Abstract
The hydrolysis of nucleoside triphosphates by enzymes is used as a regulation mechanism in key biological processes. Here, the GTP hydrolysis of the protein complex of Ras with its GTPase-activating protein is monitored at atomic resolution in a noncrystalline state by time-resolved FTIR spectroscopy. At 900 ms, after the attack of water at the γ-phosphate, there appears a H2PO4− intermediate that is shown to be hydrogen-bonded in an eclipsed conformation to the β-phosphate of GDP. The H2PO4− intermediate is in a position where it can either reform GTP or be released from the protein in 7 s in the rate-limiting step of the GTPase reaction. We propose that such an intermediate also occurs in other GTPases and ATPases.
Keywords: proteins, reaction mechanism, FTIR spectroscopy, signal transduction
The guanine nucleotide-binding protein Ras regulates several signal transduction processes involved in cell growth and differentiation (1). Ras serves as a prototype for the superfamily of GTPases that cycle between the active GTP-bound state and the inactive GDP-bound state. The switching-off of signal transduction is accomplished by GTP hydrolysis, which is a phosphoryl transfer from GTP to water. This process is slow for Ras·GTP, allowing further control of this process by GTPase-activating proteins (GAPs), which stimulate the reaction by several orders of magnitude (2). The Ras protein has been extensively studied by various methods, including x-ray crystallography (3–5), NMR (6), computation (7–10), and FTIR spectroscopy (11–15). For the slow intrinsic reaction, a substrate-assisted mechanism has been inferred from biochemical and computational studies, whereby γ-phosphate acts as a general base to activate the nucleophilic water (16, 17). Accumulating intermediates in GAP-catalyzed reactions have recently been observed for both the Ras·RasGAP and the Rap·RapGAP reaction, and these intermediates can either decompose to the products GDP and Pi or regenerate GTP (11, 18, 19). Recently, a crystal structure of an intermediate of an enzyme-catalyzed phosphoryl transfer reaction was reported (20). This intermediate was analyzed as a pentacovalent phosphate structure, but there is considerable controversy concerning this interpretation (21–23).
Time-resolved FTIR (trFTIR) difference spectroscopy monitors protein reactions at the atomic level in real time (24, 25). In the present application, Ras in the presence of saturating amounts of NF1-333, the catalytic GAP domain of neurofibromin, was loaded with caged GTP, which cannot be hydrolyzed by the Ras·RasGAP system. Ras·GTP was generated by a laser flash, and the subsequent hydrolysis reaction was monitored by tr-FTIR with a time resolution of milliseconds. The phosphate vibrations were assigned by using 18O-labeled caged GTP (12). A global multiexponential kinetic analysis of the trFTIR data obtained at 260 K was performed by using a sum of three exponential functions for the GAP-catalyzed GTPase reaction of Ras (11).
 |
The first process, with the rate constant k1 (data not shown), describes the appearance of GTP from the precursor caged GTP (11). At the second rate (rate constant k2), GTP disappears, and an intermediate appears that is assigned to protein-bound Pi, initially without implication for its structure. Pi is released from the protein in the rate-limiting step, which is described by the third rate (rate constant k3) (11).
Here, we determine the protonation state and the location of the protein-bound Pi, both of which have important implications for the molecular mechanism.
Results and Discussion
Assignment of the β,γ-Bridging Vibration.
