Abstract
Only a fraction of subjects exposed to asbestos develop malignant mesothelioma (MM), suggesting that additional factors may render some individuals more susceptible. We tested the hypothesis that asbestos and Simian virus (SV40) are cocarcinogens. Asbestos and SV40 in combination had a costimulatory effect in inducing ERK1/2 phosphorylation and activator protein-1 (AP-1) activity in both primary Syrian hamster mesothelial cells (SHM) and primary human mesothelial cells (HM). Ap-1 activity caused the expression and activation of matrix metalloprotease (MMP)-1 and MMP-9, which in turn led to cell invasion. Experiments using siRNA and chemical inhibitors confirmed the specificity of these results. The same effects were observed in HM and SHM. Experiments in hamsters showed strong cocarcinogenesis between asbestos and SV40: SV40 did not cause MM, asbestos caused MM in 20% of hamsters, and asbestos and SV40 together caused MM in 90% of hamsters. Significantly lower amounts of asbestos were sufficient to cause MM in animals infected with SV40. Our results indicate that mineral fibers and viruses can be cocarcinogens and suggest that lower amounts of asbestos may be sufficient to cause MM in individuals infected with SV40.
Keywords: activator protein-1, ERK1/2, matrix metalloprotease, environmental carcinogenesis, viral oncology
Malignant mesothelioma (MM) is a malignancy of the mesothelial cells that form the serosal membranes that cover the chest and abdominal cavities. Median survival is ≈12 months, and MM causes 2,000–3,000 deaths per year in the USA and ≈1,000 deaths per year in the U.K (1). The continuing increase in the incidence of MM has been associated to the widespread use of asbestos in the past century. The mechanisms of asbestos carcinogenesis have been linked to the extracellular response regulated kinase ERK1/2 and activator protein-1 (AP-1) pathways (2), to the activation of proinflammatory cytokines and NFκB (3), and to the production of reactive mutagenic oxygen species by nearby lung macrophages exposed to asbestos (4).
Only a fraction (≈5%) of subjects exposed to high levels of asbestos develop MM. This finding suggests that additional factors, such as SV40 infection and genetic predisposition, may render some individuals more susceptible to asbestos carcinogenicity (1, 5). SV40 is a monkey DNA virus that induces MM in hamsters (6). Following this observation, numerous laboratories have detected SV40 in MM biopsies although the prevalence of SV40 varied in different studies from ≈6% to 60%, and some studies did not detect SV40 (7–11). SV40 contaminated human polio vaccines worldwide from 1955 until ≈1961. Epidemiological studies comparing cohorts born before or after 1961 detected an increased relative risk of 3 for MM in pre-1961 cohorts that included many individuals vaccinated with contaminated polio vaccines (12). However, the overall epidemiological evidence was considered inconclusive because of differences in ages among the cohorts studied and because it was uncertain that cohorts born after 1961 were not exposed to SV40 (12). Recent data supported these concerns, showing that polio vaccines prepared in Eastern Europe and distributed in many countries contained infectious SV40 at least until 1978 (13). Because hundreds of millions of people have been exposed to infectious SV40 until recently, it is important to study whether SV40 contributes to human MM (7–11).
It seems unlikely that SV40 contributes to the MM epidemic independently of asbestos because humans are much more resistant than rodents to viral carcinogens and because most MM develop in asbestos-exposed individuals. We previously reported that SV40 and crocidolite asbestos were cocarcinogens in causing malignant transformation of HM in tissue culture (14). A recent study demonstrated that SV40 activates the AKT pathway in primary human mesothelial cells (HM), inducing cell survival and thus making HM less sensitive to asbestos cytotoxicity (15). An epidemiological study has suggested that SV40 infection may increase the risk of MM in asbestos-exposed individuals (16).
In this study, we tested the hypothesis that SV40 and asbestos are cocarcinogens. The activation of the ERK/AP-1 pathway by asbestos and other agents has been linked to cell proliferation and transformation (2, 17). These same signaling pathways are also influenced by SV40 (18), suggesting a possible mechanism of interaction. Therefore, we focused on these pathways and downstream genes. The tissue culture experiments were performed on both human and hamster primary mesothelial cells to verify the relevance of the experimental hamster model to study MM pathogenesis. The results obtained with hamster and human mesothelial cells were almost identical. The biological significance of the results obtained in vitro was tested in vivo in the hamster MM model.
Results
Comparison of ERK1/2 and AP-1 Expression in HM and Primary Syrian Hamster Mesothelial Cells (SHM) Exposed to Asbestos and Infected with SV40.
HM and SHM were infected with SV40 dl883 (see Materials and Methods for a rationale of using this virus) and exposed to doses of crocidolite asbestos [often considered the most carcinogenic type of asbestos (1)] ranging from 0.1 to 2.5 μg/cm2 dish. The effects on ERK1/2 phosphorylation and AP-1 components were measured by using Western blotting and the ELISA-based Trans AM AP-1 Family assay, respectively. The most consistent and reproducible costimulatory effects were observed at 48 h by using 0.1 μg/cm2 asbestos. Therefore, all subsequent experiments were performed by using 0.1 μg/cm2 asbestos at 48 h. The highest stimulation of p-ERK1/2 was in HM and in SHM infected with SV40 dl883 and exposed to crocidolite (2.1-fold increase, Fig. 1A).
