Abstract
Fibrocytes are a distinct population of bloodborne cells that share markers of leukocytes as well as mesenchymal cells. We hypothesized that CCR7-positive fibrocytes migrate into the kidney in response to secondary lymphoid tissue chemokine (SLC/CCL21) and contribute to renal fibrosis. To investigate this hypothesis, renal fibrosis was induced by unilateral ureteral obstruction in mice. A considerable number of fibrocytes dual-positive for CD45 and type I collagen (ColI) or CD34 and ColI infiltrated the interstitium, reaching a peak on day 7. Most fibrocytes were positive for CCR7, and CCL21/CCR7 blockade reduced the number of infiltrating fibrocytes. CCL21 and MECA79 dual-positive vessels were also detected in the interstitium. The blockade of CCL21/CCR7 signaling by anti-CCL21 antibodies reduced renal fibrosis, which was confirmed by a decrease in fibrosis in CCR7-null mice with concomitant reduction in renal transcripts of pro α1 chain of ColI and TGF-β1. The number of F4/80-positive macrophages decreased along with renal transcripts of monocyte chemoattractant protein 1 (MCP-1/CCL2) after the blockade of CCL21/CCR7 signaling. These findings suggest that CCR7-positive fibrocytes infiltrate the kidney via CCL21-positive vessels, thereby contributing to the pathogenesis of renal fibrosis. Thus, the CCL21/CCR7 signaling of fibrocytes may provide therapeutic targets for combating renal fibrosis.
Keywords: kidney, CD45
Fibrosis is a hallmark of progressive organ diseases, resulting in organ failure. Despite varied etiologies, renal diseases progress to end-stage renal failure characterized by glomerulosclerosis and interstitial fibrosis (1, 2). In addition, renal fibrosis determines the prognosis of renal diseases independent of their etiologies (3, 4). The histological picture of renal fibrosis is characterized by tubular atrophy and dilation, interstitial leukocyte infiltration, accumulation of fibroblasts, and increased interstitial matrix deposition (5). Currently, resident fibroblasts, epithelial–mesenchymal transition (EMT)-derived fibroblasts/myofibroblasts, and monocytes/macrophages are thought to be participants in the pathogenesis of renal fibrosis (6–9). However, the precise pathogenic mechanisms of renal fibrosis remain to be determined.
A circulating bone marrow-derived population of fibroblast-like cells (termed fibrocytes) was first identified a decade ago (10). Fibrocytes comprise a minor fraction of the circulating pool of leukocytes (<1%) and share the markers of leukocytes (e.g., CD45 and CD34) as well as mesenchymal cells [e.g., type I collagen (ColI) and fibronectin] (11, 12). Fibrocytes are present in experimental fibrosis associated with conditions such as pulmonary fibrosis, bronchial asthma, and skin wounds (13–15). Furthermore, fibrocytes are detected in human fibrosing diseases including nephrogenic fibrosing dermopathy and burns (16, 17). CD34-positive spindle cells are also reported to be present in the interstitium in patients with glomerulonephritis (18). In addition, fibrocytes express chemokine receptors such as CCR7, CXCR4, and CCR2 (12, 13). Recent studies demonstrated that chemokine receptors on fibrocytes are involved in the recruitment of circulating fibrocytes to sites of fibrosis (12, 13). However, the roles of fibrocytes in the pathogenesis of renal fibrosis and their trafficking into diseased kidneys have not been fully investigated.
A ligand for CCR7, secondary lymphoid tissue chemokine (SLC/CCL21), is a member of the CC chemokine family, the first two cysteine residues of which are adjacent to each other. CCL21 contains six cysteines and is a potent chemoattractant for T cells, B cells, and dendritic cells (19–21). In addition, CCL21 also acts as a chemotactic stimulus for fibrocytes (15). In humans as well as in mice, CCL21 is constitutively abundant in lymphoid tissues, particularly in the lymph nodes and spleen. It is of note that CCL21 is also expressed at lower levels in some nonlymphoid tissues, including the lung (20). CCL21 expression has been shown to be localized in high endothelial venules (HEVs) in lymph nodes under physiological conditions (20) as well as in nonlymphoid tissues under inflammatory conditions (22).
These findings prompted us to examine whether the contribution of fibrocytes to renal fibrosis depends on CCL21/CCR7 signaling. To address this issue, we evaluated renal fibrosis induced by unilateral ureteral obstruction (UUO), a well known renal fibrosis model (23, 24), in mice treated with specific neutralizing anti-CCL21 antibodies and in CCR7-null mice. We report here that fibrocytes were pivotally involved in the pathogenesis of renal fibrosis and that blockade of CCL21/CCR7 signaling represented a beneficial therapeutic approach to progressive fibrosis of the kidney in this mouse model.
Results
Effect of Inhibition of CCL21/CCR7 Signaling on Renal Fibrosis and ColI Expression.
