Abstract
We have generated mice from a N-ethyl-N-nitrosourea mutagenesis screen that carry a mutation in the translation initiation codon of Gata-1, termed Plt13, which is equivalent to mutations found in patients with acute megakaryoblastic leukemia and Down syndrome. The Gata-1 locus is present on the X chromosome in humans and in mice. Male mice hemizygous for the mutation (Gata-1Plt13/Y) failed to produce red blood cells and died during embryogenesis at a similar stage to Gata-1-null animals. Female mice that carry the Plt13 mutation are mosaic because of random inactivation of the X chromosome. Adult Gata-1Plt13/+ females were not anemic, but they were thrombocytopenic and accumulated abnormal megakaryocytes without a concomitant increase in megakaryocyte progenitor cells. Gata-1Plt13/+ mice contained large numbers of blast-like colony-forming cells, particularly in the fetal liver, but also in adult spleen and bone marrow, from which continuous mast cells lines were readily derived. Although the equivalent mutation to Gata-1Plt13 in humans results in production of GATA-1s, a short protein isoform initiated from a start codon downstream of the mutated initiation codon, Gata-1s was not detected in Gata-1Plt13/+ mice.
Keywords: N-ethyl-N-nitrosourea mutagenesis, platelet
Gata-1 is a transcription factor that is essential for appropriate development of multiple hematopoietic lineages including erythroid, megakaryocytic, eosinophilic, and mast cells (1–4). In megakaryocyte development, Gata-1 and the transcriptional cofactor Friend of Gata-1 (Fog-1) regulate expression of genes that are critical for megakaryocyte maturation and platelet function, including the receptor for thrombopoietin (c-mpl), platelet factor 4 (pf4), cell-surface integrin GPIbα, and transcription factor NF-E2 (nuclear factor erythroid 2) (5–8). Mice that lack Gata-1 expression only in the megakaryocyte lineage (ΔneoΔHS) are viable but display marked thrombocytopenia and abnormal megakaryocytopoiesis (9).
In humans, GATA1 mutations have been associated with X-linked anemia and thrombocytopenia. Recently, GATA1 mutations have been identified in patients with acute megakaryoblastic leukemia accompanying Down syndrome (AML-M7 DS) (10, 11). The mutations occur in the second exon of GATA1 and are either small insertions or deletions that introduce premature stop codons or mutations that disrupt splicing or the start codon of GATA1 (10–13). These mutations all prevent translation of full-length GATA1 and result in the synthesis of a shorter GATA1 isoform (GATA1s) that is initiated at Met-84.
The N terminus of Gata-1 contains a highly acidic region, deletion of which reduces transactivation activity (14). Nevertheless, expression of Gata-1s at high levels is able to drive megakaryocytic maturation (7, 15, 16), and mice engineered to express Gata-1s using the endogenous Gata-1 promoter (Gata-1Δe2) show no signs of anemia or thrombocytopenia in adult life (17). These mice accumulate hyperproliferative megakaryocytic progenitors in fetal life, an abnormality that resolves spontaneously at birth. This phenotype resembles the transient myeloproliferative disorder associated with trisomy 21 that resolves shortly after birth and is followed later in life by AML-M7 DS in a proportion of affected children (18).
We report here a N-ethyl-N-nitrosourea (ENU)-induced mutation in the translation initiation codon of Gata-1, designated Plt13, which is analogous to mutations detected in patients with AML-M7 DS and transient myeloproliferative disorder (10–13). Gata-1Plt13/Y mice failed to produce red blood cells and died during embryogenesis at a similar stage to Gata-1-null animals. Female mice that carry the Plt13 mutation, mosaic because of lyonization of the X chromosome, were born at the expected frequency. Gata-1Plt13/+ mice displayed thrombocytopenia and accumulation of abnormal megakaryocytes. No significant increase in megakaryocyte progenitor cell numbers was observed in Gata-1Plt13/+ mice, but accumulation of abnormal blast-like colony-forming cells was evident in the fetal liver and persisted throughout adult life in the bone marrow and spleen. Molecular analyses showed that, unlike cells in humans bearing mutations in the initiation codon of GATA1, Gata-1Plt13/+ mice expressed no detectable Gata-1s protein.
Results
Plt13, an ENU-Induced Mutation in Gata-1.