Fig. 1.
Crocidolite and SV40 dl 883 have a costimulatory effect in inducing ERK1/2 and AP-1 activity. (A) Western blot pERK1/2 phosphorylation. (B) Western blot, Fra2 expression. Antibodies against total ERK1/2 and Histone 1 (H1) were used as controls. The fold increase of ERK1/2 phosphorylation and Fra2 expression in HM (light gray bars) and in SHM (dark gray bars) is indicated below each Western blot. (C) AP-1 DNA-binding activity of the members of the AP-1 family in HM and SHM infected with SV40 dl883 and/or exposed to asbestos. We used the TransAM methodology to quantify AP-1 activity (measured at OD450). +, Cells exposed to the indicated agent, −, not exposed. ∗, Significant difference compared with untreated cells; #, significant difference compared with cells exposed to both SV40 dl883 and crocidolite. Results are the average of three independent experiments (±SD).
We found that asbestos and SV40 activated several members of the AP-1 complex. These carcinogens in combination increased the binding activity of Fra2 2.2-fold above basal levels, and of c-Jun, JunB, JunD, and Fra1 of 1.2- to 1.5-fold (Fig. 1 B and C). The results were confirmed by Western blot (Fig. 1B; only the Western for Fra2 is shown). We did not detect any costimulatory effect of these agents on c-Fos and FosB expression and binding activity (data not shown). Very similar results were obtained in SHM and HM.
Because the most notable stimulation was on the expression of Fra2, which is bound primarily to members of the Jun-family (19), we focused on this AP-1 component. We verified the apparent increase in Fra2 expression by immunoprecipitation with anti-c-Jun antibodies and subsequent Western blot for Fra2 and c-Jun and vice versa (Fig. 6, which is published as supporting information on the PNAS web site).
Crocidolite and SV40 Increase DNA Binding and Transactivation Activity of AP-1.
Next, we tested whether the binding activity of AP-1 was influenced by crocidolite and SV40 dl883 infection. AP-1 and mutated AP-1 oligonucleotides (AP-1 CA→TG) from the matrix metalloprotease (MMP)-1 promoter (see Materials and Methods and Fig. 7, which is published as supporting information on the PNAS web site) were labeled with [32P]ATP and incubated with nuclear extracts from HM and SHM. The complexes of the AP-1 oligonucleotides were immunoprecipitated with a monoclonal anti-c-Jun conjugated with agarose, and the amount of the immunoprecipitated radiolabeled AP-1 was measured. The results showed a synergistic effect of crocidolite and SV40 dl883 on AP-1 DNA binding (P < 0.001 for both HM and SHM). In SHM and in HM exposed to both crocidolite and SV40 dl883, there were 2.6- and 3-fold increases, respectively, in AP-1-binding activity compared with 1.3- to 1.5-fold increases induced by crocidolite or SV40 dl883 alone (Fig. 2A). AP-1 binding activity to a mutated Ap-1 DNA binding sequence was not influenced by the treatment with SV40 or crocidolite (Fig. 2A, negative control). These results supported a synergistic effect of crocidolite and SV40 dl883 on AP-1-binding activity.
Fig. 2.
Exposure of HM and SHM to crocidolite and SV40 dl883 infection increases AP-1 DNA-binding and transactivation activity. (A) Nuclear extracts from HM and SHM (30 μg) were assayed for c-Jun binding to the AP-1 consensus site derived from the MMP-1 proximal promoter. A 32P-radiolabeled AP-1 oligonucleotide or a mutated oligonucleotide that does not bind AP-1 (AP-1 CA→TG, negative control) was used as probe (see Fig. 7). (B) HM and SHM were transiently cotransfected with pGL3-MMP-1P and pRL, or with pGL3-RR and pRL vectors. Twenty-four hours after transfection, the cells were treated as indicated, and luciferase activity was measured. Values were normalized to cotransfection with pLR carrying Renilla luciferase and the amount of induction to that of pGL3-RR. Results are average of three independent experiments (±SD). +, Cells exposed to the indicated agent; −, nonexposed. ∗, Significant difference compared with untreated cells; #, significant difference compared with cells exposed to both SV40 dl883 and crocidolite. There was a significant interaction between the effects of SV40 dl883 and crocidolite on binding to the AP-1 site in both HM and SHM (P < 0.001).
Changes in AP-1-binding activity do not necessarily mirror AP-1 transcriptional activity. Therefore, we measured the capacity of AP-1 to activate the expression of an AP-1-dependent reporter gene. The pGL3-MMP-1P construct containing the MMP-1 proximal promoter (Fig. 7) was transfected into HM and SHM (Renilla luciferase was the internal control). Twenty-four hours after transfection, the cells were infected with SV40 dl883 for 48 h and/or exposed to crocidolite for 1 h. A pGL3-RR construct with luciferase and no regulatory region was used as negative control. The luciferase activity increased significantly in HM and SHM exposed to crocidolite (1.5- to 1.6-fold), or to SV40 dl883 (1.3- to 1.4-fold), and further increased (2- to 2.1-fold) in cells exposed to asbestos and infected with SV40 dl883 (Fig. 2B).
