Abstract
Previous studies have shown that DNA methyltransferase (Dnmt) 1 is required for maintenance of bulk DNA methylation and is essential for mouse development. However, somatic disruption of DNMT1 in the human cancer cell line HCT116 was not lethal and caused only minor decreases in methylation. Here, we report the identification of a truncated DNMT1 protein, which was generated by the disruption of DNMT1 in HCT116 cells. The truncated protein, which had parts of the regulatory N-terminal domain deleted but preserved the catalytic C-terminal domain, was present at different levels in all DNMT1 single-knockout and DNMT1/DNMT3b double-knockout cell lines tested and retained hemimethylase activity. DNMT1 RNAi resulted in decreased cell viability in WT and knockout cells and further loss of DNA methylation in DNMT1 knockout cells. Furthermore, we observed a delay in methylation after replication and an increase in hemimethylation of specific CpG sites in cells expressing the truncated protein. Remethylation studies after drug-induced hypomethylation suggest a putative role of DNMT1 in the de novo methylation of a subtelomeric repeat, D4Z4, which is lost in cells lacking full-length DNMT1. Our data suggest that DNMT1 might be essential for maintenance of DNA methylation, proliferation, and survival of cancer cells.
Keywords: DNA methylation, epigenetic
The biological roles of the major mammalian DNA methyltransferase (Dnmt), DNMT1, have been enigmatic. Although gene-targeting studies in mice have clearly demonstrated an essential function of Dnmt1 in embryonic development, cell survival, and tumorigenesis (1), there have been controversial reports regarding the function of this enzyme in human cancer cells. In mice, Dnmt1 has been implicated in maintaining the majority of bulk DNA methylation, differentiation of ES cells, and imprinting (2, 3). Furthermore, deletion of Dnmt1 in mouse embryonic fibroblasts caused a decrease in genomic methylation, p53-dependent apoptosis, and deregulation of transcription (4). Heterozygosity for Dnmt1 in combination with administration of the DNMT inhibitor 5-aza-2′deoxycytidine (5-aza-CdR) greatly reduced the number of polyps in a mouse model for intestinal neoplasia (5).
A series of RNAi experiments for DNMT1 have been described for various human cancer cell lines, which have shown apparently inconsistent results in regard to DNA methylation of tumor suppressor genes (6–10). The differences might be attributed to the techniques used but also to distinct sensitivities of individual cell lines (10). Furthermore, RNAi may not cause a complete depletion of the protein, so residual protein might still be available and capable of maintaining DNA methylation.
Rhee et al. (11, 12) generated a widely used series of HCT116 colon cancer cells with homozygous deletions for DNMT1 (DNMT1−/−) (11), DNMT3b (DNMT3b−/−) (12), or both DNMT1 and DNMT3b (12) [double knockout (DKO)]. Surprisingly, somatic disruption of DNMT1 resulted in only a 20% decrease in overall genomic methylation with no discernible changes in methylation of specific loci, whereas DKO cells lost >95% of total DNA methylation. Interestingly, one of the eight DKO clones generated (DKO8) retained ≈50% of WT methylation and exhibited less growth retardation when compared with the other DKO cell lines (12). The targeting strategy to disrupt DNMT1 resulted in deletion of exons 3, 4, and 5 of WT DNMT1. These deleted exons code for part of the DMAP1 (DNMT1-associated protein) interaction domain, which is a transcriptional repressor shown to recruit histone deacetylase HDAC2 and DNMT1 (13), and the proliferating cell nuclear antigen (PCNA) interaction domain, which is essential in targeting DNMT1 to the replication fork (14).
Here, we show that the cell lines generated are not complete knockouts for DNMT1 but have retained a hypomorphic allele and express a catalytically active, truncated DNMT1 protein. Intriguingly, the DKO8 cell line, which had appeared aberrant in the original work in that it maintained a higher level of DNA methylation (12), expressed higher levels of this truncated protein, and our data suggest a function for this variant of DNMT1 for cell viability and maintenance of DNA methylation.
Results
DNMT1 Transcripts and Protein in HCT116 Knockout Cells.