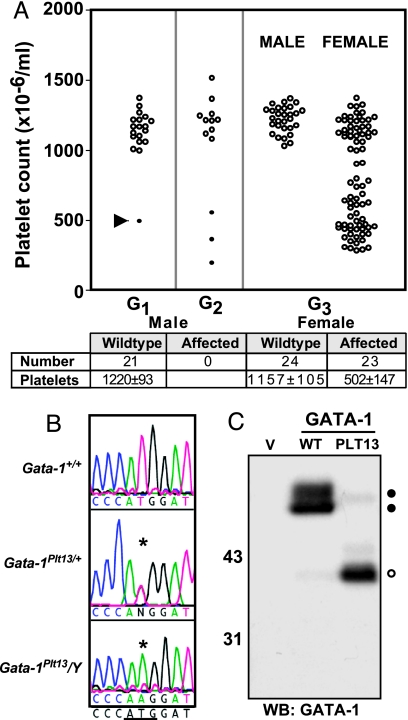
The founder of the PLT13 pedigree was a thrombocytopenic female mouse (platelet count of 495 × 106 per milliliter) identified among the G1 offspring of ENU-treated male BALB/c mice. Several female offspring of this founder exhibited a similarly reduced platelet count, and expansion of the pedigree by breeding affected G2 females with wild-type BALB/c mice revealed that whereas ≈50% of female mice were thrombocytopenic, no affected males were produced (Fig. 1A). This result established the heritability of the thrombocytopenia and suggested that the Plt13 mutation was X-linked, potentially with hemizygous lethality.
Fig. 1.
The Plt13 mutation affects the translation initiation codon of Gata-1. (A) The PLT13 founder (G1, arrow) was identified because of a low platelet count (495 × 106 per milliliter) and bred to test the heritability of the phenotype. G2 mice that displayed a reduced platelet count (filled circles) were selected for breeding, and data from their offspring (G3) were separated by sex to demonstrate that the phenotype was X-linked. Platelet counts from G3 mice were tabulated (mean ± SD), and mice that had a platelet count <800 × 106 per milliliter were considered affected. (B) DNA sequence from exon 2 of Gata-1 is shown for wild-type (Gata-1+/+) and affected (Gata-1Plt13/+ and Gata-1Plt13/Y) animals. The translation initiation codon of Gata-1 is underlined. (C) 293T cells were transfected with expression constructs for wild-type Gata-1 (WT), Gata-1Plt13 (PLT13), or vector alone (V). Lysates were separated by SDS/PAGE, and a Western blot was performed with an antibody that recognizes the C terminus of Gata-1. Filled circles mark full-length Gata-1, and an open circle marks truncated Gata-1.
In human populations, mutations in GATA1 have been associated with X-linked thrombocytopenia (19–22). Accordingly, genomic DNA was isolated from affected Gata-1Plt13/+ mice for analysis of Gata-1 by DNA sequencing. A single base pair change (T→A transversion) was identified in the second exon of Gata-1 in affected mice, which disrupts the translation initiation codon (ATG→AAG) (Fig. 1B). This mutation was not detected in unaffected littermates and was absent in stock BALB/c mice as well as other inbred mouse strains including C57BL/6 and 129/Sv. In transfected 293T cells, the Gata-1Plt13 cDNA directed synthesis of a 40-kDa protein that was recognized by an antibody directed against the C terminus of Gata-1 but which was ≈10 kDa smaller than Gata-1 expressed from a wild-type cDNA (Fig. 1C). Unlike wild-type Gata-1, the Gata-1Plt13 protein did not react with an antibody that recognizes the N terminus of the protein (data not shown), and no protein of wild-type Gata-1 size was detected in transfected cells expressing the mutant allele. These data suggest that the Plt13 mutation prevents expression of full-length Gata-1, analogous to mutant GATA1 alleles in patients with AML-M7 DS (10, 11).
The Gata-1Plt13 Mutation Results in Hemizygous Lethality.
Gata-1Plt13/Y embryos were present at the expected Mendelian frequency at embryonic day 10.5 [Gata-1+/Y (10), Gata-1Plt13/Y (7), Gata-1+/+ (8), and Gata-1Plt13/+ (8)]; however, by embryonic day 13.5 Gata-1Plt13/Y embryos were present at a reduced frequency [Gata-1+/Y (15), Gata-1Plt13/Y (6), Gata-1+/+ (17), and Gata-1Plt13/+ (12)], were smaller than wild-type embryos, were pallid consistent with severe anemia, and upon histological examination exhibited regions of necrosis and little or no fetal liver (data not shown). Whereas no Gata-1Plt13/Y mice survived to birth, female heterozygote (Gata-1Plt13/+) embryos exhibited no evidence of anemia or other abnormalities and were born at the expected frequency.
Abnormal Megakaryocytopoiesis in Gata-1Plt13/+ Mice.