MMP-1 and MMP-9 Expression Is Induced in Cells Exposed to Crocidolite and/or Infected with SV40.
AP-1 regulates the expression of MMPs that have an AP-1-binding site, such as MMP-1, -3, -7, -9, -10, -12, and -13 (20, 21). MMPs influence tumor cell invasion and metastasis by digesting basement membranes and the extracellular matrix (21). MM invasion has been linked to MMP-1, MMP-2, and MMP-9 expression (20, 21). We measured whether the observed stimulation of p-ERK1/2 and AP-1 activity caused by crocidolite and SV40 dl883 led to changes in MMP-1 and MMP-9 expression. Both crocidolite exposure and SV40 dl883 infection induced significant increases in the amount of MMP-1 and MMP-9 in HM (P = 0.007) and in SHM (P < 0.001); the maximum increase was in cells exposed to both agents (2.3- to 2.5-fold, Fig. 3A and B).
Fig. 3.
MMP-1 and MMP-9 expression and activity increases in HM and SHM exposed to both crocidolite and SV40 dl883. Western blot of cytoplasmic extracts for MMP1 (A) and for MMP9 (B). GAPDH, loading control. Shown is the substrate zymography using the whole cell lysate (C) and the tissue culture medium (D). The gelatin digestion is seen as white bands. The increased expression and activity of MMP-1 and MMP-9 are shown in the graph below each Western blot and zymogram: light gray bars, HM; dark gray bars, SHM. Results are the average of three independent experiments (±SD). +, Cells exposed to the indicated agent; −, nonexposed. ∗, Significant difference compared with untreated cells; #, significant difference compared with cells exposed to both SV40 dl883 and crocidolite.
The increases in MMP-1 and MMP-9 expression detected by Western blotting were verified by zymography. The amount of cellular MMP-1 (pro-MMP-1) increased 2.4- to 2.5-fold in cells exposed to crocidolite and infected with SV40 dl883 (Fig. 3C) and the costimulatory effects were significant for both HM (P = 0.009) and SHM (P = 0.013), but MMP-1 remained undetectable in the tissue culture medium. Cellular MMP-9 had a similar increase; furthermore, the amount of MMP-9 in the tissue culture medium increased 2.6- to 2.7-fold (Fig. 3D) and showed a significant costimulatory effect for HM (P = 0.008). The increase in MMP-1 and MMP-9 caused by asbestos exposure alone or by SV40 dl883 infection alone was less pronounced (1.3- to 1.5-fold increase). MMP-2 expression and activity (negative control) were not influenced by either asbestos or SV40 dl883, consistent with the notion that MMP-2 does not have an AP-1-binding site in its promoter (Fig. 3D shows MMP-2 in HM; similar results were seen in SHM).
MMP-1 and MMP-9 Expression Is Linked to HM Invasion.
The invasion assay is an in vitro test used to measure the ability of cells to invade a matrix gel. The invasiveness in this test is linked to the expression of MMPs (22). Using this test, we found that exposure to crocidolite, or SV40 dl883 infection of HM, caused a 20% increase in the number of invading cells. The increase reached 44% in HM that were exposed to crocidolite and infected with SV40 dl883 (Fig. 8, which is published as supporting information on the PNAS web site). All these increases were statistically significant. When in parallel experiments using the same HM we blocked ERK1/2 phosphorylation with the chemical PD98059, which inhibits MEK-1 (MEK1 is a kinase upstream of ERKs) (23), or when we blocked AP-1 activity transfecting A-Fos (a dominant-negative Fos, see below) (24), we suppressed the stimulatory effect of asbestos and SV40 dl883 on cell invasion (Fig. 8). We did not observe any appreciable difference in invasiveness among HM transfected with an empty vector (pcDNA3, control for the A-Fos) and nontransfected HM.
Crocidolite Exposure and SV40 dl883 Infection Induce Phosphorylation of ERK1/2, Which Leads to MMP-1 and MMP-9 Expression.
p-ERK1/2 stimulate MMP-1 and MMP-9 expression through AP-1 activation (17, 21). We found that SV40 dl883- and asbestos-induced ERK1/2 phosphorylation was abrogated when HM were treated with the MEK1 inhibitor PD98059 (Fig. 9, which is published as supporting information on the PNAS web site). The amount of total ERK1/2 did not change regardless of treatment, supporting specificity. These results were further confirmed by using siRNA for ERK1 (Fig. 4A). Twenty-four hours after transfection with siRNA directed against ERK1, the cells were infected with SV40 dl883 for 48 h and/or exposed to crocidolite for 1 h. Phosphorylated ERK1/2 was markedly reduced in HM treated with ERK1 siRNA, but it was not affected in HM treated with the control siRNA. Accordingly, the expression of c-Jun and of MMP-9, which requires ERK1 activity, was abolished in HM transfected with the ERK1 siRNA (Fig. 4A).
Fig. 4.
Inactivation of ERK1/2 by siRNA for ERK1 and inhibition of the AP-1 pathway with JDP2 blocks MMP-1 and MMP-9 expression. (A) Cell lysates were tested by Western blotting for p-ERK1/2, c-Jun, and MMP-9 in HM transfected with ERK1 siRNA and with control siRNA. (B) Western blot of cell lysates from HM transfected with JDP2 or empty vector. GAPDH, loading control. +, cells exposed to the indicated agent; −, nonexposed.
