engineering design that is used in bolt cutters, typewriter keys, heavy construction equipment and many other human-engineered machines that precisely transfer force or motion. Linkage mechanics are summarized here by the KT ratio of the linkage system, a measure of output velocity in feeding motions (the inverse estimates force). Maxillary KT is a core rotational variable of the linkage and results show a wide range of velocity transmission abilities that reflect their diverse feeding habits (Electronic Appendix, part B).
Labrids have on several occasions evolved fundamentally different linkage designs. The most spectacular of these changes in functional design is the jaw mechanism of the sling-jaw wrasse, which has several novel joints, a six-bar linkage and the highest jaw protrusion ever measured among fishes (Westneat & Wainwright 1989). Many parrotfish also possess a unique anterior jaw linkage with an intramandibular joint for increased control of the lower jaw (Bellwood 1994). These taxa with additional joints and links in the transmission system represent engineering breakthroughs in cranial design that are associated with widespread distribution (the sling-jaw occurs from Hawaii to the Red Sea) or speciation (parrotfish are a small radiation unto themselves with 88 species).
(c) Evolution of skull biomechanics
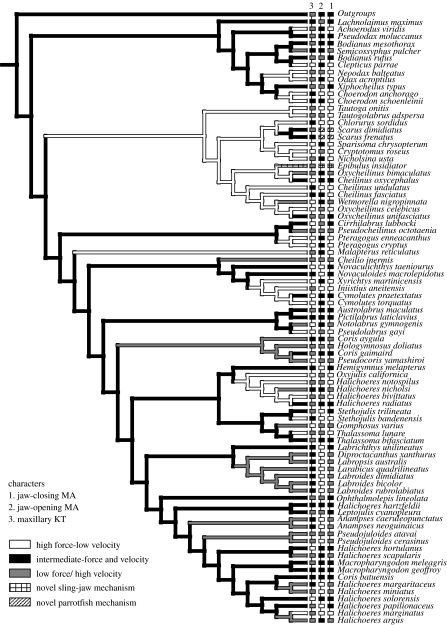
We found repeated patterns of evolutionary divergence and convergence in skull function of labrid fishes (figure 4). Each labrid subgroup contains species that show the forceful, intermediate, or fast strategy for both jaw levers and maxillary KT. Examination of gap-coded discrete states shows that jaw-closing advantage (figure 4; character 1) undergoes 35 changes on the tree and has a consistency index of just 0.14, demonstrating high levels of independent origin and reversal of states. Jaw-opening advantage and maxillary KT show similar values (figure 4), with 34 and 37 changes, and consistency indices of 0.14 and 0.11, respectively. Forceful jaw mechanisms are the most common ancestral state in the family, and in some clades this state has been retained, whereas others show more frequent evolutionary change to fast planktivorous or piscivorous forms (figure 4). Intermediate force transmission has repeatedly evolved along a forceful or more velocity-adapted trajectory of functional design. In addition, by manipulating and collapsing the tree structure to test for alternative character reconstructions, we found that these divergence and convergence patterns are robust to collapse of weakly supported nodes of the phylogeny. Labrid fishes have undergone extensive radiation along axes of cranial biomechanics in response to the demands or opportunities that diverse prey types present in shallow marine habitats. Future analysis of correlations between functional characters and ecological characters such as natural diet will enable us to test the interdependence of feeding biomechanics and ecology.
Figure 4.
Local divergence and global convergence of biomechanical traits in labrid fishes. The mechanical advantage of jaw closing (character 1) is optimized on the Bayesian MAP phylogeny to show branch reconstructions, with changes in all three characters shown in character boxes 1–3. High-velocity jaws (grey characters) have evolved 15–17 times in the three gap-coded characters. Functional characters show 34–37 total changes on the tree and have remarkably low consistency indices (about 0.10), reflecting their local divergence and global convergence. Biomechanical characters show a low level of character congruence (species showing velocity in one system matched with force in other systems) suggesting that feeding mechanisms maximize force in some features and speed in others. Divergence and convergence patterns are robust to collapse of weakly supported nodes of the phylogeny.