Analysis of peripheral blood from a cohort of female Gata-1Plt13/+ mice confirmed a 60% reduction in the number of circulating platelets when compared with wild-type littermates, but there was no significant increase in mean platelet volume (Table 1). Although Gata-1 is an essential regulator of red blood cell maturation, affected mice displayed normal numbers of red blood cells and a normal hematocrit. White blood cell counts were within the normal range (Table 1).
Table 1.
Peripheral blood profile of Gata-1Plt13/+ mice
| Blood cell parameter | Genotype |
|
|---|---|---|
| Gata-1+/+ | Gata-1Plt13/+ | |
| Platelet count (×10−6 per ml) | 1,009 ± 139 | 473 ± 143* |
| Mean platelet volume, fl | 7.1 ± 1.0 | 8.3 ± 0.8 |
| Red cell count (×10−9 per ml) | 11.1 ± 0.3 | 10.4 ± 0.8 |
| Hematocrit, % | 53.3 ± 1.7 | 52.4 ± 3.9 |
| White cell count (×10−6 per ml) | 9.2 ± 1.6 | 9.1 ± 1.9 |
| No. of neutrophils | 1.3 ± 0.3 | 1.3 ± 0.4 |
| No. of lymphocytes | 7.6 ± 1.4 | 7.5 ± 1.6 |
| No. of monocytes | 0.06 ± 0.02 | 0.06 ± 0.02 |
| No. of eosinophils | 0.16 ± 0.02 | 0.10 ± 0.04 |
Means ± SD are shown (n = 54–58 mice per group). Statistical analysis was by ANOVA with correction for multiple testing (* P < 0.001).
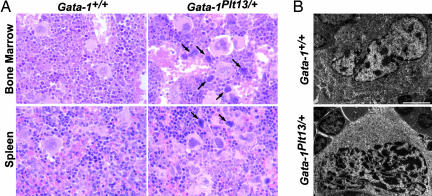
The spleen and bone marrow of Gata-1Plt13/+ mice demonstrated a significant accumulation of megakaryocytes (Figs. 2A and 3A), and a uniform finding in all Gata-1Plt13/+ mice was the presence of small numbers of single megakaryocytes in the liver and lungs of adult mice. The majority of megakaryocytes appeared abnormal, commonly with hyperchromatic nuclei surrounded by scant areas of cytoplasm. Electron microscopic examination revealed abnormalities in the demarcation membrane system with mutant megakaryocytes containing large disorganized clumps of membrane or lacking a demarcation membrane system entirely (Fig. 2B). Modal DNA ploidy in bone marrow megakaryocytes was increased from 16 N in wild-type mice to 32 N in Gata-1Plt13/+ females, and the mutant mice showed an increased abundance of megakaryocytes with high DNA (64 N and 128 N) content (Fig. 3B).
Fig. 2.
Gata-1Plt13/+ mice exhibit megakaryocytosis and abnormal megakaryocyte maturation. (A) Accumulation of megakaryocytes in the bone marrow and spleen of Gata-1Plt13/+ mice. In Gata-1Plt13/+ tissues a significant number of megakaryocytes demonstrated hyperchromatic nuclei and a reduced amount of cytoplasm (arrows). (B) Electron micrographs of megakaryocytes from wild-type mice demonstrate the expansive cytoplasm and complex demarcation membrane system that are characteristic of these cells. Gata-1Plt13/+ megakaryocytes have large, multilobed nuclei but generally have reduced cytoplasm with a poorly developed demarcation membrane system. (Scale bar: 5 μm.)
Fig. 3.
The number and DNA content of megakaryocytes are increased in Gata-1Plt13/+ mice. (A) The number of megakaryocytes enumerated from microscopic examination of histological sections of bone marrow (BM) and spleen from wild-type (open bars) and Gata-1Plt13/+ (filled bars) mice expressed as the number of megakaryocytes per 10 high-power microscopic fields (×600 for bone marrow and ×200 for spleen). (B) The DNA content of wild-type (open bars) and Gata-1Plt13/+ (filled bars) bone marrow megakaryocytes was analyzed by flow cytometry using CD41 staining in combination with propidium iodide. Mean values are presented from two to four mice per point, and error bars represent standard deviations. ∗, P < 0.05; ∗∗, P < 0.001. Statistical analysis was by ANOVA and pairwise comparison with Bonferroni's correction for multiple testing.
