Abstract
Prior research has shown memory is enhanced for emotional events. Key brain areas involved in emotional memory are the amygdala and hippocampus, which are also recruited during aversion and its anticipation. This study investigated whether anticipatory processes signaling an upcoming aversive event contribute to emotional memory. In an event-related functional MRI paradigm, 40 healthy participants viewed aversive and neutral pictures preceded by predictive warning cues. Participants completed a surprise recognition task directly after functional MRI scanning or 2 weeks later. In anticipation of aversive pictures, bilateral dorsal amygdala and anterior hippocampus activations were associated with better immediate recognition memory. Similar associations with memory were observed for activation of those areas in response to aversive pictures. Anticipatory activation predicted immediate memory over and above these associations for picture viewing. Bilateral ventral amygdala activations in response to aversive pictures predicted delayed memory only. We found that previously reported sex differences of memory associations with left amygdala for women and with right amygdala for men were confined to the ventral amygdala during picture viewing and delayed memory. Results support an established animal model elucidating the functional neuroanatomy of the amygdala and hippocampus in emotional memory, highlight the importance of anticipatory processes in such memory for aversive events, and extend neuroanatomical evidence of sex differences for emotional memory.
Keywords: aversion, expectancy, functional MRI, neuroimaging, recognition memory
Memories for emotional events are more persistent and vivid than other memories (1–3). Previous research has shown that the amygdala and hippocampus are necessary for the enhanced memory observed for emotional material and contexts (4–12). Recent neuroimaging studies have shown that amygdala and hippocampus activation during encoding of emotional stimuli is related to better recollection of those stimuli (13–23). Other studies have shown that these areas already are recruited in anticipation of aversive emotional stimuli (24–29). Moreover, emotional arousal and emotional influences on attention and perception, which are emphasized in the literature on emotional memory (2–7, 30, 31), are key features of anticipating aversion. Specifically, the anticipation of aversive events is associated with heightened arousal, as indexed by startle (25, 32–35) and the modulation of perception and attention (36–39) that, in turn, is important for memory encoding (40–42).
The present functional MRI (fMRI) study tested whether activation of the amygdala and hippocampus during the anticipation of aversive events is related to subsequent memory of these events. Based on previous findings in our laboratory (24), we expected the dorsal amygdala and anterior hippocampus to be activated both in anticipation of and response to aversive pictures, consistent with the idea that there is one system that governs both processes (26, 43). Work highlighting the role of the amygdala, especially the dorsally located central nucleus of the amygdala (CeA), in modulating attention and perception of motivationally salient events (2, 3, 7, 44–52) leads to the prediction that dorsal amygdala activation in anticipation of and response to aversive pictures may show a particularly strong association with encoding and immediate recognition of those pictures. Research establishing the hippocampus as a primary neural substrate for episodic memory (53–57) suggests that anterior hippocampus activation in this study would predict both immediate and delayed recognition. Finally, based on the large body of literature implicating the basolateral amygdala (BLA; refs. 44, 47, 58, and 59) in consolidating emotional memories via modulation of the hippocampal system (2, 5, 6, 12, 16, 18), we expected to find ventral amygdala activation to be associated with delayed recognition.
Although two very recent studies have implicated anticipatory brain processes in subsequent memory (60, 61), neither examined the amygdala or memory of emotional stimuli. Designed to test contributions of anticipatory brain function to memory of aversive stimuli, this study synthesized two distinct lines of neuroscience work on anticipating aversion and emotional memory. We also directly assessed dorsal and ventral amygdala contributions to memory that follow from the aforementioned nonhuman animal research on the dorsally located CeA in attentional mechanisms linked with memory encoding and the ventrally located BLA in modulation of memory consolidation. The emphasis on anticipatory processes and sectors of the amygdala also enabled us to assess the specificity of previously reported sex differences for the left and right amygdala in subsequent memory for emotional events (14, 15, 21, 62).
Results
Memory and Rating Data.
Because Pr and d′ were strongly correlated for both aversive (r = 0.94, P < 0.001) and neutral (r = 0.90, P < 0.001) memory and showed highly similar effects for all analyses conducted, results reported here concentrate on Pr. For a repeated-measures valence (aversive, neutral) × sex (male, female) × memory session (time 1, time 2) multivariate ANOVA (MANOVA), the only effect was a valence × sex interaction, F(1,26) = 6.83, P = 0.01, η2 = 0.21. Separate MANOVAs for hit rate and false alarm rate indicated that this sex difference was present for hit rate, F(1,26) = 6.70, P = 0.02, η2 = 0.20, but not false alarm rate, F(1,26) = 1.47, P = 0.24, η2 = 0.05. The valence main effect for hit rate, F(1,26) = 9.94, P = 0.004, η2 = 0.28, indicating better recognition for aversive [mean (M) = 0.67, SD = 0.24] than neutral (M = 0.57, SD = 0.25) pictures, was particularly pronounced for women, F(1,26) = 14.86, P = 0.001, η2 = 0.36. The valence main effect for false alarm rate, F(1,26) = 16.72, P < 0.001, η2 = 0.39, indicated more false alarms for aversive (M = 0.35, SD = 0.24) than neutral (M = 0.20, SD = 0.19) pictures. The only other effect was for memory session in the MANOVA on hit rate, F(1,26) = 7.41, P = 0.01, η2 = 0.22, with more hits at time 1 than time 2.
A key variable contributing to emotional memory is arousal. In fact, a common strategy in previous neuroimaging research on emotional memory has been to categorize stimuli by subjects’ arousal ratings of the presented stimuli (13, 14, 20, 21). A valence (aversive, neutral) × sex (male, female) × memory session (time 1, time 2) multivariate ANOVA revealed a valence main effect, F(1,27) = 133.94, P < 0.001, η2 = 0.83, with aversive pictures (M = 4.09, SD = 1.11) rated as more highly arousing than neutral pictures (M = 1.40, SD = 1.02). There were no other significant main effects or interactions.
