Abstract
Cooperative behaviors are common among social insects such as bees, wasps, ants, and termites, but they have not been reported from insect species that use aggressive mimicry to manipulate and exploit prey or hosts. Here we show that larval aggregations of the blister beetle Meloe franciscanus, which parasitize nests of the solitary bee Habropoda pallida, cooperate to exploit the sexual communication system of their hosts by producing a chemical cue that mimics the sex pheromone of the female bee. Male bees are lured to larval aggregations, and upon contact (pseudocopulation) the beetle larvae attach to the male bees. The larvae transfer to female bees during mating and subsequently are transported to the nests of their hosts. To mimic the chemical and visual signals of female bees effectively, the parasite larvae must cooperate, emphasizing the adaptive value of cooperation between larvae. The aggressive chemical mimicry by the beetle larvae and their subsequent transport to their hosts' nests by the hosts themselves provide an efficient solution to the problem of locating a critical but scarce resource in a harsh environment.
Keywords: aggressive chemical mimicry, cooperative behavior, Habropoda pallida, Meloe franciscanus, phoresy
The phenomenon of phoresy (1–3), in which one organism is transported by another, is common among arthropods. Phoresy provides an effective means of dispersal for organisms with limited mobility, particularly in extreme environments where harsh conditions and the scarcity and patchiness of critical resources present formidable obstacles to survival. The relationship between passenger and host may become obligate, with the passenger being entirely dependent on the host for transport to suitable habitats or resources. Under such circumstances, it is to be expected that the passenger will develop behavioral, morphological, semiochemical, and life history characters that promote effective exploitation of the host. Here we report a well developed, multistep phoretic system in which the passenger species not only rides on the host but actively lures the host to it by mimicking the host's sex pheromone and subsequently parasitizes the host's nest.
Our study system was comprised of the blister beetle Meloe franciscanus Van Dyke and its host, the solitary bee Habropoda pallida Timberlake, which inhabit sand dunes in the deserts of the southwestern United States. In this extremely variable habitat, widely distributed patches of plants function as islands in a sea of sand for first-instar larvae of the beetle. The dunes support populations of the psammophytic plant Astragalus lentiginosus var. borreganus M. E. Jones, which provides nectar for the host bee. The flightless adult blister beetles also feed on Astragalus foliage, and female beetles frequently oviposit at the base of the plant.
The phoretic first-instar larvae of M. franciscanus, known as triungulins, have evolved a complex four-step mechanism for ensuring their survival, which results in the host species transporting the triungulins back to their nests. In the first step, M. franciscanus larvae emerge from eggs laid at the base of plants and immediately aggregate on vegetation in tight, dark-colored masses of 120 to >2,000 individuals that visually mimic females of the host bee species in size, color, and perching location (4) (Fig. 1A). The triungulins cooperate to remain aggregated, moving as a unit up and down the stem and to different branches until they die or are removed by male H. pallida bees.
Fig. 1.
Three steps in the Habropoda–Meloe aggressive mimicry system. (A) A typical aggregation of M. franciscanus triungulins on a grass blade. Aggregations average 6.9 ± 2.8 mm in diameter (range, 2–15 mm; mode = 10; n = 153). Of 153 triungulin aggregations measured, 55% were ≥7 mm. (B) A male H. pallida bee inspecting an M. franciscanus triungulin aggregation on the branch tip of the dune plant A. lentiginosus var. borreganus. (C) A male bee covered with M. franciscanus triungulin larvae on the dorsum.
In the second step, triungulin aggregations display remarkable proficiency at enticing male bees to “inspect” (hovering within 1–10 cm of the aggregations for ≥2 s) and contact the aggregations (pseudocopulation) (Fig. 1B). Upon contact with a bee, the triungulins attach to the male bee en masse. The attachment is extraordinarily fast and efficient, with the entire mass transferring to the male bee within 0.13–2 s. In the third step of the sequence, the triungulins transfer to the female bee when a male bee infested with triungulins copulates or simply makes contact in attempts to copulate with her (Fig. 1C). Finally, in the fourth step, the female bee transports the triungulins to her nest, where they dismount to feed and develop on the nest's pollen and nectar provisions and the bee egg. The beetles complete their development inside the host's nest, then emerge and mate, and gravid female beetles lay egg masses at the base of plants to repeat the cycle.
