Abstract
Increasing levels of CO2 in the atmosphere are expected to cause climatic change with negative effects on the earth's ecosystems and human society. Consequently, a variety of CO2 disposal options are discussed, including injection into the deep ocean. Because the dissolution of CO2 in seawater will decrease ambient pH considerably, negative consequences for deep-water ecosystems have been predicted. Hence, ecosystems associated with natural CO2 reservoirs in the deep sea, and the dynamics of gaseous, liquid, and solid CO2 in such environments, are of great interest to science and society. We report here a biogeochemical and microbiological characterization of a microbial community inhabiting deep-sea sediments overlying a natural CO2 lake at the Yonaguni Knoll IV hydrothermal field, southern Okinawa Trough. We found high abundances (>109 cm−3) of microbial cells in sediment pavements above the CO2 lake, decreasing to strikingly low cell numbers (107 cm−3) at the liquid CO2/CO2-hydrate interface. The key groups in these sediments were as follows: (i) the anaerobic methanotrophic archaea ANME-2c and the Eel-2 group of Deltaproteobacteria and (ii) sulfur-metabolizing chemolithotrophs within the Gamma- and Epsilonproteobacteria. The detection of functional genes related to one-carbon assimilation and the presence of highly 13C-depleted archaeal and bacterial lipid biomarkers suggest that microorganisms assimilating CO2 and/or CH4 dominate the liquid CO2 and CO2-hydrate-bearing sediments. Clearly, the Yonaguni Knoll is an exceptional natural laboratory for the study of consequences of CO2 disposal as well as of natural CO2 reservoirs as potential microbial habitats on early Earth and other celestial bodies.
Keywords: anaerobic oxidation of methane, chemolithotroph, CO2 disposal, CO2 hydrate, liquid CO2
The release of one-carbon compounds (i.e., CO2 and methane) into the atmosphere due to human activities has been recognized as a major factor causing dramatic climatic change on the Earth. In this century, the increasing concentrations of greenhouse gases are expected to cause warmer surface temperatures at an accelerating rate and subsequent alternation of ecosystems and biogeochemical cycles (1). The emission of massive amounts of CO2 already has altered the chemistry of surface seawater worldwide (2), and its current concentration exceeds the amount of CO2 that can be absorbed by the ocean (3). To reduce CO2 emissions into the atmosphere, a variety of options have been discussed, including the disposal of CO2 into the deep sea (4, 5). Pilot studies have been carried out to test the long-term disposal of CO2 as liquid and/or hydrates in the deep sea (6–8). However, the impact of CO2 disposal on deep-sea ecosystems remains largely unknown. Negative consequences on diversity, abundance, and functioning of deep-water communities have been predicted because the dissolution of CO2 in seawater will decrease pH considerably (4, 9). It remains unknown whether physicochemical or geobiological sinks for excess CO2 may control the pH effect, such as the formation and burial of CO2 hydrates and the assimilation of CO2 into microbial biomass.
In deep-sea hydrothermal vent and cold-seep ecosystems, CO2, methane, and sulfide emissions support unique microbial ecosystems (e.g., refs. 10–13). Some types of magmatism cause a dramatic CO2 enrichment in hydrothermal fluids (14). Natural emission of liquid and hydrated CO2 droplets was first observed at the Izena Hole of the Mid-Okinawa Trough backarc hydrothermal system (15). Buoyant fluids containing 86–91% CO2, with H2S and CH4 as residual gases, were released from the seafloor at 1,400 m depth and a bottom water temperature of 3.8°C (15). However, fishery equipment covering the Izena Hole has prevented further investigations of this intriguing site. Very recently, venting of liquid CO2 (98% CO2 in droplets) has been observed at the northwest Eifuku submarine volcanoes of the northern Mariana Arc (16). The composition of microbial communities of methane hydrate-bearing sediments has been investigated (e.g., refs. 10 and 11), nothing is known regarding the presence of microbial communities in liquid CO2 and CO2-hydrate environments. Because liquid CO2 is widely used for dry cleaning clothes for its organic solvent-like properties, the sediments vented by liquid CO2 may not be habitable. However, astrobiologists have speculated that life could be hosted by and interact with liquid and/or solid CO2 on Earth and other celestial bodies. Recently, the southern pole on Mars was found to be mainly composed of ice and solid CO2 (17) and is considered as a potential habitable zone for life on Mars because the extremely high levels of biologically damaging solar UV radiation may be significantly reduced at the polar caps (18). Here we report studies of the diversity and biogeochemistry of the microbial community inhabiting liquid CO2/CO2-hydrate-bearing deep-sea sediments at the Yonaguni Knoll IV hydrothermal field of the southern Okinawa Trough.
Results and Discussion
Sample Collection at the Yonaguni Knoll IV Hydrothermal Field.