Activation for the Anticipation of and Response to Aversive Pictures.
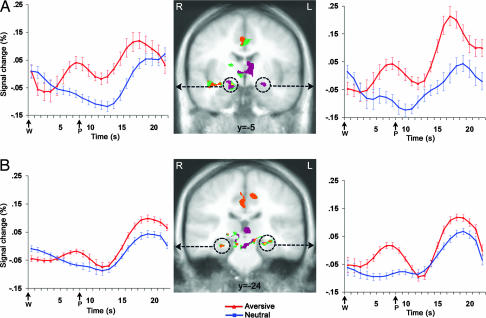
Replicating our previous work (24), both bilateral dorsal amygdala and anterior hippocampus demonstrated greater activation to the aversive trials than neutral trials (Fig. 1; see Table 1 and Fig. 4, which are published as supporting information on the PNAS web site), as indicated by the valence main effect for a period (anticipation, picture) × valence (aversive, neutral) whole-brain voxelwise ANOVA (P < 0.05, corrected). Post hoc t tests comparing aversive and neutral conditions in bilateral dorsal amygdala and anterior hippocampus for the anticipation and picture periods separately revealed more activation for the aversive than neutral anticipation period (all P values <0.006, except for the left dorsal amygdala, P < 0.09) and picture period (all P values <0.001). Additional areas activating more for the anticipation of and response to aversive pictures were similar to those found by Nitschke et al. (24) and are not central to present hypotheses for emotional memory.
Fig. 1.
Dorsal amygdala and anterior hippocampus activation during both the anticipation of and response to aversion. Circled bilateral dorsal amygdala (A) and bilateral anterior hippocampus (B) regions showed greater activation for aversive than neutral trials across anticipation and picture periods. For each brain image, all colored areas showed a valence main effect for the whole-brain period × valence ANOVA (P < 0.05, corrected; Table 1). Orange areas also showed greater activation for aversive than neutral trials during the anticipation period but not the picture period (aversive–neutral contrasts as indicated by corresponding whole-brain t tests, P < 0.05, corrected). In contrast, purple areas also showed greater activation for aversive than neutral trials during the picture period but not the anticipation period (aversive–neutral contrasts, as noted above). Yellow areas showed greater activation for aversive than neutral trials for the valence effect and for the aversive–neutral contrast for each period, whereas green areas for the valence main effect did not meet the P < 0.05 (corrected) threshold for either contrast. The view of both brains is indicated by the relevant Talairach coordinate. Time series plots of the circled clusters illustrate average percentage signal change across all time points of the aversive (red) and neutral (blue) trials. The onset of the picture (P) occurred 6–10 s after offset of the warning cue (W). Error bars for time series plots are for confidence intervals (63) around the mean after adjusting for between-subject variance (64). R, right; L, left.
Relationship Between Brain Activation and Memory.
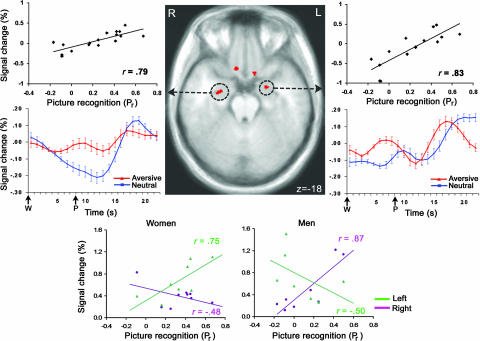
To determine whether the amygdala was specifically associated with recognition of the aversive pictures, whole-brain voxelwise regressions were run for immediate (time 1) and delayed (time 2) recognition memory. Recognition was regressed on the difference between activation to the aversive condition and activation to the neutral condition for the anticipation and picture periods separately. Greater ventral amygdala activation during the picture period correlated with delayed recognition of the aversive pictures on both the right, r = 0.79, P < 0.001, and the left, r = 0.83, P < 0.001 (Figs. 2 and 4 and Table 1; see Fig. 5, which is published as supporting information on the PNAS web site).
Fig. 2.
Ventral amygdala activation correlated with subsequent memory performance. Activation in circled left and right ventral amygdala regions was correlated with recognition 2 weeks after scanning (time 2). Whole-brain regressions at P < 0.05 (corrected) of recognition on the contrast comparing aversive to neutral pictures revealed that increased activation to the aversive pictures was associated with better recognition of the aversive pictures. The scatter plot for each circled cluster shows the relationship of recognition memory to activation for the aversive–neutral contrast used for the whole-brain regressions to identify these ventral amygdala areas. The scatter plots under the brain images for women and men separately show the relationship of recognition memory to activation for aversive pictures only, the metric used in previous neuroimaging studies showing sex differences in amygdala memory function (14, 15, 21). Description for brain images and time series plots is the same as provided in Fig. 1 legend. For right ventral amygdala cluster, n = 17 (9 women). For left ventral amygdala cluster, n = 15 (8 women) because of signal loss for two subjects (see Supporting Methods, which is published as supporting information on the PNAS web site). Ventral amygdala correlations for d′ were highly similar to those shown here for Pr (see Fig. 5).