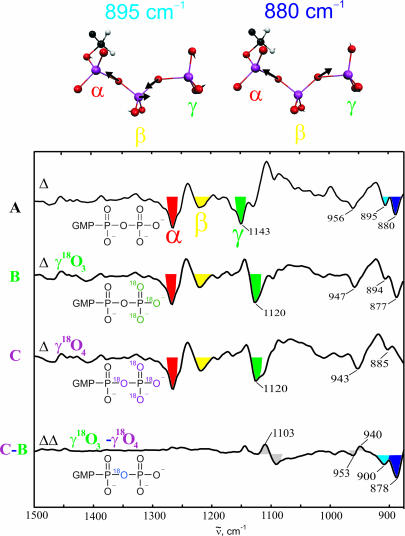
To monitor bond breakage, we needed to observe the vibrations of the bridging oxygen. According to quantum mechanics/molecular mechanics calculations by Klähn et al. (26), we expect two vibrations of the β,γ-bridging oxygen, the in-phase and out-of-phase vibrations of the Oα,β-Pβ-Oβ,γ system. The corresponding normal modes are shown in Fig. 1. By using γ-18O3- and γ-18O4-labeled GTP we could assign to these vibrations absorptions at 895 and 878 cm−1 (Fig. 1). We then followed the cleavage reaction of the γ-phosphate in the Ras·NF1 1:1 complex directly by the time dependence of the intensities of the bands, as shown in Fig. 2. The marker band for bond breakage at 878 cm−1 decreases with rate constant k2, the rate with which the intermediate is formed. Only after the intermediate is formed the final product emerges with rate constant k3, as indicated by the increase of the marker band for Pi at 1,078 cm−1, which stems from phosphate in water. Thus, we propose that in the intermediate the γ-phosphate is already cleaved from the GDP but is still within the binding pocket and linked or bound in some manner to the protein.
Fig. 1.
Band assignments. Shown are FTIR difference spectra of the hydrolysis reaction of Ras·GTP for unlabeled (spectrum A), γ-18O3- labeled (spectrum B) and γ-18O4-labeled (spectrum C) GTP. Bands facing downward belong to the Ras·GTP state, and bands facing upward are from to the Ras·GDP state. The double difference (spectrum C-B) demonstrates the differences caused by the β,γ-bridging oxygen. The normal mode vectors of the GTP vibrations in which this bridging oxygen is involved are shown according to the calculation of Klaehn et al. (26). The band at 956 cm−1 already shifts upon γ-18O3-labeling and, thus, has a mainly νs(γ-PO3) character. This assignment improves the one of Du et al. (15). Furthermore, the asymmetric stretching vibrations of the α-, β- and γ-phosphates of Ras·GTP are marked.
Fig. 2.
Time-dependent absorbance changes of marker bands of the bridging bond, the intermediate, and the product during the GAP-catalyzed GTPase reaction of Ras. The amplitudes for the intermediate formation (gray dotted lines) and product formation (black dotted lines) are given.
IR Spectrum and Kinetics of the Intermediate.
Information as to the nature of the intermediate can be gained from its FTIR spectrum. The bands are expected to shift upon γ-18O-labeling, and this is indeed found for the bands at 1,186 cm−1 (Fig. 3A) and 1,113 cm−1 (Fig. 4A), which can unequivocally be assigned to cleaved but protein-bound Pi. As shown in Fig. 3B for the 1,186 cm−1 absorption, the bands increase with the depletion of GTP (rate constant k2) and decay with the formation of GDP (rate constant k3). Figs. 3A and 4A show amplitude spectra of the intermediate formation from GTP (rate constant k2); thus, the bands of the intermediate are facing upward. The assignment is confirmed by measurements of unlabeled GTP in H218O, where these bands also shift (11). These measurements show that the nucleophilic attack of water has taken place. The bands in Figs. 3A and 4A using labeled GTP are described in Protonation State of the Phosphate and Coupling Gives Information on Structure.
Fig. 3.
Assignment of the protonation state. (A) Amplitude spectrum of the bond-breaking with rate constant k2 of the GAP-catalyzed GTPase reaction of Ras using GTP (black) or [γ-18O4]GTP (red). The positive bands are due to the protein-bound Pi. The band shifts are 16 and 32 cm−1 with approximately equal intensities. (B) Kinetics of absorptions assigned to GTP, GDP, and the intermediate. The data were fitted by a global fit with three exponential functions. The three components of each absorption are shown as dotted lines, and the sum of the components is shown as a solid line. (C) Scheme demonstrating the expected band shifts and intensity ratios for the νas vibrations of H2PO4− and HPO42−, respectively. (D) Scheme demonstrating the expected distribution of the 18O isotopes from γ-GTP for a pentacovalent or orthophosphate intermediate, respectively. The atoms participating in the vibration are shown in boldface type. (E) Scheme demonstrating that the band shifts due to the protein environment instead of water are in the expected range for H2PO4−, but not for HPO4−.