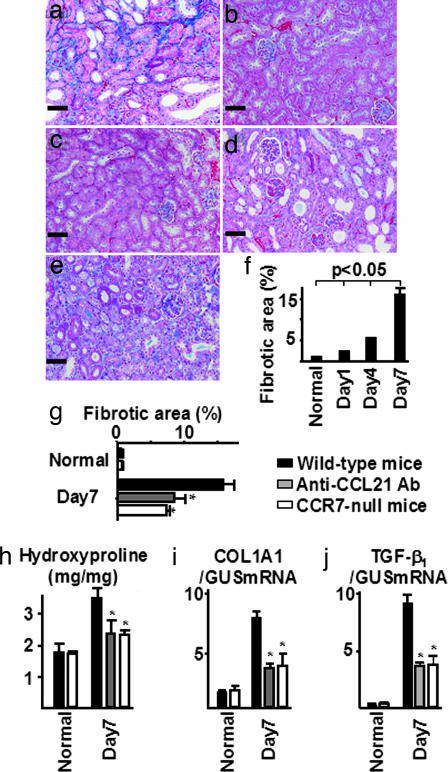
To determine the impact of CCL21/CCR7 signaling on progressive renal interstitial injury, interstitial fibrotic areas expressed as blue on Mallory–Azan-stained histological samples were examined by computer analysis. In wild-type mice, ureteral ligation caused progressive renal fibrosis in obstructed kidneys (Fig. 1a and f). In contrast, the mean interstitial fibrosis, expressed as percentage per square millimeter, was reduced in mice treated with anti-CCL21 antibodies and in CCR7-null mice compared with that in wild-type mice 7 days after UUO (Fig. 1 d, e, and g). In addition, the amount of hydroxyproline was evaluated for a more quantitative measurement to determine tissue collagen content. Similar to the computer-assisted measurement of renal fibrotic area, total tissue collagen content was significantly reduced in mice treated with anti-CCL21 antibodies and in CCR7-null mice (Fig. 1h). Furthermore, ureteral ligation enhanced the pro α1 chain of ColI (COL1A1) mRNA expression in wild-type mice, which was significantly reduced by blockade of CCL21/CCR7 signaling (Fig. 1i). Seven days after ureteral ligation, transcripts of TGF-β1, which is a profibrotic molecule, were up-regulated in wild-type mice, whereas the levels were reduced in mice treated with anti-CCL21 antibodies as well as in CCR7-null mice (Fig. 1j). Thus, CCL21/CCR7 signaling appears to play a role in the pathogenesis of renal fibrosis.
Fig. 1.
Inhibition of CCL21/CCR7 signaling reduced renal fibrosis. In wild-type mice, ureteral ligation caused progressive renal fibrosis in obstructed kidneys (a and f) compared with that in normal kidneys (b) and in contralateral kidneys (c). In contrast, the mean interstitial fibrosis was reduced in obstructed kidneys treated with anti-CCL21 antibodies (d) and in CCR7-null mice (e) compared with that in UUO-treated wild-type mice 7 days after UUO (g). Total tissue collagen content (hydroxyproline) was markedly reduced in mice treated with anti-CCL21 antibodies and in CCR7-null mice compared with that in UUO-treated wild-type mice 7 days after UUO (h). The up-regulated mRNA expression of ColI as well as TGF-β1 in diseased kidneys was reduced by CCL21/CCR7 signaling blockade (i and j). Mallory–Azan staining was done at an original magnification of ×200. Values are the mean ± SEM. ∗, P < 0.05 compared with wild-type mice on day 7. (Scale bars: 50 μm.)
Fibrocytes Infiltrated the Kidney After Ureteral Ligation.
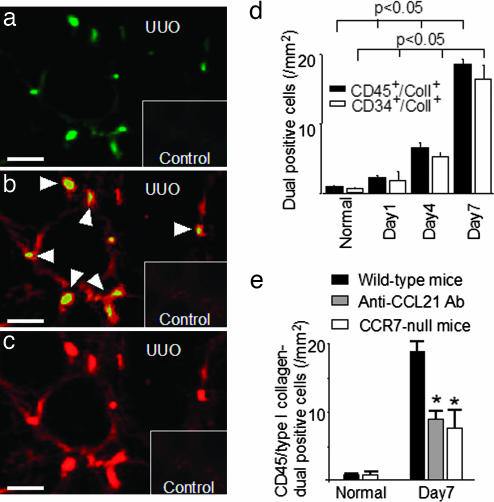
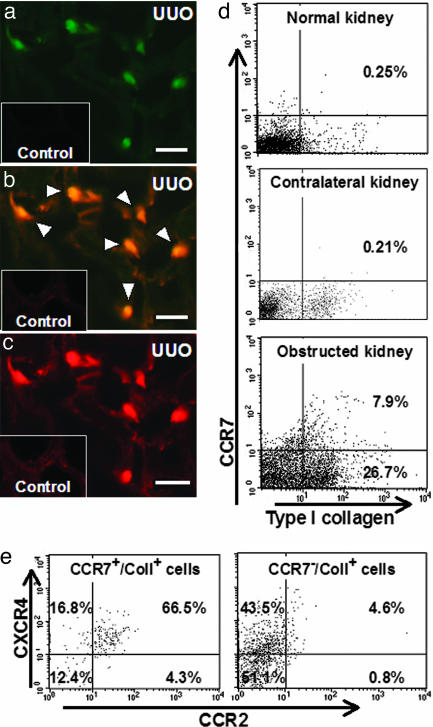
One of the unique characteristics of fibrocytes is the simultaneous expression of both leukocyte markers, such as CD45 and CD34, and ColI (12). Therefore, these cells are identified in tissue samples by double immunohistochemistry using specific antibodies against CD45 and ColI. In wild-type mice with ureteral ligation, CD45 and ColI dual-positive fibrocytes (CD45+/ColI+) infiltrated the interstitium, especially the corticomedullary regions (Fig. 2a–c). The number of infiltrating fibrocytes increased with the progression of fibrosis after ureteral ligation, reaching a peak on day 7 (19.2 ± 2.5 per square millimeter; n = 7) (Fig. 2d). To further verify the existence of fibrocytes, dual immunostainings of CD34 and ColI were also performed. The infiltration of CD34 and ColI dual-positive fibrocytes was observed in the interstitium and correlated with disease progression as determined by CD45 and ColI dual immunostainings (Fig. 2d). In addition, dual-labeling was performed by immunofluorescence immunohistochemistry to determine the presence of CCR7-expressing fibrocytes. Expressions of both CCR7 and ColI were assessed, because