The thought of liquid CO2 conjures up different things to different folks: perhaps the decaffeination of coffee beans, perhaps the recently popularized “green” method for dry cleaning, or even phase diagrams that occupied a part of one's life in past chemistry classes. What it does not conjure up is a subsurface lake at the bottom of the ocean, a lake with abundant living microbes, as reported in this issue of PNAS by Inagaki et al. (1). These authors discovered such a place near the Yonaguni Knoll in the Okinawa Trough at a depth of ≈1,400 m. The description in both words and video (see supporting movie 1 in ref. 1) is quite striking. First, because liquid CO2 at this depth is less dense than water (2, 3), so that such a lake should not be present. Second, because this is a phenomenon that few of us have ever seen, movie 1 in ref. 1 reveals a flowing stream of liquid CO2 that seems almost surreal.
The answer to the apparent conundrum surrounding the very existence of this phenomenon is that the lake is maintained in place by a surface pavement and a subpavement cap of CO2 hydrate (CO2·6H2O) that traps the low-density liquid CO2 in place. At the temperature of the seafloor at this depth, such a CO2 hydrate should be stable (4), leading to a structure similar to that shown in figure 1 of the Inagaki et al. article (1), in which a surface pavement overlies a layer of CO2 hydrate that serves as a cap for the subsurface lake. The surface pavement is quite remarkable, having a very unusual elemental sulfur content of >50%. It may well be that there are clues to the origin of the sulfur (and the role of sulfur metabolism in this system) in both the isotopic composition of the sulfur and the chemical and biological nature of the “sulfur-hydrate complex,” things that should be resolved in future studies. As discussed in a another recent article in PNAS (3), the density of liquid CO2 increases with depth, so that at depths of 3,000–3,800 m (density reaches a maximum at ≈3,500 m and decreases at greater depth), it is more dense than seawater [see figure 2 in House et al. (3)], forming a natural negative buoyancy zone, where one could rightfully expect to see lakes of liquid CO2. CO2 hydrates also form at these depths, suggesting that large subsurface lakes of liquid CO2 capped by hydrates could be excellent locations for the large-scale injection and disposal of CO2 (3). The notion that similar sites might exist as natural systems was not entertained in the House et al. article, but if they do, and are stable in the long term, then the notion that communities of microbes might be capable of adapting to such an environment becomes of great interest. Such knowledge also becomes of importance with regard to the establishment of such reservoirs in the deep sea.
Liquid CO2 in the deep ocean is not an unprecedented finding. In 1990, Sakai et al. (4) noted the release of CO2 droplets at a depth of 1,400 m and a temperature of 3.8°C in a region near the mid-Okinawa Trough, and more recently, similar observations were made in the northern Mariana Arc (5). What is new is the concept that large bodies of liquid CO2 may exist as subsurface lakes in such zones. For example, the northern Mariana is a volcanic arc with little or no sediment deposition. Thus, one does not expect to find sediment-hosted lakes such as are reported by Inagaki et al. (1). How many such “lakes” are there? How stable are they, and are they potential players in the global carbon cycle? Given our paucity of knowledge about such systems, it is fair to say that these questions remain unanswered. It may be of great interest to answer such questions for a variety of different reasons, as outlined below.
First, one of the proposed methods for disposal of CO2 (and amelioration of the associated effects on global climate) is the direct injection of CO2 into the deep sea (6–8). The expense of moving large amounts of CO2 to 3,000 m and deeper and the problems with rapidly injecting it at these depths could be substantial. Neither the biological (toxicity) nor the physical (effect on porewaters from injection of massive amounts of liquid CO2) impacts are known (3).
Do the findings of Inagaki et al. (1) offer another potential avenue for CO2 storage? Probably not. The robustness of these systems clearly depends on the formation and long-term stability of the CO2 hydrate cap, something that may not be routine to achieve. Whereas in the deeper ocean, the hydrate cap should be stable and the underlying liquid CO2 can migrate downward until it becomes neutrally buoyant and will then move only by diffusion (3), burial in zones where liquid CO2 is less buoyant than water would almost certainly have to be deeper into the sediments themselves, thus becoming subject to temperature changes caused by the geothermal gradient. Thus, the CO2 lakes reported (1) probably will not lead the way to a new avenue for shallow-water CO2 disposal. Such environments do, however, offer accessible sites where some of the other impacts of the liquid CO2/seawater interface on the environment (including the resident biota) can be studied as a naturally existing phenomenon.