Fig. 4.
Through-space coupling. (A) Amplitude spectrum of the bond breaking with rate constant k2 of the GAP-catalyzed GTPase reaction of Ras using GTP (black) or [γ-18O4]GTP (red). Bands facing upward are due to the protein-bound Pi. Additionally, the band positions for β-18O1- and β-18O4-labeled GTP are shown. (B) Calculation of the coupling element of the shown model substance as a function of the distance of the two PO2 groups. The chlorine atom was chosen because it has an electronegativity similar to oxygen, but its vibrations are decoupled from the PO2 vibrations because of its much higher mass.
Intermediate Is Not a Phosphorylated Enzyme.
The absorptions at 1,186 cm−1 and 1,113 cm−1 are similar to the band positions found for a phosphate covalently bound to Asp-351 in Ca2+-ATPase (27). Furthermore, phosphorylated Ser in phosvitin or ovalbumine absorbs at 1,194 cm−1 (28). Although it is generally accepted that GTP hydrolysis by Ras does not proceed via a phosphorylated enzyme intermediate, the absence of such a pathway has not actually been shown directly for the GAP-activated reaction. We therefore investigated this question by using [γ-32P]GTP. After rapid mixing in a quenched-flow apparatus, the reaction was quenched in trichloroacetic acid or KOH. From the FTIR results, the intermediate should accumulate several seconds after mixing. However, under the dilute solution conditions and higher temperature of the quenched-flow experiment, the reaction is known to be much faster (2), so that reaction times ranging from 2 ms to 10 s were investigated. No trace of radioactivity could be detected in either Ras or NF1 (Fig. 5), neither with a nitrocellulose filter assay nor by SDS/PAGE and PhosphorImager analysis. A control with the mutant RasA59T, which is known to undergo autophosphorylation on Thr-59 (29), shows a very strong signal (Fig. 5) that argues against the occurrence of a covalently bound phosphate. This finding is further supported by FTIR measurements through which we observe that an oxygen isotope of water is incorporated into the intermediate, which would not be observed in a phosphoryl transfer to a protein residue.
Fig. 5.
SDS/PAGE gels. M is a marker with the molecular masses as indicated. The second line shows a typical Coomassie-stained gel of the reaction mixture. The following three lines are examples from the PhosphorImager in our quenching experiments as detailed in the text. The last line is as a positive control the quenching of the reaction of RasA59T·[γ-32P]GTP.
Protonation State of the Phosphate.
The evidence presented so far suggests that in the intermediate state the β,γ-bond has been broken and that the phosphorus moiety is not covalently attached to either Ras or NF1. The remaining possibilities are that the group is present as metaphosphate or orthophosphate. The former possibility is excluded by the evidence that oxygen isotopes from water are already incorporated in the intermediate, which is not expected in a dissociative mechanism leading to metaphosphate formation. Therefore, we conclude that the intermediate harbors orthophosphate, and we can now address the question of its protonation state, which is relevant to the enzymatic mechanism not just of Ras but for general phosphoryl transfer reactions. Thus, the phosphate group could either be singly or doubly protonated.