Megakaryocytes in spleens of wild-type mice stained prominently with antibodies directed to either the N or C terminus of Gata-1 protein (Fig. 4A). Random X inactivation in Gata-1Plt13/+ females would be expected to result in half the megakaryocytes expressing the wild-type allele and half expressing Gata-1Plt13. However, only 10–20% of Gata-1Plt13/+ megakaryocytes stained with the N-terminal antibody, which detects protein from the wild-type allele. This observation suggests that the increased number of megakaryocytes in Gata-1Plt13/+ tissues was due to the selective accumulation of mutant cells. Furthermore, the majority of megakaryocytes in Gata-1Plt13/+ mice exhibited no staining with either N- or C-terminal Gata-1 antibodies (Fig. 4B), suggesting that if the truncated Gata-1s protein is expressed in mutant megakaryocytes in vivo then the amount is below detection and likely to be considerably lower than the amount of wild-type Gata-1 expressed in Gata-1+ megakaryocytes.
Fig. 4.
Analysis of Gata-1 protein expression in megakaryocytes. Spleen sections were stained with antibodies that recognize the N terminus (in cyan) and the C terminus (in red) of Gata-1. CD41 staining was used to identify splenic megakaryocytes (in green). (A) In wild-type mice megakaryocytic nuclei stain with both N- and C-terminal antibodies. (B) There is a significant accumulation of megakaryocytes in Gata-1Plt13/+ mice, and many of these appear negative for Gata-1 staining (arrows).
Hematopoietic Progenitor Cells in Gata-1Plt13/+ Mice.
Despite the increased numbers of megakaryocytes in Gata-1Plt13/+ mice, there was a reduction in the number of megakaryocyte progenitor cells in the bone marrow, and, although colony numbers were elevated in the spleen and feral liver (Table 2), colony size was unaffected. The numbers of colonies containing granulocytes, macrophages, or eosinophils were relatively normal in cultures of Gata-1Plt13/+ bone marrow stimulated by stem cell factor plus IL-3 plus erythropoietin (Table 2). Spleen cell cultures from Gata-1Plt13/+ mice revealed significantly elevated numbers of granulocyte and macrophage progenitor cells (Table 2). This extramedullary hematopoiesis was associated with a slight splenomegaly [Gata-1Plt13/+ spleen weight, 172 ± 29 mg (n = 12); Gata-1+/+ spleen weight, 106 ± 12 mg (n = 4)].
Table 2.
Hematopoietic progenitor cells in Gata-1Plt13/+ mice
| Tissue | Gata-1 genotype | No. of colonies |
|||||
|---|---|---|---|---|---|---|---|
| Blast/mast | G | GM | M | Eo | Meg | ||
| Bone marrow | +/+ | 16 ± 3 | 17 ± 10 | 5 ± 2 | 11 ± 4 | 2 ± 1 | 18 ± 3 |
| Plt13/+ | 29 ± 5 | 16 ± 3 | 9 ± 3 | 14 ± 5 | 1 ± 1 | 7 ± 2 | |
| Spleen | +/+ | 1 ± 1 | 0.2 ± 0.3 | 0 | 0.5 ± 0.5 | 0 | 0.5 ± 1 |
| Plt13/+ | 13 ± 3 | 2 ± 2 | 1 ± 2 | 5 ± 2 | 0.1 ± 0.3 | 4 ± 2 | |
| Fetal liver | +/+ | 8 ± 8 | 12 ± 8 | 7 ± 9 | 2 ± 4 | 0 | 7 ± 8 |
| Plt13/+ | 313 ± 97 | 18 ± 15 | 23 ± 17 | 93 ± 55 | 0 | 33 ± 13 | |
Data represent the means and SD of colony numbers in cultures from 3–11 mice of each genotype stimulated with stem cell factor plus IL-3 plus erythropoietin. Data have been standardized to colonies per 25,000 cultured cells. GM, granulocyte–macrophage colonies; G, granulocyte colonies; M, macrophage colonies; Eo, eosinophil colonies; Meg, megakaryocyte colonies.
Significant numbers of unusual colonies containing cells with a blast-like appearance distinguished the Gata-1Plt13/+ bone marrow and spleen cultures from those of wild-type mice, and similar colonies were found at a much higher frequency in fetal liver cultures (Table 2). These colonies differed from typical blast colonies in that none were multicentric. Individual blast-like colonies isolated from agar cultures could be maintained for at least several months as continuous cell lines in liquid cultures stimulated by stem cell factor plus IL-3, but they failed to grow in the absence of added cytokines. Cytocentrifuge preparations of cells from the continuous lines revealed a distinctive mast cell morphology (Fig. 5A). An analysis of the cell surface phenotype demonstrated that the cells were expressing c-Kit, CD9, and CD41 but were negative for other lineage markers (Ter119, B220, Gr1, and Mac1). Examination of GATA-1 mRNA from 15 cell lines confirmed expression of Gata-1Plt13 mRNA and absence of wild-type Gata-1 mRNA (Fig. 5B). As observed in primary Gata-1Plt13/+ megakaryocytes (Fig. 4B), no Gata-1 protein was detected in these cells, consistent with little or no expression of Gata-1s from the mutant allele (Fig. 5C).
