Abstract
Encephalisation is the most important characteristic in the evolutionary transition leading from protochordates to vertebrates. This event has coincided with the emergence of a transient and pluripotent structure, the neural crest (NC), which is absent in protochordates. In vertebrates, NC provides the rostral cephalic vesicles with skeletal protection and functional vascularization. The surgical extirpation of the cephalic NC, which is responsible for building up the craniofacial skeleton, results in the absence of facial skeleton together with severe defects of preotic brain development, leading to exencephaly. Here, we have analyzed the role of the NC in forebrain and midbrain development. We show that (i) NC cells (NCC) control Fgf8 expression in the anterior neural ridge, which is considered the prosencephalic organizer; (ii) the cephalic NCC are necessary for the closure of the neural tube; and (iii) NCC contribute to the proper patterning of genes that are expressed in the prosencephalic and mesencephalic alar plate. Along with the development of the roof plate, NCC also concur to the patterning of the pallial and subpallial structures. We show that the NC-dependent production of FGF8 in anterior neural ridge is able to restrict Shh expression to the ventral prosencephalon. All together, these findings support the notion that the cephalic NC controls the formation of craniofacial structures and the development of preotic brain.
Keywords: Fgf8, exencephaly, preotic vesicles, prosencephalic organizer, quail–chick chimeras
Fate mapping experiments conducted in the avian embryo and based on the construction of quail chick chimeras have led to the notion that the facial and hypobranchial skeletons are derived from neural crest (NC) cells (NCC) migrating from the mid-diencephalon down to rhombomere (r)8 (1, 2). This domain is divided into two parts. The rostral part yields the entire facial skeleton, designated facial skeletogenic NC (FSNC), in which no genes of Hox clusters are expressed, and the posterior part yields the middle and posterior parts of the hyoid bone, in which Hox genes of the four first paralogue groups are expressed (Fig. 3, which is published as supporting information on the PNAS web site). The limit between these two domains corresponds to r3, the NCC of which play a pivotal role between the rostral Hox-negative and the caudal Hox-positive NCC populations (3–6). Some r3 NCC migrate to branchial arch (BA)1, whereas others diverge to participate in BA2. The former lose their Hoxa2 expression during the migration, whereas the latter maintain it within the BA2 environment.
Experiments in which the Hox-negative neural fold (NF) was removed before NCC emigration (Fig. 1A–C) resulted in the complete absence of facial skeleton, whereas only a third of the anterior, Hox-negative territory left in situ (or grafted from quail to chick) was sufficient to generate a complete face. These and other data (7–9) led us to consider that Hox-positive cephalic NCC cannot substitute for their Hox-negative counterpart in constructing the face. Moreover, forced expression of Hox genes in the rostral domain of the NC inhibited the expression of their skeletogenic potencies (9). If gain-of-function of Hoxa2 concerned all tissues, homeotic transformations of BA1-derived skeleton ensued (10, 11).
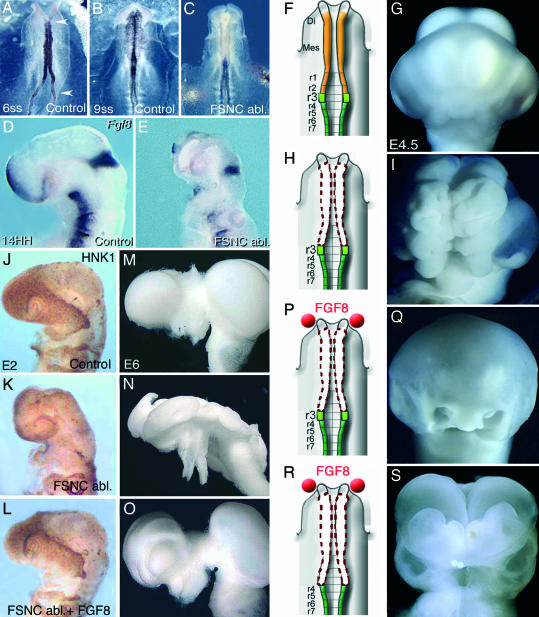
Fig. 1.