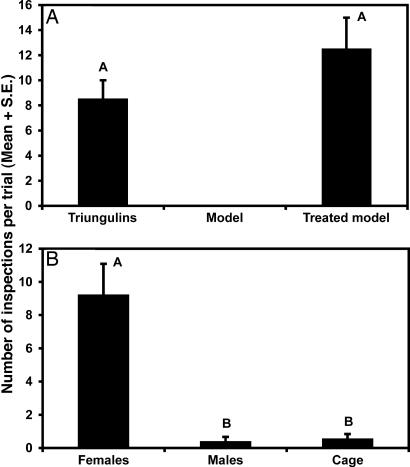
Under field conditions, female H. pallida perch on flowers and branches of plants, and we hypothesized that male bees were attracted to perching females by a sex pheromone and/or visual cues. The dark-colored triungulins form round to oval aggregations averaging 6.9 mm in diameter on branch tips of plants growing in the dune depressions, similar in female bee size and perching locations. Based on preliminary observations of male bees responding to triungulin aggregations from downwind, we postulated that triungulins also use volatile chemical cues to attract male bees. Thus, we tested the attraction of male bees to triungulin aggregations, to unscented visual models of the aggregations, and to models treated with triungulin extract. We also tested the attraction of males to caged male and female bees and dead males and females. Visual mimicry of a female bee (size, color, and location) (4) was not sufficient to entice males to interact with triungulin aggregations. Here we report that aggregations of triungulins attract male bees by mimicking the sex pheromone of the female bees, exhibiting cooperative aggressive chemical mimicry.
Results
In the first series of field experiments, male bees frequently inspected (hovering within ≤10 cm for ≥2 s) triungulin aggregations, whereas visual models of triungulin masses received no visits (Fig. 2A). In contrast, visual models of aggregations treated with hexane extracts of triungulin masses were as attractive to male bees as the aggregations themselves (Fig. 2A), proving that a volatile chemical cue was the primary means of attraction. Furthermore, the treated models elicited patterns of approach and inspection (12 close inspections, hovering within 1 cm, of 36 observed inspections of lures; n = 6) analogous to those exhibited by males toward female bees (28 male–female bee contacts made during 354 observed male inspections of female bees) or triungulin aggregations (14 pseudocopulations during 69 observed inspections of aggregations) observed during the duration of the study (see Movies 1 and 2, which are published as supporting information on the PNAS web site).
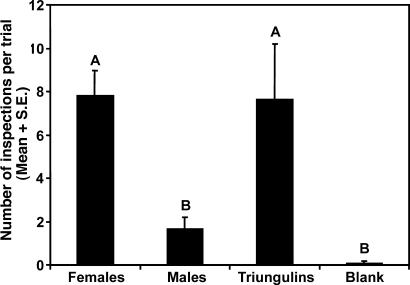
Fig. 2.
Results of bioassays to characterize the nature of male bee attraction to triungulin aggregations or female bees. (A) Male bee inspection visits of triungulin aggregations, visual models of aggregations, and models treated with extracts of triungulins (n = 3) (χ2 = 1.53, P = 0.22). Visual models received no visits and were not included in the data analysis. (B) Inspection visits of male bees to caged female bees, caged male bees, and empty cage controls (n = 9). Two-way ANOVA: treatment, F = 27.45, P = 0.0001, df = 2,17; trial, F = 1.63, P = 0.024, df = 5,17. Bars marked by different letters are significantly different (Student–Newman–Keuls procedure, P < 0.05).
Mating status did not affect the attractiveness of female bees to males. Mated female H. pallida begin carrying pollen masses on their legs at the onset of nesting. Mated females carrying a pollen mass received an average of 13.4 ± 14.8 inspections per hour from male bees (range, 1–28; n = 5). Dead females pinned to plants also attracted males (5 ± 2.6 inspections per hour; 3 ± 2.9 contacts per hour; n = 4), whereas equal numbers of replicates of dead males and controls (visual models) received no inspections or contacts.