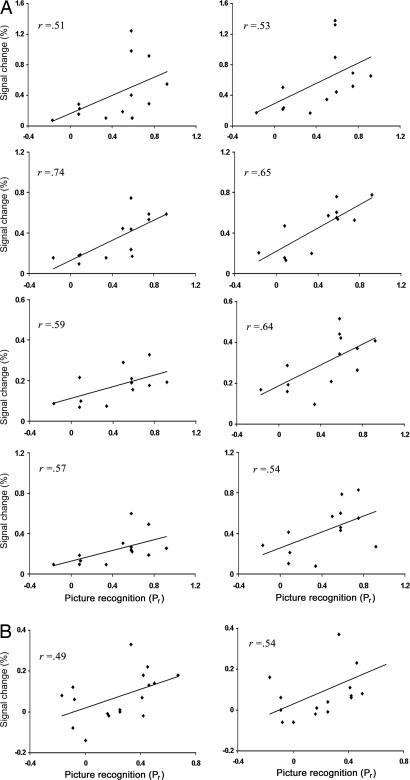
In addition, the bilateral dorsal amygdala and anterior hippocampus activations to aversion found for the voxelwise ANOVA valence main effect were correlated with immediate and delayed recognition of the aversive pictures. Greater aversive than neutral activation was calculated by subtracting activation to the neutral condition from activation to the aversive condition in the statistically defined regions of interest (ROIs) for each period on a single subject basis. Greater bilateral dorsal amygdala and anterior hippocampus activations both in anticipation of and response to the aversive pictures were correlated with better recognition of the aversive pictures directly after the scan (Fig. 3A; see Table 2, which is published as supporting information on the PNAS web site). Moreover, hierarchical regressions revealed that activation during the anticipation period showed unique associations with immediate memory, accounting for 9–20% of variance in Pr (18–25% of variance in d′) beyond the variance explained by activation during picture viewing across the bilateral dorsal amygdala and hippocampus ROIs. In addition, further analyses revealed a reliable dissociation between the dorsal and ventral amygdala activations predicting memory. Anticipatory activation in the left dorsal amygdala (r = 0.74, P = 0.004) showed a stronger correlation than the left ventral amygdala (r = 0.32, P = 0.28) with immediate memory for Pr, t(10) = 1.78, P = 0.11 (for d′, t(10) = 2.33, P = 0.04). Conversely, for picture viewing, ventral amygdala activation showed stronger correlations with delayed memory than dorsal amygdala activation on the right, t(14) = 3.07, P = 0.008, and the left, t(12) = 2.63, P = 0.02. Two weeks after the scan, bilateral anterior hippocampus and right dorsal amygdala activation during the picture period were moderately correlated with better recognition of the aversive pictures (Fig. 3B; see Table 3, which is published as supporting information on the PNAS web site).
Fig. 3.
Dorsal amygdala and anterior hippocampus correlated with subsequent memory performance. (A) Example scatter plots of correlations between activation during aversive trials in the dorsal amygdala and anterior hippocampus clusters shown in Fig. 1 and recognition of the aversive pictures directly after the scan (time 1, n = 13). (Left) Plots demonstrate correlations between activation during the anticipation of aversive pictures and immediate memory for the aversive pictures. (Right) Plots demonstrate correlations between activation in response to aversive pictures and immediate memory for aversive pictures. Plots are, from top to bottom, right dorsal amygdala, left dorsal amygdala, right anterior hippocampus, and left anterior hippocampus. (B) Example plots of correlations between activation in response to aversive pictures for the right and left anterior hippocampus shown in Fig. 1 and recognition of the aversive pictures 2 weeks after scanning (Time 2, n = 17). As indicated in Tables 2 and 3, correlations for d′ were highly similar to those shown here for Pr.
Sex Differences in Relationship Between Brain Activation and Memory.
To test for sex differences, correlations were run separately for women and men by using the statistically defined bilateral dorsal amygdala, ventral amygdala, and anterior hippocampus clusters from the aforementioned whole-brain analyses. The only sex differences observed were in the ventral amygdala for picture viewing. Delayed recognition of the aversive pictures for women was associated primarily with left ventral amygdala activation in response to the aversive pictures, whereas delayed recognition of the aversive pictures for men was associated primarily with right ventral amygdala activation in response to the aversive pictures (Figs. 2 and 5). To assess laterality, t tests compared the correlations for the left and right clusters. For women, the correlation with the left ventral amygdala (r = 0.75, P = 0.03) was greater than with the right ventral amygdala (r = −0.48, P = 0.19), t(5) = 5.44, P = 0.003. For men, the correlation with the right ventral amygdala (r = 0.87, P = 0.005) was greater than with the left ventral amygdala (r = −0.50, P = 0.256), t(4) = 4.76, P = 0.008. Removing extreme values does not alter the patterns observed (62). Corresponding correlations for ventral amygdala activation in response to the neutral pictures were not significant (r values between −0.22 and 0.22, all P values >0.60).
We also conducted exploratory whole-brain voxelwise regressions (P < 0.01, uncorrected) for recognition memory separately for women and men. There was a correlation between women’s delayed recognition of the aversive pictures and left ventral amygdala activation only (r = 0.93, P < 0.001) and between men’s delayed recognition of the aversive pictures and right ventral amygdala activation only (r = 0.95, P < 0.001). Laterality differences between the sexes were not observed for dorsal amygdala or anterior hippocampus associations with memory for the anticipation or picture periods (Tables 2 and 3), although statistical power for detecting sex effects was compromised by the small cell sizes (n values range from 5 to 9).
Interrelationships Among Amygdala and Hippocampal Areas.
The bilateral dorsal amygdala and bilateral anterior hippocampus activations identified in the whole-brain ANOVA and the bilateral ventral amygdala activations observed in the whole-brain regressions were correlated with one another, similar to findings in a recent report (16). Consistently positive correlations were observed both within and across hemisphere and both within and across the anticipation and picture periods (Table 4, which is published as supporting information on the PNAS web site).
Discussion
This study found that the anticipation of aversion resulted in bilateral activation of the dorsal amygdala and anterior hippocampus that was associated with subsequent memory. The presentation of a simple warning cue instigated amygdala and hippocampus memory-related processes that preceded exposure to the picture that is remembered. Anticipatory activity in the dorsal amygdala and anterior hippocampus predicted immediate but not delayed memory of aversive pictures, whereas activity in those regions when viewing the pictures predicted memory at both intervals. Moreover, the anticipatory activity predicted better immediate memory above and beyond the associations for viewing the pictures. In addition, ventral amygdala activity when viewing the pictures, but not in anticipation of them, was associated with delayed memory only.