CCR7-positive T cells, B cells, and dendritic cells are not capable of producing ColI. CCR7-expressing fibrocytes positive for both CCR7 and ColI (CCR7+/ColI+) were detected in diseased kidneys 7 days after UUO in wild-type mice (Fig. 3a–c). Furthermore, flow cytometry analyses of renal cells isolated from normal kidneys, obstructed kidneys, and contralateral kidneys 7 days after UUO were performed to determine the characteristics of infiltrating fibrocytes. In wild-type mice, the ratio of CCR7+/ColI+ cells in obstructed kidneys was increased to 7.9% of the total isolated renal cells compared with that in normal kidneys (0.25%) and contralateral kidneys (0.21%) (Fig. 3d). Of these CCR7-expressing fibrocytes in obstructed kidneys, 66.5% of cells were CXCR4+/CCR2+, 16.8% of cells were CXCR4+/CCR2−, 4.3% of cells were CXCR4−/CCR2+, and 12.4% of cells were CXCR4−/CCR2− (Fig. 3e). In addition, the percentage of CCR7-negative collagen-producing cells (CCR7−/ColI+) increased to 26.7% of the total isolated renal cells from obstructed kidneys (Fig. 3d). Furthermore, 48.9% of these CCR7−/ColI+ cells (13.0% of total isolated cells) were thought to be CCR2- and/or CXCR4-expressing fibrocytes (CCR7-nonexpressing fibrocytes) (Fig. 3e). As a result, 37.8% of infiltrating fibrocytes expressed CCR7 (number of CCR7+/ColI+ divided by the number of CCR7+ or CXCR4+ or CCR2+/ColI+).
Fig. 2.
Fibrocytes infiltrated the kidney after ureteral ligation. In wild-type mice, CD45+/ColI+ fibrocytes infiltrated the interstitium, especially the corticomedullary regions after ureteral ligation. (a) CD45. (b) Merge. (c) ColI. Arrowheads indicate CD45+/ColI+ fibrocytes. The number of infiltrating fibrocytes (CD45+/ColI+ and CD34+/ColI+) increased with the progression of fibrosis after ureteral ligation, reaching a peak on day 7 (d). In contrast, the number of infiltrating fibrocytes (CD45+/ColI+) was reduced in mice treated with anti-CCL21 antibodies (8.8 ± 1.8 per square millimeter; n = 7) and in CCR7-null mice (7.5 ± 1.4 per square millimeter; n = 7) compared with that in wild-type mice (19.2 ± 2.5 per square millimeter; n = 7) 7 days after ureteral ligation (e). Values are the mean ± SEM. UUO, UUO-treated wild-type mice; Control, untreated wild-type mice. ∗, P < 0.05 compared with wild-type mice on day 7. (Scale bars: 50 μm.)
Fig. 3.
Infiltrating fibrocytes were positive for CCR7 as well as CXCR4 and CCR2. Immunofluorescence immunohistochemistry and flow cytometry were performed to determine the presence of CCR7 on infiltrating fibrocytes by detecting CCR7 and ColI. Dual-positive cells for CCR7 and ColI were detected in diseased kidneys 7 days after ureteral ligation in wild-type mice in immunohistochemical studies. (a) CCR7. (b) Merge. (c) ColI. Arrowheads indicate CCR7+/ColI+ fibrocytes. In wild-type mice, flow cytometry analyses demonstrated that the proportion of CCR7+/ColI+ cells in obstructed kidneys was increased to 7.9% of the total isolated renal cells compared with that in normal kidneys (0.25%) and contralateral kidneys (0.21%) (d). Of these CCR7-expressing fibrocytes in obstructed kidneys, 66.5% were CXCR4+/CCR2+, 16.8% were CXCR4+/CCR2−, 4.3% were CXCR4−/CCR2+, and 12.4% were CXCR4−/CCR2− (e Left). In addition, the percentages of CCR7-negative collagen-producing cells (CCR7−/ColI+) rose to 26.7% of the isolated renal cells in obstructed kidneys (d). Of these CCR7−/ColI+ cells, 48.9% (13.0% of total isolated cells) were thought to be CCR2- and/or CXCR4-positive fibrocytes (CCR7-nonexpressing fibrocytes) (e Right). UUO, UUO-treated wild-type mice; Control, untreated wild-type mice. (Scale bars: 50 μm.)
CCL21/CCR7-Dependent Fibrocyte Infiltration in the Kidney.