In the next set of experiments, caged live female bees elicited numerous inspections by male bees, whereas caged males and empty cage controls were hardly visited at all (Fig. 2B). During these experiments, female bees were observed to masticate the screen of the cages with their mandibles, suggesting that they might be using this behavior to mark the substrate with a chemical signal. This hypothesis was supported by the fact that a visual model treated with the glandular secretions from a crushed head was attractive to male bees, receiving 11 inspections in 1 h, whereas models treated with a crushed thorax or crushed abdomen received no inspections. Together, these data showed that mate location behaviors of H. pallida bees are mediated by chemical signals present in the heads of females.
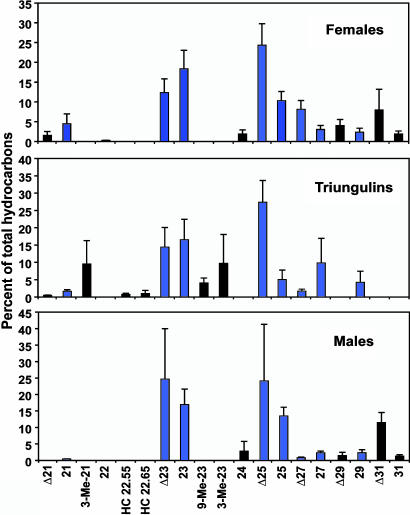
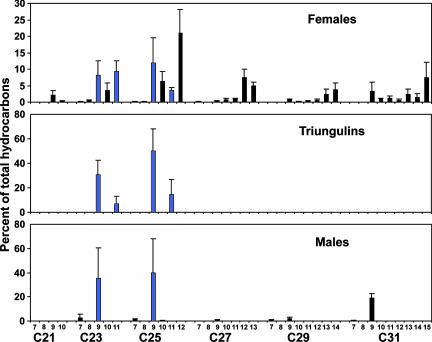
Analyses of hexane extracts of heads of bees and whole-body extracts of triungulins revealed remarkable correspondence in the profiles of chemicals extracted (Fig. 3). Extracts of both the host and the parasite species were dominated by C23 and C25 straight-chain alkanes and alkenes, with lesser amounts of homologs. Superficially, the profiles of male and female bees looked similar, with no obvious indication of female-specific compounds that might constitute a sex pheromone. However, more detailed analyses of the isomeric composition of extracts showed that the alkene fraction of males consisted almost exclusively of (Z)-9-alkenes, particularly (Z)-9-tricosene and (Z)-9-pentacosene, whereas the blend from females consisted of mixtures of (Z)-alkenes with double bonds in positions 9–15 (Fig. 4). In contrast, the alkene profile of triungulins comprised a subset of the alkene blend produced by the female bees, consisting almost exclusively of (Z)-9- and (Z)-11-tricosenes and pentacosenes (Fig. 4 Middle).
Fig. 3.
Composition of extracts (mean percentages ± SD) from male (n = 12) and female (n = 14) bee heads and whole-body extracts of triungulin aggregations (n = 6). Numbers indicate hydrocarbon chain length, alkenes are indicated by Δ, and methyl branches are indicated with a numerical prefix. HC 22.55 and HC 22.65 were two unidentified hydrocarbons.
Fig. 4.
Positional isomeric composition (mean percentages ± SD) of the alkene fractions of the extracts of heads of male and female bees and of triungulin aggregations. Trace amounts of (Z)-9-C21 and (Z)-11-C21 also were detected in two of the six triungulin extracts.
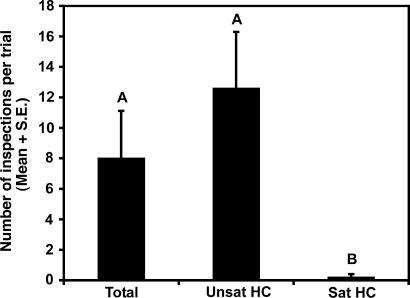
Reconstructions of the extracts of triungulins and bee heads were applied to visual models and tested in field bioassays. A mixture mimicking the blend of alkenes found in an extract of a triungulin aggregation was as attractive to male bees as the complete blend of eight components (alkenes plus alkanes), whereas the three-component alkane fraction was minimally attractive (Fig. 5). With these data suggesting that the alkenes were most important, further bioassays were carried out with reconstructions of the alkene fractions (Fig. 4) of male and female bees and of triungulins. The alkene blend from female bees and the triungulins blend were equally attractive to males, whereas the blend from males was no different from the solvent control (Fig. 6). Of 36 observed inspection visits by males to the triungulin lure blend, 12 (33%) resulted in very close inspection (hovering within 1 cm), whereas 35 observed inspection visits to the female lure blend resulted in 12 (34%) very close inspections (n = 6 for both treatments). These data indicate that the triungulins use a subset of components of the female bee pheromone to attract male bees in an effective aggressive mimicry of the pheromone.