Rhee et al. (11) previously generated a DNMT1 knockout construct in which exons 3, 4, and 5 of human DNMT1 were replaced with a hygromycin resistance gene. First, we performed RT-PCR analyses to identify putative DNMT1 transcripts in HCT116 WT and various knockout cells. We detected PCR products of the expected sizes spanning exons 1–6 of DNMT1 in WT and DNMT3b−/− cells (Fig. 1 A and B, exons 1–6). Sequencing of PCR products revealed different shorter transcripts in the knockout cell lines, in which the cDNAs had deleted exons 3–5 in DNMT1−/− and DKO1 cells, as predicted by the knockout targeting. Interestingly, the transcript detected in the DKO8 cells carried an additional deletion of exon 2, which had most likely been generated by alternative splicing. This transcript was of specific interest, because it could potentially give rise to a truncated DNMT1 protein in the same reading frame as WT DNMT1. To specifically amplify this transcript, we designed a forward RT-PCR primer covering both exon 1 and exon 6 and a reverse primer lying in exon 10 of WT DNMT1. We were able to identify such alternative transcripts in both the DNMT1−/− and the DKO1 cell lines, although at lower levels than in the DKO8 cells (Fig. 1B, exons 1/6–10), which were not detected in the initial PCR spanning exons 1–6, most likely because there was a preference for the amplification of the transcript with a deletion of exons 3–5. Sequencing of RT-PCR products of exons 32–34 showed that mRNAs containing intact catalytic domains were present in all cell lines (Fig. 1B, exons 32–34, and data not shown). Therefore, all knockout cell lines expressed truncated DNMT1 transcripts, which could give rise to a shortened DNMT1 protein. Indeed, we were able to detect a faster migrating protein as compared with full-length DNMT1 on Western blots of nuclear extracts of the different knockout cell lines probed with an antibody directed against the C terminus of DNMT1 (Fig. 1C Upper). We quantified the levels of the truncated protein to ≈20% of WT DNMT1 in the DNMT1−/− and DKO1 cell lines and to ≈40% in the DKO8 cells (Fig. 1D), which was in line with the amount of transcript detected in the different cell lines (Fig. 1B, exons 1/6–10). The deletion of ≈17 kDa encoded part of the DMAP1 interaction domain and the PCNA interaction domain. However, the truncated protein retained the nuclear localization signal and other regulatory N-terminal domains, as well as the catalytic C-terminal domain (1) (Fig. 1A). Thus, the knockout had generated a hypomorphic allele, which yielded a truncated DNMT1 protein in DNMT1−/− cells and two individual DKO cell lines. The availability of the DKO8 clone, which expressed higher levels of the hypomorphic protein than the DKO1 or DNMT1−/− cells, allowed us to titrate the enzyme levels and facilitated the characterization of the potential biological function of the truncated protein.
Fig. 1.
DNMT1 expression in different HCT116 cell lines. (A Upper) Genomic map of WT DNMT1. Black vertical boxes indicate exons; black horizontal bars drawn below indicate deletions determined by direct sequencing of transcripts in different knockout cell lines. (A Lower) DNMT1 protein domain structure. DMAP1 domain, amino acids 1–120; PCNA domain, amino acids 163–174; NLS, nuclear localization signal domain, amino acids 177–205; DNA replication foci-targeting domain, amino acids 331–550; ZN D, zinc finger region, amino acids 646–692; KEN, KEN box (KENxxxR), amino acids 644–650; BAH1 and BAH2, bromo-adjacent homology domains, amino acids 755–880 and 972–1100, respectively; KG, 6 × 2-aa tandem repeats of K-G, amino acids 1109–1120; catalytic domain, amino acids 1139–1616. Note that illustrations are not drawn to scale. (B) RT-PCR in different WT and knockout cell lines was performed by using primers located in exons 1 and 6 of WT DNMT1 (E1–6), half exons 1 and 6 and exon 10 of DNMT1 (E1/6–10), or exons 32 and 34 (E32–34). As a control, primers against β-actin were used (Actin). (C) Western blot analysis of various HCT116 cell lines with C-terminal DNMT1 (Upper) or PCNA antibody as a loading control (Lower). (D) Quantitation of the Western blot shown in C for DNMT1 protein levels, normalized to PCNA expression. The WT expression level was arbitrarily set as 1. WT, HCT116 WT; 1KO, DNMT1−/− cells; 3bKO, DNMT3b−/− cells; DKO8 and DKO1, two independent DNMT1 and DNMT3b DKO clones, respectively.
DNMT inhibitors such as 5-aza-CdR are incorporated into DNA and inhibit DNMT activity by forming covalent complexes with the catalytic domains of DNMTs, leading to their depletion from protein extracts (15–17). Interestingly, 5-aza-CdR treatment caused depletion of both the full-length and truncated DNMT1 proteins, suggesting the presence of a functional catalytic domain in the shortened protein (Fig. 2A). Immunoprecipitation experiments and DNMT1 activity assays using hemimethylated or unmethylated DNA fragments corresponding to a genomic p16 sequence showed that the truncated enzyme was active (Fig. 2B). Both the WT and truncated proteins showed preferences for the naturally occurring hemimethylated DNA target. We could not detect any activity against an unmethylated sequence in either DNMT1 WT or knockout cell lines. Thus, even though the overall level of Dnmt activity had been highly reduced (11, 12), a demonstrable maintenance activity was still present in DNMT1−/− and DKO cells.
Fig. 2.
DNMT1 activity. (A) Western blot analysis of cells treated with 0.3 μM 5-aza-CdR or left untreated for 24 h. The blot was sequentially probed with an antibody against the C terminus of DNMT1 and HDAC1 as a loading control. (B) DNMT1 was immunoprecipitated with the same DNMT1 antibody as in A and incubated with hemimethylated or unmethylated DNA fragments corresponding to a 428-bp-long p16 intron 1 sequence and 160 μM S-adenosylmethionine for 3 h at 37°C. The percentage of methylation was determined by quantitative Ms-SNuPE (33). IP, immunoprecipitation with DNMT1 antibody; Pre, control immunoprecipitation with whole rabbit serum.
DNMT1 RNAi.
