Abstract
Antiretroviral therapy (ART) is becoming available in South Africa. Demand will exceed supply; thus, difficult decisions will have to be made in allocating ART. The majority of those treated for HIV are likely to be in cities, because health infrastructure and personnel are concentrated in urban centers. We predict the epidemiological impact of drug allocation strategies (DAS) by using a spatially explicit model that links urban and rural epidemics. We parameterize our model by using data from the KwaZulu-Natal province in South Africa. We model the South African government's treatment plan from 2004–2008, and we predict the consequences of one DAS that allocates drugs only to Durban and of two DAS that allocate drugs to both urban and rural areas. All three strategies would treat 500,000 people by 2008. Not surprisingly, the Durban-only DAS would prevent the greatest number of infections (an additional 15,000 infections by 2008). However, it may have been expected that this DAS would generate the highest levels of transmitted resistance, because it concentrates ART in one location. Paradoxically, we found that this DAS would generate the lowest levels of transmitted resistance. Concentrating treatment in Durban would also avert the greatest number of AIDS-related deaths. We discuss the difference between using the principle of treatment equity versus using the principle of utilitarianism/efficiency to allocate ART. Decisions about allocating scarce drugs should consider treatment equity as well as epidemiological consequences. Notably, a Durban-only DAS would lead to new disparities in healthcare between urban and rural areas in KwaZulu-Natal.
Keywords: antiretroviral therapy rationing, epidemiology, HIV, South Africa
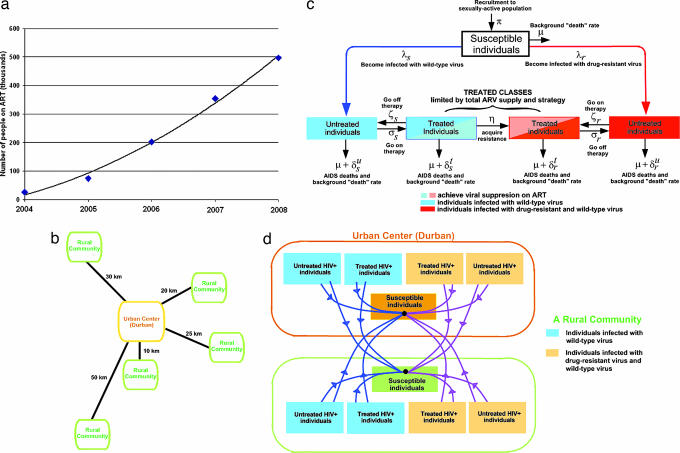
South Africa has more people living with HIV/AIDS (PLWHA) than any other country. KwaZulu-Natal is the South African province with the largest population (9.4 million) and the highest prevalence of PLWHA [≈21% of all South African cases (ref. 1 and www.avert.org/safricastats.htm)]. The majority (56%) of the population of KwaZulu-Natal live in rural communities where the prevalence of HIV is less than in urban areas [9% versus ≈13% (ref. 1 and www.avert.org/safricastats.htm)]. In April 2004, the South African government launched a strategic treatment plan for HIV/AIDS (2). Their first-line drug regime consists of stavudine (D4T), lamivudine (3TC), and efavirenz (EFV) [with nevirapine (NVP) replacing EFV for pregnant women]. The government plans to treat ≈500,000 people in KwaZulu-Natal by 2008, with numbers treated increasing approximately quadratically with time (Fig. 1a). This plan will result in 50% of PLWHA receiving treatment by 2008. To predict the epidemiological consequences of different drug allocation strategies (DAS), we modeled the South African government's treatment plan from 2004–2008 (2) (Fig. 1a). The World Health Organization has outlined four ethical principles that should govern the rollout of antiretrovirals in Africa; all four of these ethical principles are important for consideration in decision making by government health officials. We predict the epidemiological impact of DAS if one of these ethical principles is considered: namely, the ethical principle of utilitarianism/efficiency. We evaluated three DAS: two urban–rural strategies (strategies 1 and 2) and an urban-only strategy that would treat only PLWHA in Durban (strategy 3). We evaluated which of the DAS would have the maximum impact on reducing the HIV epidemic by 2008 in terms of reducing the incidence rate, minimizing transmitted resistance, and decreasing the AIDS-related death rate.
Fig. 1.
Conceptual overview of mathematical model. (a) Data for the planned number of cases on ART in KwaZulu-Natal based on the South African government plan (3). We used a fitted interpolating curve to calculate the number of cases on ART each year; this treatment rate was then used as an input to make predictions by using our spatially explicit model. (b) Conceptual diagram of the spatial urban–rural model; distance between the urban center and each rural community determines the amount of sexual cross-mixing. (c) A schematic flow diagram of the HIV epidemic in each community. (d) Curves represent transmission between compartments and act to link the transmission source with the group of susceptible individuals that can become infected.
We modeled heterogeneity in HIV transmission dynamics by developing a spatially explicit model of KwaZulu-Natal that linked the urban epidemics with the surrounding rural epidemics (Fig. 1b); for the mathematical structure of our model and our analytical techniques, see Methods. Our spatially explicit model builds to some degree on our earlier models (3–7) but is substantially different. Unlike our previous models, our new model is much more detailed and (i) is a spatial model, (ii) allows us to examine urban–rural differences, (iii) is an epidemiological model combined with a drug allocation model, (iv) is specific for KwaZulu-Natal, and (v) includes many more biological processes. In our new model we include a mechanism for delaying therapy, and we use the World Health Organization's criteria for establishing the timing for treatment-eligibility (8). Our new model is parameterized specifically to apply to KwaKulu-Natal by using demographic, clinical, and epidemiological data, including birth rates, natural death rates, and AIDS-related death rates from KwaZulu-Natal. We also parameterized our model such that the initial epidemiological conditions reflected the current prevalence of HIV in the province. The model tracks uninfected individuals, untreated individuals (infected with either wild-type or drug-resistant strains), and treated individuals (infected with either wild-type or drug-resistant strains) (Fig. 1c). Infected individuals both initiate and discontinue therapy (Fig. 1c); survival time depends on treatment status (specifically, treatment increases survival time). Patients respond differentially to antiretroviral therapy (ART) (some achieve viral suppression, whereas others do not); we also include heterogeneity in treatment response in terms of viral suppression and disease progression rates (Fig. 1b).