Fig. 5.
Cell lines derived from Gata-1Plt13/+ mice have a mast cell phenotype. (A) Cells from a continuous mast cell line derived from a blast-like colony from Gata-1Plt13/+ bone marrow were cytocentrifuged and stained with May–Grunwald–Giemsa. (B) RT-PCR was performed on RNA extracted from continuous cell lines and from bone marrow (BM) with primers that flank the site of the Plt13 mutation (468-bp product). Digestion with NcoI cleaves the wild-type PCR product, resulting in two fragments (381 bp and 87 bp), but not the product from the Gata-1Plt13 allele. (C) Lysates from cell lines were immunoprecipitated with an antibody that binds to the C terminus of Gata-1 (+) or a control IgG from goat (−). Western blotting with the C-terminal M20 antibody demonstrated that Gata-1s protein (open circle) was absent in mast cell lines from Gata-1Plt13/+ mice, whereas the protein was detectable in lysates from transfected 293T cells. IgG heavy chain is marked by an arrow.
Discussion
The founder of the Plt13 pedigree was identified in an ENU mutagenesis screen because of its significantly reduced platelet count. Upon sequencing, the Plt13 mutation was found to disrupt the translation initiation codon of Gata-1. Mutations that disrupt the initiation codon of the human GATA1 mRNA result in the production of a truncated GATA1 protein, GATA1s, which lacks the N-terminal transactivation domain (23). Patients with such mutations exhibit clonal accumulation of megakaryoblasts that resolves shortly after birth. However, in ≈20% of such cases, AML-M7 DS develops within 4 years of life (18). Mice engineered to express Gata-1s in place of full-length Gata-1 (Gata-1Δe2 mice) are born at the expected frequency and exhibit normal red blood cell production. Consistent with the transient myeloproliferative disorder in humans, Gata-1Δe2 mice transiently accumulate large numbers of hyperproliferative megakaryocyte progenitor cells in the yolk sac and fetal liver. This abnormality resolves, and adult Gata-1Δe2 mice display normal megakaryocytopoiesis and platelet numbers (17).
In contrast, although Gata-1Plt13/+ mice display myeloproliferation in the fetal liver, this phenotype is associated with accumulation of blast-like colony-forming cells with mast cell potential rather than excessive numbers of megakaryocyte progenitors. In adult hematopoietic tissues, the abnormal blast-like colony-forming cells persist in Gata-1Plt13/+ mice and are accompanied by an accumulation of megakaryocytes without significant expansion of megakaryocyte progenitors. The Gata-1Plt13 megakaryocytes exhibit morphological abnormalities and higher than normal modal DNA ploidy, and the mice were thrombocytopenic, suggesting that the dysmorphic megakaryocytes are unable to efficiently release platelets. Inefficient platelet production may result from reduced expression of Gata-1 target genes including NF-E2 and GpIbα, both of which are known to have a critical role in megakaryocyte maturation and platelet shedding (24, 25). The thrombocytopenia and megakaryocytosis in Gata-1Plt13/+ mice resemble more the phenotype of mice lacking Gata-1 expression in megakaryocytes, such as Gata-1ΔneoΔHS and Gata-1low, than that of mice that express Gata-1s. However, the excessive number of megakaryocytes in Gata-1ΔneoΔHS and Gata-1low mice is due to the presence of hyperproliferative megakaryocyte progenitors, and the megakaryocytes that accumulate are immature with low DNA content (6, 9), whereas in Gata-1Plt13/+ mice megakaryocyte progenitor cell numbers are not elevated, and the megakaryocytes that accumulate have large and complex nuclei with a high DNA content. The blast-like colonies observed in Gata-1Plt13/+ mice failed to grow in the presence of thrombopoietin, were uniformly negative for acetylcholinesterase activity, and had mast cell morphology when grown in liquid culture. Highly proliferative mast cell progenitors have also been identified in Gata-1low mice; however, unlike the Gata-1Plt13/+ progenitors, the colonies derived from Gata-1low mice also contained megakaryocytes and erythroid cells (2).
High-level expression of the Gata-1Plt13 mRNA in 293T cells confirmed that full-length Gata-1 protein could not be produced from this allele but resulted instead in the production of a truncated protein predicted to initiate from Met-84 that was equivalent in size to Gata-1s. Analysis of Gata-1 protein from the endogenous Gata-1Plt13 mRNA in cells derived from Gata-1Plt13/+ mice demonstrated no detectable expression of Gata-1s in megakaryocytes or in cell lines derived from blast-like colonies, suggesting that translation is initiated at Met-84 with low efficiency in vivo in Gata-1Plt13/+ mice, or that the truncated protein is unstable and rapidly degraded. Consistent with little or no expression of Gata-1 from the Gata-1Plt13 allele, Gata-1Plt13/Y males died at a similar stage to Gata-1-null embryos (1, 3) and exhibited no signs of fetal red blood cell production.