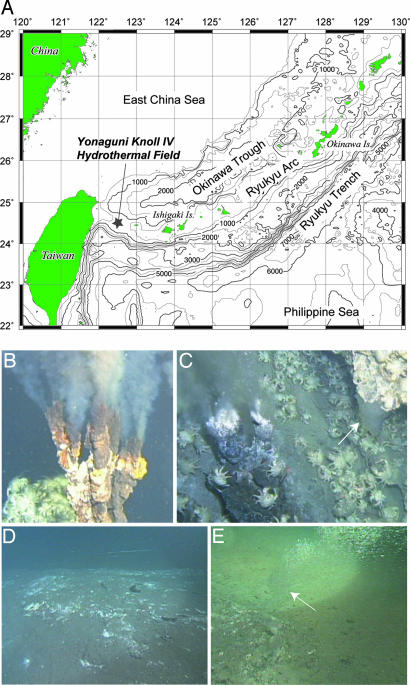
During the YK03-05 and YK04-05 expeditions in 2003 and 2004, we investigated the Yonaguni Knoll IV hydrothermal field (24°50.7′N, 122°42.1′E; 1,370–1,385 m water depths) by using the R/V Yokosuka and the manned submersible Shinkai 6500 (Fig. 1A). The hydrothermal field is characterized by two active black smoker vents (Tiger and Lion chimneys) (Fig. 1B), with vent fluid temperatures up to 323°C. Near the black smokers, vapor-rich clear venting fluids and small liquid CO2 droplets were observed emanating from the sediment (Fig. 1C). Approximately 50 m southward from the hydrothermal vents, down a gentle slope, we found a liquid CO2 lake below a 20–40 cm thick cover of sediments (Movie 1). On this seafloor structure, we observed white patches and pavements from which small droplets of liquid CO2 were leaking, so-called “CO2-hydrate zone” (>200 m2, Fig. 1D). Benthic fauna was rarely observed. After the retrieval of a sediment core from one of the white patches, the continuous emission of liquid CO2 through the cored hole was observed (Fig. 1E). The in situ temperature in this area increased from 3.9°C in the overlying bottom water to 9.9°C at 35 cm sediment depth, indicating the migration of hydrothermal fluids (Fig. 5, which is published as supporting information on the PNAS web site). At 1,380 m depth, liquid CO2 is less dense than water (6). Hence, the question arises as to how gaseous/liquid CO2 can accumulate into the lake. At 13.5 MPa pressure, hydrate stability is reached at temperatures <10°C (6, 9, 15). Hence, at in situ bottom temperatures of 4°C, liquid CO2 may react with seawater to form a solid ice-like hydrate (CO2·6H2O) that, because of its higher density, can cap the liquid CO2 reservoir (15). Indeed, our visual inspection indicated a layer of CO2 hydrates (<10 cm thickness) below the sediment cover, which likely acts as a cap structure for the underlying liquid CO2 lake (Fig. 2A and Movie 1). To investigate the distribution and composition of microbial communities in the liquid CO2/CO2-hydrate-bearing sediments, representative samples of sediments (core 819NK, 35 cm in length) and seafloor pavement (819p, 10 cm in thickness), covering the liquid CO2/CO2-hydrate transition zone (Fig. 2A), were collected for geochemical and microbiological analyses. Additionally, a sediment core was taken 50 m away from the CO2-hydrate zone as a reference (core 818).
Fig. 1.
Overview of the Yonaguni Knoll IV hydrothermal field at the southern Okinawa Trough. (A) Location map of the Yonaguni Knoll IV. (B) “Lion chimney,” one of the most active black smoker vents in this field. (C) “Crystal chimney,” one of the vapor-rich clear smoker vents adjacent to the Tiger black smoker. The emission of liquid CO2 droplets was observed in close proximity. (D) White patchy area, “CO2-hydrate zone,” ≈50 m southward from the Tiger chimney. (E) Continuous emission of liquid CO2 droplets from the “CO2 lake” overlying hydrates. White arrows in C and E indicate the emission of liquid CO2 droplets. The video of these hydrothermal events recorded by DSV Shinkai 6500 is available as Movie 1, which is published as supporting information on the PNAS web site.
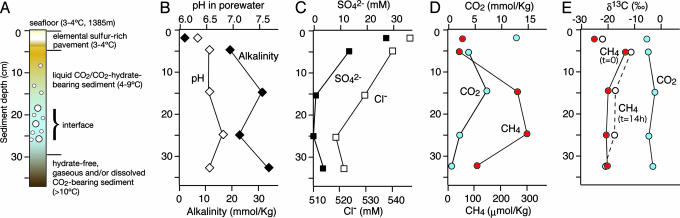
Fig. 2.