Absence of cephalic NC entails exencephaly. (A–C) Snail2 expression in cephalic NCCs. (A) Embryo at 6ss. The NC territory subjected to surgical extirpation is delineated with red arrowheads. (B) In a 9ss unoperated embryo, NCC migrated dorsolaterally and is evidenced with Snail2 transcript detection. (C) In a stage-matched FSNC-ablated embryo, the lack of Snail2 expression accounts for the absence of migrating NCC in the developing head. (D and E) Fgf8 expression in 24ss control (D) and FSNC-deprived (E) embryos showing the loss of Fgf8 expression in ANR as a consequence of FSNC ablation. (F–I) Gross anatomy of E4.5 control (F and G) or FSNC-deprived (H and I) embryos. Shown is an extended exencephaly in the operated embryo (I), compared with the stage-matched control (G). (J–L) Whole-mount HNK1 immunolabeling of an E2.5 control (J); FSNC-deprived (K); or FGF-supplemented; FSNC-deprived (L) embryos. Although the facial processes of the FSNC-ablated embryo are devoid of NCC mesenchyme (except in trigeminal root ganglia), when supplemented with FGF8 in ANR, nasofrontal bud benefits from a massive stream of NCC that arise from the margin of the excised territory and migrate along the upper aspect of anterior cephalic vesicles. (M–O) Whole-mount brain preparations dissected out from control (M); FSNC-deprived (N); and FGF8-supplemented, FSNC-deprived (O) embryos at E6. (N) In the absence of cephalic NCC, brain is exencephalic and partitions into telencephalon, diencephalon, and mesencephalon are no longer recognizable. (O) When stimulated with exogenous FGF8 in ANR, neural tube closure occurs and brain regionalization is restored. (P–S) Role of NCC in dorsal NT closure. In FSNC-ablated embryos supplemented with FGF8 beads (in red) (P), the development of the upper face and brain is restored (Q), whereas, if NC excision is extended down to r3 (R), embryos fail to develop facial structures and exhibit exencephaly (S).
In addition to the effect on the facial skeleton, ablation of the FSNC at the five- to six-somites stage (5–6ss) in chick or quail embryos resulted in major perturbations of forebrain and midbrain development characterized by failure of neural tube (NT) closure and exencephaly (Fig. 1 F–I). In the operated embryos observed at embryonic day (E)2 and E3, Fgf8 expression was nearly abolished in the anterior neural ridge (ANR), both in the neuroepithelium and superficial ectoderm, and absent in the ectoderm of BA1 (Fig. 1 D and E) (5). The abnormalities appearing after FSNC ablation could be significantly corrected by the implantation of FGF8-soaked beads bilaterally on the presumptive territories of BA1 ectoderm. This FGF8 supply induced a cell outflow from r3 that filled up BA1. An upper and lower jaw developed as well as part of the nasal septum. In addition, closure of the NT occurred and brain development tended to normalize. In the present work, we show that the NCC exert a critical effect on the patterning of forebrain and midbrain. The action of NCC is in part direct because it is mediated by a still unknown factor of NC origin. Moreover, an additional indirect effect is due to FGF8, whose production by the ANR and the BA ectoderm is induced and maintained by NCC. We show here that this factor restricts the expression of Shh to the forebrain basal plate.
Results
Rescue of Facial Structures Depends on the Location of the Source of Exogenous FGF8-Soaked Beads.
As previously described, the phenotype resulting from the ablation of FSNC was the absence of facial skeleton and severe defects in forebrain and midbrain development (Fig. 1 A–I). In these embryos, the loss of endogenous FGF8 production in BA1 was first substituted for by exogenous sources of the recombinant protein (i.e., FGF8-soaked heparin acrylic beads) placed on the presumptive BA1 ectoderm. We have previously shown (5) that NCC arising from the remaining r3 (at the limit of the excised territory, which normally provides a few cells only to BA1) expanded considerably and migrated to the maxillomandibular region, where they generated a nearly complete jaw skeleton. In the present work, we have followed the migration flow of NCC of r3 origin in various experimental conditions throughout E2–E3, when NCC colonize the facial processes. After FSNC excision, r3 was replaced by its quail counterpart, and the FGF8 beads were placed either on BA1 ectoderm or at the presumptive level of the ANR (Fig. 4A, which is published as supporting information on the PNAS web site).
In the absence of FGF8 beads, the very few NCC arising from r3 line the root of the trigeminal nerve (Fig. 1 J and K). As shown previously (5) in embryos where FGF8 beads were placed on BA1 ectoderm, NCC arose massively from r3 and predominantly invaded BA1. When the exogenous source of FGF8 was placed on the ANR (Fig. 1P), the direction of the migration stream of NCC was strikingly different. Although originating from the same source (r3) as in the previously described experiment, the r3-derived NCC progressed rostrally along the lateral margins of the mesencephalon and prosencephalon to reach and encompass the upper aspect of the optic vesicle (n = 7) (Fig. 1L). Thus, the FGF8 beads implanted in contact to the ANR were able to drive the NCC progression toward the frontonasal region. As a result and in contrast with the outcome of experiments where the FGF8 beads were placed on BA1 presumptive ectoderm, the lower jaw was only partly rescued, the upper beak was shortened, and the maxillary process was reduced (n = 8) (Fig. 4 A–D). The mandible contained a short cartilage rod that can be interpreted as a rudimentary Meckel's cartilage (Fig. 4C). This bone was always made up of quail cells when the chick r3 had been replaced by its quail counterpart (n = 4) (Fig. 4D).
Exogenous FGF8 Rescues Brain Development.