At higher phylogenetic levels, feeding characters show strong convergence (figure 4). The three biomechanical characters exhibit from 14 to 17 independent origins of a velocity-specialized strategy (figure 4). Interestingly, some functional characters evolve largely independently of one another, whereas others are strongly correlated. Analysis of biomechanical variables as continuous quantitative characters with independent contrasts shows that some jaw functions are independent of others. Independent contrasts tests of character correlation (Purvis & Rambaut 1995; Martins & Hansen 1997) show that jaw opening and closing lever ratios are uncorrelated (p=0.5) and closing lever is not correlated with maxillary KT (p=0.1). However, the inverse of open lever advantage and maxillary KT (two measures of opening speed) are significantly correlated (p<0.0001). Independent character change emerges as a strong phylogenetic pattern reflective of low correlations among multiple variables of the labrid skull (Wainwright et al. 2004). Patterns of character independence or congruence in multiple biomechanical characters permits the coupling or decoupling of speed and force in components of the feeding system. We conclude that independent evolution of biomechanical traits in complex skulls is a major source of functional diversity in reef fish feeding systems.
In labrid fishes, convergence on biomechanical design is a higher-level phenomenon that emerges from repeated divergence in function among lower-level clades. Such ‘local’ phylogenetic divergence in feeding mechanisms is often seen within smaller clades of other diverse fish families such as cichlids (Rüber et al. 1999). The divergence of closely related species in shallow-water reef environments along axes of feeding biomechanics may be one of the driving forces behind the radiation of reef fishes into such a varied array of forms. In many cases, there is evidence for broadly overlapping distribution of close relatives, and these patterns may provide evolutionary biologists with historical evidence for underlying processes of speciation such as character displacement (Schluter 2000a,b) that have been invoked to explain the divergence among close relatives in diverse clades. Equipped with a precise mechanical explanation of what divergence in jaw dimensions means, we have a biomechanically relevant approach to quantitative traits that may promote analysis of the genetic basis for functional evolution (Albertson et al. 2003). Skull mechanisms such as levers and linkages are subject to physical constraints (Westneat 2003), which may only be broken when a fundamentally new engineering system for feeding arises. The macroevolutionary history of labrid fishes on global reefs has involved species divergence in skull structure within a range of mechanically feasible forms, creating an unparalleled higher-level pattern of convergence that is occasionally punctuated by major transitions in engineering design.
Acknowledgments
Numerous individuals helped with fieldwork and tissue collections, including Terry Bertozzi, Di Bray, Kent Carpenter, Jim Cooper, Jeff Janovetz, Jeff Leis, Mark MacGrouther, Randy Mooi, Luz Regis, Aaron Rice, Luiz Rocha, Todd Streelman, Tom Trinski, Jeff Williams and Brad Wright. Thanks to Erin Loomis and Michael Montague for help with DNA sequencing. Melina Hale, Aaron Rice and Tristan Stayton made useful comments on the manuscript. This work was supported by the National Science Foundation and the Australian Research Council.
Supplementary Material
References
- Albertson R.C, Streelman J.T, Kocher T.D. Directional selection has shaped the oral jaws of Lake Malawi cichlid fishes. Proc. Natl Acad. Sci. USA. 2003;100:5252–5257. doi: 10.1073/pnas.0930235100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autumn K, Ryan M.J, Wake D.B. Integrating historical and mechanistic biology enhances the study of adaptation. Q. Rev. Biol. 2002;77:383–408. doi: 10.1086/344413. [DOI] [PubMed] [Google Scholar]