Thus, unlike cells in humans bearing mutations in the translation initiation codon of GATA1 or Gata-1Δe2 mice, which express Gata-1s, there is no detectable expression of full-length Gata-1 or Gata-1s from the Gata-1Plt13 allele, and this is highly likely to account for the differences in the phenotypes observed between these distinct mouse and human models. In the Gata-1Δe2 mice, deletion of exon 2 removes two additional AUG codons that are upstream of the Met-84 translation initiation site for Gata-1s (17). These start sites are out of frame with the Gata-1 ORF and may reduce the efficiency of translation of Gata-1s in Gata-1Plt13/+ mice. Interestingly, only one of these upstream AUG codons is evident in the human GATA-1 mRNA, potentially explaining the capacity of human GATA1 alleles with mutations in the usual translation initiation codon to express GATA1s. A germ-line mutation in the second exon of GATA1 has recently been described in a family with heritable macrocytic anemia in the absence of trisomy 21 (26). In contrast to the murine model (Gata-1Δe2), this finding would suggest that the production of GATA-1s, in the context of GATA1 mutation, is unable to support normal erythropoiesis in humans. Thus, species differences and precise levels of expression of Gata-1 and Gata-1s appear critical to the specific effects of Gata-1 mutations on regulation of megakaryocytopoiesis and erythropoiesis and the potential for development of megakaryoblastic leukemia. The development of mouse models with diverse mutations in Gata-1, in which these variables are differentially affected, have established powerful tools for dissecting the mechanism of Gata-1 action in blood cell regulation in health and disease.
Materials and Methods
ENU Mutagenesis.
Male BALB/c mice were injected with 200–400 mg/kg ENU (Sigma, St. Louis, MO) and then mated to untreated BALB/c females to produce G1 offspring. At 7 weeks of age G1 mice were bled and platelet numbers were determined by using an Advia 120 automated hematological analyzer (Bayer, Tarrytown, NY).
DNA Sequencing and Genotyping.
Genomic DNA was isolated from Gata-1Plt13/+ mice, and exonic regions were amplified by PCR in reactions containing 20 ng of DNA, 2 mM MgCl2, 50 μM dNTPs, 2 units of Taq polymerase, and 10 pmol of each primer (for exon two of Gata-1: forward, 5′-TCTCAGTGACAGATTCGGAGAA-3′; reverse, 5′-GCTAACCATCTCTATGGCAACC-3′). PCR products were sequenced by using Big Dye Terminator V3.1 (Applied Biosystems, Carlsbad, CA) before processing on an ABI 3700 sequence analyzer.
RT-PCR.
The Gata-1 ORF was amplified by PCR from bone marrow cDNA and cloned into the pEFBOS mammalian expression vector incorporating a C-terminal Flag epitope, and a Plt13 mutant version was generated via PCR mutagenesis. To distinguish between wild-type and mutant mRNA, RT-PCR primers were designed to flank the second exon of Gata-1 (forward, 5′-CTTGGGATCACCCTGAACTC-3′; reverse, 5′-GCTCTTCCCTTCCTGGTCTT-3′), and the resulting PCR products were digested with NcoI.
Immunoprecipitation and Western Blotting.
293T cells grown in DMEM with 10% FCS were transfected with expression constructs by using FuGENE 6 reagent (Roche Diagnostics, Indianapolis, IN). Cells were lysed after 48 h in KALB buffer (1 mM EDTA/150 mM NaCl/50 mM Tris/1% Triton X-100, pH 7.4), and proteins were separated by SDS/PAGE. Protein was transferred to a PVDF membrane and blotted with an antibody directed against the C terminus of Gata-1 (M-20, Santa Cruz Biotechnology, Santa Cruz, CA). Lysates prepared in KALB buffer were immunoprecipitated overnight with biotinylated goat anti-human GATA-1 (R & D Systems, Minneapolis, MN).
Hematology.
Manual or automated counts were performed on blood collected into EDTA. In vitro colony assays for enumeration of hematopoietic progenitors were performed as described (27). Bone marrow (2.5 × 104), spleen (5 × 104), or fetal liver (2.5 × 103) cells were cultured for 7 days in 1 ml of 0.3% agar in DMEM supplemented with 20% FCS and recombinant cytokines. Individual colonies were isolated then maintained in liquid culture in DMEM with 10% FCS and recombinant cytokines.