Fig. 5.
Attraction of male bees to hydrocarbon blends mimicking fractions of an extract of a triungulin mass. “Unsat HC” treatment consisted of (Z)-9-C21 (0.025 mg), (Z)-9-C23 (0.860 mg), (Z)-11-C21 (0.100 mg), (Z)-9-C25 (1.25 mg), and (Z)-11-C25 (0.252 mg); “Sat HC” treatment consisted of C21 (0.089 mg), C23 (1 mg), C25 (0.252 mg), and C27 (0.704 mg); “Total” treatment consisted of Unsat HC plus Sat HC (n = 5). Two-way ANOVA: treatment, F = 20.37, P = 0.0007, df = 2,14; trial, F = 4.01, P = 0.045, df = 4,14. Bars marked by the same letter are not significantly different (Student–Newman–Keuls procedure, P = 0.05).
Fig. 6.
Attraction of male bees to hydrocarbon blends mimicking the alkene fractions of female bees, male bees, and triungulins as determined from analyses of Figs. 3 and 4 and a solvent control (n = 12). Two-way ANOVA: treatment, F = 21.28, P < 0.0001, df = 3,47; trial, F = 1.61, P = 0.14, df = 11,47. Bars marked by the same letter are not significantly different (Student–Newman–Keuls procedure, α = 0.05). “Females” treatment consisted of (Z)-9-C21 (0.090 mg), (Z)-9-C23 (0.340 mg), (Z)-10-C23 (0.145 mg), (Z)-11-C23 (0.390 mg), (Z)-9-C25 (0.500 mg), (Z)-10-C25 (0.270 mg), (Z)-11-C25 (0.150 mg), (Z)-12-C25 (0.880 mg), (Z)-12-C27 (0.315 mg), (Z)-13-C27 (0.205 mg), (Z)-14-C29 (0.160 mg), (Z)-9-C31 (0.140 mg), and (Z)-15-C31 (0.310 mg). “Males” treatment consisted of (Z)-9-C23 (0.438 mg), (Z)-9-C25 (0.500 mg), and (Z)-9-C31 (0.234 mg). “Triungulin” treatment consisted of (Z)-9-C23 (0.305 mg), (Z)-11-C23 (0.068 mg), (Z)-9-C25 (0.500 mg), and (Z)-11-C25 (0.143 mg). Control treatment consisted of 50 μl of hexane.
The advantages of cooperation by the triungulins in the production of a chemical signal of sufficient strength were suggested by a dose–response bioassay in which male bees were less attracted to a 1:9 dilution of a female bee blend (6.7 ± 4.1 inspection visits per hour, mean ± SD) than to the undiluted blend (11.8 ± 6.0 inspection visits per hour) (n = 6) (two-way ANOVA: treatment effect, F = 5.26, P = 0.07, df = 1,11; trial effect, F = 2.77, P = 0.14, df = 5,11). Female beetles lay their eggs in batches averaging 761 eggs (n = 8; SD = 533; range, 106–1,365 eggs), and in the laboratory all eggs laid in a group by a single female emerged together and aggregated together (n = 17). Larvae cooperated to stay aggregated throughout their lifespan (n = 50), moving up and down and to different branches together (n = 42). In addition, male bees carrying large numbers of triungulins (n = 10 males with triungulin masses) attracted and were contacted by other males (n = 6), presumably because they smelled like females. These male–male contacts, lasting 1–15 s, may serve to enhance triungulin dispersal, suggesting a further benefit of cooperative behavior by the beetle larvae.