Scheme of the examined sediment sample from the “CO2-hydrate zone” and related geochemical depth profiles. (A) Location of liquid CO2/CO2-hydrate interface, sediment cover, and pavement in core 819NK, and in situ temperatures. (B) Profiles of pH (open diamonds) and alkalinity (as CaCO3, filled diamonds) in pore waters. (C) Concentrations of SO42− (filled squares) and Cl− (open squares) in pore water. (D) Concentration of methane and CO2 in headspace. (E) Carbon isotopic compositions of methane (red circles, t = 0; white circles, t = 14 h) and CO2 (light blue circles) in headspace. The data from top layer (single points) and sediment column (connected by lines) correspond to 819 pavement and 819NK sediment core, respectively, both obtained from the CO2-hydrate zone.
Chemical Characteristics.
The pore water chemistry of the cores collected from the sediments overlying the CO2 lake showed distinct differences to sediments taken outside the hydrate/liquid CO2 zone. Alkalinity in core 819NK (>20 mmol/kg) was one order of magnitude higher than that in the reference cores (<3 mmol/kg) (Fig. 2B), reflecting the migration of CO2-rich fluids into the sediment cover of the CO2 lake. Also, the concurrent decrease in sulfate and chloride pore water concentrations of core 819NK with depth (Fig. 2B) indicates upward or lateral fluid migration but also could be due to melting of CO2 hydrates upon recovery. The pH measured onboard in pore water of core 819NK and pavement samples were 6.6 and 6.3, respectively (Fig. 2B), in contrast to a pH of ≈7.3 outside of the CO2-hydrate zone in the Yonaguni Knoll IV hydrothermal field (data not shown). However, in situ pH at the liquid CO2 interface below the sediment cover may be considerably lower than onboard results and theoretically may reach pH 4 taking into account the alkalinity, CO2 concentration, and ambient pressure of the system (19). We measured >300 mM CO2 in vent fluids and >20 mM CO2 in pore water of sediments at the Yonaguni Knoll hydrothermal system (ref. 20; see Fig. 2D). The emanating liquid CO2 droplets collected in situ by a gas-tight water sampling system (21) were composed mostly of CO2 containing a small amount of methane (CO2, 85.10 ± 2.12%; CH4, 13.95 ± 2.05%; n = 2), with hydrogen concentrations below the detection limit (<0.01 mM). The stable carbon isotopic compositions of CO2 and CH4 in these droplets were −7.0‰ and −26.4‰, respectively, which were very similar to those from the Tiger chimney vent fluids (−7.6‰ and −26.3‰, respectively). These similar isotopic values indicate that both CO2 and CH4 in the CO2-hydrate zone have the same origin as in the hydrothermal fluids and that they have not been altered significantly by microbial activities [e.g., by methanogenesis, and/or anaerobic oxidation of methane (AOM)].
X-ray diffraction (XRD) analysis showed that the sediments and pavements in the CO2-hydrate zone consisted mainly of elemental sulfur, quartz, and montmorillonite. They were devoid of carbonates, probably due to the low pH of pore water in sediments of the CO2-hydrate zone. Average organic carbon content in pavement and sediment core samples was 1.12 ± 0.30% and 0.76 ± 0.02% [wt/wt], respectively. XRD analysis and carbon, nitrogen, and sulfur analysis with an elemental analyzer showed large amounts of elemental sulfur [15≈60% (wt/wt) as total sulfur compounds; Fig. 5] in the samples, which may have been deposited by hydrothermal processes and associated CO2 migration. The microbial oxidation of hydrothermally derived hydrogen sulfide also may contribute to sulfur deposition in the sediment cover above the CO2 reservoir. Further investigations are needed to understand whether microbial processes contribute to sediment consolidation and pavement formation and, hence, to the capping of the liquid CO2 reservoir in this zone.
Cell Abundance and Archaea Population.
Acridine orange direct counts of microbial cells revealed that the pavement sample harbored >109 cells·cm−3 integrated over a 10-cm sediment depth. In contrast, sediments at the liquid CO2/CO2-hydrate interface in core 819NK contained only ≈107 cells·cm−3. Notably, all microbial parameters indicate a decline toward the liquid CO2 interface (Fig. 5). Quantitative real-time PCR analysis of 16S rRNA genes by using a specific probe and primer set for Archaea (22) indicated that archaeal 16S rRNA genes were abundant in the upper core 819NK sediment and pavement samples (maximum 30% of total 16S rRNA genes; see Fig. 5) but decreased considerably toward the interface with liquid CO2/CO2 hydrates.
Diversity of Microbial Community.