To elucidate the role of CCL21/CCR7 interaction in the infiltration of fibrocytes into the kidney, anti-CCL21 antibody-treated mice and CCR7-null mice were used. The number of infiltrating fibrocytes (CD45+/ColI+) was reduced both in mice treated with anti-CCL21 antibodies (8.8 ± 1.8 per square millimeter; n = 7) and in CCR7-null mice (7.5 ± 1.4 per square millimeter; n = 7) compared with that in wild-type mice (19.2 ± 2.5 per square millimeter; n = 7) 7 days after UUO (Fig. 2e). It was further noted that the number of CCR7+/ColI+ was also decreased in mice treated with anti-CCL21 antibodies (4.8 + 1.8 per square millimeter; n = 7) (P < 0.05 vs. wild-type) compared with that in wild-type mice (10.2 + 2.5 per square millimeter; n = 7) 7 days after UUO. Furthermore, the infiltration of CCR2+/ColI+ was significantly reduced both in mice treated with anti-CCL21 antibodies (2.9 + 1.1 per square millimeter; n = 7) (P < 0.05 vs. wild-type) and in CCR7-null mice (3.4 + 0.9 per square millimeter; n = 7) (P < 0.05 vs. wild-type) compared with that in wild-type mice (5.6 + 1.3 per square millimeter; n = 7) 7 days after UUO, whereas there was no difference in the number of CXCR4+/ColI+ between wild-type mice (8.4 + 2.3 per square millimeter; n = 7), anti-CCL21 antibody-treated mice (9.3 + 1.7 per square millimeter; n = 7), and CCR7-null mice (8.6 + 3.1 per square millimeter; n = 7).
Detection of CCL21-Positive HEV-Like Vessels in Fibrotic Kidney.
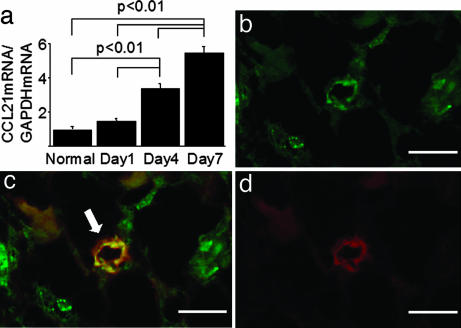
RT-PCR was performed to determine the renal expression of CCL21 during fibrogenesis. The expression of CCL21 mRNA in diseased kidneys was up-regulated with the progression of fibrosis in wild-type mice after ureteral ligation (Fig. 4a). Furthermore, dual immunostainings were performed to determine the CCL21 expression by HEV-like vessels in diseased kidneys. MECA79 antibody was used immunohistochemically to detect HEV-like vessels in the kidney, because the MECA79-reactive antigen is closely associated with the carbohydrate ligands for L-selectin, which are expressed on HEVs in lymphoid tissues and at sites of chronic inflammation (25). In UUO-treated wild-type mice, CCL21 protein colocalized with MECA79-positive vessels in the corticomedullary regions on day 7 (Fig. 4 b–d). The increase in the number of CCL21 and MECA79 dual-positive vessels correlated with the progression of fibrosis after ureteral ligation, reaching a peak on day 7 [7.5 ± 1.1/all fields on day 7 (n = 7) (P < 0.05) vs. 0.3 ± 0.2/all fields on day 0 (n = 7)].
Fig. 4.
CCL21-positive HEV-like vessels were detected in fibrotic kidney. The expression of CCL21 mRNA in diseased kidneys was up-regulated with progression of fibrosis in wild-type mice (a). Furthermore, the colocalization of CCL21 protein with MECA79-positive vessels was detected in the corticomedullary junction on day 7. (b) CCL21. (c) Merge. (d) MECA79. Arrow, CCL21 and MECA79 dual-positive vessel. Values are the mean ± SEM. (Scale bars: 50 μm.)
Effect of Blockade of CCL21/CCR7 Signaling on Expression of Renal Monocyte Chemoattractant Protein-1 (MCP-1/CCL2) and Infiltration of F4/80-Positive Macrophages.
CCL2 is thought to be a profibrotic mediator in various organs (9, 12). Transcription of CCL2 was faintly detected in UUO-untreated kidneys by real-time RT-PCR. Seven days after UUO, the expression of CCL2 mRNA was up-regulated in obstructed kidneys in wild-type mice. In contrast, expression was reduced both in mice treated with anti-CCL21 antibodies and in CCR7-null mice compared with that in UUO-treated wild-type mice (see Fig. 6, which is published as supporting information on the PNAS web site). In addition, the number of infiltrated F4/80-positive macrophages in the interstitium was reduced in mice treated with anti-CCL21 antibodies and in CCR7-null mice 7 days after UUO compared with that in UUO-treated wild-type mice (see Fig. 6). In contrast, the cell number in the glomeruli did not differ at any time point after ureteral ligation (data not shown).
Discussion
In this study we demonstrated that circulating CD45+/ColI+ fibrocytes infiltrated the diseased kidneys in a murine UUO model. CCR7 was also expressed on the infiltrating fibrocytes. In contrast, the inhibition of CCL21/CCR7 signaling reduced the severity of fibrocyte infiltration and the extent of fibrosis in this model. Concomitantly, the blockade of CCL21/CCR7 signaling reduced the renal expression of TGF-β1 and ColI. Thus, we conclude that fibrocytes contribute to renal fibrosis by the production of ColI and that this process requires CCL21/CCR7 signaling.