The second issue at hand relates to the potential toxicity of CO2 to various forms of marine life. Indeed, it has been argued from many points of view that injection of CO2 may have dire consequences on the deep sea biota. It is argued that the combined effects of CO2 itself and the lowered pH that goes along with it will have minor to major impacts on marine life, including microbial life (9). The potential impact of CO2 in liquid form may be much less extreme (3), but for the moment this remains unknown. Given that liquid CO2 is decidedly nonpolar and behaves like an organic solvent, it might be a rather harsh environment itself, regardless of any pH-associated effects. To this end, it should also be noted that both pH and alkalinity were measured onboard after degassing had occurred during recovery of the samples. Thus, one expects the in situ pH to be lower and the alkalinity to be higher, stressing the importance of in situ measurements in future studies.
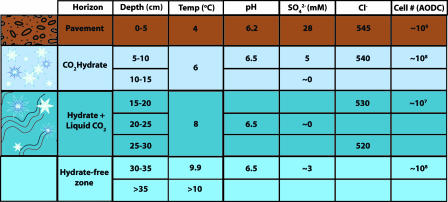
Inagaki et al. (1) studied both the chemistry of the environment and the populations of microbes present in both the sediments above the liquid CO2 environment and the CO2 lake itself (Fig. 1). The pH of the environment changes very little as one proceeds from the overlying sediment to the CO2–hydrate/liquid CO2 interface, being stable at ≈6.5, whereas the alkalinity increases from 20 to 30 mmol/kg. Alongside these rather stable values, the authors observed that the cell number (as judged from microscopic counts) declined from ≈109 per ml in the overlying pavement to 107 per ml in the liquid CO2 zone and then increased again in the zone below the liquid CO2 (Fig. 1). Although a 100-fold decrease is a large drop, the fact that 107 cells per ml of intact cells remain is quite remarkable, given the potentially hostile nature of this nonpolar solvent. What are these cells? What are they doing? Are these new and unusual organisms, or are they just survivors that can tolerate this environment? For the moment, these questions cannot all be answered. Molecular methods were used to identify the major microbial groups, which individually looked familiar in terms of their phylogentic affiliations; similar microbes have been seen in deep-sea and methane-seep environments (10–13). However, the combinations of microbes present (i.e., the community composition) did not appear to make a story that could be easily related to any other deep-sea site examined so far. That is, this report revealed the presence of archaea previously identified in zones of anaerobic methane oxidation (the so-called ANME-2c group), and the Eel-2 group of Deltaproteobacteria, associated with sulfate reduction but not previously known as major components of anaerobic methane-oxidizing consortia. Furthermore, no members of the DSS group, a group of sulfate-reducing Deltaproteobacteria that are usually found with ANME-2 cells (10, 11), were detected. Thus, if there is a consortium driving anaerobic methane oxidation, it may well be something new. With the members of this environment now partially identified, it should be possible in future work to examine the consortium by fluorescence in situ hybridization (FISH) and other similar techniques to resolve these issues.
Fig. 1.
Data from the CO2 lake zone, showing the vertical dimensions of the lake and overlying sediment, its general properties in terms of temperature, pH, sulfate, chlorinity, and cell number, as determined by acridine orange direct counts (AODC). The pavement is located down slope from a large black smoker, at a water depth of ≈1,400 m (1).
At depths of 3,000–3,800 m, CO2 is more dense than seawater.
To further complicate the story, although both methane consumption activity measurements and stable isotope analyses of bacterial and archael lipids suggested that methane oxidation and perhaps CO2 fixation were occurring in the liquid CO2 zone, neither the predicted organisms nor the genes coding for the needed enzymes (as detected by gene probes) were abundant in this environment. This, of course, raises the very exciting possibility that both the methane oxidation measured and the lipid fractionation observed are the result of activities of entirely new types of organisms that may have eluded detection by standard molecular probe analyses. The resolution of this possibility will await the application of more detailed studies, including fluorescence labeling, substrate labeling, stable isotopic probing, and metagenomic community analysis.