Discussion
The use of chemical mimicry by arthropods as a means of camouflage is ubiquitous in nature (5–7), whereas aggressive chemical mimicry appears to be much less common. Aggressive chemical mimicry has been most thoroughly described in bolas spiders in the genus Mastophora (8–10) and in sexually deceptive orchids in the genus Ophrys (11, 12) and Chiloglottis (13). Here we have documented cooperative aggressive chemical mimicry in the Insecta. M. franciscanus larvae have evolved a suite of complementary semiochemical and behavioral characters (4) in response to the challenge of locating their hosts' nests in a changeable environment in which host bees, bee nests, and bee floral sources are both patchy and ephemeral. The sand dune habitat represents a formidable barrier to dispersal and host location by the small (≈2 mm in length) and flightless triungulins, and, to circumvent this barrier, the triungulins have commandeered their hosts' sexual communication system.
Several factors may have contributed to the evolution of larval sociality, phoresy, and aggressive chemical mimicry in M. franciscanus. For example, adult female beetles lay large batches of eggs from which the neonate larvae eclose synchronously. The triungulins are positively phototactic and negatively thermotactic to surface radiated heat (14), factors that predispose them to climb to the tips of plants. Formation of aggregations may have several functions in addition to attracting male bees, such as slowing desiccation of individuals in the group (15–17) and mutual protection from predation with their chemical defense, cantharidin (18). Triungulins need a high-quality nutrient resource of long duration on which to feed and complete their development. Ecological constraints, such as high spatial and temporal patchiness of essential resources (19, 20) (the host bee, bee nests, and bee nectar resources), high temperatures, and low humidity limit larval survival, longevity, and mobility, providing strong selection for traits that maximize the probability of being picked up by a host. In addition, the triungulins in an aggregation are typically siblings from a single egg mass, so the degree of relatedness within an aggregation is high. As exemplified by the social insects, closely related individuals are more likely to exhibit cooperative behaviors than are more distantly related individuals (16, 21, 22).
Slow-motion replay of a video recording of a male bee approaching a triungulin aggregation revealed a further cooperative behavioral adaptation that increases the chances of being picked up by a host. Rather than waiting passively as a male bee approaches, the triungulins rapidly rearrange themselves to reach out toward the approaching bee. Thus, even though a male bee may only intend a close-range inspection of an aggregation rather than contact, he may still be ensnared by a mass of triungulin larvae as they actively extend out toward him. The mechanisms by which the triungulins detect the approach of a male bee are not known, but they might include air movement, visual cues, or acoustic cues associated with the flight of the bee.
The fact that mated and even dead females remained attractive to males demonstrates that the sex pheromone is persistent, as would be expected from the chemical nature of the long-chain hydrocarbons that constitute the pheromone. The presence of the pheromone blend in the head extracts is analogous to what has been found with several other bees in the family Apidae, including Xylocopa sulcatipes, Bombus terrestris, and Apis mellifera, for which sex attractant pheromones have been found in extracts of the cuticle and mandibular glands (23).
Evidence that both receptive and unreceptive females are attractive to males also has been reported for a congeneric species. Barthell and Daly (24) found that male Habropoda depressa repeatedly attempted copulation with unreceptive females, and it appeared that males could not distinguish between receptive and unreceptive females. We found this to be the case with H. pallida as well. For example, L.S.S.-G. observed five consecutive mating attempts by a persistent male H. pallida, in which the unreceptive female was grabbed with such vigor that the pair fell to the ground. Each time, the female shook off the male but he returned for another unsuccessful copulation attempt.
There are remarkable parallels between the chemistry of the Habropoda–Meloe system reported here and the chemical mimicry used by a sexually deceptive orchid to achieve pollination. The orchid Ophrys sphegodes is pollinated by males of the solitary bee Andrena nigroaenea. The attractant produced by the orchid closely mimics the pheromone of the female bee, which consists of a blend of straight-chain alkanes and alkenes with 21–29 carbons, similar to the blend of hydrocarbons produced by H. pallida females (12). However, in contrast to the Ophrys–A. nigroaenea system in which the orchid matches the entire hydrocarbon blend produced by female A. nigroaenea, the triungulins produce only a subset of the total blend of hydrocarbons produced by H. pallida females.
Phoresy, low vagility, and survival in unpredictable habitats are often correlated (25). Thus, other meloid species may have evolved host location mechanisms similar to the one reported here. Phoresy, which was first reported in Meloe by Siebold in 1841 (1), is believed to have evolved independently at least seven times within the family (26), occurring in 42 species in 21 genera. Furthermore, triungulin aggregation behavior has been reported for two other meloid species, in the genera Meloe and Hornia, both of which are nest parasites of bees (2, 4, 27–29), suggesting that analogous aggressive chemical mimicry also may be found within these systems.