Referring to the bands discussed above, the corresponding vibrations of H2PO4− in water (Fig. 3E) are at 1,156 cm−1 (asymmetric stretching mode) and 1,077 cm−1 (symmetric stretching mode) (30). Following the general tendency for PO vibrations, we would expect these bands to be blue-shifted in the protein environment, and shifts of ≈30 cm−1 are indeed observed. These shifts are very similar to those observed for the PO-stretching vibrations of GDP (α and β) and GTP (α and γ), comparing water and the Ras environment or the shift of the PO2 groups of DNA upon hydration (31). In contrast, the protein-induced shifts for a HPO42− moiety would be 106 and 125 cm−1, which is greater than expected. Further information was obtained by the pattern of the shift of the 1,186 cm−1 band shown in Fig. 3A: Three of the four oxygen atoms of the Pi are from the γ-phosphorus of GTP and, thus, are 18O-labeled, but one is from the attacking water and, therefore, is unlabeled. We observe two bands of approximately equal intensity, and the shifts are 16 and 32 cm−1, respectively. This is expected only for a PO2 vibration as in H2PO4−, as demonstrated in Fig. 3C. If we assume that proton transfer can occur between the individual oxygen atoms at a rate that is rapid compared with the lifetime of the intermediate, there is a 50% probability that the unlabeled oxygen is protonated only in the case of H2PO4−. In this case, both oxygen atoms of the PO2 group are labeled and give rise to the full shift of 32 cm−1. In the other 50% of the molecules, only one of these two oxygen atoms is labeled, giving rise to exactly half of the shift, which is in agreement with the observation. In the PO3 vibration expected for HPO42−, the probability for protonation of the unlabeled oxygen is 25%; thus, only 25% of the molecules should be subject to the full shift of 32 cm−1. The remaining 75% of the molecules have a P18O216O vibration, which should show two-thirds of the full shift. Thus, both the intensity ratio and the shift ratio are clearly in line with the PO2 vibration of H2PO4− but not with the PO3 vibration of HPO42−.
Coupling Gives Information on Structure.
Interestingly, the band at 1,113 cm−1 also shifts in the case of β-GTP labeling as shown in Fig. 4A. Given the fact that the bridging bond is already cleaved, through-space coupling is the only source for the band shift. This coupling decreases with the distance r of the dipoles by r−3 in the near-field approximation (32). Thus, the distance of the Pi from the GDP must still be small in the intermediate. To estimate the maximum distance for observing this coupling, we calculated the coupling element of a model system (Fig. 4B) with two PO2 vibrations in an eclipsed conformation. One PO2 group simulates the β-phosphate of GDP; the other group models the PO2 group of the cleaved γ-phosphate. These density functional theory calculations [BP86/6-311++G(d, p)] give a distance dependency of r−3.7 of the coupling element, similar to the findings of Hahn et al. (32). In this model system, the conditions for coupling are ideal (dihedral angle of 0° and complete degeneracy); therefore, the result can be taken as an upper limit for the coupling. In the protein, the vibrations of the groups are probably not completely degenerated, and the groups might not be completely eclipsed. Even in the model system, the coupling becomes negligible at distances of >5 Å. Thus, the distance of the phosphorus atoms of the β-phosphate and the cleaved Pi must be <5 Å, which means that the Pi is probably hydrogen-bonded to GDP.
The Intermediate Has No Pentacovalent Structure.
We have so far implicitly excluded the possibility that the intermediate has a pentacovalent structure (Fig. 3D); however, we can now add further arguments against this. This point is important because such an intermediate is often considered for phosphoryl transfer reactions (33, 34), and crystallographic evidence for such a mechanism has recently been presented in the case of β-phosphoglucomutase (20). In such an intermediate, the bond order of the P–O bonds is reduced to 1 so that the absorption bands of such a species are expected at wave numbers lower than 1,000 cm−1. Furthermore, we observe two vibrations at 1,170 and 1,154 cm−1 upon γ-18O-labeling. This coupling pattern excludes a pentacovalent intermediate, because a pentacovalent intermediate cannot show a P16O18O vibration given that an exchange of the equatorial and axial positions is not possible; therefore, the oxygen of the attacking water would remain in the axial position, and we would observe only the P18O2 vibration at 1,154 cm−1 (compare with Fig. 3D). Note that a PO2 vibration would be possible only if one of the equatorial oxygen atoms were protonated.