In the absence of FGF8 beads, the brain of the FSNC-ablated embryos was wide open and the preotic brain vesicles were no longer visible as shown at E6 (n = 6) in Fig. 1N. FGF8 supplementation with beads, whether placed laterally on BA1 ectoderm or on the ANR, restored the closure of the brain vesicles, whereas the development of the prosencephalon and mesencephalon tended to normalize. The most complete rescues of brain development were obtained when exogenous FGF8 was delivered on the ANR (n = 5). In these cases, the growth of the telencephalic vesicles increased over their normal size up to E7 and later on normalized (n = 5) (Fig. 1O). It is remarkable that 4.5 h after FSNC excision (i.e., at 9ss), the rostral NT is normally closed in spite of the absence of the NF (n = 5) (see Fig. 1 A–C; see also Fig. 5, which is published as supporting information on the PNAS web site); this closure, however, was unstable because later on the forebrain and midbrain exhibited typical exencephaly (Fig. 1 I and N).
Respective Roles of the NCC and FGF8 in the Restoration of Brain Development.
We have shown so far that FGF8 beads placed either on the ANR or on BA1 ectoderm stimulate the expansion of r3-derived NCC and provide guidance cues for their migration. The problem was then raised as to whether a stable closure of the forebrain and midbrain NT depends on either the FGF8 supply or a specific action of the NCC. The other alternative being that both factors play a joint role in this process.
To answer this question, we eliminated the r3 source of NCC while providing exogenous FGF8 to FSNC-deprived embryos through beads placed on the ANR (Fig. 1 P–S). The source of regenerating NCC being eliminated, the question was to see whether closure of the NT could be maintained. These embryos exhibited an “open” brain and had no facial development (n = 23) (Fig. 1 R and S). It was interesting to compare the migration flux of NCC in this situation with that prevailing when r3 is present. In the absence of r3 NF, the r4 produced NCC that, instead of migrating exclusively to BA2 (n = 3), also formed a small rostral stream of cells that failed, however, to reach the level of BA1 and of the forebrain (n = 4) (Fig. 4 I–M). This result shows that the source of FGF8 placed on the ANR is not sufficient by itself to induce NT closure and that the NCC play an essential role in this process.
We examined whether NCC were participating in roof plate formation in prosencephalon and mesencephalon in embryos subjected to FSNC excision followed by exogenous FGF8 supply on ANR. In these embryos, r3 NF was replaced by its quail counterpart (Fig. 4A). In this context r3-derived NCC, although giving rise to mesenchymal cells in the forehead, did not take part in the formation of the roof plate along the preotic cephalic vesicles (n = 5) (Fig. 4 E–H). These data indicate that migrating NCC operate in this process by providing the cephalic neuroepithelium with critical diffusible signals for the maintenance of NT closure rather than with a cellular contribution to the roof plate.
To further study the roles played by the NCC in NT closure, we next studied the patterns of gene expression in the developing forebrain and midbrain in normal and experimental conditions.
Comparative Gene Expression Patterns of the Developing Head in Normal and Experimental Embryos.
We have investigated the changes in expression of genes that had been previously shown to play a major role in patterning the ventrodorsal and anteroposterior organization of the brain.
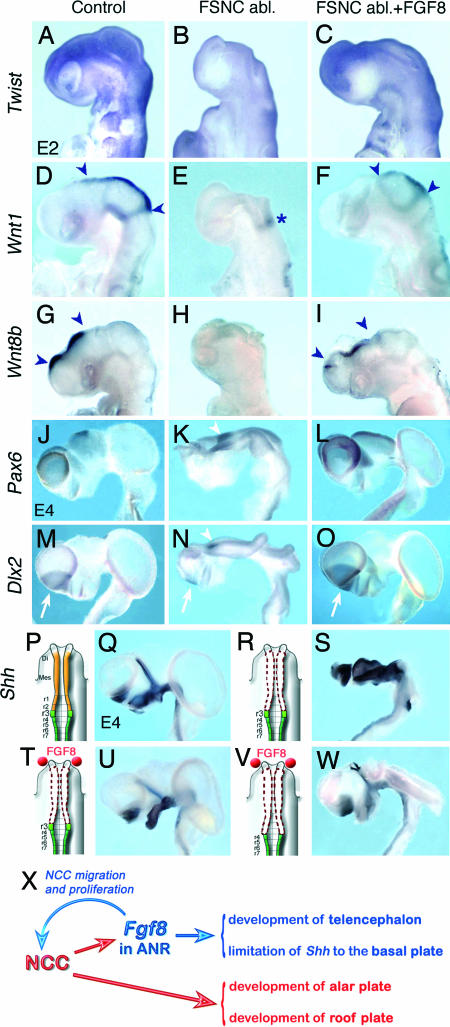
Expression of Twist appears as a critical step in cephalic neurulation given that its null mutation results in the failure of the rostral NT closure (12). Moreover, Twist is involved in Fgf-dependent epitheliomesenchymal interactions taking place in several morphogenetic processes (13). In normal avian embryos at 24ss, Twist transcripts are present in the cephalic NCC that have populated the BAs and the frontonasal primordia (n = 8) (Fig. 2A). In addition, Twist-labeled cells, fated to participate in the development of the calvarium (not stained with HNK1 Mab), are normally present on the dorsolateral aspects of the cephalic vesicles. The abnormally developed heads of stage-matched FSNC-ablated embryos were devoid of Twist transcripts in both presumptive BA1 and precalvarial domains (n = 7) (Fig. 2B). This finding is in agreement with the absence of NCC in these regions. When subjected to FGF8 supplementation administered on the ANR, FSNC-ablated embryos exhibited a strong expression of Twist in the frontonasal region together with an expanded expression domain laterodorsally to the telencephalic and diencephalic vesicles (n = 7) (Fig. 2C). Thus, r3-derived NCC, which developed under the influence of exogenous FGF8, exhibited a normal pattern of Twist expression.