Having demonstrated that the truncated DNMT1 protein possessed in vitro DNMT activity, we asked whether this protein would have in vivo functions as well. Three independent RNAi knockdown experiments were performed by using three rounds of transient transfections with a double-stranded RNA oligonucleotide whose sequence had been previously known to specifically knock down DNMT1 (7). We obtained substantial reductions of DNMT1 protein levels in both DNMT1 WT and knockout cell lines (Fig. 3A), which were accompanied by a reduction in cell number, with <50% viable cells in WT, DNMT1−/−, and DNMT3b−/− cells (Fig. 3B). The DKO8 cells were less affected by the additional reduction of DNMT1 protein, probably because of their slow proliferation rates or because they had already been selected to survive with low levels of Dnmts. Furthermore, after the reduction of DNMT1 protein levels, only DNMT1−/− cells sustained a significant loss in DNA methylation at specific loci, whereas a mild effect was observed in DNMT1 WT and DKO8 cells (Fig. 3C). These results indicate that even trace amounts of DNMT1 may therefore be sufficient to maintain some methylation at specific loci. The observed decrease in DNA methylation in the DNMT1−/− cells after RNAi was accompanied by a weak induction of genes normally silenced by hypermethylation, such as the tumor suppressor TIMP3 or the testis-specific antigen MAGE A1 (data not shown).
Fig. 3.
DNMT1 siRNA. (A) Western blot of HCT116 WT and knockout cells untransfected (0) or transiently transfected with negative control siRNA (C) or DNMT1 siRNA (RNAi) and probed with a DNMT1 or PCNA antibody. (B) Percentage of viability was calculated as the ratio of the number of DNMT1 RNAi-transfected cells to control-transfected cells. (C) Ms-SNuPE assays of untransfected, control, and DNMT1 RNAi cells for different loci. D4Z4, subtelomeric repeat, CpG island; RUNX1, CpG-poor region in intron 1; MAGE A1, CpG-poor promoter region; TIMP3, CpG island promoter region. Data shown are representatives of at least three independently repeated experiments. NS, P value not significant; *, P value significant; T, P value shows a tendency toward significance.
Hemimethylation in HCT116 Cells.
Many experiments have demonstrated that DNMT1 preferentially methylates hemimethylated DNA and functions at the replication fork to copy methylation patterns while anchored to PCNA (1, 14, 18, 19). Because the DNMT1 hypomorphic cell line had presumably lost the ability to interact with PCNA and therefore might not be able to fully methylate its target sequences during S phase, it might be anticipated that the cells would show an increased level of hemimethylation after depletion of WT DNMT1. We therefore measured the level of hemimethylation within three regions of the p16 locus by using an assay we have previously described that couples methylation-sensitive restriction enzyme digestion with bisulfite treatment and methylation-sensitive single-nucleotide primer extension (Ms-SNuPE) analysis (20, 21). The ability of the assay to detect hemimethylation at three sites [within the 5′ region of the p16 gene (H1), at a CpG-poor site (H2), and at a CpG-rich site in intron 1 (H3)] was first validated by using T24 cells after 24-h treatment with 5-aza-CdR (20). Results shown in Fig. 4 clearly demonstrate an increase in the proportion of hemimethylated sites in cells that were hypomorphic for DNMT1. The increase in hemimethylation was especially prominent at the CpG-poor site tested and seemed to correlate with the levels of the truncated DNMT1 protein, demonstrating the importance of DNMT1 for the maintenance of methylation of CpG-poor regions (compare also Fig. 5). Thus, failure to localize DNMT1 to the replication fork by deletion of the PCNA-binding site within the enzyme substantially increased the percentage of sites that were hemimethylated within the hypomorphic cells.
Fig. 4.
Hemimethylation assay. Hemimethylation was assessed in the different HCT116 cell lines at three different sites within the p16 gene (H1, H2, and H3). H1 is located in the promoter, H2 is in a CpG-poor region within intron 1, and H3 is in a CpG-rich region of intron 1. Percentage of hemimethylation was defined as h/(h + f), where h represents hemimethylated sites (only one strand methylated) and f represents fully methylated sites (both strands methylated).
Fig. 5.
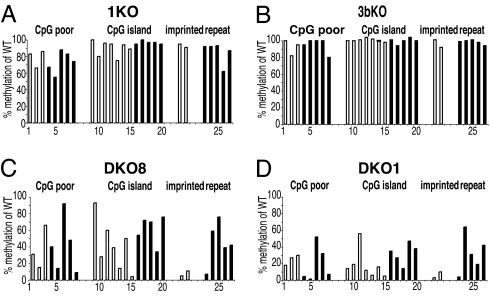
Methylation of various regions in HCT116 WT and knockout cells. Methylation levels (average of two to three CpG sites at each locus) were determined by Ms-SNuPE analyses and graphed as a percentage of WT methylation levels. Open bars represent promoter regions, and filled bars indicate nonpromoter regions. The numbering of regions is as follows. CpG-poor regions: 1, RUNX3 promoter; 2, MAGE A1 promoter; 3, NANOG promoter; 4, p16 intron 1; 5, PAX3 intron; 6, RUNX1 intron; 7, BDNF intron; 8, PCNA intron. CpG islands: 9, p16 promoter; 10, ENDRB promoter; 11, ATBF1 promoter; 12, XIST promoter; 13, TIMP3 promoter; 14, TPEF promoter; 15, DNMT3a2 promoter; 16, microRNA-127; 17, p16 exon 1; 18, p16 intron 4; 19, M4-4 single-copy sequence, chromosome 16q22; 20, p16 exon 2. Imprinted regions: 21, H19; 22, SNRPN. Repeats: 23, D4Z4; 24, p53-Alu; 25, p16-Alu; 26, LINE global; 27, Alu global.