DNA Ploidy.
Bone marrow was collected from femurs and tibias into 1 ml of CATCH buffer (Hank's balanced salt solution with 3% BSA, 1.3 mM sodium citrate, 1 mM adenosine, 2 mM theophylline, and 3% FCS) and stained with FITC-conjugated anti-CD41 antibody (Becton Dickinson, Franklin Lakes, NJ) on ice. Samples were incubated with propidium iodide (0.05 mg/ml in 3.4 mM sodium citrate) for 1 h on ice. Cells were washed twice in CATCH buffer, aggregates were removed with a 100-μm sieve, and samples were treated with 50 μg/ml RNase H for 30 min at room temperature before analysis on a FACScan flow cytometer (Becton Dickinson).
Immunofluorescence Microscopy.
Frozen spleen sections (5 μm) were fixed sequentially in 4% paraformaldehyde and methanol, washed with PBS/1% BSA, and incubated for 1 h with 2 μg/ml rabbit anti-C-terminal GATA1 (Sigma) or 1 μg/ml rat anti-N-terminal GATA1 (Santa Cruz Biotechnology). Slides were washed and incubated with 2 μg/ml Alexa Fluor 546-conjugated anti-rabbit antibody (Invitrogen, Carlsbad, CA) and 1 μg/ml Cy5-conjugated anti-rat antibodies (Jackson ImmunoResearch, West Grove, PA) for 1 h then washed, blocked with 1 μg/ml purified rat IgG2a (Becton Dickinson), and incubated with 1 μg/ml FITC-conjugated anti-CD41 antibody (Becton Dickinson) and 0.5 μg/ml DAPI (Sigma) for 30 min. Purified rabbit IgG (2 μg/ml; Zymed, San Francisco, CA), rat IgG2a (1 μg/ml; Becton Dickinson), and FITC-conjugated rat IgG1 (1 μg/ml; Becton Dickinson) were used as isotype control primary antibodies. Images were captured with a TCS-SP2 confocal microscope (Leica, Bannockburn, IL).
Electron Microscopy.
After perfusion of mice with 2% glutaraldehyde, 2.5% paraformaldehyde, 0.1 M sodium cacodylate, and 2 mM calcium chloride (pH 7.4), femurs were immersion-fixed for a further 4 h before rinsing in PBS and decalcifying in 270 mM EDTA (pH 7.4) for 7 days at 4°C. Bones were washed three times in PBS/5% sucrose for 15 min then postfixed in 2% osmium tetroxide in PBS for 1 h and rinsed in distilled water before dehydration through graded concentrations of ethanol before infiltration and embedding in Spurr's resin. Ultra thin sections were stained with both methanolic uranyl acetate and lead citrate before viewing in a transmission electron microscope at 60 kV (Hitachi, Tokyo, Japan). Images were collected using a Megaview-III CCD camera (Soft Imaging Systems, Münster, Germany).
Acknowledgments
We thank Erin Salt, Marc Sacco, Kelly Trueman, and Katya Gray for excellent animal husbandry and the staff of the microscopy core at the Peter MacCallum Cancer Centre for technical assistance. This work was supported by The Cancer Council Victoria and the Australian National Health and Medical Research Council (Program Grant 257500). I.J.M. is supported by an Australian Postgraduate Award.
Abbreviations
- ENU
N-ethyl-N-nitrosourea
- AML-M7 DS
acute megakaryoblastic leukemia and Down syndrome.
Footnotes
The authors declare no conflict of interest.