Fig. 2.
NCC progression in the forehead territory restores brain patterning. Gene expression in NCC and cephalic neuroepithelium in control (A, D, G, J, M, and Q); FSNC-ablated (B, E, H, K, N, and U); and FGF8-treated, FSNC-ablated (C, F, I, L, O, and S) embryos. (A) At 24ss, Twist is activated in NC that populate BA and nasofrontal primordium along with the presumptive calvarial domain. In addition, a fringe of Twist-labeled cells likely devoted to the development of the calvarium was present on the dorsolateral aspects of the cephalic vesicles. (B) In stage-matched FSNC-ablated embryos, no Twist transcript is evidenced in the developing head. (C) When supplemented with exogenous FGF8, embryos exhibit Twist expression in the nasofrontal region and an expanded expression domain laterodorsally to the telencephalic and diencephalic vesicles. (D–F) At 24ss, Wnt1 expression in the dorsal mesencephalon and thalamus (D, arrowheads) is lost in the absence of FSNC except at the level of the isthmus (E, asterisk) but is restored when NCC progression is stimulated by FGF8 in ANR (F). (G–I) Expression of Wnt8b in dorsal diencephalon and telencephalon (G, arrowheads) is abolished in the absence of FSNC (H) but partly restored if embryos are treated with FGF8 (I). (J–L) At E4.5, Pax6 expression in pallium and dorsal diencephalon (J) is reduced in telencephalon but up-regulated in P3 (K, arrowhead) in the absence of FSNC, whereas treating with FGF8 rescues normal pattern of expression in dorsal prosencephalic vesicles (L). (M and N) At this stage, Dlx2 expression in ganglionic eminence (M, arrow) is severely perturbed in FSNC-deprived embryos (N). (N) Dlx2 remains as a small focus in telencephalon (N, arrow) and is overexpressed in P3 (N, arrowhead). (O) Exogenous FGF8 restores Dlx2 in telencephalon (arrow). (P and Q) In normal development, Shh is expressed in the prosencephalic basal plate. (R and S) In FSNC-deprived embryos (R), Shh transcript accumulation is expanded at the expense of the alar plate (S). (T) With FGF8 supply, Shh expression tends to normalize. (V and W) Suppressing r3 together with FSNC (V) in FGF8-treated embryos does not severely perturb the pattern of Shh expression while brain is exencephalic (W). (X) Schematic representation of the role of NCC in brain development.
Similar observations were made for Wnt5a. In mammals, the activation of Wnt5a signaling in head ectomesenchyme correlates with the outgrowth of the frontonasal and maxillomandibular processes. In null Wnt5a mouse mutants, nasal capsule and jaw apparatus are absent (14). In normal E2.5 chick embryos, Wnt5a is expressed in BA1 and in the ventral aspect of the frontonasal process. Wnt5a transcripts were absent in FSNC-ablated embryos but present if the embryos were supplemented with FGF8 beads (data not shown).
We then explored the expression of genes shown to play a role in alar plate patterning in the preotic brain. Wnt1 transcripts are present in midbrain–hindbrain transition (15). In E2.5 normal embryos (n = 4), Wnt1 is expressed as a ring of cells abutting to the isthmic domain. From this point up to the diencephalon, Wnt1 is also expressed along the dorsal mesencephalic midline except at the level of r1 (Fig. 2D). In FSNC-deprived embryos, the absence of NC-derived mesenchyme in the developing head coincided with the loss of Wnt1 expression at the dorsal midline, whereas some neuroepithelial cells, closely associated with the isthmus, remained weakly positive (n = 7) (Fig. 2E). FGF8 supplementation at the level of the ANR partly restored Wnt1 expression in the dorsal diencephalic and mesencephalic neuroepithelium (n = 13) (Fig. 2F).
More rostrally, Wnt8b expression was described in the dorsal thalamus (16). In E2.5 normal chick embryos (n = 10), Wnt8b expression was accordingly detected in the dorsal prosencephalic neuroepithelium and in the dorsal thalamus (Fig. 2G). In FSNC-deprived embryos, Wnt8b expression was lost (n = 8) (Fig. 2H). By contrast, FGF8 supplementation at the level of the ANR restored a normal pattern of Wnt8b in the dorsal thalamus (n = 9) (Fig. 2I). Therefore, it turns out that the rostral deployment of NCC along the preotic encephalic vesicles is required for Wnt gene expression at the disencephalic and mesencephalic dorsal midline.