We suggest that the cooperation and chemical communication exhibited by triungulins for mutual benefit transcends aggregation behavior to become social behavior. In the context of insects, the term “social” often has been used interchangeably with “eusocial” and has been defined narrowly as requiring parental involvement in brood care (20, 30). As such, this narrow definition excludes a large array of cooperative behaviors in diverse taxa from formal recognition as social behaviors (16, 30, 31). However, M. franciscanus larvae can be termed “social” following Wilson's broader use of the word “social” (“a group of individuals that belong to the same species … organized in a cooperative manner” with “reciprocal communication of a cooperative nature”) (16). Thus, the cooperative behaviors described here, along with other recent reports of cooperative behaviors in organisms as diverse as amoeba (32), bacteria (22), and caterpillars (20), provide insights into the evolution of social behavior.
Methods
Study Animals.
All field experiments were conducted at Kelso Dunes in the Mojave National Preserve in San Bernardino County, CA, from March to May 2000–2005. H. pallida and M. franciscanus specimens used for chemical analyses were collected at this site. M. franciscanus triungulin aggregations composed of individuals measuring an average of 2.0 ± 0.1 mm long were collected from their natural aggregation sites on the branches of plants. Individual aggregations were extracted in a glass vial with 1 ml of hexane for 1 h. The resulting extracts were transferred to clean vials and used for bioassays or stored at −20°C until they were analyzed by coupled GC-MS. Individual male and female H. pallida bees were collected with a sweep net, held in 20-ml glass vials, and transported to the laboratory. After brief chilling, each bee was divided into head, thorax, and abdomen. Each body section was extracted individually with 1 ml of hexane for 1 h, and the extracts were transferred to clean vials and stored at −20°C until they were used in bioassays or analyses.
Chemical Analysis.
Extracts of triungulins and heads of male and female H. pallida were analyzed by coupled GC-MS by using a Hewlett–Packard 6890 GC interfaced to a Hewlett–Packard 5973 mass selective detector (Hewlett–Packard, Palo Alto, CA) operated in electron impact ionization mode (70 eV). The GC was equipped with a DB-5MS column (20 m × 0.2 mm ID, 0.25 μm film; J&W Scientific, Folsom, CA), with helium carrier gas, injector and transfer line temperatures of 300°C, and a temperature program of 100°C at 0 min with a 15°C/min increase to 300°C and hold for 30 min. Sample aliquots (1 μl) were injected in splitless mode with the split valve opened after 1 min.
To determine double bond positions of alkenes, 100-μl aliquots of extracts were transferred to 1.5-ml vials with Teflon-lined screw-cap lids. One drop of a solution of iodine in ether (20 mg/ml) and one drop of dimethyldisulfide was added to each vial, and vials were sealed and heated to 50°C overnight. After cooling, the mixtures were worked up by addition of 50 μl of 5% aqueous Na2S2O3 and pentane (100 μl) with vortexing until the iodine color was discharged. The pentane layer was then transferred to a clean vial, concentrated under a gentle stream of nitrogen, and analyzed as described above.
To determine the stereochemistry of the alkene double bonds, 100-μl aliquots of extracts were concentrated to dryness under a stream of nitrogen and treated with five drops of meta-chloroperbenzoic acid in methylene chloride (2 mg/ml). After standing at room temperature for 2 h, each mixture was treated with 10 drops of 5% NaHCO3 in water and 0.5 ml of pentane. After stirring for 15 min, the upper organic layer was transferred to a clean vial, concentrated under a stream of nitrogen, and analyzed as described above. Standards of (Z)-alkenes and (E)-alkenes derivatized under identical conditions gave the corresponding epoxides that were separated to baseline, with the (E)-isomers eluting first in all cases.
Straight-chain saturated hydrocarbon standards were obtained from commercial sources. (Z)-9-Heneicosene and (Z)-9-tricosene were purchased from Sigma Chemical (St. Louis, MO) and Lancaster Synthesis (Pelham, NH), respectively. Other (Z)-alkenes were synthesized by (Z)-selective Wittig reactions and purified by recrystallization from hexane at −20°C as previously described (33).