Sequence Specificity of Residual Methylation.
We next measured the residual methylation within CpG-poor regions and CpG islands of the genome and separated out the analysis of imprinted loci and repeat sequences in the different HCT116 cell lines to study whether WT or truncated DNMT1 was required for the maintenance of methylation at specific loci (Fig. 5). Quantitative Ms-SNuPE analyses of specific CpG-poor regions in the DNMT1−/− cells showed that these sequences lost ≈30% of their methylation in the hypomorphic cells independently of whether they were located within the promoters or transcribed regions of genes (Fig. 5A, nos. 1–8). On the other hand, CpG islands were less sensitive to DNMT1 depletion, and most of these islands were maintained at ≈90% of the level found in the WT cells (Fig. 5A, nos. 9–20). Once again, there were no clear differences in the decreased methylation levels within promoters relative to nonpromoter regions. The imprinted loci examined were hardly affected by depletion (Fig. 5A, nos. 21 and 22), and the methylation of repeats, including long interspersed nuclear elements and Alus in general and specific Alu sequences located within genes and the subtelomeric region D4Z4, showed an ≈10% decrease in DNA methylation (Fig. 5A, nos. 23–27). These data were quite different from data obtained from studies in mice (2, 3), indicating a potential role of the truncated protein in maintaining the methylation of certain regions. Overall, DNMT1 appeared to play a more prominent role in maintaining the methylation of CpG-poor regions rather than CpG island regions, because the decrease in methylation was significant in CpG-poor regions as compared with CpG islands (P = 0.003).
Interestingly, there was little effect of knocking out DNMT3b with respect to the level of residual methylation in any of the sequences analyzed (Fig. 5B). Of particular interest was the fact that D4Z4, a subtelomeric repeat, was hardly affected by disruption of this enzyme even though it was shown to be demethylated in patients with ICF syndrome (immunodeficiency, centromeric instability, and facial anomalies syndrome), which is characterized by mutations in DNMT3b (22).
By far the most dramatic effects on residual DNA methylation were seen in the two DKO strains (Fig. 5 C and D). Interestingly, the amount of residual truncated DNMT1 protein seemed to correlate with the level of residual DNA methylation, in such a way that the DKO8 cells, which expressed ≈2-fold the amount of the truncated DNMT1 protein as the DKO1 cells, also retained an overall higher level of DNA methylation at most loci examined, which has been observed previously for the total methylation content of these cells (12) (compare Fig. 5 C and D). It has been reported previously that the DKO1 cells lose ≈95% of their 5-methylcytosine content (12), and a methylation screen revealed a massive loss in hypermethylation of CpG islands in the same cells (23). Although we observed a loss in methylation both in CpG-poor regions and CpG islands, we also detected several regions that had still maintained up to 50% of WT methylation levels in DKO1 cells and up to 90% in the higher DNMT1-expressing DKO8 cells (Fig. 5 C and D). Of particular interest was the complete demethylation of the CpG island promoter of DNMT3a2 (Fig. 5, no. 15), a DNMT3a variant, which is expressed predominantly during embryogenesis (24) and which might be expected to be up-regulated in DKO cells. As had been reported earlier (12, 25), we found that imprinted regions and repetitive elements such as D4Z4 were strongly demethylated in DKO cells, although specific Alu sequences located in the p53 and p16 genes retained substantial residual methylation. Overall, the methylation pattern in these cells, which was maintained predominantly by DNMT3a together with the truncated DNMT1, was determined by factors other than the sequence itself. The correlation between the amount of residual DNMT1 protein and the level of methylation retained in the DKO cells suggests a strong impact of the truncated protein on maintenance of methylation at most loci tested. The methylation of regions that lost all of their methylation in DKO cells might have been maintained by the cooperative action of both WT DNMT1 and DNMT3b, as suggested before (12).
Kinetics of Methylation of Newly Synthesized DNA.
Methylation of newly synthesized DNA has to be synchronized with the process of DNA replication and chromatin assembly and is an inherently complex process. We used BrdU pulse–chase experiments to follow the kinetics of methylation of newly synthesized DNA as described in ref. 20.