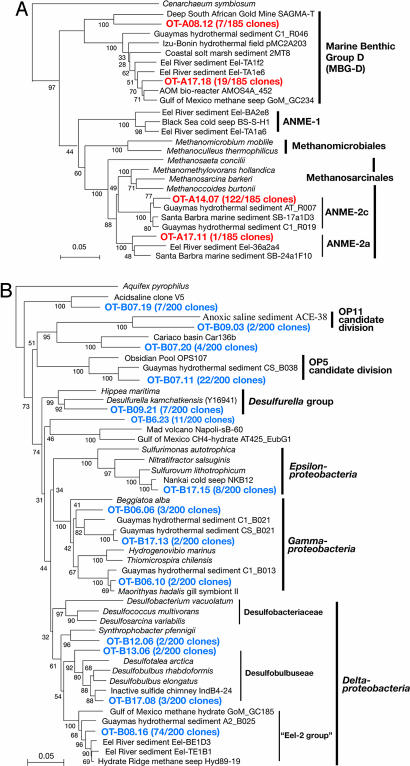
Sequence analysis revealed that the most frequently detected phylotypes belonged to the ANME-2 group archaea and sulfate-reducing bacteria (SRB) belonging to a cluster of Deltaproteobacteria previously found associated with cold methane-seep environments (10, 12, 23, 24). The representative sequence of OT-A14.07 (122 of 185 total archaea clones with similarity cutoff at 97%) was affiliated with the ANME-2c group and that of OT-B08.16 (74 of 200 total bacteria clones with similarity cut off at 97%) belonged to the Eel-2 group (alternatively classified as Seep SRB-2 group) within the Deltaproteobacteria (ref. 24; Fig. 3). Sequences related to the genus Desulfobulbus, which contains potential AOM-associated sulfate-reducing bacterias (25), also were detected, albeit as minor percentages of the total bacterial sequences. Interestingly, the composition of the sulfate reducer guild in sediments containing liquid CO2/CO2 hydrate substantially was different from that previously reported for cold-seep sediments accompanied with high methane fluxes. For example, the members of the Eel-2 group, which dominated here have been only minor components of cold-seep communities (25–27), and sequences affiliated with the Desulfococcus/Desulfosarcina group, which is the typical consortium partner of ANME I and II cells (10, 12, 23–27), never were detected.
Fig. 3.
Phylogenetic trees of archaeal (A) and bacterial (B) 16S rRNA gene sequences from the liquid CO2-hydrate hydrothermal system as marked in red and blue, respectively. The trees were inferred by neighbor-joining analysis by using manually aligned homologous positions of 16S rRNA sequences. The number of related clones with a 97% similarity sequence cutoff are indicated in parentheses. The results of 100 bootstrap trials are shown at each phylogenetic branch. (Scale bars: 0.05-nt substitution per sequence position.)
We also studied the methyl coenzyme M reductase α-subunit gene (mcrA), a key gene of methanogenesis and anaerobic methanotrophy (12, 28). Compared with the 16S rRNA gene library, the analysis of the mcrA gene clone library revealed a higher diversity of ANME phylotypes, most of which belonged to ANME-2 mcrA group-d and -e (ref. 28; see phylogenetic tree in Fig. 6, which is published as supporting information on the PNAS web site). Neither 16S rRNA nor mcrA sequences related to hydrogenotrophic methanogens were detected from the CO2-hydrate zone. We tried to cultivate hydrogenotrophic methanogens; however, no growth was observed. These microbiological results are consistent with the fact that the hydrogen concentration in the liquid CO2 droplets was less than the detection limit.
A number of other phylotypes representing relatively minor components of the microbial community were retrieved from the liquid CO2/CO2-hydrate-bearing sediments. The second most abundant archaeal phylotype based on 16S rRNA gene sequences belonged to the Marine Benthic Group-D (MBG-D) (Fig. 3A), which is a phylotype that is commonly found to co-occur with ANME sequences in methane-driven seep systems. However, the function of members of this group remains unknown (10, 12, 25). In the bacterial clone libraries, sequences of Gamma- and Epsilonproteobacteria, the OP5 and OP11 candidate divisions, and a few unknown groups were detected. Some of these phylotypes are closely related to previously cultured chemolithotrophs (Fig. 3B). For example, the Epsilonproteobacteria sequences were related closely to sulfur-oxidizing chemolithoautotrophic bacterium Sulfurovum lithotrophicum (29) that uses the reductive tricarboxylic acid cycle for carbon assimilation (30, 31). Gammaproteobacteria sequences were related to sulfur- and/or hydrogen-oxidizing chemolithoautotrophs (or chemolithomixotrophs) such as Beggiatoa, Hydrogenovibrio, and Thiomicrospira. In addition, the ribulose 1,5-bisphosphate carboxylase large subunit gene (cbbL), which is a key gene for chemoautotrophic CO2 assimilation via the Calvin-Benson cycle, was amplified successfully from surface sediments of core 819NK sediment and pavement samples. The sequence of OT-cbbL1.07 was the most abundant cbbL phylotype (24 of 32 total cbbL clones), which is related to the obligatory chemolithoautotrophic sulfur-oxidizing Gammaproteobacteria Thioalkalivibrio thiocyanoxidans and Hydrogenovibrio marinus (Fig. 7, which is published as supporting information on the PNAS web site). These molecular results suggest the co-occurrence of a diverse group of sulfur-metabolizing chemolithotrophs with the AOM community in the liquid CO2/CO2-hydrate-bearing marine sediments.
AOM Activity.