A-Fos and c-Jun Dimerization Protein (JDP2) Inhibit AP-1-Binding Activity and MMP-1 and MMP-9 Expression.
We tested the specificity of the results using A-Fos and JDP2 to inhibit AP-1-binding activity and MMP-1 and MMP-9 expression. A-Fos is a biologically inactive Fos mutant with a high affinity for the Jun-family members. A-Fos interferes with the formation of AP-1 complexes containing either c-Fos, FosB, Fra1, or Fra2 by displacing these proteins and binding to Jun-proteins (24). When A-Fos binds to Jun, the AP-1 activity is inhibited (24). Using A-Fos in EMSA assays, we found that we inhibited AP-1-binding activity and that this effect in turn inhibited MMP-1 and MMP-9 expression in mesothelial cells exposed to crocidolite and infected with SV40 dl883. Western blot analyses confirmed that the levels of MMP-1 and MMP-9 in these same A-Fos-transfected cells were significantly reduced compared with nontransfected HM or HM transfected with the empty vector and tested in parallel (Fig. 9).
To further verify the results, we used an expression vector containing JDP2 (25). JDP2 inhibits AP-1 activity because it binds to and blocks c-Jun protein activity, which in turn leads to an overall decrease of c-Jun expression (25). Twenty four hours after transfection with JDP2, HM were infected with SV40 dl883 and 47 h later were exposed to crocidolite for 1 h (0.1 μg/cm2). In parallel, nontransfected HM from the same donor and HM transfected with an empty vector were used as controls. Nuclear extracts were prepared and evaluated by EMSA. We found that, in HM transfected with JDP2, the AP-1-binding activity was inhibited, regardless of type of exposure (data not shown). Western blot analyses confirmed that c-Jun expression was inhibited in JDP2-transfected HM and that in the same cells MMP-1 and MMP-9 expression levels were markedly reduced compared with nontransfected HM or HM transfected with the empty vector and tested in parallel (Fig. 4B). Together, these results confirmed the specificity of the results and of the pathways studied.
Animal Experiments to Test for Cocarcinogenesis.
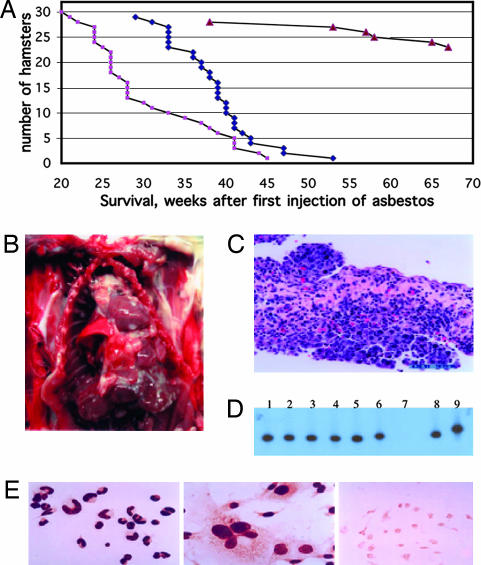
To verify in vivo the cocarcinogenic effects observed in vitro with SV40 and asbestos, we injected 21-day-old hamsters with SV40 dl883 into the left ventricle (to expose most cell types and organs to the virus). The same hamsters were also injected intrapleurally with 0.4 mg of crocidolite and with a total of 4 mg intraperitoneally (see Material and Methods and Supporting Materials and Methods, which is published as supporting information on the PNAS web site, for details). Additional groups included hamsters injected with either crocidolite or SV40 dl883 alone, or with tissue culture medium (control). The mean tumor latency [i.e., the time from injection to the time when the tumor became detectable or the animals became ill (which was also the time when the hamsters were euthanized)] was 30.2 weeks for the SV40 dl883 plus crocidolite group and 37.6 weeks for the crocidolite only group (Fig. 5). Crocidolite alone caused MM in 20% of hamsters (Fig. 5). In addition, crocidolite induced lymphomas, sarcomas, and liver carcinomas (Table 1), supporting previous observations (1, 26). MM did not develop in hamsters only injected with SV40 dl883 (no asbestos), although a few animals developed lymphomas and sarcomas after a prolonged latency (Table 1), as observed previously (6). Coinjection of SV40 dl883 and asbestos caused a significantly higher incidence of MM (Table 1, 90% MM incidence) and a significantly shorter tumor latency (Fig. 5A). Pleural MMs developed only in the coinjected group, suggesting that, in the presence of viral infection, lower amounts of asbestos (0.4 mg) are sufficient to cause MM. No tumors developed in the control group.
Fig. 5.