To further define brain defects taking place in the absence of FSNC, we looked at the normal expression patterns of transcription factors Emx2, Pax6, Dlx2, and Otx2 at E4.5. These genes have been shown to play a critical role in brain development (17–21).
In normal brains (n = 7), Pax6 is expressed in the dorsal telencephalon (corresponding to the pallium) and in the dorsal diencephalon (Fig. 2J). In absence of FSNC (n = 8), Pax6 transcripts were detected in a limited caudal site in the remaining telencephalic structure and overexpressed in the diencephalon at the level of prosomere 3 (P3) (22), i.e., in the part of the diencephalon normally fated to give rise to the prethalamus that extends dorsally in embryos exhibiting an open brain (Fig. 2K). In FGF8-rescued brains, Pax6 expression was restored in the dorsal telencephalon and diencephalon (n = 7) (Fig. 2L).
Emx2 is expressed in the dorsal telencephalon (fated to yield the pallium) and in a sharply delineated focus of positive cells in the hypothalamus of normal embryos (n = 7) (Fig. 6 A and D, which is published as supporting information on the PNAS web site). In the FSNC-ablated embryos, Emx2 transcripts were present in the anterior lateral margin of the open telencephalic vesicle (n = 8) (Fig. 6 B and E). Additionally, Emx2 expression was also present in the region corresponding to P3 (Fig. 6F). In FGF8-rescued brains, Emx2 expression was detected in the dorsal telencephalon, thus restoring a normal pattern of transcript accumulation (n = 8) (Fig. 6 C and F).
Dlx2 is expressed in the ventral telencephalon (i.e., in the ganglionic eminences corresponding to the striatum and pallidum), in the ventral thalamus (P3) alar plate, and in the hypothalamus of normal brains (n = 7) (Fig. 2M). In FSNC-ablated embryos, Dlx2 remained weakly expressed in a small focus restricted to the basal open neural plate (n = 6) (Fig. 2N). Dlx2 also was found to be overexpressed in P3, like Pax6 and Emx2. FGF8 treatment allowed Dlx2 expression to be partly rescued in the ventral telencephalon, and it normalized the expression pattern in P3 (n = 6; Fig. 2O).
In normal embryos, Otx2 transcripts are present in the mesencephalic neuroepithelium and, to a lesser extent, in the dorsal diencephalon (n = 7) (Fig. 6G). In FSNC-ablated embryos, Otx2 expression was up-regulated and uniformly present rostrally to the isthmus in the mesencephalic and diencephalic neural plates (n = 6) (Fig. 6H). After FGF8-soaked bead implantation, Otx2 expression was recovered in the mesencephalic vesicle as well as in the dorsal diencephalon, where it remained stronger than normal (n = 6) (Fig. 6I).
These data indicate that NCC deployment along the cephalic vesicles is responsible for signals essential for preotic alar plate patterning. To figure out the role of the NC in this process, we hypothesized that NCC act by repressing ventral cues originating from the basal plate.
Shh expression is restricted to the basal plate of the mesencephalon and prosencephalon in normal developing brain at E4.5 (n = 6). In addition, Shh also is expressed in the zona limitans intrathalamica as a strand of alar plate cells that demarcate the posterior diencephalon (the pretectum and thalamus, corresponding to P1 and P2, respectively) from the anterior diencephalon (the prethalamus, corresponding to P3) (Fig. 2 P–Q). It has been shown that mispatterning of zona limitans intrathalamica results in forebrain defects (23, 24). In FSNC-deprived embryos, the accumulation of Shh transcripts was considerably expanded at the expense of the prosencephalic alar plate territory (n = 5) (Fig. 2 R–S). In contrast, in these embryos, FGF8 supply in contact to the ANR allowed the pattern of Shh gene expression to normalize (n = 5) (Fig. 2 V and W). The question was raised of the gene expression status of the brains of embryos deprived of FSNC and r3 NC but subjected to FGF8 supplementation on the ANR. Although in operated embryos the preotic brain remains exencephalic (as shown in Fig. 1S), Shh transcript accumulation is not affected by the absence of migrating NCC in the forehead (n = 6).
This result indicates that the limitation of Shh expression to the basal plate in our experiments is constrained by the source of FGF8 in ANR and that the NCC are not directly involved in this process. In normal development, NCC act by inducing FGF8 production by ANR (5).
Structural Telencephalic and Thalamic Defects Caused by FSNC Excision Can Be Corrected by FGF8 Supplementation.
In E8 FSNC-ablated embryos (i.e., 7 days after the operation), the telencephalon was very reduced in size. Only a small part of the ventral telencephalon subsisted but showed a poor histological organization (Fig. 7, which is published as supporting information on the PNAS web site). The diencephalon and mesencephalon displayed important alterations at the dorsal level: The neuroepithelium exhibited an exencephalic organization, and the thalamic and pretectal nuclei failed to develop. The dorsal defects that encompassed the optic tectum ended in the isthmic domain where the roof plate was present. However, the cerebellum showed weak structural defects, midline fusion, and important depletion of external granular cells (Fig. 7).