To determine whether methane is being produced or consumed, we anaerobically incubated freshly collected sediments from the CO2-hydrate zone on board and monitored changes in carbon isotopic composition (δ13C) of methane over time. In both 819NK sediment core and pavement samples, the headspace methane became slightly enriched in 13C (–1.12 ± 1.15‰: n = 5) after a 14-h incubation at 4°C (Fig. 2E), indicating that methane is oxidized anaerobically in these sediments. We also measured in vitro anaerobic methane consumption coupled to sulfate reduction by incubating sediment slurries diluted with methane-enriched medium for sulfate-reducing bacteria with 35SO4 and 14CH4 tracers at 4°C (32). Significant but low AOM [1–8 nmol·g of dry weight (gdw)−1·d−1] and sulfate reduction rates (1–15 nmol·gdw−1·d−1) were measured in the examined sediments. These combined results indicate that methane oxidation indeed occurs in liquid CO2/CO2-hydrate-bearing sediments. The effect of pH and CO2-fluid migration on AOM and the growth and distribution of methanotrophic consortia above the CO2 lake needs further investigation.
Carbon Isotopic Composition of Lipid Biomarkers.
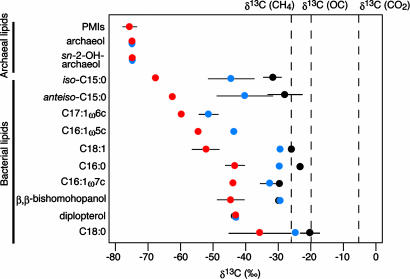
Further evidence for the in situ assimilation of CH4/CO2 by the microbial community inhabiting the liquid CO2/CO2-hydrate-bearing sediments was provided by the stable carbon isotopic composition of specific lipid biomarkers. The δ13C values of components of archaeal origin such as archaeol, sn-2-hydroxyarchaeol and 2,6,10,15,19-pentamethylicosanes were strongly 13C-depleted (Fig. 4), indicating that methane plays a major role for the carbon and energy metabolisms of archaea in this habitat (10, 23, 27, 34–36). A similar 13C depletion, albeit slightly smaller, also was observed for the δ13C values of i-C15, ai-C15, and C17 fatty acids (Fig. 4), which are known to be abundant in sulfate-reducing bacteria (37, 38). Matching the occurrence of Desulfobulbus sequences, we also detected their typical biomarker C17:1ω6c (37) in the pavement samples with δ13C values of –59.8‰. In accordance with the depth profiles of bacterial counts by acridine orange direct counts, the concentrations of lipid biomarkers derived from organisms associated with AOM was significantly higher in the pavement samples than in the underlying liquid CO2-bearing sediments (Fig. 8, which is published as supporting information on the PNAS web site). This decrease in abundance of AOM-related lipid biomarkers could be related to decreasing sulfate availability but also to other factors such as decreasing pH levels in the liquid CO2-vented sediments.
Fig. 4.
Carbon isotopic compositions of archaeal and bacterial lipids extracted from 819p pavements (red) and 819NK core sediments (blue) in the CO2-hydrate zone and 818 core sediments (black) obtained from outside of the CO2-hydrate zone (>50 m). Carbon isotopic compositions of CO2 and methane were obtained from liquid CO2 droplets collected in situ by the gas-tight sampler. The δ13C value of algal organic carbon (OC) was calculated from the δ13C value of phytol from core 818, assuming an isotopic offset between phytoplanktonic biomass and phytol of +4‰ (33). Bars indicate SDs (n = 2–6).
Diplopterol, a bacterial biomarker, was detected as a major component in all sediment samples. The δ13C values of diplopterol were ≈–43‰ (Fig. 4), which is 13C-depleted relative to in situ CO2 and methane by 36‰ and 17‰, respectively. The 13C-depleted diplopterol could be derived either from aerobic methanotrophs or yet-unidentified chemolithotrophs (39). However, an aerobic methanotrophic origin for diplopterol is unlikely based on the genetic and organic geochemical data. No phylotypes capable of aerobic methane oxidation were observed, and no sequences related to the methane monooxygenase (pmoA and mmoX) genes (15), the key genes in aerobic methanotrophy, were detected. Consequently, we infer that the diplopterol is most likely derived from yet-unidentified chemolithotrophs. Even C16:0, which is a major fatty acid in many bacteria and eukaryotes, was depleted in 13C (–45‰) relative to CO2 and methane (Fig. 4). These isotopic values were notably different from those in sediments obtained from outside of the CO2-hydrate zone (core 818, see Fig. 4). The strong 13C depletion of archaeal and bacterial lipids indicates the dominance of microbial populations adapted to exploiting hydrothermal carbon sources in the form of CH4 and CO2.