Recently, it was reported that expressions of certain chemokine receptors, such as CCR7, CXCR4, and CCR2, are detectable on fibrocytes isolated from humans and mice (12, 13). Therefore, flow cytometry analyses were performed in this study to characterize the infiltrating fibrocytes based on expressions of chemokine receptors. In wild-type mice in this study, 37.8% of the infiltrating fibrocytes expressed CCR7 after ureteral ligation. Of these CCR7-expressing fibrocytes, 66.5% of cells were positive for both CXCR4 and CCR2, and 21.1% of cells were positive for either CXCR4 or CCR2. Based on the finding that treatment with anti-CCL21 antibodies or CCR7 deficiency resulted in a >50% reduction in the number of CD45+/ColI+ fibrocytes, CCL21/CCR7 signaling is thought to be the major pathway attracting fibrocytes into the kidney in this particular model. Moreover, blockade of CCL21/CCR7 signaling reduced the number of CCR7-expressing fibrocytes as well as CCR2-expressing fibrocytes in immunohistochemical studies. A recent study reported that CCL2/CCR2 signaling mediated recruitment of CCR2-expressing fibrocytes to the alveolar space after administration of fluorescein isothiocyanate, resulting in pulmonary fibrosis (12). In this study, renal CCL2 expression in obstructed kidneys was also reduced by the CCL21/CCR7 blockade. Fibrocytes have been reported to be capable of producing CCL2 under pathological fibrotic conditions (11). Therefore, inhibiting the infiltration of CCR7-expressing fibrocytes appears to decrease the infiltration of CCR2-expressing fibrocytes through suppression of CCL2 production, thereby contributing to more effective protection from renal fibrosis (Fig. 5). Thus, the CCL21/CCR7 pathway strongly contributes to the trafficking of fibrocytes into the kidney, leading to renal fibrosis. In contrast, the infiltration of CXCR4-positive fibrocytes was not reduced by the blockade of CCL21/CCR7. In another study, CXCR4-positive fibrocytes migrated in response to CXCL12, a ligand for CXCR4, and trafficked to the lungs in a murine model of bleomycin-induced pulmonary fibrosis (13). Furthermore, treatment of bleomycin-exposed animals with specific neutralizing anti-CXCL12 antibodies inhibited infiltration of CXCR4-positive fibrocytes and attenuated lung fibrosis (13). Therefore, these findings suggest that other chemokine/chemokine receptor pathways may also be involved in the recruitment and activation of fibrocytes, resulting in progressive fibrosis. Further studies will be required to elucidate the precise mechanisms of fibrocyte trafficking into target organs.
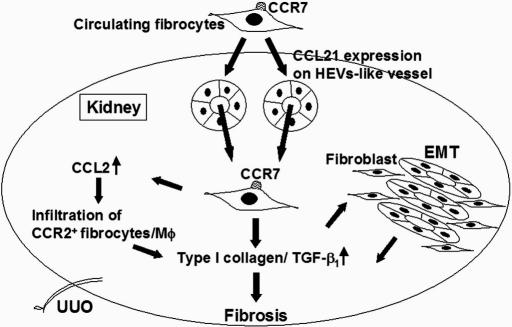
Fig. 5.
Schema for CCL21/CCR7-dependent infiltration and activation of fibrocytes in fibrosis. We propose that CCR7-expressing circulating fibrocytes infiltrate in the kidney via CCL21-positive HEV-like vessels as illustrated. In addition, fibrocytes might be involved in the pathogenesis of fibrosis not only by synthesizing collagen but also by regulating the infiltration and activation of macrophages through CCL2 production. In addition, it is possible that fibrocytes regulate EMT through the production of TGF-β1.
Our study demonstrates that CCL21-positive HEV-like vessels were induced in diseased kidneys, mainly in the corticomedullary regions. The number of CCL21 and MECA79 dual-positive HEV-like vessels as well as renal expression of CCL21 mRNA increased with disease progression after ureteral ligation. HEVs are specialized venules that allow rapid and selective lymphocyte trafficking from the blood into lymph nodes and Peyer’s patches under physiological conditions (26). HEVs express certain chemokines, such as CCL21 (20) and EBI1-ligand chemokine/CCL19 (27), that can activate CCR7-expressing cells. However, HEV-like vessels, which are observed in chronically inflamed nonlymphoid tissues, are thought to play an important role in the pathogenesis of various inflammatory diseases, such as rheumatoid arthritis and Graves’ disease (28, 29). In addition, CCL21 and MECA79 dual-positive vessels were found in synovial tissues from patients with rheumatoid arthritis (30). With regard to human kidney diseases, MECA79-positive HEV-like vessels located at the corticomedullary junction were detected and associated with interstitial leukocyte infiltration in human glomerulonephritis, whereas MECA79-positive vessels were not detected in normal kidneys (31). In this study, the number of infiltrating CCR7-positive fibrocytes was markedly reduced by the blockade of CCL21/CCR7 signaling. Taken together, these findings suggest that CCR7-expressing circulating fibrocytes infiltrate the kidney via CCL21-positive HEV-like vessels as illustrated in Fig. 5, resulting in the contribution to renal fibrosis.
In this study, fibrocytes dual-positive for CCR7 and ColI infiltrated fibrotic kidneys. However, the precise intracellular signal transduction pathway for collagen production in fibrocytes has not been fully defined. A recent report showed that CCL21/CCR7 signaling activates MAPK family members including ERK, JNK/stress-activated protein kinase, and p38 MAPK, which control chemotaxis and the migratory speed of CCR7-positive dendritic cells (32). Of note, the expression of ColI is also regulated by the MAPK family in vitro (33). Therefore, the interaction of CCR7 on circulating fibrocytes with CCL21 expressed on HEV-like vessels during fibrocyte extravasation might induce the activation of a signal transduction pathway such as that involving the MAPK family, resulting in the production of collagen and the development of fibrosis.