The methylation of a CpG-poor region (RUNX1) was almost complete after a 30-min pulse with BrdU in both WT and DNMT3b−/− cells (Fig. 6A). In contrast, in cells lacking a fully functional DNMT1 protein, the methylation of this region was substantially delayed with respect to DNA synthesis in both DNMT1−/− and DKO8 cells. Interestingly, the delay was greater in the DNMT1−/− cells compared with the DKO8 cells, which might have been due to the different levels of the truncated protein present in the two cell lines. Similarly, the methylation of the subtelomeric D4Z4 repeat sequence, which is embedded in a CpG island, showed substantial delays in the DNMT1−/− cells, whereas the sequence was almost completely methylated immediately after replication in the WT and DNMT3b−/− cells (Fig. 6B). Because this region was not methylated in the DKO8 cells, no change in methylation was seen after DNA synthesis. Therefore, in cells lacking a fully functional DNMT1, neither the RUNX1 nor the D4Z4 region acquired its full level of methylation until a considerable time after the DNA had left the replication fork. We interpret this result to suggest that, in the absence of an adequate level of DNMT1 at the replication fork, because of the lack of the PCNA interaction domain, the truncated enzyme completes the methylation process on DNA, which is already assembled into chromatin.
Fig. 6.
Methylation kinetics of newly synthesized DNA. The different cell lines were pulsed for 1 h with BrdU and subsequently chased with thymidine for 0, 15, or 30 min or 8 h. DNA that had incorporated BrdU was isolated by anti-BrdU immunoprecipitation. Methylation levels were determined by Ms-SNuPE analyses at the indicated time points. (A) Methylation kinetics of a CpG-poor region within the RUNX1 intron. (B) Methylation levels of the CpG-rich subtelomeric repeat D4Z4 at different time points of the pulse–chase.
Remethylation of Target Regions After 5-aza-CdR Treatment.
We next treated the different cell lines for a 24-h period with the demethylating agent 5-aza-CdR to depress the methylation level and then grew the cells in the absence of further drug treatment and measured the kinetics of remethylation. We examined the D4Z4 and p16 exon 2 regions before and after drug treatment by genomic bisulfite sequencing to assess in a precise manner how the methylation patterns were reestablished (Fig. 7). Both WT and DNMT1−/− cells showed almost complete methylation of the D4Z4 sequence before treatment, with very substantial demethylation being induced 4 days after exposure to 0.3 μM 5-aza-CdR (Fig. 7A). The methylation increased 32 days after treatment in the WT cells but was far more sporadic than that seen in the untreated controls, showing that it was difficult for this region to regain methylation once it had been removed. In the DNMT1 knockout cells, however, many copies of the D4Z4 sequence examined remained completely unmethylated even 32 days after treatment. The fact that some strands were fully methylated 32 days after treatment in the DNMT1−/− cells suggests that the methylation in this particular sequences was inherited and indeed copied by some process. The slow remethylation in DNMT1 WT cells as compared with the almost complete lack of remethylation in the DNMT1−/− cells suggests that full-length DNMT1 might be able to bring about some sporadic de novo methylation in DNMT1 WT cells, whereas the truncated protein has lost this ability. Similarly, almost complete methylation was seen in untreated WT or DNMT1 knockout cells at the p16 exon 2 region (Fig. 7B). Substantial demethylation was apparent shortly after drug treatment, and almost complete remethylation was present 32 days after treatment in the WT cells. Cells lacking a full-length DNMT1 protein also showed substantial remethylation, suggesting that this region was maintained by the truncated DNMT1 protein, perhaps in addition to another protein such as DNMT3a. It also appeared that the single-knockout cells were demethylated to a higher degree after 5-aza-CdR treatment on day 4 at both loci tested, which might account to some extent for the lower levels of methylation in the single-knockout cells at day 32.
Fig. 7.
Remethylation after 5-aza-CdR treatment. HCT116 WT and knockout cells were treated with 0.3 μM 5-aza-CdR for 24 h and released from the drug thereafter. Methylation levels were determined by bisulfite sequencing in untreated cells and on days 4 and 32 after treatment. Each line with circles indicates an individual DNA molecule. Open circles represent unmethylated cytosines, and filled circles represent methylated cytosines within a CpG dinucleotide context. (A) Bisulfite sequencing of D4Z4. (B) Sequencing results for a p16 exon 2 region.
We also analyzed the kinetics of remethylation of the D4Z4 and p16 exon 2 region for 50 days after 5-aza-CdR treatment by quantitative Ms-SNuPE (Fig. 8, which is published as supporting information on the PNAS web site). As seen before with bisulfite sequencing, the remethylation of D4Z4 occurred very slowly after treatment in HCT116 WT cells. DNMT3b−/− cells showed remethylation kinetics similar to WT cells, whereas remethylation was completely inhibited in DNMT1−/− cells, indicating a strong dependency of this region on WT DNMT1. On the other hand, p16 exon 2 was remethylated rapidly and with similar kinetics both in DNMT1 WT and knockout cell lines, most likely because of a strong influence of DNMT3a on this region.