Field Bioassays.
Bioassays were conducted in dune depressions (≈750 m elevation) in March and April from 9:00 a.m. to 5:00 p.m. PST, 2000–2005. The research site is dominated in spring by several species of native and exotic grasses (Panicum urvilleanum, Pleuraphis rigida, Achnatherum hymenoides, and Schismus barbatus), A. lentiginosus var. borreganus, and bare branches of the perennials Petalonyx thurberi and Croton californicus var. mohavensis. These species are found predominantly in the depressions of the dunes, leaving the ridges and peaks comparatively bare. Distances between depressions with vegetation ranged from ≈70 to 400 m. Vegetation at the base of the dune field was dominated by creosote bush scrub (Larrea tridentata).
The duration of each individual trial was 60 min, consisting of three or four treatments in each replicate, depending on the bioassay. Positions of treatments were randomized within a replicate. Because only one replicate could be run at any one time, replicates were accumulated over a number of days of observations. An inspection or “inspection visit” is defined as a male bee approaching a female bee, triungulin aggregation, or lure and hovering within 10 cm for ≥2 s. This term follows the “hovering inspection” terminology devised by Kullenberg (34). For bioassays, models consisting of uniform 1-cm squares of brown felt were treated with hexane extracts of bee heads or hexane solutions of synthetic compounds (typical dose, 0.5 or 1 mg of the major component, corresponding amounts of the minor components, approximately equal to 5 or 10 female bee head equivalents, respectively). Control lures were treated with an equivalent volume of hexane. Triungulin models were made of crushed aluminum foil shaped to resemble an oval triungulin aggregation, then painted with brown tempera paint and treated with triungulin extract. Controls consisted of models treated only with hexane. Models were placed at the tops of plant stems in patches of plants located in dune depressions. The number of bee inspections of each lure, the behavior of bees around the lures, and the sex of each bee were recorded by visual observations supplemented with video recordings. Subsets of responding animals were caught with a sweep net to verify their sex. Bioassays with dead female and male bees were conducted with specimens attached to branches by pinning them from below with a #3 insect pin. Bioassays of live females or males were carried out with individual bees placed in cubic cages of 10 cm per side constructed from aluminum window screen. Each cage was used once and discarded. Controls consisted of empty cages. In bioassays of females with and without pollen masses, cages were covered with three layers of additional screening to obscure any visual cues from the test animals. Bioassay data were tested for normality, then analyzed by appropriate statistical tests as described in the figure legends.
Supplementary Material
Acknowledgments
We thank N. Gershenz for field and laboratory assistance; J. S. McElfresh for statistical analyses and preparation of graphics; J. D. Pinto and P. Fiedler for advice and insightful discussions; R. Fulton of the Desert Studies Center of California State University (Fullerton, CA) for invaluable discussions, resources, and logistical support; J. Andre of the Jack and Marilyn Sweeney Granite Mountain Desert Research Center for use of their facilities; K. Will, J. McNeil, D. Janzen, R. Dowell, M. Moffett, and the reviewers for critical reading and comments that improved the manuscript; and Debra Hughson and the Mojave National Preserve for permission to conduct this research.