- Barber P.H, Bellwood D.R. Biodiversity hotspots: evolutionary origins of biodiversity in wrasses (Halichoeres: Labridae) in the Indo-Pacific and new world tropics. Mol. Phylogenet. Evol. 2005;35:235–253. doi: 10.1016/j.ympev.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Basolo A.L. Female preference predates the evolution of the sword in swordtail fish. Science. 1990;250:808–810. doi: 10.1126/science.250.4982.808. [DOI] [PubMed] [Google Scholar]
- Bellwood D.R. A phylogenetic study of the parrotfish family Scaridae (Pisces: Labroidei), with a revision of genera. Rec. Aust. Mus. 1994;20(Suppl.) [Google Scholar]
- Bellwood D.R, Wainwright P.C. The history and biogeography of fishes on coral reefs. In: Sale P.F, editor. Coral reef fishes: dynamics and diversity in a complex ecosystem. Academic Press; Orlando, FL: 2002. pp. 5–32. [Google Scholar]
- Burns K.J, Hackett S.J, Klein N.K. Phylogenetic relationships and morphological diversity in Darwin's finches and their relatives. Evolution. 2002;56:1240–1252. doi: 10.1111/j.0014-3820.2002.tb01435.x. [DOI] [PubMed] [Google Scholar]
- Clements K.D, Alfaro M.E, Fessler J.L, Westneat M.W. Relationships of the temperate Australasian labrid fish tribe Odacini (Perciformes; Teleostei) Mol. Phylogenet. Evol. 2004;32:575–587. doi: 10.1016/j.ympev.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Gomon M.F. Relationships of fishes of the labrid tribe Hypsigenyini. Bull. Mar. Sci. 1997;60:789–871. [Google Scholar]
- Hanel R, Westneat M.W, Sturmbauer C. Phylogenetic relationships, evolution of broodcare behavior, and geographic speciation in the wrasse tribe Labrini. J. Mol. Evol. 2002;55:776–789. doi: 10.1007/s00239-002-2373-6. [DOI] [PubMed] [Google Scholar]
- Harmon L.J, Schulte J.A, II, Larson A, Losos J.B. Tempo and mode of evolutionary radiation in iguanian lizards. Science. 2003;301:961–964. doi: 10.1126/science.1084786. [DOI] [PubMed] [Google Scholar]
- Huelsenbeck J.P, Ronquist F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics. 2001;17:754–755. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- Hulsey C.D, Wainwright P.C. Projecting mechanics into morphospace: disparity in the feeding system of labrid fishes. Proc. R. Soc. B. 2002;269:317–326. doi: 10.1098/rspb.2001.1874. doi:10.1098/rspb.2001.1874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman L.S, Liem K.F. Fishes of the suborder Labroidei (Pisces: Perciformes): phylogeny, ecology and evolutionary significance. Breviora. 1982;472:1–19. [Google Scholar]
- Lauder G.V, Liem K.F. The role of historical factors in the evolution of complex organismal function. In: Wake D.B, Roth G, editors. Complex organismal functions: integration and evolution in vertebrates. Wiley Press; Chichester: 1989. pp. 63–78. [Google Scholar]
- Lovette I.J, Bermingham E, Ricklefs R.E. Clade-specific morphological diversification and adaptive radiation in Hawaiian songbirds. Proc. R. Soc. B. 2002;269:37–42. doi: 10.1098/rspb.2001.1789. doi:10.1098/rspb.2001.1789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins E.P, Hansen T.F. Phylogenies and the comparative method: a general approach to incorporating phylogenetic information into the analysis of interspecific data. Am. Nat. 1997;149:646–667. [Google Scholar]
- Parenti P, Randall J.E. An annotated checklist of the species of the labroid fish families Labridae and Scaridae. Bull. J.L.B. Smith Inst. Ichthyol. 2000;68:1–97. [Google Scholar]
- Purvis A, Rambaut A. Comparative-analysis by independent contrasts (CAIC)—an Apple–Macintosh application for analyzing comparative data. Cabios. 1995;11:247–251. doi: 10.1093/bioinformatics/11.3.247. [DOI] [PubMed] [Google Scholar]
- Randall J.E. Food habits of reef fishes of the West Indies. Stud. Trop. Oceanogr. 1967;5:655–847. [Google Scholar]