Discussion
The generation of HCT116 colon cancer cells with disruption of DNMT1, DNMT3b, or both has been an important step in elucidating the role of DNMTs in cancer cells. The finding that unique truncated DNMT1 isoforms, which were generated by the knockout strategy (11, 12), are expressed in all independent DNMT1 and DKO cell lines suggests that DNMT1 expression may in fact be essential for cell survival. We detected transcripts with deletions of exons 2–5 in all DNMT1 knockout cell lines. Interestingly, these transcripts are very similar to a naturally occurring, alternative DNMT1 mRNA (GenBank accession no. AF180682), which splices from exon 1 to exon 5 of WT DNMT1, although no corresponding protein has been described as yet. Previous studies have demonstrated that a deletion of up to 580 aa from the N terminus of human DNMT1 did not inhibit its methyltransferase activity in vitro (26). It will be interesting to test in the future whether these transcripts are present and translated into a protein in a natural setting in vivo.
The slightly smaller protein present in DNMT1−/− and DKO cells corresponded to the size predicted by the mRNA. The deletion covered most of the DMAP1 interaction domain, a region that is also deleted in the murine oocyte-specific isoform Dnmt1o. Deletion of this region produced no abnormal phenotype in mice, which expressed the shortened protein in all somatic tissues instead of full-length Dnmt1 (27). In fact, this protein could maintain normal levels of DNA methylation and appeared to be more stable than the full-length form of Dnmt1 containing the DMAP1 interaction domain (27).
The truncated protein showed preferential DNMT activity in vitro against a hemimethylated target, suggesting that it might also be active in vivo. However, the fact that a substantial increase in the percentage of hemimethylation was seen in the knockout cells suggests that there was not sufficient DNMT1 protein to complete maintenance methylation. The deletion of the PCNA interaction domain in the hypomorphic protein could account for a failure of tethering the truncated protein to the replication machinery and for a loss of immediate methylation of hemimethylated DNA (14, 18, 20). Indeed, a considerable delay in methylation was seen, suggesting that the reduced amount of non-PCNA-targeted enzyme acted over a longer period to achieve methylation. Interestingly, ≈50% of the newly synthesized DNA was methylated immediately after replication (time 0), which might be due to the fact that the truncated protein still possesses a replication foci-targeting domain (amino acids 331–550 of WT DNMT1) and therefore might be targeted to regions of active replication. The observed delay could therefore simply be a consequence of limited amounts of DNMT1 protein, which is also strengthened by the fact that the DKO8 cells, with double the amount of truncated DNMT1 compared with the DNMT1−/− cells, show higher levels of methylation immediately after replication.
In their original work, Rhee et al. (12) described eight independent DKO clones, seven of which grew very slowly and had lost ≈95% of their 5-methylcytosine content. However, one clone, DKO8, retained higher methylation levels and proliferated more rapidly. Our analyses revealed that this clone expresses higher levels of the truncated DNMT1 protein, both at the RNA and protein levels, and retains considerably higher levels of DNA methylation at various loci tested. Likewise, further depletion of the truncated protein by RNAi caused a measurable decrease in methylation of various loci in the DNMT1−/− cells and a decrease in cell viability in both WT and knockout cell lines. This finding suggests that a certain threshold of DNMT1 protein is necessary to confer survival of the cells. The DKO cells have been selected for survival with low levels of DNMT1 and might also compensate the loss with expression of a different methyltransferase. A candidate would be the embryonic isoform DNMT3a2, which is expressed from a CpG island that was methylated in all cell lines except the DKOs. Taken together, the data suggest to us that the truncated protein is indeed functional in vivo and might help to maintain DNA methylation and also play a role in cell proliferation or even survival.
Although DNMT1 is viewed as a maintenance enzyme, it has been speculated that it might confer some de novo methyltransferase activity (1, 28). In vitro, DNMT1 shows a clear preference for hemimethylated DNA; nevertheless, it can methylate completely unmethylated CpG pairs with a higher affinity than the classic de novo enzymes DNMT3a and DNMT3b (29, 30). Yet this finding is still questionable in vivo. Our data suggest that DNMT1 can confer some de novo activity against a demethylated subtelomeric repeat, D4Z4. After treatment with 5-aza-CdR, WT and DNMT3b−/− cells slowly regain their methylation, whereas DNMT1−/− cells are impaired in their remethylation. This failure could be caused by loss of cooperativity with DNMT3b or by direct loss of de novo activity. Because DNMT3b cells show the same remethylation kinetics as WT cells, we speculate that the latter might be the case.
In summary, the knockout cells generated by Rhee et al. (11, 12) are useful tools for the study of DNMT function in cancer cells. However, the fact that they are hypomorphs for DNMT1 masks the real phenotype that DNMT1 disruption might have, and it might be anticipated that a complete null will not produce viable cells, as seen before in mouse studies.
Materials and Methods
Tissue Culture and Drug Treatment.
HCT116 cells were kept in McCoy's 5A modified medium supplemented with 10% FCS. For 5-aza-CdR treatment, cells were incubated with 0.3 μM 5-aza-CdR for 24 h; then, the medium was changed, and cells were harvested at the indicated time points.
RNA Isolation and RT-PCR.
Total RNA was isolated by using TRIzol reagent (Invitrogen, Carlsbad, CA). Total RNA (2–5 μg) was transcribed with SuperScript III (Invitrogen). Primer sequences can be found in Table 1, which is published as supporting information on the PNAS web site.
Protein Extraction and Western Blotting.