Conclusion and Prospects
This investigation shows microbial diversity and function at the interface of liquid CO2 reservoir covered by CO2 hydrates in deep-sea sediments. Our findings demonstrate that this extreme habitat can be populated by an indigenous community of microbes assimilating one-carbon compounds and suggest that methane and sulfur oxidations are the main energy providing processes in the methane-containing liquid CO2/CO2-hydrate system. However, we observed a strong decline in cell numbers and abundance of specific lipid biomarkers toward the liquid CO2 interface on a scale of decimeters, indicating that these communities and their functioning may be affected by variations in CO2 concentrations and in situ pH. Critical parameters for further investigations are the stability of the liquid/solid CO2 interface and the role of fluid flow in pH, mass transport, and mineralogy of this hydrothermal system.
Materials and Methods
Sample Collection.
During the YK04-05 cruise in May 2004, the liquid CO2/CO2-hydrate-bearing sediments were collected by 35- or 50-cm-long push cores equipped with multiple temperature probes (interval 5 cm). Release of CO2 gas bubbles from the sediment cores was observed during the submersible rise to the sea surface as previously described by Sakai et al. (15). Emission of liquid CO2 was observed from a crack in the white-yellowish pavement and was collected by the submersible by using a WHATS gas-tight bottle sampler (21). Sediment cores and pavement samples were subsampled immediately by using 50-ml tip-cut sterilized syringes at 5-cm depth intervals and then prepared for microbiological and geochemical studies in the onboard laboratory.
Characterization of Chemistry in Pore Water and Liquid CO2.
Pore water was obtained from sediments within 3 h after recovery. The innermost part of a sediment core was transferred immediately into airtight 50-ml plastic syringes, and then pore water was extracted by pressure filtration through a 0.45-μm pore-size filter by using a stainless steel clamp at 4°C. Cl− and SO42− were measured with an ion chromatograph as described in refs. 12 and 15. Compound-specific concentrations and stable carbon isotopic compositions of CO2 and methane were measured by an isotope ratio-monitoring gas chromatograph-mass spectrometer (MAT252; Thermo Finnigan, Bremen, Germany) as described in ref. 40.
For incubation of core sediments, ≈3 cm3 sediment samples were collected by tip-cut syringe into 69 cm3 glass bottles with N2 gas in headspace. Each bottle then was sealed with a rubber stopper and incubated at 4°C for 14 h. Before and after incubation, the samples were poisoned with HgCl2 (6% wt/vol) and stored at 4°C until the measurement of the content and carbon isotopic composition of methane in the headspace.
DNA Extraction and Molecular Analyses.
DNA was extracted from 10 g of wet sediment by using MoBio Mega-prep Soil DNA Kit (Mo Bio Laboratories, Inc., Solana Beach, CA) according to the manufacturer's instructions and then purified (11). 16S rRNA, mcrA, cbbL, pmoA, and mmoX genes were amplified by PCR, and then phylogenetic analyses were carried out (11, 12, 30). The relative abundances of archaeal 16S rRNA genes among total prokaryotic 16S rRNA genes were estimated by the quantitative real-time PCR with domain Archaea-specific TaqMan probe and primer sets according to the protocol described in ref. 22.
AOM Activity.
To confirm AOM activity in sediments, we measured AOM rates in vitro by using anoxic sediment slurries stored at 4°C for a year. The stored slurry samples [≈10–15 ml containing 10% (vol/vol) sediment] were amended with anoxic artificial seawater containing 28 mM sulfate and 300 KPa methane in the headspace and then horizontally incubated at 4°C. After 1 month of preincubation, a significant production of sulfide (0.3–2.3 mM) was observed. The activated slurry samples then were subjected to the measurement of AOM and sulfate reduction rates according to protocols described in refs. 32 and 41. The rates were calculated based on one to six replicates depending on the total slurry volume available.
Total Organic Carbon, Total Sulfur, and Lipid Analyses.
Contents of total organic carbon and total sulfur were determined by using a carbon, nitrogen, and sulfur analyzer (Carlo Erba, Milan, Italy). The freeze-dried sediments were extracted ultrasonically with 2× methanol/2× dichloromethane:methanol [1:1(vol/vol)]/2× dicholoromethane to obtain the total lipid extracts. Aliquots of the total extracts were saponified after the addition of an internal standard (containing 1-nonadecanol, nonadecanoic acid, 5-cholestane, and hexatriacontane) with aqueous 0.5 M KOH in methanol (3 h at 80°C). Nonsaponifiable (neutral lipids) and acid fractions were sequentially extracted with hexane at pH ≈14 and 2, respectively. The neutral fractions were silylated with N,O-bis(trimethylsilyl)trifluoroacetamide in pyridine and analyzed by GC-MS (Trace GC-MS; Thermo Finnigan) for lipid identification and quantification. Repeated concentration measurements were within ±10%. Compound-specific δ13C analyses were performed by using an isotope ratio-monitoring GC-MS system (Delta Plus XP; Thermo Finnigan). The δ13C values for individual compounds are the means of duplicate runs (σ = ±0.3 to 0.6) expressed versus VPDB.