But where are the organisms residing in this fascinating environment? Given the nature of liquid CO2, it would seem likely that the resident population is in fact taking advantage of the presence of water/hydrate interface as the niche of choice. Such a microhabitat should provide a microbe with an acceptable place for life. Although laboratory experiments could establish this as a possibility, it is clear that in situ studies will be needed to locate microbes in their natural habitats.
Inagaki et al. (1) end their article with an interesting speculation as to the potential for such environments to exist on other solar-system bodies. If follow-up in situ studies to this work show that microbial life is capable of existing in the liquid CO2 domain and what kinds of metabolism are consistent with such a habitat, it could well open up a new area with regard to the search for life both on Earth and elsewhere. As the authors explain: “the Yonaguni Knoll is an exceptional natural laboratory for the study of consequences of CO2 disposal as well as of natural CO2 reservoirs as potential microbial habitats on early Earth and other celestial bodies” (1). This is one of those rare times when the statement, “this system deserves more study” is surely true, with the immediate issues at hand being the identification of the microbes in the various substrata and the determination of the activities actually occurring in the liquid CO2 environment.
Footnotes
The author declares no conflict of interest.
See companion article on page 14164.
References
- 1.Inagaki F, Kuypers MMM, Tsunogai U, Ishibashi J-i, Nakamura K-i, Treude T, Ohkubo S, Nakaseama M, Gena K, Chiba H, et al. Proc Natl Acad Sci USA. 2006;103:14164–14169. doi: 10.1073/pnas.0606083103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brewer PG, Friederich G, Peltzer ET, Orr FMJ. Science. 1999;284:943–945. doi: 10.1126/science.284.5416.943. [DOI] [PubMed] [Google Scholar]
- 3.House KZ, Schrag DP, Harvey CF, Lackner KS. Proc Natl Acad Sci USA. 2006;103:12291–12295. doi: 10.1073/pnas.0605318103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sakai H, Gamo T, Kim E-S, Tsutumi M, Tanaka T, Ishibashi J, Wakita H, Yamano M, Oomori T. Science. 1990;248:1093–1096. doi: 10.1126/science.248.4959.1093. [DOI] [PubMed] [Google Scholar]
- 5.Lupton J, Butterfield D, Lilley M, Evans L, Nakamura K, Chadwick WJ, Resing J, Embley R, Olson E. Geochem Geophys Geosys. 2006;7:Q08007. [Google Scholar]
- 6.National Research Council. Novel Approaches to Carbon Management: Separation, Capture, Sequestration, and Conversion to Useful Products. Washington, DC: Natl Acad Press; 2003. [Google Scholar]
- 7.Hanisch C. Environ Sci Technol. 1999;33:66A–70A. doi: 10.1021/es992680f. [DOI] [PubMed] [Google Scholar]
- 8.Caldeira K, Akai M, Brewer P, Chen B, Haugan P, Iwama T, Johnston P, Kheshgi H, Li Q, Ohsumi T, et al. In: Carbon Dioxide Capture and Storage: A Special Report of IPCC Working Group III. Metz B, Davidson O, editors. Cambridge, UK: Cambridge Univ Press; 2005. pp. 279–307. [Google Scholar]
- 9.Seibel BA, Walsh PJ. Science. 2001;294:319–320. doi: 10.1126/science.1065301. [DOI] [PubMed] [Google Scholar]
- 10.Inagaki F, Nunoura T, Nakagawa S, Teske A, Lever M, Lauer A, Suzuki M, Takai K, Delwiche M, Colwell FS, et al. Proc Natl Acad Sci USA. 2006;103:2815–2820. doi: 10.1073/pnas.0511033103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inagaki F, Tsunogai U, Suzuki M, Kosaka A, Machiyama H, Takai K, Nunoura T, Nealson KH, Horikoshi K. Appl Environ Microbiol. 2004;70:7445–7455. doi: 10.1128/AEM.70.12.7445-7455.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kato C, Li L, Tamaoka J, Horikoshi K. Extremophiles. 1997;1:117–123. doi: 10.1007/s007920050024. [DOI] [PubMed] [Google Scholar]
- 13.Orphan V, House C, Hinrichs K-U, McKeegan KD, DeLong EF. Proc Natl Acad Sci USA. 2002;99:7663–7668. doi: 10.1073/pnas.072210299. [DOI] [PMC free article] [PubMed] [Google Scholar]