Footnotes
The authors declare no conflict of interest.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Siebold C. Stettiner Entomologische Zeitung. 1841;2:130–136. [Google Scholar]
- 2.Newport G. Proc Linn Soc London; 1851. pp. 297–320. [Google Scholar]
- 3.Askew RR. Parasitic Insects. New York: American Elsevier; 1971. [Google Scholar]
- 4.Hafernik J, Saul-Gershenz L. Nature. 2000;404:35–36. doi: 10.1038/35011129. [DOI] [PubMed] [Google Scholar]
- 5.Stowe MK. In: Chemical Mediation of Coevolution. Spencer KC, editor. San Diego: Academic; 1988. pp. 513–580. [Google Scholar]
- 6.Howard RW, Akre RD. In: Chemical Ecology of Insects. Cardé RT, Bell WJ, editors. Vol 2. New York: Chapman & Hall; 1995. pp. 365–424. [Google Scholar]
- 7.Haynes KF, Yeargan KV. Ann Entomol Soc Am. 1999;92:960–970. [Google Scholar]
- 8.Eberhard WG. Science. 1977;198:1173–1175. doi: 10.1126/science.198.4322.1173. [DOI] [PubMed] [Google Scholar]
- 9.Stowe MK, Tumlinson JH, Heath RR. Science. 1987;236:964–967. doi: 10.1126/science.236.4804.964. [DOI] [PubMed] [Google Scholar]
- 10.Haynes KF, Gemeno C, Yeargan KV, Millar JG, Johnson KM. Chemoecology. 2002;12:94–105. [Google Scholar]
- 11.Borg-Karlson AK, Tengö J. J Chem Ecol. 1986;12:1927–1941. doi: 10.1007/BF01041856. [DOI] [PubMed] [Google Scholar]
- 12.Schiestl FP, Ayasse M, Paulus HF, Löfstedt C, Hansson BS, Ibarra F, Francke W. Nature. 1999;399:421–422. [Google Scholar]
- 13.Schiestl FP, Peakall R, Mant JG, Ibarra F, Schulz C, Franke S, Francke W. Science. 2003;302:437–438. doi: 10.1126/science.1087835. [DOI] [PubMed] [Google Scholar]
- 14.Erickson EH, Werner FG. Ann Entomol Soc Am. 1974;67:903–908. [Google Scholar]
- 15.Allee WC. J Exp Zool. 1926;145:255–277. [Google Scholar]
- 16.Wilson EO. Insect Societies. Cambridge, MA: Belknap/Harvard Univ Press; 1971. [Google Scholar]
- 17.Klok CJ, Chown SL. Funct Ecol. 1999;13:417–427. [Google Scholar]
- 18.Carrel JE, Eisner T. Science. 1974;183:755–756. doi: 10.1126/science.183.4126.755. [DOI] [PubMed] [Google Scholar]
- 19.Strassmann JE, Queller DC. In: The Genetics of Social Evolution. Breed M, Page R, editors. Boulder, CO: Westview; 1989. pp. 81–101. [Google Scholar]
- 20.Costa JT, Pierce NE. In: The Evolution of Social Behavior in Insects and Arachnids. Choe JC, Crespi BJ, editors. Cambridge, UK: Cambridge Univ Press; 1997. pp. 407–442. [Google Scholar]
- 21.Hamilton WD. J Theor Biol. 1964;7:1–52. doi: 10.1016/0022-5193(64)90038-4. [DOI] [PubMed] [Google Scholar]
- 22.Griffin AS, West SA, Buckling A. Nature. 2004;430:1024–1027. doi: 10.1038/nature02744. [DOI] [PubMed] [Google Scholar]
- 23.Ayasse M, Paxton RJ, Tengö J. Annu Rev Entomol. 2001;46:31–78. doi: 10.1146/annurev.ento.46.1.31. [DOI] [PubMed] [Google Scholar]
- 24.Barthell JF, Daly HV. Pan-Pac Entomol. 1995;71:149–156. [Google Scholar]
- 25.Houck MA, O'Connor AC. Annu Rev Entomol. 1991;36:611–636. [Google Scholar]
- 26.Bologna MA, Pinto JD. Syst Entomol. 2001;26:33–72. [Google Scholar]
- 27.Rau P. Psyche. 1930;XXXVII:155–156. [Google Scholar]
- 28.McSwain JW. Univ of California Publ Entomol. 1956;12:1–182. [Google Scholar]
- 29.Mayer DF, Johansen CA. Proc Wash State Entomol Soc; 1979. pp. 1–46. [Google Scholar]
- 30.Wheeler WM. The Social Insects: Their Origin and Evolution. New York: Harcourt Brace; 1928. [Google Scholar]
- 31.Michener CD. Proc 10th Int Congr Entomol; 1958. pp. 441–447. [Google Scholar]
- 32.Strassmann JE, Zhu Y, Queller DC. Nature. 2000;408:965–967. doi: 10.1038/35050087. [DOI] [PubMed] [Google Scholar]
- 33.Ginzel MD, Moreira JA, Ray AM, Millar JG, Hanks LM. J Chem Ecol. 2006;32:435–451. doi: 10.1007/s10886-005-9010-y. [DOI] [PubMed] [Google Scholar]
- 34.Kullenberg B. Zoon. 1973;(Suppl 1):31–41. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.