Supplementary Material
Acknowledgments
We acknowledge shipboard scientific parties of YK03-05 and YK04-05 cruises and crew and operation teams R/V Yokosuka and DSV Shinkai 6500 for helping us to collect deep-sea samples and thank K. Fujikara, M. Suzuki, G. Klockgether, and J. Wulf for technical assistances and D. Wolf-Gladrow, K. Knittel, and K. H. Nealson for useful discussions. Support of F.I. was provided in part by a research fellowship of the Alexander von Humboldt Foundation, Germany.
Abbreviation
- AOM
anaerobic oxidation of methane.
Footnotes
Conflict of interest statement: No conflicts declared.
Data deposition: The 16S rRNA, mcrA, and cbbL gene sequences reported in this paper have been deposited in the DNA Data Bank of Japan/European Molecular Biology Laboratory/GenBank databases (accession nos. AB252422–AB252455).
See Commentary on page .
References
- 1.Fung IY, Doney SC, Lindsay K, John J. Proc Natl Acad Sci USA. 2005;102:11201–11206. doi: 10.1073/pnas.0504949102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brewer PB. Geophys Res Lett. 1997;24:1367–1369. [Google Scholar]
- 3.Houghton JT, Jenkins GJ, Ephraums JJ. Climate Change: The IPCC Scientific Assessment. Cambridge, UK: Cambridge Univ Press; 1990. [Google Scholar]
- 4.Caldeira K, Akai M, Brewer P, Chen B, Haugan P, Iwama T, Johnston P, Kheshgi H, Li Q, Ohsumi T, et al. In: IPCC Special Report of Carbon Dioxide Capture and Storage, Metz B, Davidson O, de Coninck H, Loos M, Meyer L, editors. Cambridge, UK: Cambridge Univ Press; 2005. pp. 227–317. Chapter 6. [Google Scholar]
- 5.Holloway S. Ann Rev Ener Environ. 2001;26:145–166. [Google Scholar]
- 6.Brewer PG, Friederich G, Peltzer ET, Orr FM., Jr Science. 1999;284:943–945. doi: 10.1126/science.284.5416.943. [DOI] [PubMed] [Google Scholar]
- 7.Riestenberg DE, Tsouris C, Brewer PG, Peltzer ET, Waltz P, Chow AC, Adams EE. Environ Sci Technol. 2005;39:7287–7293. doi: 10.1021/es050125+. [DOI] [PubMed] [Google Scholar]
- 8.Brewer PG, Peltzer E, Aya I, Haugan P, Bellerby R, Yamane K, Kojima R, Walz P, Nakajima Y. J Oceanograph. 2004;60:751–758. [Google Scholar]
- 9.Brewer PG, Orr FM, Jr, Friederich G, Kvenvolden KA, Orange DL. Energy Fuels. 1998;12:183–188. [Google Scholar]
- 10.Boetius A, Ravenschlag K, Schubert CJ, Rickert D, Widdel F, Gieseke A, Amann R, Jørgensen BB, Witte U, Pfannkuche O. Nature. 2000;407:623–626. doi: 10.1038/35036572. [DOI] [PubMed] [Google Scholar]
- 11.Inagaki F, Nunoura T, Nakagawa S, Teske A, Lever M, Lauer A, Suzuki M, Takai K, Delwiche M, Colwell FS, et al. Proc Natl Acad Sci USA. 2006;103:2815–2820. doi: 10.1073/pnas.0511033103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inagaki F, Tsunogai U, Suzuki M, Kosaka A, Machiyama H, Takai K, Nunoura T, Nealson KH, Horikoshi K. Appl Environ Microbiol. 2004;70:7445–7455. doi: 10.1128/AEM.70.12.7445-7455.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakagawa S, Takai K, Inagaki F, Chiba H, Ishibashi J, Kataoka S, Hirayama H, Nunoura T, Horikoshi K, Sako Y. FEMS Microbiol Ecol. 2005;54:141–155. doi: 10.1016/j.femsec.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 14.Von Damm KL. Ann Rev Earth Planet Sci. 1990;18:173–204. [Google Scholar]
- 15.Sakai H, Gamo T, Kim E-S, Tsutumi M, Tanaka T, Ishibashi J, Wakita H, Yamano M, Oomori T. Science. 1990;248:1093–1096. doi: 10.1126/science.248.4959.1093. [DOI] [PubMed] [Google Scholar]
- 16.Lupton J, Butterfield D, Lilley M, Evans L, Nakamura K, Chadwick W, Jr, Resing J, Embley R, Olson E, Proskurowski G, et al. Geochim Geophys Geosys, 2006 Aug 10; doi: 10.1029/2005GC001152. [DOI] [Google Scholar]