Progressive organ fibrosis is pathologically characterized by the presence of infiltrating macrophages and accumulation of extracellular matrix, including ColI (1). Currently, macrophages are thought to be involved in the development of fibrosis by secreting various cytokines and growth factors including TGF-β1 (34). Furthermore, recent studies reported that the CCL2/CCR2 signaling pathway is involved in the progression of fibrosis through the recruitment and activation of macrophages in various fibrotic diseases (9, 35–40). CCL2 is reported to be produced by tubular epithelial cells and infiltrating cells in fibrotic kidneys (37). Recently, the expression of CCL2 mRNA was shown to be enhanced in fibrocytes under fibrotic circumstances (11). In addition, the current study demonstrated that renal expression of CCL2 mRNA and the infiltration of F4/80-positive macrophages as well as CCR7-expressing fibrocytes were significantly reduced in mice treated with anti-CCL21 antibodies and in CCR7-null mice after ureteral ligation compared with that in UUO-treated wild-type mice. Our previous reports demonstrated that monocytes/macrophages also contribute to renal fibrosis because the blockade of CCL2/CCR2 signaling resulted in a 30% reduction of renal fibrosis after ureteral ligation (9, 39). In contrast, fibrosis and infiltration of fibrocytes in the kidneys were reduced up to 50% by the inhibition of CCL21/CCR7 signaling in this study. Taken together, these findings suggest that CCR7-expressing fibrocytes are involved in the pathogenesis of fibrosis not only by secreting collagen but also by regulating the infiltration and activation of macrophages through CCL2 production (Fig. 5).
To date, participants involved in the pathogenesis of renal fibrosis have been considered resident fibroblasts, EMT-derived fibroblasts/myofibroblasts, and monocytes/macrophages (6–9). TGF-β1 is a well characterized inducer of EMT in renal tubular epithelial cells (7), whereas bone morphogenic protein 7 counteracts TGF-β1-induced EMT, resulting in improvement of renal fibrosis and renal function in experimental progressive renal diseases (7, 41). Recently, fibrocytes have been demonstrated to be capable of producing TGF-β1 under fibrotic conditions (11). Therefore, it is possible that fibrocytes regulate EMT through the production of TGF-β1 (Fig. 5). In addition, a recent report has demonstrated that 36% of renal fibroblasts found in a UUO model were derived from EMT and that 15% were derived from CD34-negative fibroblasts in bone marrow (8). However, fibrocytes detected in this study were positive for leukocyte markers, such as CD34 and CD45; therefore, it is suggested that the origin of fibrocytes may be different from that of CD34-negative fibroblasts. Collectively, fibroblasts derived from EMT and CD34-negative fibroblasts in bone marrow, as well as circulating fibrocytes, may be involved in organ fibrosis. Further investigations are needed to elucidate the origin and mechanisms of differentiation of precursor cells to fibrocytes.
In summary, these findings suggest that CCR7-positive fibrocytes infiltrate the kidney via CCL21-positive HEV-like vessels, thereby contributing to the pathogenesis of renal fibrosis. Regulating the recruitment and activation of fibrocytes may provide a therapeutic approach to combating organ fibrosis.
Materials and Methods
Details are presented in Supporting Materials and Methods, which is published as supporting information on the PNAS web site. In brief, inbred male BALB/c mice, aged 8 wk, were obtained from Charles River Japan (Atsugi, Kanagawa, Japan). CCR7-null mice, aged 8 wk, were also used (42).
All procedures used in the animal experiments complied with the standards set out in the Guidelines for the Care and Use of Laboratory Animals of the Takara-machi campus of Kanazawa University. Unilateral ureteral ligation was performed as previously described (5). To evaluate the impact of CCL21/CCR7 signaling on renal fibrosis as well as infiltrates in diseased kidneys, 600 μg of rabbit anti-CCL21 polyclonal antibodies (22) in 0.6 ml of normal saline or preimmunized rabbit IgG as a negative control was administered by tail vein 1 h before ureteral ligation. This antibody was obtained by immunizing 3-kg New Zealand White rabbits with 100–200 μg of recombinant murine CCL21 as previously described (22). The mean interstitial fibrotic area, expressed as blue on Mallory–Azan staining, was determined from the whole area of cortex and outer medulla in the individual complete sagittal kidney section and is expressed as the percentage per square millimeter of the field using Mac Scope version 6.02 (Mitani Shoji, Fukui, Japan).
Fibrocytes were identified in tissue samples by double immunohistochemistry using specific antibodies against CD45 (Santa Cruz Biotechnology, Santa Cruz, CA) or CD34 (BD Pharmingen, San Diego, CA) and ColI (Chemicon International, Temecula, CA) as previously described (12). Photoshop (Adobe Systems, San Jose, CA) was used for image handling, and three-color channels were handled separately. A preparation of isolated renal cells, including renal resident cells and infiltrated cells, was obtained from normal kidneys, obstructed kidneys, and contralateral kidneys 7 days after UUO in wild-type mice (5). After lysis of erythrocytes with FACS lysing solution (Becton Dickinson, San Jose, CA), cells were stained with FITC-labeled goat anti-mouse CCR2 polyclonal antibodies (Santa Cruz Biotechnology), PerCP-Cy5.5-conjugated rat anti-mouse CXCR4 monoclonal antibody (BD Pharmingen), allophycocyanin-conjugated rat anti-mouse CCR7 monoclonal antibody (BioLegend, San Diego, CA), and R-phycoerythrin-labeled rabbit anti-mouse ColI polyclonal antibodies (Chemicon International), and then analyzed on a FACSCalibur flow cytometer using CELLQuest software (Becton Dickinson). Cells incubated with irrelevant isotype-matched antibodies (BD Pharmingen) and unstained cells were used as controls. The cutoffs were set according to the findings in controls.