Nuclear extracts were prepared as described in ref. 31; see also Supporting Materials and Methods, which is published as supporting information on the PNAS web site. Antibodies used were DNMT1 (Santa Cruz Biotechnology, Santa Cruz, CA), histone deacetylase HDAC1 (Upstate Biotechnology, Lake Placid, NY), and PCNA (Santa Cruz Biotechnology). For quantitation of Western blots, we used a Fluor-S MultiImager (Bio-Rad, Hercules, CA).
In Vitro DNMT1 Assay.
Immunoprecipitated DNMT1 was incubated with 1 μl (30 ng) of hemimethylated or unmethylated oligonucleotide (corresponding to an endogenous p16 sequence), 0.5 μl of BSA, and 0.25 μl of S-adenosylmethionine for 3 h at 37°C. DNA methylation was determined after bisulfite conversion and PCR amplification by Ms-SNuPE analysis. A detailed protocol for the assay can be found in Supporting Materials and Methods.
Quantitation of DNA Methylation Levels.
Genomic DNA was bisulfite-converted by using high-speed conversion as described in ref. 32. Methylation levels were measured by Ms-SNuPE (33) or bisulfite sequencing (see details in Supporting Materials and Methods).
DNMT1 RNAi.
RNAi experiments were performed by transiently transfecting 100 nM, 21-nt-long double-stranded RNA oligonucleotides (Silencer Custom siRNA; Ambion, Austin, TX) into the different HCT116 cell lines by using Oligofectamine (Invitrogen). (For details, see Supporting Materials and Methods).
Hemimethylation Assay.
Hemimethylation was measured as described in ref. 20.
BrdU Pulse–Chase.
BrdU pulse–chase experiments were performed as described in ref. 20. For details, also see Supporting Materials and Methods.
Statistics.
For details about statistical calculations, see Supporting Materials and Methods.
Supplementary Material
Acknowledgments
We thank Drs. Bert Vogelstein (Howard Hughes Medical Institute, The Johns Hopkins University, Baltimore, MD) and Stephen Baylin (The Johns Hopkins University) for kindly providing the HCT116 cell lines and constructive discussions and Dong Yun Yang (Norris Comprehensive Cancer Center Statistics Core Facility) for performing statistical analyses. This work was supported by National Institutes of Health Grants R01 CA 82422 and R01 CA 83867, National Institutes of Health Training Grant 5251143701 (to T.W.H.L.), and the Max Kade Foundation (G.E.).
Abbreviations
- Dnmt
DNA methyltransferase
- DKO
double knockout
- 5-aza-CdR
5-aza-2′deoxycytidine
- PCNA
proliferating cell nuclear antigen
- DMAP1
DNMT1-associated protein
- Ms-SNuPE
methylation-sensitive single-nucleotide primer extension.
Footnotes
The authors declare no conflict of interest.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Goll MG, Bestor TH. Annu Rev Biochem. 2005;74:481–514. doi: 10.1146/annurev.biochem.74.010904.153721. [DOI] [PubMed] [Google Scholar]
- 2.Li E, Bestor TH, Jaenisch R. Cell. 1992;69:915–926. doi: 10.1016/0092-8674(92)90611-f. [DOI] [PubMed] [Google Scholar]
- 3.Li E, Beard C, Forster AC, Bestor TH, Jaenisch R. Cold Spring Harbor Symp Quant Biol. 1993;58:297–305. doi: 10.1101/sqb.1993.058.01.035. [DOI] [PubMed] [Google Scholar]
- 4.Jackson-Grusby L, Beard C, Possemato R, Tudor M, Fambrough D, Csankovszki G, Dausman J, Lee P, Wilson C, Lander E, Jaenisch R. Nat Genet. 2001;27:31–39. doi: 10.1038/83730. [DOI] [PubMed] [Google Scholar]
- 5.Laird PW, Jackson-Grusby L, Fazeli A, Dickinson SL, Jung WE, Li E, Weinberg RA, Jaenisch R. Cell. 1995;81:197–205. doi: 10.1016/0092-8674(95)90329-1. [DOI] [PubMed] [Google Scholar]
- 6.Robert MF, Morin S, Beaulieu N, Gauthier F, Chute IC, Barsalou A, MacLeod AR. Nat Genet. 2003;33:61–65. doi: 10.1038/ng1068. [DOI] [PubMed] [Google Scholar]
- 7.Leu YW, Rahmatpanah F, Shi H, Wei SH, Liu JC, Yan PS, Huang TH. Cancer Res. 2003;63:6110–6115. [PubMed] [Google Scholar]