Cocarcinogenesis among SV40 dl883 and crocidolite in causing hamster MMs. (A) Tumor latency in the different groups of animals injected with the following: light colored squares, crocidolite and SV40 dl883; dark diamonds, crocidolite only; triangles, SV40 dl883 only. Survival was analyzed by using the log-rank (Mantel–Cox) test. P < 0.001, crocidolite versus SV40 dl883; P < 0.001, crocidolite plus SV40 dl883 versus SV40 dl883; P = 0.003, crocidolite versus crocidolite plus SV40 dl883. (B) Macroscopic appearance of MM in hamster no. 2 (injected with SV40 dl883 and crocidolite). Shown are multiple whitish tumor nodules on the lungs, pericardium, and chest wall. The main tumor mass is seen above the heart around the superior vena cava. (C) Histology of the tumor shown in B. Shown is biphasic MM. (D) Representative Southern blot hybridization with a SV40-specific probe showing the presence of SV40 in hamster tumors and derived cell lines. SV40-specific sequences were amplified by PCR. Lanes 1 and 2, lymphoma and derived cell line (SV40 dl883 injected-hamster); lanes 3–6, two separate MM (lanes 3 and 5) and corresponding derived cell lines (lanes 4 and 6) (SV40 dl883-crocidolite coinjected hamsters); lane 7, normal peritoneal cells from the hamster with the pleural MM shown in lane 5 do not contain SV40; lane 8, SV40 dl883 DNA (control); lane 9, SV40 wild-type (strain 776) DNA (control). The different DNA size in lanes 5, 6, and 8 seems to be an electrophoresis artifact because sequencing did not reveal differences. (E) SV40 Tag nuclear staining of tumor-derived cell cultures. (Left) Lymphoma (myeloid) cells in culture, same cells as lane 2 of D. (Center) MM cells in culture, same cells as lane 6 of D. (Right) Normal peritoneal cells, same as lane 7 of D, Tag negative.
Table 1.
Tumor types and/or cause of death observed in different groups of animals exposed to the indicated agents
| SV40 dl883 | Crocidolite | SV40 dl883 + crocidolite | |
|---|---|---|---|
| Pleural MM | 0/28 | 0/29 | 13/30* |
| Peritoneal MM | 0/28 | 6/29† | 11/30‡ |
| Pleural/peritoneal MM | 0/28 | 0/29 | 3/30§ |
| Peritoneal fibrosis | 0/28 | 11/29¶ | 3/30 |
| Lymphoma/THL | 2/28 | 6/29‖ | 0/30 |
| Liver carcinoma | 0/28 | 3/29 | 0/30 |
| Sarcoma | 4/28** | 6/29†† | 0/30 |
Groups of 30 hamsters were injected. Some groups have fewer hamsters because some died within 1 day from injection. Chronic inflammation was frequent in the peritoneum of animals injected with asbestos, and several developed and died of intestinal occlusion caused by peritoneal fibrosis. Most of those injected with both SV40 dl883 and crocidolite died of MM at an earlier time before they could develop extensive fibrosis and intestinal occlusion. Tumor histology subtype and cause of death are shown.
*Two epithelial MM, two sarcomatoid MM, nine biphasic MM (prevalently sarcomatoid).
†Five epithelial MM, 1 biphasic MM.
‡Four epithelial MM, two sarcomatoid MM, five biphasic MM (prevalently sarcomatoid).
§One sarcomatoid MM, two biphasic MM (prevalently sarcomatoid).
¶Intestinal occlusion caused by asbestos-mediated chronic inflammation.
‖Two hamsters had two tumors, lymphoma and leiomyosarcoma and lymphoma and fibrosarcoma.
**Two malignant fibrous histiocytoma (MFH), one fibrosarcoma, and one myxofibrosarcoma.
††Two MFH, two leiomyosarcomas, one dedifferentiated liposarcoma, and one fibrosarcoma.
Macroscopically, most MM surrounded the lungs and/or the pericardium or spread along the peritoneum, and MM nodules studded both the pleural and the peritoneal cavities (Fig. 5B). The histology of these MMs was identical to human MM. Most MM in the coinjected group showed a sarcomatoid or biphasic morphology (Fig. 5C). MMs in hamsters injected only with crocidolite were mostly of the “epithelial type,” and the tumors showed a more bland, well differentiated tubular-papillary morphology.
All tumors and derived cell lines from the SV40 dl883-injected group and from the coinjected group contained SV40 dl883 DNA corresponding to the input virus (Fig. 5D) and expressed SV40 tumor antigens (Fig. 5E). In parallel experiments, tumors that developed in hamsters injected with crocidolite but not exposed to SV40 tested SV40-negative (data not shown). These results indicated a strong cocarcinogenic effect in causing MM among SV40 dl883 and crocidolite asbestos in hamsters.
Discussion
We investigated the hypothesis that SV40 and asbestos are cocarcinogens by studying possible mechanisms of interaction in vitro and testing the cocarcinogenesis hypothesis in vivo. As in previous experiments in tissue culture (14), we used both wild-type SV40 and SV40 dl883. Both viruses induced the same biochemical effects: only the results obtained by using SV40 dl883 are presented here because of space constraints and because the results are easier to quantify as this virus does not induce cell lysis and cellular transformation in vitro, and MM in hamsters (6, 18).