The expression of COL1A1, TGF-β1, and CCL2 in the whole kidneys was determined by quantitative real-time RT-PCR using the ABI Prism 7900HT Sequence Detection System (Applied Biosystems, Foster City, CA). Transcripts of CCL21 in diseased kidneys were also estimated by semiquantitative RT-PCR. Statistical analyses were performed by using the Wilcoxon rank-sum test, the Kruskal–Wallis test, and ANOVA.
Supplementary Material
Acknowledgments
We thank Drs. Yuko Ishida and Toshikazu Kondo (Wakayama Medical University, Wakayama, Japan) for technical advice in the measurement of hydroxyproline contents and Dr. Joost J. Oppenheim (National Cancer Institute) for critical review of the manuscript. T.W. is the recipient of a grant-in-aid from the Ministry of Education, Science, Sports, and Culture in Japan. This work was supported in part by a grant-in-aid from the Ministry of Health, Labor, and Welfare of Japan.
Abbreviations
- HEV
high endothelial venule
- UUO
unilateral ureteral obstruction
- EMT
epithelial–mesenchymal transition
- ColI
type I collagen.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office. R.B. is a guest editor invited by the Editorial Board.
References
- 1.Wada T, Razzaque MS, Matsushima K, Taguchi T, Yokoyama H. In: Fibrogenesis: Cellular and Molecular Basis. Razzaque MS, editor. Washington, DC: Landes Bioscience Eurekah; 2004. pp. 9–26. [Google Scholar]
- 2.Bohle A, Muller GA, Wehrmann M, Mackensen-Haen S, Xiao JC. Kidney Int Suppl. 1996;49:S2–S9. [PubMed] [Google Scholar]
- 3.Risdon RA, Sloper JC, de Wardener HE. Lancet. 1968;2:363–366. doi: 10.1016/s0140-6736(68)90589-8. [DOI] [PubMed] [Google Scholar]
- 4.Nath KA. Kidney Int. 1998;54:992–994. doi: 10.1046/j.1523-1755.1998.00079.x. [DOI] [PubMed] [Google Scholar]
- 5.Vielhauer V, Anders HJ, Mack M, Cihak J, Strutz F, Stangassinger M, Luckow B, Grone HJ, Schlöndorff D. J Am Soc Nephrol. 2001;12:1173–1187. doi: 10.1681/ASN.V1261173. [DOI] [PubMed] [Google Scholar]
- 6.Strutz F, Zeisberg M, Ziyadeh FN, Yang CQ, Kalluri R, Muller GA, Neilson EG. Kidney Int. 2002;61:1714–1728. doi: 10.1046/j.1523-1755.2002.00333.x. [DOI] [PubMed] [Google Scholar]
- 7.Zeisberg M, Hanai J, Sugimoto H, Mammoto T, Charytan D, Strutz F, Kalluri R. Nat Med. 2003;9:964–968. doi: 10.1038/nm888. [DOI] [PubMed] [Google Scholar]
- 8.Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG. J Clin Invest. 2002;110:341–350. doi: 10.1172/JCI15518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kitagawa K, Wada T, Furuichi K, Hashimoto H, Ishiwata Y, Asano M, Takeya M, Kuziel WA, Matsushima K, Mukaida N, Yokoyama H. Am J Pathol. 2004;165:237–246. doi: 10.1016/S0002-9440(10)63292-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bucala R, Spiegel LA, Chesney J, Hogan M, Cerami A. Mol Med. 1994;1:71–81. [PMC free article] [PubMed] [Google Scholar]
- 11.Chesney J, Metz C, Stavitsky AB, Bacher M, Bucala R. J Immunol. 1998;160:419–425. [PubMed] [Google Scholar]
- 12.Moore BB, Kolodsick JE, Thannickal VJ, Cooke K, Moore TA, Hogaboam C, Wilke CA, Toews GB. Am J Pathol. 2005;166:675–684. doi: 10.1016/S0002-9440(10)62289-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Phillips RJ, Burdick MD, Hong K, Lutz MA, Murray LA, Xue YY, Belperio JA, Keane MP, Strieter RM. J Clin Invest. 2004;114:438–446. doi: 10.1172/JCI20997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmidt M, Sun G, Stacey MA, Mori L, Mattoli S. J Immunol. 2003;171:380–389. doi: 10.4049/jimmunol.171.1.380. [DOI] [PubMed] [Google Scholar]
- 15.Abe R, Donnelly SC, Peng T, Bucala R, Metz CN. J Immunol. 2001;166:7556–7562. doi: 10.4049/jimmunol.166.12.7556. [DOI] [PubMed] [Google Scholar]
- 16.Hauser C, Kaya G, Chizzolini C. Dermatology. 2004;209:50–52. doi: 10.1159/000078587. [DOI] [PubMed] [Google Scholar]
- 17.Yang L, Scott PG, Giuffre J, Shankowski HA, Ghahary A, Tredget EE. Lab Invest. 2002;82:1183–1192. doi: 10.1097/01.lab.0000027841.50269.61. [DOI] [PubMed] [Google Scholar]
- 18.Okon K, Szumera A, Kuzniewski M. Am J Nephrol. 2003;23:409–414. doi: 10.1159/000074298. [DOI] [PubMed] [Google Scholar]