When FGF8-soaked beads were implanted in contact with the ANR after NC ablation, telencephalic development in E8 embryos was basically restored because the telencephalon exhibited normal histological domains, including midline structures as the choroidal plexus (Fig. 7). The diencephalon developed normal prethalamic (P3), thalamic (P2), and pretectal (P1) structures, including the posterior commissure (Fig. 7), but the diencephalic choroidal plexus did not develop normally.
Discussion and Conclusions
The results presented here show that the cephalic NF corresponding to the posterior diencephalon, the mesencephalon, and the anterior rhombencephalon (r1 and r2), in which no genes of the Hox family are expressed and which is responsible for the development of the facial skeleton (9), is essential for the development of forebrain and midbrain. The embryos in which the NF from this level is removed exhibit exencephaly with an open NT in which the dorsal cephalic structures fail to grow and differentiate. Moreover, these embryos are deprived of their entire facial skeleton.
We have previously shown that early removal of the FSNC results in the loss of Fgf8 in the ANR (5). This structure, which differentiates from the margin of the neural plate rostral to the limit where the NF produces NCC, has been demonstrated to play a critical role in vertebrate brain development. In mouse and zebrafish, this zone was shown to produce signals influencing telencephalic development and was considered a prosencephalic organizer. Fgf8 was identified to participate in this signal (23, 25, 26).
Here, we demonstrate that the negative effect of the removal of the Hox-negative NC on brain development can be overcome by treatment of the operated embryos with exogenous FGF8. Beads of FGF8 placed either on the ANR or on the presumptive BA1 ectoderm at 5–6ss induced the proliferation of the NCC exiting from r3. These r3-derived NCC were able to regenerate facial skeletal structures and migrated in distinct flows according to the position of the FGF8 source. When the FGF8-soaked beads were located on the presumptive BA1 ectoderm, the NCC migrated ventrolaterally, hence replenishing the facial processes with skeletogenic mesenchymal cells. If FGF8 beads were implanted on the ANR, the progression of NCC arising from the edge of the resected territory was directed not only to BA1 but also toward the frontonasal process. Thus, r3-derived cells migrated along the preotic cephalic vesicles as shown by HNK1 and Twist labeling, respectively. This observation indicates that FGF8 diffusion from its source, the ANR, extends down to the level of the mid-rhombencephalon, given that it increases significantly the number of NCC produced by r3. Moreover, the fact that the directionality of the NCC stream varies according to the position of the FGF8 beads means that they constitute a chemoattractive cue for these cells.
Interestingly, as shown in our previous study (5), NCC exert a positive effect on the production of FGF8 by the superficial ectoderm and the neuroepithelium in the ANR as well as by the BA1 ectoderm.
As far as the brain is concerned, the exogenous source of FGF8 and the rostral migration of r3-derived NCC allow closure of the NT to be maintained and the development of the telencephalon and of the alar plate derivatives of the mesencephalic and diencephalic vesicles to proceed. r3-derived NCC, which carry the quail marker, have never been found to participate in the roof plate. Therefore, both alar plate and roof plate development depend on a signal provided by the cephalic NCC. If the FSNC ablation includes r3-NCC, the embryos exhibit a wide-open brain even when supplemented with exogenous FGF8. In these embryos, r4 NF becomes the rostralmost source of NCC. r4-derived NCC are induced to migrate rostrally but fail to reach either BA1 or the frontonasal bud. As a consequence, the lack of migrating NCC in the head precludes the dorsal closure of the NT.
The role of the NCC in cephalic roof plate development has been further documented by looking at the pattern of genes normally expressed in the prosencephalic and mesencephalic primordium, such as Wnt1 (expressed from the mid-diencephalon to the posterior mesencephalic level) and Wnt8b (expressed more anteriorly). After ablation of FSNC, exencephalic brains are devoid of Wnt transcripts (except for the accumulation of Wnt1 mRNA, which is maintained rostrally to the isthmus). In the telencephalic primordium, the absence of NCC also impedes the expression of Pax6, Emx2, and Dlx2; activation of these genes merely subsists as reminiscent autonomous foci, meaning that the structures in which they are normally expressed either do not develop or do not mature properly. At the diencephalic level, Otx2 expression is up-regulated as if this structure were posteriorized. Concurrently, a striking dorsalization of Shh transcript distribution occurs in diencephalon and mesencephalon, increasing the prosencephalic basal plate at the expense of the alar plate. Severe perturbations in gene expression at E4.5 foreshadow long-term structural defects recorded at E8. It turns out that the removal of NC ends up with the loss of dorsal telencephalon and the agenesis of the thalamic and pretectal nuclei.