Our tissue culture experiments revealed that asbestos and SV40 dl883 cooperate in inducing ERK1/2 phosphorylation that in turn caused an increase in AP-1 binding and transactivation activity, primarily mediated through Fra-2-c-Jun heterodimers. AP-1 activity caused an increase in MMP-1 and MMP-9 expression and activity that led to cell invasion. The specificity of this pathway is supported by the observations that blocking the ERKs pathways with siRNA and the MEK-1 inhibitor PD98059, or blocking AP-1 with A-Fos (a dominant negative Fos mutant) and JDP-2 (a protein that binds and inhibits c-Jun expression), abrogates the stimulatory effect of SV40 dl883 and asbestos on MMPs expression and on cell invasion.
These results provide mechanistic evidence for cocarcinogenesis between SV40 dl883 and asbestos in both HM and SHM and elucidate numerous steps in the ERK1/2–AP-1 pathway that are influenced by crocidolite and SV40 in HM. The relevance of the ERK/AP-1 pathway to asbestos pathogenesis has been proposed previously in a rat-MM experimental model (2), and it is confirmed here in human and in hamster mesothelial cells. Moreover, we show that SV40 has a costimulatory effect with asbestos in inducing these pathways. We hope that it will be possible to use this knowledge to design novel drugs for MM that specifically target these pathways.
Because asbestos induced the same biochemical changes in hamsters and in human mesothelial cells, we tested the possible biological relevance of the in vitro experiments in our hamster-MM model (6). Doses of asbestos <1 mg are usually insufficient to cause MM in animals (27). We found that SV40 dl883 did not cause MM; 4 mg of crocidolite caused peritoneal MM in 20% of hamsters, but the same hamsters injected with 0.4 mg intrapleurally did not develop pleural MM. SV40 dl883 and crocidolite together caused MM in 90% of hamsters: these results strongly support cocarcinogenesis. Moreover, hamsters exposed to both carcinogens developed pleural MM with significantly shorter latency. Of particular interest was the finding that ≈50% of hamsters exposed to SV40 dl883 infection and to 0.4 mg of crocidolite injected intrapleurally developed MM, but that hamsters injected only with 0.4 mg of crocidolite intrapleurally or only with SV40 dl883 intracardially did not. These results revealed that SV40 infection lowers the threshold of crocidolite required to cause MM and may have relevance when considering the threshold limit of exposure below which asbestos exposure is considered “acceptable.” Such supposedly “safe” levels of exposure may vary among individuals depending on history of exposure.
The finding that 20% of hamsters exposed to a total of 4 mg of crocidolite i.p. developed MM confirmed that crocidolite induces MM when a sufficient dose is administered (27). In hamsters and in rodents, the incidence of MM is proportional to the amount of asbestos exposure. In humans, there is not a clear dose-response curve (1). We propose that in animals we can control the type and amount of exposure, whereas humans are exposed to different carcinogens during their lifetime that, together with genetics (5), can influence their sensitivity to asbestos. In summary, our findings do not call into question the ability of these carcinogens to cause MM independently of each other. Instead, our results show that mineral fibers and viruses can interact in carcinogenesis, a finding that may lead to novel preventive and therapeutic strategies.
The pathogenesis of MM remains controversial, and it is still debated whether all types of asbestos cause MM, whether there is a threshold amount below which asbestos does not cause MM, etc. Similarly, the association of SV40 with human MM has been controversial: >40 different laboratories have confirmed the association, some have not or have minimized the pathogenic role of SV40 (7–12). We suggested that some of the discrepancies were caused by technical issues (28), or by geographical differences linked to contaminated polio vaccines (13).
We propose here that, rather than looking at an all or none effect for SV40 and asbestos individually in MM, we should also consider cocarcinogenesis. Low amounts of asbestos or SV40 may be harmless in certain individuals but may be sufficient to cause MM in people exposed to both agents. This hypothesis is of particular relevance to current trends in MM. Although in the past most MM patients were miners, insulators, and shipyard workers heavily exposed to asbestos, presently most MM patients are individuals with relatively limited exposures. SV40 infection, by lowering the threshold amounts of asbestos required for inducing MM, might be one explanation for these developments. Thus, it will be important to study whether low doses of these two widely distributed cocarcinogens interact in causing human MM, as suggested by our results and by a recent molecular-epidemiological study (16). This situation is somewhat reminiscent of aflatoxin-B1 exposure and hepatitis B virus (HBV) infection in causing hepatocellular carcinoma. Both agents increase the risk, but together they have a multiplicative carcinogenic effect (29). In MM, rather than a viral infection (HBV) and a toxin (aflatoxin-B1), we have a viral infection (SV40) and mineral fibers (asbestos) that together increase the risk of malignant transformation of mesothelial cells.
Materials and Methods
Reagents.
For information on cells, SV40, asbestos, and antibodies, see Supporting Materials and Methods.
SV40.
Wild-type SV40 can cause HM transformation in tissue culture and MM in hamsters independently of asbestos; therefore, it would have been difficult to study the mechanisms underlying the cocarcinogenesis of crocidolite and SV40 (14, 18). However, neither crocidolite alone nor SV40 viruses that do not express the small t antigen (tag), such as SV40 dl883, can cause malignant transformation of HM (14). Moreover, SV40 dl883 does not cause MM when injected into hamsters (6). Therefore, to study cocarcinogenesis with crocidolite, we used this virus. SV40 virions with deletions of the tag as well as strains of SV40 with different regulatory regions have been detected in human tumors and MM (30, 31).