Present results replicate our prior findings (24) that the anticipation of and response to aversion activates the amygdala and hippocampus, consistent with the idea that the brain mechanisms recruited upon exposure to aversion also are recruited in anticipation of aversion (26, 43). A large corpus of work has pointed to the critical role of the dorsal amygdala in threat detection and vigilance (24, 44, 65, 66). The dorsal amygdala activation found here, and in previous work in our laboratory (24), likely reflects the adaptive benefit of increased attention when preparing for imminent aversive circumstances (2, 3, 7, 24, 26, 44). In relating the dorsal/ventral distinction to the amygdala nuclei (47, 67), the CeA is by far the most prominent nucleus in the dorsal amygdala, whereas the ventral amygdala is predominantly composed of the BLA. Rodent work has demonstrated that the CeA receives input from the basal nucleus, hippocampus, and sensory cortices (68). For the aversive pictures and the visual cues that precede them, the most likely pathway of afferent information is into the lateral nucleus, then to the basal nucleus, and finally to the CeA, with additional information coming from visual areas. Although both the BLA and hippocampus project to the CeA, these connections are not reciprocal (68), whereas those with primary and secondary visual cortices are (69). Direct projections to sensory cortices likely contribute to the impact of aversion in enhancing attention and perception (2, 3, 7, 39, 51, 52). In addition, prior work indicates that efferent projections from the CeA to the basal forebrain and eventually to the prefrontal cortex via the cholinergic system may lead to increased perceptual attention (5, 46, 70) and, in turn, influence selective attention and vigilance for threatening stimuli (25, 44). Consistent with our findings for immediate memory, increased recruitment of these areas via dorsal amygdala activation both in anticipation of and response to aversion may reflect enhanced attention to and subsequent encoding of the aversive pictures.
In accord with recent neuroimaging studies implicating the ventral amygdala in the consolidation of emotional memories in humans (13–23), we found increased bilateral ventral amygdala activation in response to the aversive pictures was correlated with better delayed memory for the aversive pictures. Accruing evidence indicates that interactions between the amygdala and hippocampus are critical for enhancing emotional memory (2–7, 12, 16, 18). McGaugh (5) proposed a modulation hypothesis stating that the BLA enhances emotional memory by modulating the activity of other medial temporal lobe structures, especially the hippocampus, via β-adrenergic projections activated within the amygdala by norepinephrine release from the adrenal medulla and glucocorticoids (e.g., cortisol and corticosterone) from the adrenal cortex (31, 71). An extensive and elegant body of nonhuman animal work detailing amygdala connections has shown that the BLA, particularly the basal nucleus, has stronger connections with the hippocampal formation than any other nuclei (68). Modulation of the hippocampus by the BLA enhances consolidation of the emotional material, both at an initial stage of consolidation and continually through long-term potentiation via reciprocal connections between the BLA and hippocampus (5, 6, 72). Finally, evidence here that hippocampal recruitment in anticipation of and response to aversive pictures are associated with subsequent memory recognition of those pictures is consistent with prior work establishing its role in both encoding (16, 73, 74) and consolidation (4, 5, 75).
Testing sex differences revealed that left but not right ventral amygdala activation when viewing aversive pictures was associated with delayed memory of those pictures in women, whereas the converse pattern of right but not left activation was associated with delayed memory in men. In addition to replicating prior reports of this sex difference in samples with both sexes (14, 15, 21), this pattern is consistent with findings from studies on female humans only (19, 20) and male rats only (76). Addressing the specificity of sex differences in amygdala memory function, sex differences were not observed for the dorsal amygdala, anticipatory activation, or immediate memory. Future research, including neuroimaging studies with large samples for both men and women, is needed to further characterize the boundaries of lateralized sex differences in emotional memory and to determine whether these sex differences are related to consolidation processes or other factors (2, 15).
A key conceptual issue that warrants attention is the fact that recent studies examining emotional memory have focused on the highly arousing nature of emotional stimuli or experimental contexts as the key component contributing to enhanced memory (13–23, 31, 77–79). Several studies have demonstrated that the highly arousing nature of emotional stimuli and not their unpleasant valence, as self-reported by subjects, promotes enhanced emotional memory (16, 22, 77, 80). Using established norms (81), our study used aversive pictures selected to be highly arousing and highly unpleasant. Subjects invariably rated the aversive pictures as more arousing than the neutral pictures, with no sex differences. Two studies on emotional memory that reported sex differences for arousal ratings used only pictures rated as most highly arousing (15, 21).
In conclusion, anticipatory activity in the amygdala and hippocampus plays a central role in emotional memory. Building on prior human research on emotional memory that has often confounded the effects of attention, encoding, and consolidation, a recent report found that postencoding emotion enhanced consolidation of neutral memoranda (78, 79). Findings here highlight that attention-related anticipation and encoding processes of the dorsal amygdala make a contribution to emotional memory that is distinct from consolidation processes modulated by the ventral amygdala. Our results also suggest that lateralized sex differences are specific to ventral amygdala and memory consolidation. Understanding the dual functioning of the amygdala and hippocampus in aversion and emotional memory may improve our clinical understanding of the multiple cognitive/affective abnormalities identified in people with anxiety and mood disorders, especially those involving emotional memory (e.g., traumatic memories and memory biases) and exhibiting sex differences. Future research examining activation of the amygdala and hippocampus in people with anxiety and mood disorders (65, 82–84) may better inform the role of emotional memory in the pathophysiology of these disorders.
Experimental Procedures
Subjects.
Subjects were 40 right-handed healthy undergraduate students (18 women and 22 men; M = 20.65, SD = 1.53) who responded to flyers posted in campus buildings at the University of Wisconsin-Madison. They were free of any medical or neurological problems, took no medications, and had no current psychiatric diagnoses. All subjects gave informed consent in accord with study approval by the Human Subjects Committee of the University of Wisconsin Medical School and were paid for their participation.
Experimental Design and Stimuli.