- 19.Campbell JJ, Bowman EP, Murphy K, Youngman KR, Siani MA, Thompson DA, Wu L, Zlotnik A, Butcher EC. J Cell Biol. 1998;141:1053–1059. doi: 10.1083/jcb.141.4.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gunn MD, Tangemann DK, Tam C, Cyster JG, Rosen SD, Williams LT. Proc Natl Acad Sci USA. 1998;95:258–263. doi: 10.1073/pnas.95.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ogata M, Zang Y, Wang Y, Itakura M, Zang YY, Harada A, Hashimoto S, Matsushima K. Blood. 1999;93:3225–3232. [PubMed] [Google Scholar]
- 22.Itakura M, Tokuda A, Kimura H, Nagai S, Yoneyama H, Onai N, Ishikawa S, Kuriyama T, Matsushima K. J Immunol. 2001;166:2071–2079. doi: 10.4049/jimmunol.166.3.2071. [DOI] [PubMed] [Google Scholar]
- 23.Wright F. Semin Nephrol. 1982;2:5–16. [Google Scholar]
- 24.Klahr S. Kidney Int. 1983;23:414–426. doi: 10.1038/ki.1983.36. [DOI] [PubMed] [Google Scholar]
- 25.Michie SA, Streeter PR, Bolt PA, Butcher EC, Picker LJ. Am J Pathol. 1993;143:1688–1698. [PMC free article] [PubMed] [Google Scholar]
- 26.Kraal G, Mebius RE. Adv Immunol. 1997;65:347–395. [PubMed] [Google Scholar]
- 27.Baekkevold ES, Yamanaka T, Palframan RT, Carlsen HS, Reinholt FP, von Andrian UH, Brandtzaeg P, Haraldsen G. J Exp Med. 2001;193:1105–1112. doi: 10.1084/jem.193.9.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dinther-Janssen ACHM, Pals ST, Scheper R, Breedveld F, Meijer CJLM. J Rheumatol. 1990;17:11–17. [PubMed] [Google Scholar]
- 29.Kabel PJ, Voorbij HAM, Haan-Meulman M, Pals ST, Drexhage HA. J Clin Endocrinol Metab. 1989;68:744–751. doi: 10.1210/jcem-68-4-744. [DOI] [PubMed] [Google Scholar]
- 30.Weninger W, Carlsen HS, Goodarzi M, Moazed F, Crowley MA, Baekkevold ES, Cavanagh LL, von Andrian UH. J Immunol. 2003;170:4638–4648. doi: 10.4049/jimmunol.170.9.4638. [DOI] [PubMed] [Google Scholar]
- 31.Takaeda M, Yokoyama H, Segawa-Takaeda C, Wada T, Kobayashi K. Am J Nephrol. 2002;22:48–57. doi: 10.1159/000046674. [DOI] [PubMed] [Google Scholar]
- 32.Riol-Blanco L, Sanchez-Sanchez N, Torres A, Tejedor A, Narumiya S, Corbi AL, Sanchez-Mateos P, Rodriguez-Fernandez JL. J Immunol. 2005;174:4070–4080. doi: 10.4049/jimmunol.174.7.4070. [DOI] [PubMed] [Google Scholar]
- 33.Sato M, Shegogue D, Gore EA, Smith EA, McDermott PJ, Trojanowska M. J Invest Dermatol. 2002;118:704–711. doi: 10.1046/j.1523-1747.2002.01719.x. [DOI] [PubMed] [Google Scholar]
- 34.Border WA, Noble NA. N Engl J Med. 1994;331:1286–1292. doi: 10.1056/NEJM199411103311907. [DOI] [PubMed] [Google Scholar]
- 35.Suga M, Iyonaga K, Ichiyasu H, Saita N, Yamasaki H, Ando M. Eur Respir J. 1999;14:376–382. doi: 10.1034/j.1399-3003.1999.14b23.x. [DOI] [PubMed] [Google Scholar]
- 36.Lehmann MH, Kuhnert H, Muller S, Sigusch HH. Cytokine. 1998;10:739–746. doi: 10.1006/cyto.1998.0354. [DOI] [PubMed] [Google Scholar]
- 37.Wada T, Furuichi K, Segawa C, Shimizu M, Sakai N, Takeda S, Takasawa K, Kida H, Kobayashi K, Mukaida N, et al. Kidney Int. 1999;56:995–1003. doi: 10.1046/j.1523-1755.1999.00646.x. [DOI] [PubMed] [Google Scholar]
- 38.Moore BB, Paine R, III, Christensen PJ, Moore TA, Sitterding S, Ngan R, Wilke CA, Kuziel WA, Toews GB. J Immunol. 2001;167:4368–4377. doi: 10.4049/jimmunol.167.8.4368. [DOI] [PubMed] [Google Scholar]
- 39.Wada T, Furuichi K, Sakai N, Iwata Y, Kitagawa K, Ishida Y, Kondo T, Hashimoto H, Ishiwata Y, Mukaida N, et al. J Am Soc Nephrol. 2004;15:940–948. doi: 10.1097/01.asn.0000120371.09769.80. [DOI] [PubMed] [Google Scholar]
- 40.Wada T, Yokoyama H, Furuichi K, Kobayashi K, Harada K, Naruto M, Su SB, Akiyama M, Mukaida N, Matsushima K. FASEB J. 1996;12:1418–1425. [PubMed] [Google Scholar]
- 41.Hruska KA, Guo G, Wozniak M, Martin D, Miller S, Liapis H, Loveday K, Klahr S, Sampath TK, Morrissey J. Am J Physiol. 2000;280:F130–F143. doi: 10.1152/ajprenal.2000.279.1.F130. [DOI] [PubMed] [Google Scholar]
- 42.Forster R, Schubel A, Breitfeld D, Kremmer E, Muller IR, Wolf E, Lipp M. Cell. 1999;99:23–33. doi: 10.1016/s0092-8674(00)80059-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.