As previously mentioned, when the FSNC-deprived embryos were supplemented with FGF8-soaked beads placed on the ANR, FGF8 supply stimulated the proliferation and the migration of r3 NCC to the forehead. The progression of NCC driven by FGF8 was accompanied by the restoration of Wnt1 and Wnt8b expressions. This result suggests that Wnt activation in the anterior roof plate relies on NC-dependant signals. By contrast, specification of the dorsal midline structures, such as choroidal plexus, are only incompletely restored in these experiments. Together with the restoration of roof plate identity characterized by expression of Wnt genes, NCC also concur to the patterning of the pallial and subpallial territories. NCC deployment is accompanied by the recovery of gene expression at these levels, meaning that NCC are likely to impart signals required for the proper patterning of telencephalic markers.
In conclusion, the cephalic NC coordinates the formation of craniofacial structures and the development of forebrain and midbrain. Its various roles are schematized in Fig. 2X. The NC is at the origin of the still unknown signals that trigger and maintain the expression of Fgf8 in the ANR. FGF8 of ANR origin exerts a positive effect on the proper patterning of the basal plate in the diencephalon and mesencephalon, as shown by the restriction of Shh expression to the ventral midline and zona limitans intrathalamica. In contrast, the NC has a direct effect on the development of the alar plate in the diencephalon and mesencephalon and on patterning the roof plate.
Materials and Methods
Quail and chick embryos were operated at 5-6ss, corresponding to 24–29 h of incubation at 38°C (i.e., Hamburger and Hamilton's stage 8) (27). Bilateral excision of the FSNC were performed according to the fate map of the NF (Supporting Materials and Methods, which is published as supporting information on the PNAS web site) (28). FGF8-soaked beads (Sigma, St. Louis, MO) were prepared as described in ref. 5 and implanted in contact with the ANR.
Hybridizations with Snail2, Fgf8, Twist, Wnt1, Wnt5a, Wnt8b, Emx2, Pax6, Dlx2, and Otx2 probes (19–21, 29–31) were performed as described in refs. 32 and 33.
Supplementary Material
Acknowledgments
We thank Christine Vincent and Monica Rodenas for technical assistance and Drs. Boncinelli, Delfini, Gruss, McMahon, Martin, Puelles, and Simeone for graciously providing probes for in situ hybridization. Work in N.M.L.D.'s laboratory was supported by Program Project Grant 3149 from Association pour la Recherche contre le Cancer at the Centre National de la Recherche Scientifique. Work in S.M.'s laboratory was supported by Grants UE QLRT-1999-31625, QLRT-2000-02310, FCT/MCES, DIGESIC-MEC PM98-0056, BFI2002-02979, and GV CTDIA/2002/91. S.E.C. was the recipient of a fellowship from Fondation Bettencourt–Schueller.
Abbreviations
- ANR
anterior neural ridge
- BA
branchial arch
- En
Embryonic day n
- NC
neural crest
- FSNC
facial skeletogenic NC
- NCC
NC cell(s)
- NF
neural fold
- NT
neural tube
- P3
prosomere 3
- rn
rhombomere n
- nss
n-somites stage.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Le Lièvre CS, Le Douarin NM. J Embryol Exp Morphol. 1975;34:125–154. [PubMed] [Google Scholar]
- 2.Couly GF, Coltey P, Le Douarin NM. Development (Cambridge, UK) 1993;117:409–429. doi: 10.1242/dev.117.2.409. [DOI] [PubMed] [Google Scholar]
- 3.Graham A, Heyman I, Lumsden A. Development (Cambridge, UK) 1993;119:233–245. doi: 10.1242/dev.119.1.233. [DOI] [PubMed] [Google Scholar]
- 4.Graham A, Francis-West P, Brickell P, Lumsden A. Nature. 1994;372:684–686. doi: 10.1038/372684a0. [DOI] [PubMed] [Google Scholar]
- 5.Creuzet S, Schuler B, Couly G, Le Douarin NM. Proc Natl Acad Sci USA. 2004;101:4843–4847. doi: 10.1073/pnas.0400869101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kulesa P, Ellies DL, Trainor PA. Dev Dyn. 2004;229:14–29. doi: 10.1002/dvdy.10485. [DOI] [PubMed] [Google Scholar]