As shown in Fig. 6, which is published as supporting information on the PNAS web site, each trial consisted of a warning cue (“X,” circle, or question mark) presented for 1 s, followed by a black screen presented for 6, 7, 8, 9, or 10 s. Each trial then continued with the presentation of an aversive or neutral picture for 1 s and another black screen presented for 6, 7, 8, 9, or 10 s. For aversive trials, an X cue always was followed by an aversive picture. For neutral trials, a circle was always followed by a neutral picture. For ambiguous trials, a question mark was followed by either an aversive or neutral picture. Subjects were instructed regarding this cue-picture pairing before scanning. All warning cues were white and presented on a black background and were geometrically similar. Trial order was pseudorandomized, with the stipulation that no trial type (aversive, neutral, or ambiguous) be presented more than twice in a row. Trial length varied from 14 to 22 s, with an average trial length of 18 s. The intertrial interval (ITI) was randomized after both the warning cue and picture presentation. Using a variable ITI for both epochs allowed for better separation and estimation of the hemodynamic response for both the anticipation and response periods.
There were three functional runs, each consisting of eight aversive trials, eight neutral trials, and eight ambiguous trials. Each functional scan began with a 30-s black screen, resulting in scan lengths of 8:52, 9:00, and 8:46, respectively. Using a response box during the fMRI experiment, subjects were instructed to push a button after each cue and after each picture. Subjects were instructed to press a different button if they saw a square in place of the cue or picture. There were two trials with a square in the first functional run, three in the second run, and two in the third run. These trials were used to help maintain subjects’ attention to the cue and picture stimuli and were excluded from analyses.
During the fMRI experiment, subjects viewed 75 pictures (three on trials with a square in place of the cue) from the International Affective Picture Set (81) at a resolution of 800 × 600 pixels, with no picture being shown more than once. Of the remaining 72 pictures, 36 were aversive (24 for aversive trials and 12 for ambiguous trials) and 36 were neutral (24 for neutral trials and 12 for ambiguous trials). Based on published norms (81), pictures with the most unpleasant valence ratings and highest arousal ratings comprised the aversive set, which primarily included photographs of mutilated bodies and attack scenes. Neutral pictures (e.g., household items) selected had neutral valence ratings and low arousal ratings.
Directly after the scan, half of the subjects completed a surprise recognition task. All subjects completed a surprise recognition task 2 weeks after the scan. Subjects completing the recognition task directly after the scan were the time 1 group (n = 20, 13 men and 7 women), and their data from the second recognition task were not included. Subjects completing the recognition task only 2 weeks after the scan were the time 2 group (n = 20, 9 men and 11 women). Four subjects were dropped from analyses: two women (one from time 1 and one from time 2) because of technical difficulties with fMRI data acquisition, one man from time 1 because of excessive movement, and one man from time 1 on account of failure to follow task instructions. Resultant sample sizes for fMRI analyses were 17 (11 men and 6 women) for the time 1 group and 19 (9 men and 10 women) for the time 2 group. One woman from time 2 was dropped from all memory analyses because of insufficient memory data. Five additional subjects (three men from time 1, one woman from time 1, and one man from time 2) were dropped from all memory analyses and arousal rating analyses because of insufficient data for the arousal ratings described below. These subjects were dropped to make our results comparable with previous neuroimaging studies on emotional memory that have categorized pictures according to subjects’ arousal ratings of the pictures (13–22). Resultant sample sizes for all analyses involving memory data were 13 (8 men and 5 women) for the time 1 group and 17 (8 men and 9 women) for the time 2 group.
For the recognition task, subjects were escorted to a small room with a computer and informed that they would be seeing a series of pictures similar to those seen during the experimental scanning session. Subjects were instructed to press a “Y” if they recognized the picture from the scan and an “N” if they did not recognize the picture from the scan. Each picture was followed by 7-point rating scales for valence (0, unpleasant; 3, neutral; 6, pleasant) and arousal (0, low; 6, high). Subjects responded to each scale by pressing the corresponding number key. There was no time constraint on subjects’ responses. Text and scales were in white font with a black background, and pictures were again 800 × 600 pixels.
Subjects saw a total of 72 pictures during the recognition task, with 48 pictures from the scan (12 aversive pictures from aversive trials, 12 aversive pictures from ambiguous trials, 12 neutral pictures from neutral trials, and 12 neutral pictures from ambiguous trials) and 24 novel pictures (12 aversive and 12 neutral). All subjects saw the same pictures in the recognition task, but the conditions that the pictures came from varied across subjects to eliminate effects due to specific pictures. Three different versions for each of the functional runs were created, such that each aversive picture was in an aversive trial, ambiguous trial, or shown as a novel picture in the recognition task. Similarly, each neutral picture was in a neutral trial, ambiguous trial, or shown as a novel picture in the recognition task. The three versions were counterbalanced across subjects. For the recognition task, picture order was randomized, and pictures were selected equally from all three runs. For the purposes of this report, memory and fMRI data for the ambiguous trials were not analyzed because they address different theoretical and conceptual questions.
fMRI Data Acquisition and Analysis.
Anatomical and functional data were collected on a General Electric (Waukesha, WI) 3.0 tesla system by using data-acquisition parameters selected to minimize signal loss in the amygdala and orbitofrontal cortex, areas vulnerable to the differential magnetic susceptibility coefficients of bone/air/tissue boundaries (see Supporting Methods). All fMRI data processing was done with Analysis of Functional Neural Images (AFNI) version 2.41 software (85) (see Supporting Methods).
Initial analyses focused on identifying regions associated with both anticipating and responding to the aversive as compared with neutral pictures. A whole-brain voxelwise period (anticipation, picture) × valence (aversive, neutral) repeated-measures ANOVA at a threshold of P < 0.005 (uncorrected) identified relevant brain regions of activation. Using AlphaSim in AFNI, we applied a correction for multiple comparisons by using Monte Carlo simulations within a region of interest defined by the anatomical boundaries of the amygdala and hippocampus, the focus of study hypotheses (24, 85). The spatial correlation of the input data and an uncorrected P value threshold of 0.005 resulted in a minimum cluster size of 99 mm3 for this repeated-measures ANOVA to achieve a corrected mapwise P < 0.05. For statistically defined clusters meeting this threshold criterion for the valence main effect, the average percentage signal change value was extracted for each condition on a single-subject basis. Each subject’s average percentage signal change values for the neutral anticipation and picture periods was subtracted from each subject’s values for the aversive anticipation and picture periods. These absolute and difference values were subsequently correlated with recognition of the aversive pictures both at time 1 and time 2.