References
- 1.Fujiwara Y, Browne CP, Cunniff K, Goff SC, Orkin SH. Proc Natl Acad Sci USA. 1996;93:12355–12358. doi: 10.1073/pnas.93.22.12355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Migliaccio AR, Rana RA, Sanchez M, Lorenzini R, Centurione L, Bianchi L, Vannucchi AM, Migliaccio G, Orkin SH. J Exp Med. 2003;197:281–296. doi: 10.1084/jem.20021149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weiss MJ, Keller G, Orkin SH. Genes Dev. 1994;8:1184–1197. doi: 10.1101/gad.8.10.1184. [DOI] [PubMed] [Google Scholar]
- 4.Yu C, Cantor AB, Yang H, Browne C, Wells RA, Fujiwara Y, Orkin SH. J Exp Med. 2002;195:1387–1395. doi: 10.1084/jem.20020656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamaguchi Y, Zon LI, Ackerman SJ, Yamamoto M, Suda T. Blood. 1998;91:450–457. [PubMed] [Google Scholar]
- 6.Vyas P, Ault K, Jackson CW, Orkin SH, Shivdasani RA. Blood. 1999;93:2867–2875. [Google Scholar]
- 7.Muntean AG, Crispino JD. Blood. 2005;106:1223–1231. doi: 10.1182/blood-2005-02-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stachura DL, Chou ST, Weiss MJ. Blood. 2006;107:87–97. doi: 10.1182/blood-2005-07-2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shivdasani RA, Fujiwara Y, McDevitt MA, Orkin SH. EMBO J. 1997;16:3965–3973. doi: 10.1093/emboj/16.13.3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wechsler J, Greene M, McDevitt MA, Anastasi J, Karp JE, Le Beau MM, Crispino JD. Nat Genet. 2002;32:148–152. doi: 10.1038/ng955. [DOI] [PubMed] [Google Scholar]
- 11.Xu G, Nagano M, Kanezaki R, Toki T, Hayashi Y, Taketani T, Taki T, Mitui T, Koike K, Kato K, et al. Blood. 2003;102:2960–2968. doi: 10.1182/blood-2003-02-0390. [DOI] [PubMed] [Google Scholar]
- 12.Rainis L, Bercovich D, Strehl S, Teigler-Schlegel A, Stark B, Trka J, Amariglio N, Biondi A, Muler I, Rechavi G, et al. Blood. 2003;102:981–986. doi: 10.1182/blood-2002-11-3599. [DOI] [PubMed] [Google Scholar]
- 13.Hirose Y, Kudo K, Kiyoi H, Hayashi Y, Naoe T, Kojima S. Leukemia. 2003;17:2250–2252. doi: 10.1038/sj.leu.2403121. [DOI] [PubMed] [Google Scholar]
- 14.Martin DI, Orkin SH. Genes Dev. 1990;4:1886–1898. doi: 10.1101/gad.4.11.1886. [DOI] [PubMed] [Google Scholar]
- 15.Visvader JE, Crossley M, Hill J, Orkin SH, Adams JM. Mol Cell Biol. 1995;15:634–641. doi: 10.1128/mcb.15.2.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shimizu R, Takahashi S, Ohneda K, Engel JD, Yamamoto M. EMBO J. 2001;20:5250–5260. doi: 10.1093/emboj/20.18.5250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Z, Godinho FJ, Klusmann JH, Garriga-Canut M, Yu C, Orkin SH. Nat Genet. 2005;37:613–619. doi: 10.1038/ng1566. [DOI] [PubMed] [Google Scholar]
- 18.Hitzler JK, Zipursky A. Nat Rev Cancer. 2005;5:11–20. doi: 10.1038/nrc1525. [DOI] [PubMed] [Google Scholar]
- 19.Balduini CL, Pecci A, Loffredo G, Izzo P, Noris P, Grosso M, Bergamaschi G, Rosti V, Magrini U, Ceresa IF, et al. Thromb Haemostasis. 2004;91:129–140. doi: 10.1160/TH03-05-0290. [DOI] [PubMed] [Google Scholar]
- 20.Nichols KE, Crispino JD, Poncz M, White JG, Orkin SH, Maris JM, Weiss MJ. Nat Genet. 2000;24:266–270. doi: 10.1038/73480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu C, Niakan KK, Matsushita M, Stamatoyannopoulos G, Orkin SH, Raskind WH. Blood. 2002;100:2040–2045. doi: 10.1182/blood-2002-02-0387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mehaffey MG, Newton AL, Gandhi MJ, Crossley M, Drachman JG. Blood. 2001;98:2681–2688. doi: 10.1182/blood.v98.9.2681. [DOI] [PubMed] [Google Scholar]
- 23.Calligaris R, Bottardi S, Cogoi S, Apezteguia I, Santoro C. Proc Natl Acad Sci USA. 1995;92:11598–11602. doi: 10.1073/pnas.92.25.11598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ware J, Russell S, Ruggeri ZM. Proc Natl Acad Sci USA. 2000;97:2803–2808. doi: 10.1073/pnas.050582097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shivdasani RA, Rosenblatt MF, Zucker-Franklin D, Jackson CW, Hunt P, Saris CJ, Orkin SH. Cell. 1995;81:695–704. doi: 10.1016/0092-8674(95)90531-6. [DOI] [PubMed] [Google Scholar]
- 26.Hollanda LM, Lima CS, Cunha AF, Albuquerque DM, Vassallo J, Ozelo MC, Joazeiro PP, Saad ST, Costa FF. Nat Genet. 2006;38:807–812. doi: 10.1038/ng1825. [DOI] [PubMed] [Google Scholar]
- 27.Alexander WS, Roberts AW, Nicola NA, Li R, Metcalf D. Blood. 1996;87:2162–2170. [PubMed] [Google Scholar]