- 17.Córdoba-Jabonero C, Zorzano M-P, Selsis F, Patel MR, Cockell CS. Icarus. 2005;175:360–371. doi: 10.1016/j.icarus.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 18.Bibring J-P, Langevin Y, Poulet F, Gendrin A, Gondet B, Berthé M, Soufflot A, Drossart P, Combes M, Bellucci G, et al. Nature. 2004;428:627–630. doi: 10.1038/nature02461. [DOI] [PubMed] [Google Scholar]
- 19.Zeebe RE, Wolf-Gladrow D. Elsevier Oceanography Series. Vol 65. Amsterdam: Elsevier; 2002. p. 346. [Google Scholar]
- 20.Konno U, Tsunogai U, Nakagawa F, Nakashima M, Ishibashi J, Nunoura T, Nakamura K. Geophys Res Lett, August 19 2006 doi: 10.1029/2006GL026115. [DOI] [Google Scholar]
- 21.Tsunogai U, Toki T, Nakayama N, Gamo T, Kato H, Kaneko S. Chikyukagaku. 2003;37:101–109. (Japanese with English abstract) [Google Scholar]
- 22.Takai K, Horikoshi K. Appl Environ Microbiol. 2000;66:5066–5072. doi: 10.1128/aem.66.11.5066-5072.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hinrichs K-U, Hayes JM, Sylva SP, Brewer PG, DeLong EF. Nature. 1999;398:802–805. doi: 10.1038/19751. [DOI] [PubMed] [Google Scholar]
- 24.Orphan VJ, House CH, Hinrichs K-U, McKeegan KD, DeLong EF. Appl Environ Microbiol. 2001;67:1922–1934. doi: 10.1128/AEM.67.4.1922-1934.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knittel K, Lösekann T, Boetius A, Kort R, Amann R. Appl Environ Microbiol. 2005;71:467–479. doi: 10.1128/AEM.71.1.467-479.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Teske A, Hinrichs K-U, Edgcomb V, Gomez AV, Kysela D, Sylva SP, Sogin ML, Jannasch HW. Appl Environ Microbiol. 2002;68:1994–2007. doi: 10.1128/AEM.68.4.1994-2007.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knittel K, Boetius A, Lemke A, Eilers H, Lochte K, Pfannkuche O, Linke P. Geomicrobiol J. 2003;20:269–294. [Google Scholar]
- 28.Hallam SJ, Girguis PR, Preston CM, Richardson PM, DeLong EF. Appl Environ Microbiol. 2003;69:5483–5491. doi: 10.1128/AEM.69.9.5483-5491.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inagaki F, Takai K, Nealson KH, Horikoshi K. Int J Syst Evol Micobiol. 2004;54:1477–1482. doi: 10.1099/ijs.0.03042-0. [DOI] [PubMed] [Google Scholar]
- 30.Takai K, Campbell BJ, Cary SC, Suzuki M, Oida H, Nunoura T, Hirayama H, Nakagawa S, Suzuki Y, Inagaki F, et al. Appl Environ Microbiol. 2005;71:7310–7320. doi: 10.1128/AEM.71.11.7310-7320.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suzuki Y, Sasaki T, Suzuki M, Nogi Y, Miwa T, Takai K, Nealson KH, Horikoshi K. Appl Environ Microbiol. 2005;71:5440–5450. doi: 10.1128/AEM.71.9.5440-5450.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Treude T, Boetius A, Knittel K, Wallmann K, Jørgensen BB. Mar Ecol Prog Ser. 2003;264:1–14. [Google Scholar]
- 33.Bidgare RR, Hanson KL, Buesseler KO, Wakeham SG, Freeman KH, Pancost RD, Millero FJ, Steinberg P, Popp B, Latasa M, et al. Paleoceanography. 1999;14:589–595. [Google Scholar]
- 34.Hayes JM. Rev Min Geochem. 2001;43:225–278. [Google Scholar]
- 35.Hinrichs K-U, Summons RE, Orphan V, Sylva SP, Hays JM. Org Geochem. 2000;31:1685–1701. [Google Scholar]
- 36.Pancost RD, Damste JSS, de Lint S, van der Maarel MJ, Gottschal JC, The Medinaut Shipboard Scientific Party Appl Environ Microbiol. 2000;66:1126–1132. doi: 10.1128/aem.66.3.1126-1132.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaneda T. Microbiol Rev. 1991;55:288–302. doi: 10.1128/mr.55.2.288-302.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elvert M, Boetius A, Knittel K, Jørgensen BB. Geomicrobiol J. 2003;20:403–419. [Google Scholar]
- 39.Hinrichs K-U, Hmelo LR, Sylva SP. Science. 2003;299:1214–1217. doi: 10.1126/science.1079601. [DOI] [PubMed] [Google Scholar]
- 40.Tsunogai U, Yoshida N, Ishibashi J, Gamo T. Geochim Cosmochim Acta. 2000;64:2439–2452. [Google Scholar]
- 41.Kallmeyer J, Ferdelman TG, Weber A, Fossing H, Jørgensen BB. Limnol Oceanogr Methods. 2004;2:171–180. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.