Animal Experiments.
Twenty-one-day-old, female Syrian Golden hamsters were injected (under radiological guidance, to place the needle in the pleural space) with 0.4 mg of crocidolite suspended in PBS. Only one injection was performed in the pleura because the procedure is associated with a high risk of mortality. Crocidolite was also injected in the peritoneum of these same hamsters: 0.4 mg every 2 weeks, for a total of 4 mg of total asbestos load in the peritoneum. SV40 (diluted in DMEM, with 2% FBS) was injected into the left ventricle (108.5 pfu per animal). Additional details are provided in Supporting Materials and Methods.
Western Blotting.
Western blotting was as described in ref 14. The intensities of the bands were determined by using the rectangular box method from TIFF files imported into Scion Image 1.62c densitometry program of the public domain software NIH image (available at http://rsb.info.nih.gov/nih-image/) using three separate Western blots/experiments.
Binding of c-Jun to AP-1-Binding Site.
This assay was optimized from ref. 32. We used the AP-1 double-stranded oligonucleotide, 5′-GTTATTAAAGCATGAGTCAGACACC-3′ and AP-1 mutant, 5′-GTTATTAAAGCATGAGTTGGACACC-3′ (the AP-1-binding site is underlined and the mutation is in bold; only the sense strand is reported here) from the MMP-1 promoter (Fig. 7).
MMP-1 and MMP-9 activity assays, gene reporter constructs, expression vectors, transfections, reporter gene assays, and cell invasion assays, were according to standard procedures. For experimental details, see Supporting Materials and Methods.
Statistical Analysis.
All experiments were performed three or more times, and all of the data were independently analyzed by a statistician (P.V.) and expressed as mean values ± SD. For in vitro experiments, two-way analysis of variance was used to assess the individual and combined effects of crocidolite and SV40, as well as to test for their interaction. The interaction test was used to determine whether the combined effects of crocidolite were synergistic (greater than the sum of their individual effects). Pair-wise comparisons between treatment groups were done by using the Student–Newman–Keuls procedure with a significance level of 0.05 to adjust for multiple testing. Survival data from the animal experiment were analyzed by using the log-rank (Mantel–Cox) test. All statistical tests were two-sided, and differences were considered statistically significant at P < 0.05.
Supplementary Material
Acknowledgments
We thank Drs. C. Vinson [National Cancer Institute (NCI), Bethesda, MD], M. Diaz (Loyola University Chicago), and A. Aronheim (B. Rappaport Faculty of Medicine, Haifa, Israel) for the expression vectors; S. Eliasz for assistance with Western blots; and Dr. Antonio Pannuti for critical reading of the manuscript. This work was entirely supported by NCI Grants R01 CA092657, R01 CA106567, and ACS-National RSGCCE-106924 (to M.C.) and P01 CA114047 (to M.C., B.T.M., and H.I.P.).
Abbreviations
- AP-1
activator protein 1
- HM
primary human mesothelial cells
- MM
malignant mesothelioma
- MMP
matrix metalloprotease
- SHM
primary Syrian hamster mesothelial cells
- JDP2
c-Jun dimerization protein
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Pass HI, Vogelzang NJ, Hahn SM, Carbone M. In: Cancer Principles & Practice of Oncology. Pass HI, Vogelzang N, Carbone M, editors. VII Ed. Philadelphia: Lippincot Williams and Wilkins; 2005. pp. 1687–1715. [Google Scholar]
- 2.Ramos-Nino ME, Timblin CR, Mossman BT. Cancer Res. 2002;62:6065–6069. [PubMed] [Google Scholar]
- 3.Yang H, Bocchetta M, Kroczynska B, Elmishad GA, Chen Y, Liu Z, Bubici C, Mossman BT, Pass HI, Testa JR, et al. Proc Natl Acad Sci USA. 2006;103:10397–10402. doi: 10.1073/pnas.0604008103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu A, Zhou H, Yu DZ, Hei TK. Environ Health Perspect. 2002;110:1003–1008. doi: 10.1289/ehp.021101003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dogan AU, Baris YI, Dogan M, Emri S, Steele I, Elmishad AG, Carbone M. Cancer Res. 2006;66:5063–5068. doi: 10.1158/0008-5472.CAN-05-4642. [DOI] [PubMed] [Google Scholar]