Additionally, whole-brain voxelwise regressions for recognition data on activation during the anticipation and picture periods were run at a threshold of P < 0.005 (uncorrected). The aforementioned procedure by using Monte Carlo simulations to correct for multiple comparisons resulted in a minimum cluster size of 81 mm3 for this regression analysis to achieve a corrected mapwise P < 0.05. Separate regressions were conducted for each period, memory metric (Pr and d′), and memory session (time 1 and time 2). Whole-brain data were composed of the difference between activation to the respective aversive and neutral condition.
Analysis of Behavioral Data.
Recognition was calculated by using two separate metrics, Pr and d′ (see Supporting Methods). Calculations were computed for both recognition measures because although previous neuroimaging studies on emotional memory have used Pr, no neuroimaging study to date has been able to calculate d′ because of experimental restraints. Further, Cahill et al. (15) called for the use of d′ in future neuroimaging research on recognition memory. All analyses for the memory and arousal rating data were conducted at an α level of 0.05. Two-tailed tests were used throughout, including for the directional hypotheses that amygdala and hippocampus activation would predict better memory for aversive pictures.
Supplementary Material
Acknowledgments
We thank Andrew Alexander, Michael Anderle, Krista Bluske, Ron Fisher, John Herrington, Tom Johnstone, Roberta Koch, Hillary Schaefer, Eric Steege, and Mai Lor for their contributions to this project. J.B.N. was supported by National Institute of Mental Health Grants R01-MH74847 and K08-MH63984. The research reported in this publication also was supported by National Institute of Child Health and Human Development Grant P30-HD03352 to the Waisman Center.
Abbreviations
- BLA
basolateral amygdala
- CeA
central nucleus of the amygdala
- fMRI
functional MRI
- M
mean.
Footnotes
The authors declare no conflict of interest.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Christianson SA. The Handbook of Emotion and Memory: Research and Theory. Hillsdale, NJ: Lawrence Earlbaum Associates; 1992. [Google Scholar]
- 2.Phelps EA. Curr Opin Neurobiol. 2004;14:198–202. doi: 10.1016/j.conb.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 3.Phelps EA. Annu Rev Psychol. 2006;57:27–53. doi: 10.1146/annurev.psych.56.091103.070234. [DOI] [PubMed] [Google Scholar]
- 4.McGaugh JL. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- 5.McGaugh JL. Annu Rev Neurosci. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- 6.McGaugh JL, Roozendaal B. Curr Opin Neurobiol. 2002;12:205–210. doi: 10.1016/s0959-4388(02)00306-9. [DOI] [PubMed] [Google Scholar]
- 7.Phelps EA, LeDoux JE. Neuron. 2005;48:175–187. doi: 10.1016/j.neuron.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 8.Packard MG, Cahill L, McGaugh JL. Proc Natl Acad Sci USA. 1994;91:8477–8481. doi: 10.1073/pnas.91.18.8477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adolphs R, Tranel D, Denburg N. Learn Mem. 2000;7:180–186. doi: 10.1101/lm.7.3.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cahill L, Babinsky R, Markowitsch HJ, McGaugh JL. Nature. 1995;377:295–296. doi: 10.1038/377295a0. [DOI] [PubMed] [Google Scholar]
- 11.LaBar KS, Phelps EA. Psychol Sci. 1998;9:490–493. [Google Scholar]
- 12.Richardson MP, Strange BA, Dolan RJ. Nat Neurosci. 2004;7:278–285. doi: 10.1038/nn1190. [DOI] [PubMed] [Google Scholar]
- 13.Cahill L, Haier RJ, Fallon J, Alkire MT, Tang C, Keator D, Wu J, McGaugh JL. Proc Natl Acad Sci USA. 1996;93:8016–8021. doi: 10.1073/pnas.93.15.8016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cahill L, Haier RJ, White NS, Fallon J, Kilpatrick L, Lawrence C, Potkin SG, Alkire MG. Neurobiol Learn Mem. 2001;75:1–9. doi: 10.1006/nlme.2000.3999. [DOI] [PubMed] [Google Scholar]
- 15.Cahill L, Uncapher M, Kilpatrick L, Alkire MT, Turner J. Learn Mem. 2004;11:261–266. doi: 10.1101/lm.70504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dolcos F, LaBar KS, Cabeza R. Neuron. 2004;42:855–863. doi: 10.1016/s0896-6273(04)00289-2. [DOI] [PubMed] [Google Scholar]
- 17.Dolcos F, LaBar KS, Cabeza R. Proc Natl Acad Sci USA. 2005;102:2626–2631. doi: 10.1073/pnas.0409848102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Strange BA, Dolan RJ. Proc Natl Acad Sci USA. 2004;31:11454–11458. doi: 10.1073/pnas.0404282101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Canli T, Zhao Z, Brewer J, Gabrieli JDE, Cahill L. J Neurosci. 2000;20:RC99. doi: 10.1523/JNEUROSCI.20-19-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Canli T, Zhao Z, Desmond JE, Glover G, Gabrieli JDE. Psychobiology. 1999;27:441–452. [Google Scholar]