That this intermediate is not a pentacovalent phosphorus structure does not rule out the possibility of such a structure occurring as an intermediate preceding the one characterized here. The lack of experimental evidence for the existence of such a species, which is actually favored in a reaction mechanism involving proton transfer from the nucleophile to the γ-phosphate substrate (16, 17), could arise from the fact that it does not accumulate during the reaction, i.e., that its decay is much faster than its production.
Insights into the Mechanism.
The arguments presented make it most likely that the intermediate identified by FTIR is H2PO4− (Fig. 6), which is probably directly bound via a hydrogen bond bridge to the β-phosphate of GDP. In this case, the distance would be short enough for the observed coupling. Such a scenario is in excellent agreement with a recent x-ray structure of Rab11 Q70L containing Pi, in which the Pi interacts via a proposed low-barrier hydrogen bond to GDP (35). In the reported structure, it is not clear whether the identified intermediate arises directly from cleavage of GTP or is generated by the long incubation during crystallization at very high phosphate concentration. In the results reported here, it can only arise directly from the cleavage reaction and is therefore a genuine intermediate. An attractive explanation for the production of this species is the extensively discussed possibility of transfer (direct or indirect) of a proton from the attacking water molecule to an oxygen of the γ-phosphate group (9, 16, 17, 35–37), a mechanism that has been called substrate-assisted catalysis (16). Such proton transfer can be direct or mediated via water molecules, as has been discussed for Ras and the F1 ATPase reaction (4, 38). After attack of the generated hydroxyl ion on phosphorus and cleavage of the β,γ-bond, H2PO4− would be generated as the initial product. Thus, if proton transfer occurs before the cleavage reaction, it would favor an associative transition state (39). In support of this possibility, proton transfer to the γ-phosphate has been recognized as a crucial element of the reciprocal GTPase stimulation of the signal recognition particle and its receptor (40, 41).
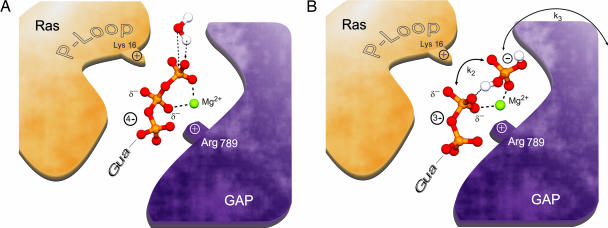
Fig. 6.
Reaction mechanism. (A) Schematic illustrations of GTP within the Ras·RasGAP complex, in which negative charge is transferred toward the β-phosphate. The attacking water is shown in the position of the ground state. The attaching water might conceivably transfer a proton to GTP as the first step toward hydrolysis. (B) Schematic illustrations of the intermediate according to results and interpretation given in the text. The phosphate is already cleaved from the GDP. Despite the absence of a covalent bond to the phosphate, it is retained in the binding pocket via a hydrogen bond and electrostatic interactions with the magnesium atom. We propose that the phosphate group is doubly protonated.
Conclusion
We have shown that the GAP-catalyzed GTPase reaction of Ras proceeds via an identifiable intermediate in which phosphate has not yet dissociated from Ras. We have assigned the vibrations of the β,γ-bridging oxygen of GTP bound to Ras and demonstrated that this bridge is already broken in the intermediate. This finding is confirmed in studies with isotopically labeled GTP. A strong through-space coupling between GDP and the protein-bound Pi indicates a small distance (<5 Å between the phosphorus atoms) between the two species. The close juxtaposition of the GDP and Pi in the intermediate state is in harmony with the finding that a considerable amount of back reaction to GTP takes place not only in Ras·RasGAP but also in the RapGAP reaction (18) and might be a general phenomenon of this type of reactions. We have ruled out the possibility that the intermediate represents a pentacovalent phosphorus structure or a phosphorylated protein. Analysis of the FTIR bands suggests that the protein-bound Pi is a H2PO4− species, possibly bound by a proton to the GDP, as shown in Fig. 6, supports a mechanism involving protonation of an oxygen of the γ-phosphate group to form an associative transition state. Our results allow the arrangement of crystallographic data in a sequential order of events.