- 7.Couly G, Grapin-Botton A, Coltey P, Ruhin B, Le Douarin NM. Development (Cambridge, UK) 1998;125:3445–3459. doi: 10.1242/dev.125.17.3445. [DOI] [PubMed] [Google Scholar]
- 8.Couly G, Creuzet S, Bennaceur S, Vincent C, Le Douarin NM. Development (Cambridge, UK) 2002;129:1061–1073. doi: 10.1242/dev.129.4.1061. [DOI] [PubMed] [Google Scholar]
- 9.Creuzet S, Couly G, Vincent C, Le Douarin NM. Development (Cambridge, UK) 2002;129:4301–4313. doi: 10.1242/dev.129.18.4301. [DOI] [PubMed] [Google Scholar]
- 10.Grammatopoulos GA, Bell E, Toole L, Lumsden A, Tucker AS. Development (Cambridge, UK) 2000;127:5355–5365. doi: 10.1242/dev.127.24.5355. [DOI] [PubMed] [Google Scholar]
- 11.Pasqualetti M, Ori M, Nardi I, Rijli FM. Development (Cambridge, UK) 2000;127:5367–5378. doi: 10.1242/dev.127.24.5367. [DOI] [PubMed] [Google Scholar]
- 12.Chen ZF, Behringer BR. Genes Dev. 1995;9:686–699. doi: 10.1101/gad.9.6.686. [DOI] [PubMed] [Google Scholar]
- 13.Zuniga A, Quillet R, Perrin-Schmitt F, Zeller R. Mech Dev. 2002;114:51–59. doi: 10.1016/s0925-4773(02)00048-5. [DOI] [PubMed] [Google Scholar]
- 14.Yamagushi TP, Bradley A, McMahon AP, Jones S. Development (Cambridge, UK) 1999;126:1211–1223. doi: 10.1242/dev.126.6.1211. [DOI] [PubMed] [Google Scholar]
- 15.McMahon AP, Bradley A. Cell. 1990;69:581–595. doi: 10.1016/0092-8674(92)90222-x. [DOI] [PubMed] [Google Scholar]
- 16.Garcia-Lopez R, Vieira C, Echevarria D, Martinez S. Dev Biol. 2004;268:514–530. doi: 10.1016/j.ydbio.2003.12.038. [DOI] [PubMed] [Google Scholar]
- 17.Walther C, Gruss P. Development (Cambridge, UK) 1991;113:1435–1449. doi: 10.1242/dev.113.4.1435. [DOI] [PubMed] [Google Scholar]
- 18.Simeone A, Acampora D, Gulisano M, Stornaiuolo A, Boncinelli E. Nature. 1992;358:687–690. doi: 10.1038/358687a0. [DOI] [PubMed] [Google Scholar]
- 19.Bally-Cuif L, Gulisano M, Broccoli V, Boncinelli E. Mech Dev. 1995;49:49–63. doi: 10.1016/0925-4773(94)00301-3. [DOI] [PubMed] [Google Scholar]
- 20.Smith-Fernandez A, Pieau C, Repérant J, Boncinelli E, Wassef M. Development (Cambridge, UK) 1998;125:2099–21111. doi: 10.1242/dev.125.11.2099. [DOI] [PubMed] [Google Scholar]
- 21.Puelles L, Kuwana E, Puelles E, Bulfone A, Shimamura K, Keleher J, Smiga S, Rubinstein JLR. J Comp Neurol. 2000;424:409–438. doi: 10.1002/1096-9861(20000828)424:3<409::aid-cne3>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 22.Rubinstein JLR, Martinez S, Shimamura K, Puelles L. Science. 1994;266:578–580. doi: 10.1126/science.7939711. [DOI] [PubMed] [Google Scholar]
- 23.Houart C, Westerfield M, Wilson SW. Nature. 1998;391:788–792. doi: 10.1038/35853. [DOI] [PubMed] [Google Scholar]
- 24.Braun MM, Etheridge A, Bernard A, Robertson CP, Roelink H. Development (Cambridge, UK) 2003;130:5579–5587. doi: 10.1242/dev.00685. [DOI] [PubMed] [Google Scholar]
- 25.Shimamura K, Rubinstein JL. Development (Cambridge, UK) 1997;124:2709–2718. doi: 10.1242/dev.124.14.2709. [DOI] [PubMed] [Google Scholar]
- 26.Heisenberg CP, Brennan C, Wilson SW. Development (Cambridge, UK) 1999;126:2129–40. doi: 10.1242/dev.126.10.2129. [DOI] [PubMed] [Google Scholar]
- 27.Hamburger V, Hamilton HL. J Morphol. 1951;88:49–92. [PubMed] [Google Scholar]
- 28.Couly G, Grapin-Botton A, Coltey P, Le Douarin NM. Development (Cambridge, UK) 1996;122:3393–3407. doi: 10.1242/dev.122.11.3393. [DOI] [PubMed] [Google Scholar]
- 29.Nieto MA, Sargent MG, Wilkinson DG, Cooke J. Science. 1994;264:835–839. doi: 10.1126/science.7513443. [DOI] [PubMed] [Google Scholar]
- 30.Crossley PH, Minowada G, McArthur CA, Martin GR. Cell. 1996;84:127–136. doi: 10.1016/s0092-8674(00)80999-x. [DOI] [PubMed] [Google Scholar]
- 31.Holliday M, McMahon JA, McMahon AP. Mech Dev. 1995;52:9–52. doi: 10.1016/0925-4773(95)00385-e. [DOI] [PubMed] [Google Scholar]
- 32.Henrique D, Adam J, Myat A, Chitnis A, Lewis J, Ish-Horowicz D. Nature. 1995;375:787–790. doi: 10.1038/375787a0. [DOI] [PubMed] [Google Scholar]
- 33.Etchevers HC, Vincent C, Le Douarin NM, Couly GF. Development (Cambridge, UK) 2001;128:1059–1168. doi: 10.1242/dev.128.7.1059. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.