- Rüber L, Verheyen E, Meyer A. Replicated evolution of trophic specializations in an endemic cichlid fish lineage from Lake Tanganyika. Proc. Natl Acad. Sci. USA. 1999;96:10 230–10 235. doi: 10.1073/pnas.96.18.10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruedi M, Mayer F. Molecular systematics of bats of the genus Myotis (Vespertilionidae) suggests deterministic ecomorphological convergences. Mol. Phylogenet. Evol. 2001;21:436–448. doi: 10.1006/mpev.2001.1017. [DOI] [PubMed] [Google Scholar]
- Ryan M.J. Sexual selection, receiver biases, and the evolution of sex differences. Science. 1998;281:1999–2003. doi: 10.1126/science.281.5385.1999. [DOI] [PubMed] [Google Scholar]
- Schluter D. Ecological character displacement in adaptive radiation. Am. Nat. 2000a;156:S4–S16. [Google Scholar]
- Schluter D. Oxford University Press; 2000b. The ecology of adaptive radiation. [Google Scholar]
- Stiassny M.L.J, Jensen J.S. Labroid interrelationships revisted: morphological complexity, key innovations, and the study of comparative diversity. Bull. Mus. Comp. Zool. 1987;151:269–319. [Google Scholar]
- Stoddard P.K. Predation enhances complexity in the evolution of electric fish signals. Nature. 1999;400:254–256. doi: 10.1038/22301. [DOI] [PubMed] [Google Scholar]
- Streelman J.T, Karl S.A. Reconstructing labroid evolution with single-copy nuclear DNA. Proc. R. Soc. B. 1997;264:1011–1020. doi: 10.1098/rspb.1997.0140. doi:10.1098/rspb.1997.0140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streelman J.T, Alfaro M.E, Westneat M.W, Karl S.A. Evolutionary history of the parrotfish: biogeography, ecomorphology, and comparative diversity. Evolution. 2003;56:961–971. doi: 10.1111/j.0014-3820.2002.tb01408.x. [DOI] [PubMed] [Google Scholar]
- Swofford D.L. Sinauer Associates, Inc; Sunderland, MA: 2000. Paup*: phylogenetic analysis using parsimony (* and other methods), version 4.0. [Google Scholar]
- Taylor W.R, Van Dyke G.C. Revised procedures for staining and clearing small fishes and other vertebrates for bone and cartilage study. Cybium. 1985;9:107–119. [Google Scholar]
- Verheyen E, Salzburger W, Snoeks J, Meyer A. Origin of the superflock of cichlid fishes from Lake Victoria, East Africa. Science. 2003;300:325–329. doi: 10.1126/science.1080699. [DOI] [PubMed] [Google Scholar]
- Wainwright P.C, Bellwood D.R. Ecomorphology of feeding in coral reef fishes. In: Sale P.F, editor. Coral reef fishes: dynamics and diversity in a complex ecosystem. Academic Press; Orlando, FL: 2002. pp. 33–55. [Google Scholar]
- Wainwright P.C, Richard B.A. Predicting patterns of prey use from morphology of fishes. Environ. Biol. Fish. 1995;44:97–113. [Google Scholar]
- Wainwright P.C, Bellwood D.R, Westneat M.W, Grubich J.R, Hoey A.S. A functional morphospace for the skull of labrid fishes: patterns of diversity in a complex biomechanical system. J. Linn. Soc. 2004;82:1–25. [Google Scholar]
- Westneat M.W. A phylogenetic hypothesis for the tribe Cheilinini (Labridae: Perciformes) Bull. Mar. Sci. 1993;52:351–394. [Google Scholar]
- Westneat M.W. Transmission of force and velocity in the feeding mechanisms of labrid fishes. Zoomorphology. 1994;114:103–118. [Google Scholar]
- Westneat M.W. Feeding, function, and phylogeny: analysis of historical biomechanics in labrid fishes using comparative methods. Syst. Biol. 1995;44:361–383. [Google Scholar]
- Westneat M.W. A biomechanical model for analysis of muscle force, power output and lower jaw motion in fishes. J. Theor. Biol. 2003;223:269–281. doi: 10.1016/s0022-5193(03)00058-4. [DOI] [PubMed] [Google Scholar]
- Westneat M.W. Evolution of levers and linkages in the feeding mechanisms of fishes. Integr. Comp. Biol. 2004;44:378–389. doi: 10.1093/icb/44.5.378. [DOI] [PubMed] [Google Scholar]
- Westneat, M. W. & Alfaro, M. E. In press. Phylogenetic relationships and evolutionary history of the reef fish family Labridae. Mol. Phylogenet. Evol. [DOI] [PubMed]
- Westneat M.W, Wainwright P.C. Feeding mechanism of Epibulus insidiator: evolution of a novel functional system. J. Morphol. 1989;202:129–150. doi: 10.1002/jmor.1052020202. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.