- 6.Cicala C, Pompetti F, Carbone M. Am J Pathol. 1993;142:1524–1533. [PMC free article] [PubMed] [Google Scholar]
- 7.Butel JS, Lednicky JA. J Natl Cancer Inst. 1999;15:119–134. doi: 10.1093/jnci/91.2.119. [DOI] [PubMed] [Google Scholar]
- 8.Jasani B, Cristaudo A, Emri SA, Gazdar AF, Gibbs A, Krynska B, Miller C, Mutti L, Radu C, Tognon M, et al. Semin Cancer Biol. 2001;11:49–61. doi: 10.1006/scbi.2000.0346. [DOI] [PubMed] [Google Scholar]
- 9.Gazdar AF, Butel JS, Carbone M. Nat Rev Cancer. 2002;2:957–964. doi: 10.1038/nrc947. [DOI] [PubMed] [Google Scholar]
- 10.Wong M, Pagano JS, Schiller JT, Tevethia SS, Raab-Traub N, Gruber J. J Natl Cancer Inst. 2002;94:1832–1836. doi: 10.1093/jnci/94.24.1832. [DOI] [PubMed] [Google Scholar]
- 11.Klein G, Powers A, Croce C. Oncogene. 2002;21:1141–1149. doi: 10.1038/sj.onc.1205173. [DOI] [PubMed] [Google Scholar]
- 12.Dang-Tan T, Mahmud SM, Puntoni R, Franco EL. Oncogene. 2004;23:6535–6540. doi: 10.1038/sj.onc.1207877. [DOI] [PubMed] [Google Scholar]
- 13.Cutrone R, Lednicky J, Dunn G, Rizzo P, Bocchetta M, Chumakov K, Minor P, Carbone M. Cancer Res. 2005;65:10273–10279. doi: 10.1158/0008-5472.CAN-05-2028. [DOI] [PubMed] [Google Scholar]
- 14.Bocchetta M, Di Resta I, Powers A, Fresco R, Tosolini A, Testa JR, Pass HI, Rizzo P, Carbone M. Proc Natl Acad Sci USA. 2000;97:10214–10219. doi: 10.1073/pnas.170207097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cacciotti P, Barbone D, Porta C, Altomare DA, Testa JR, Mutti L, Gaudino G. Cancer Res. 2005;65:5256–5262. doi: 10.1158/0008-5472.CAN-05-0127. [DOI] [PubMed] [Google Scholar]
- 16.Cristaudo A, Foddis R, Vivaldi A, Buselli R, Gattini V, Guglielmi G, Cosentino F, Ottenga F, Ciancia E, Libener R, et al. Cancer Res. 2005;65:3049–3052. doi: 10.1158/0008-5472.CAN-04-2219. [DOI] [PubMed] [Google Scholar]
- 17.Eferl R, Wagner EF. Nat Rev Cancer. 2003;3:859–868. doi: 10.1038/nrc1209. [DOI] [PubMed] [Google Scholar]
- 18.Rundell K, Parakati R. Semin Cancer Biol. 2001;11:15–13. doi: 10.1006/scbi.2000.0341. [DOI] [PubMed] [Google Scholar]
- 19.Mechta-Grigoriou F, Gerald D, Yaniv M. Oncogene. 2001;20:2378–2389. doi: 10.1038/sj.onc.1204381. [DOI] [PubMed] [Google Scholar]
- 20.Liu Z, Ivanoff A, Klominek J. Int J Cancer. 2001;91:638–643. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1102>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 21.Westermarck J, Kahari VM. FASEB J. 1999;13:781–792. [PubMed] [Google Scholar]
- 22.Sato T, Koike L, Miyata Y, Hirata M, Mimaki Y, Sashida Y, Yano M, Ito A. Cancer Res. 2002;62:1025–1029. [PubMed] [Google Scholar]
- 23.Buder-Hoffmann S, Palmer C, Vacek P, Taatjes D, Mossman B. Am J Respir Cell Mol Biol. 2001;24:405–413. doi: 10.1165/ajrcmb.24.4.4290. [DOI] [PubMed] [Google Scholar]
- 24.Olive M, Krylov D, Echlin DR, Gardner K, Taparowsky E, Vinson C. J Biol Chem. 1997;272:18586–18594. doi: 10.1074/jbc.272.30.18586. [DOI] [PubMed] [Google Scholar]
- 25.Heinrich R, Livne E, Ben-Izhak O, Aronheim A. J Biol Chem. 2004;279:5708–5715. doi: 10.1074/jbc.M307608200. [DOI] [PubMed] [Google Scholar]
- 26.Kagan E, Jacobson RJ. Am J Clin Pathol. 1983;80:14–20. doi: 10.1093/ajcp/80.1.14. [DOI] [PubMed] [Google Scholar]
- 27.Saffiotti U. In: Malignant Mesothelioma. Pass HI, Vogelzang NJ, Carbone M, editors. New York: Springer; 2005. pp. 60–86. [Google Scholar]
- 28.Carbone M, Rdzanek MA, Rudzinski JJ, De Marco MA, Bocchetta M, Ramos Nino M, Mossman B, Pass HI. Cancer Res. 2005;65:10120–10121. doi: 10.1158/0008-5472.CAN-05-1911. [DOI] [PubMed] [Google Scholar]
- 29.Pagano JS, Blaser M, Buendia MA, Damania B, Khalili K, Raab-Traub N, Roizman B. Semin Cancer Biol. 2004;14:453–471. doi: 10.1016/j.semcancer.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 30.Carbone M, Rizzo P, Procopio A, Giuliano M, Pass HI, Gebhardt MC, Mangham C, Hansen M, Malkin DF, Bushart G, et al. Oncogene. 1996;13:527–535. [PubMed] [Google Scholar]
- 31.Lednicky JA, Butel JS. Semin Cancer Biol. 2001;11:19–47. doi: 10.1006/scbi.2000.0345. [DOI] [PubMed] [Google Scholar]
- 32.Chen BK, Chang WC. Proc Natl Acad Sci USA. 2000;97:10406–10411. doi: 10.1073/pnas.180321497. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.