- 8.Suzuki M, Sunaga N, Shames DS, Toyooka S, Gazdar AF, Minna JD. Cancer Res. 2004;64:3137–3143. doi: 10.1158/0008-5472.can-03-3046. [DOI] [PubMed] [Google Scholar]
- 9.Ting AH, Jair KW, Suzuki H, Yen RW, Baylin SB, Schuebel KE. Nat Genet. 2004;36:582–584. doi: 10.1038/ng1365. [DOI] [PubMed] [Google Scholar]
- 10.Ting AH, Jair KW, Schuebel KE, Baylin SB. Cancer Res. 2006;66:729–735. doi: 10.1158/0008-5472.CAN-05-1537. [DOI] [PubMed] [Google Scholar]
- 11.Rhee I, Jair KW, Yen RW, Lengauer C, Herman JG, Kinzler KW, Vogelstein B, Baylin SB, Schuebel KE. Nature. 2000;404:1003–1007. doi: 10.1038/35010000. [DOI] [PubMed] [Google Scholar]
- 12.Rhee I, Bachman KE, Park BH, Jair KW, Yen RW, Schuebel KE, Cui H, Feinberg AP, Lengauer C, Kinzler KW, et al. Nature. 2002;416:552–556. doi: 10.1038/416552a. [DOI] [PubMed] [Google Scholar]
- 13.Rountree MR, Bachman KE, Baylin SB. Nat Genet. 2000;25:269–277. doi: 10.1038/77023. [DOI] [PubMed] [Google Scholar]
- 14.Chuang LS, Ian HI, Koh TW, Ng HH, Xu G, Li BF. Science. 1997;277:1996–2000. doi: 10.1126/science.277.5334.1996. [DOI] [PubMed] [Google Scholar]
- 15.Bouchard J, Momparler RL. Mol Pharmacol. 1983;24:109–114. [PubMed] [Google Scholar]
- 16.Taylor SM, Constantinides PA, Jones PA. Curr Top Microbiol Immunol. 1984;108:115–127. doi: 10.1007/978-3-642-69370-0_8. [DOI] [PubMed] [Google Scholar]
- 17.Santi DV, Norment A, Garrett CE. Proc Natl Acad Sci USA. 1984;81:6993–6997. doi: 10.1073/pnas.81.22.6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iida T, Suetake I, Tajima S, Morioka H, Ohta S, Obuse C, Tsurimoto T. Genes Cells. 2002;7:997–1007. doi: 10.1046/j.1365-2443.2002.00584.x. [DOI] [PubMed] [Google Scholar]
- 19.Jeltsch A. Curr Top Microbiol Immunol. 2006;301:203–225. doi: 10.1007/3-540-31390-7_7. [DOI] [PubMed] [Google Scholar]
- 20.Liang G, Chan MF, Tomigahara Y, Tsai YC, Gonzales FA, Li E, Laird PW, Jones PA. Mol Cell Biol. 2002;22:480–491. doi: 10.1128/MCB.22.2.480-491.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng JC, Weisenberger DJ, Gonzales FA, Liang G, Xu GL, Hu YG, Marquez VE, Jones PA. Mol Cell Biol. 2004;24:1270–1278. doi: 10.1128/MCB.24.3.1270-1278.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kondo T, Bobek MP, Kuick R, Lamb B, Zhu X, Narayan A, Bourc'his D, Viegas-Pequignot E, Ehrlich M, Hanash SM. Hum Mol Genet. 2000;9:597–604. doi: 10.1093/hmg/9.4.597. [DOI] [PubMed] [Google Scholar]
- 23.Paz MF, Wei S, Cigudosa JC, Rodriguez-Perales S, Peinado MA, Huang TH, Esteller M. Hum Mol Genet. 2003;12:2209–2219. doi: 10.1093/hmg/ddg226. [DOI] [PubMed] [Google Scholar]
- 24.Chen T, Ueda Y, Xie S, Li E. J Biol Chem. 2002;277:38746–38754. doi: 10.1074/jbc.M205312200. [DOI] [PubMed] [Google Scholar]
- 25.Cui H, Onyango P, Brandenburg S, Wu Y, Hsieh CL, Feinberg AP. Cancer Res. 2002;62:6442–6446. [PubMed] [Google Scholar]
- 26.Pradhan S, Esteve PO. Biochemistry. 2003;42:5321–5332. doi: 10.1021/bi034160+. [DOI] [PubMed] [Google Scholar]
- 27.Ding F, Chaillet JR. Proc Natl Acad Sci USA. 2002;99:14861–14866. doi: 10.1073/pnas.232565599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hermann A, Gowher H, Jeltsch A. Cell Mol Life Sci. 2004;61:2571–2587. doi: 10.1007/s00018-004-4201-1. [DOI] [PubMed] [Google Scholar]
- 29.Fatemi M, Hermann A, Pradhan S, Jeltsch A. J Mol Biol. 2001;309:1189–1199. doi: 10.1006/jmbi.2001.4709. [DOI] [PubMed] [Google Scholar]
- 30.Okano M, Xie S, Li E. Nat Genet. 1998;19:219–220. doi: 10.1038/890. [DOI] [PubMed] [Google Scholar]
- 31.Fatemi M, Pao MM, Jeong S, Gal-Yam EN, Egger G, Weisenberger DJ, Jones PA. Nucleic Acids Res. 2005;33:e176. doi: 10.1093/nar/gni180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shiraishi M, Hayatsu H. DNA Res. 2004;11:409–415. doi: 10.1093/dnares/11.6.409. [DOI] [PubMed] [Google Scholar]
- 33.Gonzalgo ML, Jones PA. Nucleic Acids Res. 1997;25:2529–2531. doi: 10.1093/nar/25.12.2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.