- 21.Canli T, Desmond JE, Zhao Z, Gabrieli JDE. Proc Natl Acad Sci USA. 2002;99:10789–10794. doi: 10.1073/pnas.162356599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamann SB, Ely TD, Grafton ST, Kilts CD. Nat Neurosci. 1999;2:289–293. doi: 10.1038/6404. [DOI] [PubMed] [Google Scholar]
- 23.Kensinger EA, Schacter DL. J Neurosci. 2006;26:2564–2570. doi: 10.1523/JNEUROSCI.5241-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nitschke JB, Sarinopoulos I, Mackiewicz KL, Schaefer HS, Davidson RJ. NeuroImage. 2006;29:106–116. doi: 10.1016/j.neuroimage.2005.06.068. [DOI] [PubMed] [Google Scholar]
- 25.Davis M. Annu Rev Neurosci. 1992;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- 26.LeDoux JE. Synaptic Self: How Our Brains Become Who We Are. New York: Viking; 2002. [Google Scholar]
- 27.Büchel C, Morris J, Dolan RJ, Friston KJ. Neuron. 1998;20:947–957. doi: 10.1016/s0896-6273(00)80476-6. [DOI] [PubMed] [Google Scholar]
- 28.Büchel C, Dolan RJ, Armony JL, Friston KJ. J Neurosci. 1999;19:10869–10876. doi: 10.1523/JNEUROSCI.19-24-10869.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA. Neuron. 1998;20:937–945. doi: 10.1016/s0896-6273(00)80475-4. [DOI] [PubMed] [Google Scholar]
- 30.Cahill L, McGaugh JL. Trends Neurosci. 1998;21:294–299. doi: 10.1016/s0166-2236(97)01214-9. [DOI] [PubMed] [Google Scholar]
- 31.Okuda S, Roozendaal B, McGaugh J. Proc Natl Acad Sci USA. 2004;101:853–858. doi: 10.1073/pnas.0307803100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davis M, Schlesinger LS, Sorenson CA. J Exp Psychol Anim Behav Processes. 1989;15:295–310. [PubMed] [Google Scholar]
- 33.Grillon C, Ameli R, Woods SW, Merikangas K, Davis M. Psychophysiology. 1991;28:588–595. doi: 10.1111/j.1469-8986.1991.tb01999.x. [DOI] [PubMed] [Google Scholar]
- 34.Grillon C, Ameli R, Merikangas K, Woods SW, Davis M. Psychophysiology. 1993;30:340–346. doi: 10.1111/j.1469-8986.1993.tb02055.x. [DOI] [PubMed] [Google Scholar]
- 35.Nitschke JB, Larson CL, Smoller MJ, Navin SD, Pederson AJ, Ruffalo D, Mackiewicz KL, Gray SM, Victor E, Davidson RJ. Psychophysiology. 2002;39:254–258. doi: 10.1017/S0048577202010156. [DOI] [PubMed] [Google Scholar]
- 36.Koster EH, Crombez G, Van Damme S, Verschuere B, De Houwer J. Emotion. 2004;4:312–317. doi: 10.1037/1528-3542.4.3.312. [DOI] [PubMed] [Google Scholar]
- 37.Van Damme S, Crombez G, Eccleston C. Pain. 2004;111:392–399. doi: 10.1016/j.pain.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 38.Van Damme S, Lorenz J, Eccleston C, Koster EHW, De Clercq A, Crombez G. Neurophysiol Clin. 2004;34:33–39. doi: 10.1016/j.neucli.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 39.Morris JS, Büchel C, Dolan RJ. NeuroImage. 2001;13:1044–1052. doi: 10.1006/nimg.2000.0721. [DOI] [PubMed] [Google Scholar]
- 40.Iidaka T, Anderson ND, Kapur S, Cabeza R, Craik FI. J Cognit Neurosci. 2000;12:267–280. doi: 10.1162/089892900562093. [DOI] [PubMed] [Google Scholar]
- 41.Mangels JA, Picton TW, Craik FI. Brain Res Cogn Brain Res. 2001;11:77–95. doi: 10.1016/s0926-6410(00)00066-5. [DOI] [PubMed] [Google Scholar]
- 42.Anderson ND, Iidaka T, Cabeza R, Kapur S, McIntosh AR, Craik FI. J Cognit Neurosci. 2000;12:775–792. doi: 10.1162/089892900562598. [DOI] [PubMed] [Google Scholar]
- 43.Pavlov IP. Conditioned Reflexes: An Investigation of the Physiological Activity of the Cerebral Cortex. New York: Dover; 1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Davis M, Whalen PJ. Mol Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- 45.Holland PC, Gallagher M. Trends Cognit Sci. 1999;3:65–73. doi: 10.1016/s1364-6613(98)01271-6. [DOI] [PubMed] [Google Scholar]
- 46.Holland PC, Han J, Gallagher M. J Neurosci. 2000;20:6701–6706. doi: 10.1523/JNEUROSCI.20-17-06701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee HJ, Groshek F, Petrovich GD, Cantalini JP, Gallagher M, Holland PC. J Neurosci. 2005;25:3881–3888. doi: 10.1523/JNEUROSCI.0416-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Groshek F, Kerfoot E, McKenna V, Polackwich AS, Gallagher M, Holland PC. Behav Neurosci. 2005;119:202–212. doi: 10.1037/0735-7044.119.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anderson AK, Phelps EA. Nature. 2001;411:305–309. doi: 10.1038/35077083. [DOI] [PubMed] [Google Scholar]
- 50.Amaral DG. Ann NY Acad Sci. 2003;1000:337–347. doi: 10.1196/annals.1280.015. [DOI] [PubMed] [Google Scholar]
- 51.Morris JS, Friston KJ, Büchel C, Frith CD, Young AW, Calder AJ, Dolan RJ. Brain. 1998;121:47–57. doi: 10.1093/brain/121.1.47. [DOI] [PubMed] [Google Scholar]