Materials and Methods
Wild-type Ras (residues 1–166) was prepared from Escherichia coli CK600K harboring the ptac-ras plasmid (42). NF1-333 was isolated from E. coli BL21DE3 with the pGEX-NF1-333 plasmid (2). Time-resolved FTIR measurements were performed as described in refs. 12 and 13. Amplitude spectra were obtained by fitting the data according to Eq. 1 with a sum of three exponential functions. To each rate constant kl belongs an amplitude spectrum al(ν) showing the spectral changes during the respective transition. In Figs. 3 and 4, the amplitude spectra −al(ν) are shown; thus, the bands that are due to the disappearing state face downward and the bands belonging to the evolving state face upward. In a hydrolysis spectrum (as in Fig. 1) the bands of the Ras·GTP state face downward and the bands of the Ras·GDP state face upward. [18O4]Phosphate was prepared from PCl5 (Fluka, Buchs, Switzerland) and H218O (99% 18O; Campro Scientific, Berlin, Germany). [β,γ-18O, γ-18O3]GTP (throughout the text named [γ-18O4]GTP for simplicity) was prepared from GDP (Fluka) by condensation of the phosphorimidazolidate of GDP with [18O4]phosphate (43, 44). [γ-18O3]GTP was prepared from [18O4]phosphate and GDP by means of carbamate kinase.
Quenched Flow.
[γ-32P]GTP [27 μl at 270 μCi (1 Ci = 37 GBq)] was obtained from Hartmann Analytik (Göttingen, Germany). Solutions of Ras·[γ-32P]GTP (15 μl of 10 μM Ras·[γ-32P]GTP (15 μl at 0.2 μCi)/50 mM Mes/5 mM DTT) and NF1 (15 μl of 20 μM NF1-333/50 mM Mes/5 mM DTT/20 mM MgCl2) were mixed and quenched by a denaturing agent after time delays between 2 ms and 20 s at 274 K in a quenched-flow apparatus. Acid (0.6% trifluoroacetic acid) or alkaline (0.5 M KOH) denaturing agents were used, respectively. An excess of GAP was used to assure single-turnover conditions. Alternatively, multiple turnover conditions were used. Here, a steady state of the intermediate is present for a longer time, so that manual mixing is possible. The time between mixing and quenching was varied between 2 and 20 s, and the temperature was between 260 and 293 K. The resulting solutions were investigated by a nitrocellulose assay with subsequent determination of the radioactivity by using a scintillation counter. Additionally, the two proteins were separated by SDS/PAGE, and the obtained gel was scanned by using a PhosphorImager.
Computation.
In the calculation, the coupling of two PO2 vibrations was determined. The aim was to get an upper limit for the distance for which significant coupling is expected. A chlorine atom was used to saturate the free valence of the phosphor, because its vibrations are uncoupled from the PO2 system and the electronegativity is similar to oxygen. The geometry was optimized at the BP86/6-311++G(d, p) level of theory, constraining the P–P distance by using the Gaussian 03 program (45). Frequencies were obtained by harmonic approximation using the second derivative of the potential energy.
Acknowledgments
This work was supported by Deutsche Forschungsgemeinschaft Grant SFB 642.
Abbreviation
- GAP
GTPase-activating protein.
Footnotes
The authors declare no conflict of interest.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Wittinghofer A. Biol Chem. 1998;379:933–937. [PubMed] [Google Scholar]
- 2.Ahmadian MR, Hoffmann U, Goody RS, Wittinghofer A. Biochemistry. 1997;36:4535–4541. doi: 10.1021/bi962556y. [DOI] [PubMed] [Google Scholar]
- 3.Scheffzek K, Ahmadian MR, Kabsch W, Wiesmuller L, Lautwein A, Schmitz F, Wittinghofer A. Science. 1997;277:333–338. doi: 10.1126/science.277.5324.333. [DOI] [PubMed] [Google Scholar]
- 4.Scheidig AJ, Burmester C, Goody RS. Struct Fold Des. 1999;7:1311–1324. doi: 10.1016/s0969-2126(00)80021-0. [DOI] [PubMed] [Google Scholar]
- 5.Graham DL, Lowe PN, Grime GW, Marsh M, Rittinger K, Smerdon SJ, Gamblin SJ, Eccleston JF. Chem Biol. 2002;9:375–381. doi: 10.1016/s1074-5521(02)00112-6. [DOI] [PubMed] [Google Scholar]
- 6.Spoerner M, Herrmann C, Vetter IR, Kalbitzer HR, Wittinghofer A. Proc Natl Acad Sci USA. 2001;98:4944–4949. doi: 10.1073/pnas.081441398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cavalli A, Carloni P. J Am Chem Soc. 2002;124:3763–3768. doi: 10.1021/ja015821y. [DOI] [PubMed] [Google Scholar]
- 8.Futatsugi N, Tsuda M. Biophys J. 2001;81:3483–3488. doi: 10.1016/S0006-3495(01)75979-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glennon TM, Villa J, Warshel A. Biochemistry. 2000;39:9641–9651. doi: 10.1021/bi000640e. [DOI] [PubMed] [Google Scholar]
- 10.Kosloff M, Selinger Z. J Mol Biol. 2003;331:1157–1170. doi: 10.1016/s0022-2836(03)00847-7. [DOI] [PubMed] [Google Scholar]
- 11.Allin C, Ahmadian MR, Wittinghofer A, Gerwert K. Proc Natl Acad Sci USA. 2001;98:7754–7759. doi: 10.1073/pnas.131549798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allin C, Gerwert K. Biochemistry. 2001;40:3037–3046. doi: 10.1021/bi0017024. [DOI] [PubMed] [Google Scholar]
- 13.Cepus V, Scheidig AJ, Goody RS, Gerwert K. Biochemistry. 1998;37:10263–10271. doi: 10.1021/bi973183j. [DOI] [PubMed] [Google Scholar]
- 14.Cheng H, Sukal S, Deng H, Leyh TS, Callender R. Biochemistry. 2001;40:4035–4043. doi: 10.1021/bi0021131. [DOI] [PubMed] [Google Scholar]
- 15.Du X, Frei H, Kim SH. J Biol Chem. 2000;275:8492–8500. doi: 10.1074/jbc.275.12.8492. [DOI] [PubMed] [Google Scholar]
- 16.Schweins T, Geyer M, Scheffzek K, Warshel A, Kalbitzer HR, Wittinghofer A. Nat Struct Biol. 1995;2:36–44. doi: 10.1038/nsb0195-36. [DOI] [PubMed] [Google Scholar]
- 17.Langen R, Schweins T, Warshel A. Biochemistry. 1992;31:8691–8696. doi: 10.1021/bi00152a002. [DOI] [PubMed] [Google Scholar]
- 18.Chakrabarti PP, Suveyzdis Y, Wittinghofer A, Gerwert K. J Biol Chem. 2004;279:46226–46233. doi: 10.1074/jbc.M405603200. [DOI] [PubMed] [Google Scholar]
- 19.Phillips RA, Hunter JL, Eccleston JF, Webb MR. Biochemistry. 2003;42:3956–3965. doi: 10.1021/bi027316z. [DOI] [PubMed] [Google Scholar]
- 20.Lahiri SD, Zhang G, Dunaway-Marino D, Allen KN. Science. 2003;299:2067–2071. doi: 10.1126/science.1082710. [DOI] [PubMed] [Google Scholar]
- 21.Blackburn GM, Williams NH, Gamblin SJ, Smerdon SJ. Science. 2003;301:1184. doi: 10.1126/science.1085796. [DOI] [PubMed] [Google Scholar]