- 52.Vuilleumier P, Richardson MP, Armony JL, Driver J, Dolan RJ. Nat Neurosci. 2004;7:1271–1278. doi: 10.1038/nn1341. [DOI] [PubMed] [Google Scholar]
- 53.Eichenbaum H. The Cognitive Neuroscience of Memory. New York: Oxford Univ Press; 2002. [Google Scholar]
- 54.Squire LR, Zola-Morgan S. Science. 1991;253:1380–1386. doi: 10.1126/science.1896849. [DOI] [PubMed] [Google Scholar]
- 55.Scoville WB, Milner B. J Neurol Neurosurg Psychiatry. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cohen NJ, Ryan J, Hunt C, Romine L, Wczalek T, Nash C. Hippocampus. 1999;9:83–98. doi: 10.1002/(SICI)1098-1063(1999)9:1<83::AID-HIPO9>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 57.Schacter DL, Wagner AD. Hippocampus. 1999;9:7–24. doi: 10.1002/(SICI)1098-1063(1999)9:1<7::AID-HIPO2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 58.Maren S. Trends Neurosci. 1999;22:561–567. doi: 10.1016/s0166-2236(99)01465-4. [DOI] [PubMed] [Google Scholar]
- 59.Setlow B, Holland PC, Gallagher M. Behav Neurosci. 2002;116:267–275. doi: 10.1037//0735-7044.116.2.267. [DOI] [PubMed] [Google Scholar]
- 60.Otten JJ, Quayle AH, Akram S, Ditewig TA, Rugg MD. Nat Neurosci. 2006;9:489–491. doi: 10.1038/nn1663. [DOI] [PubMed] [Google Scholar]
- 61.Adcock RA, Thangavel A, Whitfield-Gabrieli S, Knutson B, Gabrieli JDE. Neuron. 2006;50:507–517. doi: 10.1016/j.neuron.2006.03.036. [DOI] [PubMed] [Google Scholar]
- 62.Cahill L. Nat Rev Neurosci. 2006;7:477–484. doi: 10.1038/nrn1909. [DOI] [PubMed] [Google Scholar]
- 63.Cumming G, Finch S. Am Psychol. 2005;60:170–180. doi: 10.1037/0003-066X.60.2.170. [DOI] [PubMed] [Google Scholar]
- 64.Loftus GR, Masson MEJ. Psychonomic Bull Rev. 1994;1:476–490. doi: 10.3758/BF03210951. [DOI] [PubMed] [Google Scholar]
- 65.Nitschke JB, Heller W. Int Rev Neurobiol. 2005;67:1–42. doi: 10.1016/S0074-7742(05)67001-8. [DOI] [PubMed] [Google Scholar]
- 66.Pessoa L, Kastner S, Ungerleider LG. Cognit Brain Res. 2002;15:31–45. doi: 10.1016/s0926-6410(02)00214-8. [DOI] [PubMed] [Google Scholar]
- 67.Kim H, Somerville LH, Johnstone T, Polis S, Alexander AL, Shin LM, Whalen PJ. J Cognit Neurosci. 2004;16:1730–1745. doi: 10.1162/0898929042947865. [DOI] [PubMed] [Google Scholar]
- 68.Pitkänen A. In: The Amygdala: A Functional Analysis. Aggleton JP, editor. New York: Oxford Univ Press; 2000. pp. 31–116. [Google Scholar]
- 69.Freese JL, Amaral DG. J Comp Neurol. 2005;486:295–317. doi: 10.1002/cne.20520. [DOI] [PubMed] [Google Scholar]
- 70.Gallagher M. In: The Amygdala: A Functional Analysis. Aggleton JP, editor. New York: Oxford Univ Press; 2000. pp. 311–330. [Google Scholar]
- 71.Roozendaal B, Okuda S, de Quervain DJ, McGaugh JL. Neuroscience. 2006;138:901–910. doi: 10.1016/j.neuroscience.2005.07.049. [DOI] [PubMed] [Google Scholar]
- 72.Chapman PF, Chattarji S. In: The Amygdala: A Functional Analysis. Aggleton JP, editor. New York: Oxford Univ Press; 2000. pp. 117–154. [Google Scholar]
- 73.Lepage M, Habib R, Tulving E. Hippocampus. 1998;8:313–322. doi: 10.1002/(SICI)1098-1063(1998)8:4<313::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 74.Strange BA, Fletcher PC, Henson RNA, Friston KJ, Dolan RJ. Proc Natl Acad Sci USA. 1999;96:4034–4039. doi: 10.1073/pnas.96.7.4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Malin EL, McGaugh J. Proc Natl Acad Sci USA. 2006;103:1959–1963. doi: 10.1073/pnas.0510890103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.LaLumiere RT, McGaugh J. Learn Mem. 2005;12:527–532. doi: 10.1101/lm.97405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hamann S. Trends Cognit Sci. 2001;9:394–400. doi: 10.1016/s1364-6613(00)01707-1. [DOI] [PubMed] [Google Scholar]
- 78.Anderson AK, Wais PE, Gabrieli DE. Proc Natl Acad Sci USA. 2006;103:1599–1604. doi: 10.1073/pnas.0506308103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McGaugh JL. Trends Cognit Sci. 2006;10:345–347. doi: 10.1016/j.tics.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 80.Cahill L, McGaugh JL. Behav Neurosci. 1990;104:532–543. doi: 10.1037//0735-7044.104.4.532. [DOI] [PubMed] [Google Scholar]
- 81.Lang PJ, Bradley MM, Cuthbert BN. International Affective Picture System: Technical Manual and Affective Ratings. Gainesville, FL: Univ Florida; 1999. [Google Scholar]
- 82.Davidson RJ, Pizzagalli D, Nitschke JB, Putnam KM. Annu Rev Psychol. 2002;53:545–574. doi: 10.1146/annurev.psych.53.100901.135148. [DOI] [PubMed] [Google Scholar]
- 83.Drevets WC. Ann NY Acad Sci. 2003;985:420–444. doi: 10.1111/j.1749-6632.2003.tb07098.x. [DOI] [PubMed] [Google Scholar]
- 84.Rauch SL, Shin LM, Wright CI. Ann NY Acad Sci. 2003;985:389–410. doi: 10.1111/j.1749-6632.2003.tb07096.x. [DOI] [PubMed] [Google Scholar]
- 85.Cox RW. Comp Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.