- 22.Allen KN, Dunaway-Mariano D. Science. 2003;301:1184. [Google Scholar]
- 23.Tremblay LW, Zhang G, Dai J, Dunaway-Mariano D, Allen KN. J Am Chem Soc. 2005;127:5298–5299. doi: 10.1021/ja0509073. [DOI] [PubMed] [Google Scholar]
- 24.Garczarek F, Gerwert K. Nature. 2006;439:109–112. doi: 10.1038/nature04231. [DOI] [PubMed] [Google Scholar]
- 25.Kötting C, Gerwert K. ChemPhysChem. 2005;6:881–888. doi: 10.1002/cphc.200400504. [DOI] [PubMed] [Google Scholar]
- 26.Klaehn M, Schlitter J, Gerwert K. Biophys J. 2005;88:3829–3844. doi: 10.1529/biophysj.104.058644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ulbrich C. Bochum, Germany: Ruhr-Universität Bochum; 2000. Dissertation. [Google Scholar]
- 28.Sanchez-Ruiz JM, Martinez-Carrion M. Biochemistry. 1988;27:3338–3342. doi: 10.1021/bi00409a033. [DOI] [PubMed] [Google Scholar]
- 29.John J, Frech M, Wittinghofer A. J Biol Chem. 1988;263:11792–11799. [PubMed] [Google Scholar]
- 30.Klaehn M, Mathias G, Koetting C, Nonella M, Schlitter J, Gerwert K, Tavan P. J Phys Chem A. 2004;108:6186–6194. [Google Scholar]
- 31.Pohle W, Bohl M, Boehlig W. J Mol Struct. 1990;242:333–342. [Google Scholar]
- 32.Hahn S, Kwak K, Cho M. J Chem Phys. 2000;112:4553–4556. [Google Scholar]
- 33.Li G, Zhang XC. J Mol Biol. 2004;340:921–932. doi: 10.1016/j.jmb.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 34.Shurki A, Warshel A. Proteins. 2004;55:1–10. doi: 10.1002/prot.20004. [DOI] [PubMed] [Google Scholar]
- 35.Pasqualato S, Cherfils J. Structure (London) 2005;13:533–540. doi: 10.1016/j.str.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 36.Schweins T, Langen R, Warshel A. Nat Struct Biol. 1994;1:476–484. doi: 10.1038/nsb0794-476. [DOI] [PubMed] [Google Scholar]
- 37.Topol IA, Cachau RE, Nemukhin AV, Grigorenko BL, Burt SK. Biochim Biophys Acta. 2004;1700:125–136. doi: 10.1016/j.bbapap.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 38.Dittrich M, Hayashi S, Schulten K. Biophys J. 2004;87:2954–2967. doi: 10.1529/biophysj.104.046128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Florian J, Warshel A. J Phys Chem B. 1998;102:719–734. [Google Scholar]
- 40.Egea Pascal F, Shan S-O, Napetschnig J, Savage DF, Walter P, Stroud Robert M. Nature. 2004;427:215–221. doi: 10.1038/nature02250. [DOI] [PubMed] [Google Scholar]
- 41.Focia PJ, Shepotinovskaya IV, Seidler JA, Freymann DM. Science. 2004;303:373–377. doi: 10.1126/science.1090827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tucker J, Sczakiel G, Feuerstein J, John J, Goody RS, Wittinghofer A. EMBO J. 1986;5:1351–1358. doi: 10.1002/j.1460-2075.1986.tb04366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hoard DE, Ott DG. J Am Chem Soc. 1965;87:1785–1788. doi: 10.1021/ja01086a031. [DOI] [PubMed] [Google Scholar]
- 44.Hecht SM, Kozarich JW. Biochim Biophys Acta. 1973;331:307–309. doi: 10.1016/0005-2787(73)90015-4. [DOI] [PubMed] [Google Scholar]
- 45.Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JA, Jr, Montgomery J, Vreven T, Kudin KN, Burant JC, et al. Pittsburgh: Gaussian Inc; 2003. Gaussian 03. [Google Scholar]