Our model tracks both acquired and transmitted resistance. One of the most important factors that lead to acquired resistance is adherence. The level of adherence that is necessary to sustain viral suppression and prevent resistance is associated with the clinical success of ART (9, 10). However, we note that viral resistance occurs even among patients with high levels of medication adherence if the medications fail to durably suppress HIV replication (11). Even substantial, but imperfect, levels of adherence are predictors of antiretroviral resistance (12). Thus it is necessary that healthcare systems for managing patients receiving ART include potent combinations of active medications, laboratory monitoring to assess viral response to treatment, and high adherence goals to minimize the development of acquired resistance (11–13). In our model, we also include transmitted resistance; we assume (that as occurs in the “real world”) individuals infected with drug-resistant strains differ in their transmission rates and disease progression rates in comparison with individuals infected with wild-type strains. Individuals can sexually transmit infection both within their own community and to all connected communities; Fig. 1d illustrates linkages in our model among urban and rural epidemics showing who infects whom. We used our model (and uncertainty analyses) to predict the epidemiological consequences of three DAS. The two urban–rural strategies that we analyzed allocate drugs among the rural and urban communities based on either the population size of each community (strategy 1) or the HIV prevalence within each community weighted by the distance from the urban center (strategy 2).
Results
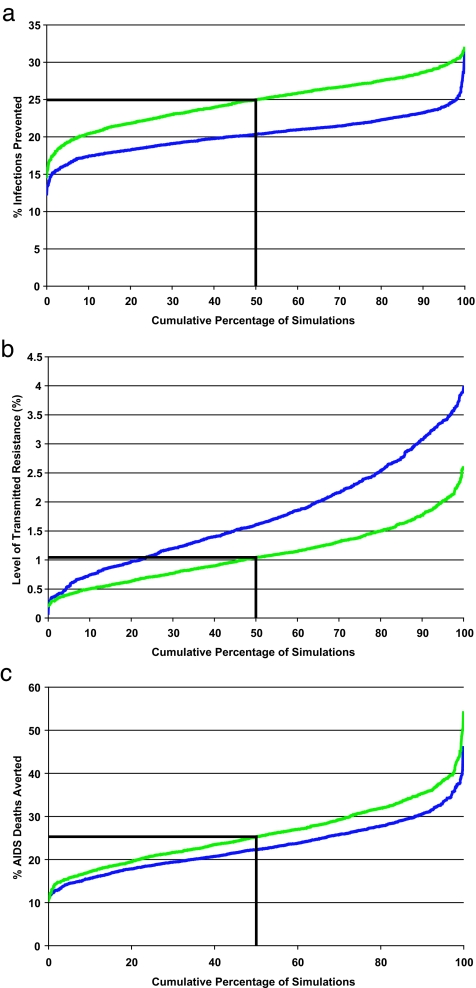
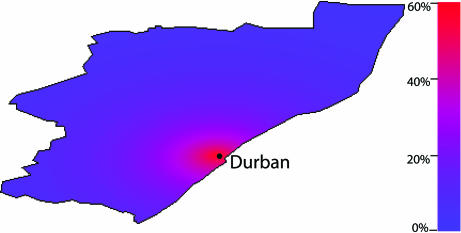
Although each of the three DAS would treat 500,000 people by 2008 (Fig. 1a), they would differentially divide the scarce antiretrovirals between the rural and urban HIV-infected populations. Surprisingly, our uncertainty analysis predictions reveal that the three DAS would lead to differences in infections prevented by 2008. The two urban–rural DAS would each similarly reduce transmission (Fig. 2). However, if all of the available drugs were allocated to Durban, the number of infections prevented would be substantially greater than if the drugs were shared between the urban and rural areas (Fig. 2a). The Durban-only DAS would reduce transmission by 25% (median) by 2008; preventing an additional 15,000 infections in comparison with either of the two urban–rural DAS. To understand these results, we quantified the heterogeneity in risk of acquiring HIV by using our spatially explicit model. We calculated individual risk of infection in rural communities standardized in comparison to the individual risk of infection in Durban (Fig. 3). The maximum standardized risk of infection in rural communities is only ≈60% of the risk of infection in Durban and disseminates sharply with distance from Durban (Fig. 3). This particular result is not surprising because it is consistent with the “city and villages” paradigm (14). Hence, allocating all drugs to Durban would maximize the number of infections prevented because the risk of infection is greatest in Durban, due to the concentration of core groups in the urban center. Thus concentrating treatment in Durban would have the maximum impact of the three DAS in decreasing transmission of HIV in KwaZulu-Natal.
Fig. 2.
Cumulative distribution functions (cdf) for predicted variables from our model. The cdf were calculated from an uncertainty analysis of our spatially explicit model and are based on 1,000 Monte Carlo simulations; results are for an urban–rural DAS (blue curve) and the urban-only DAS (green curve). The two urban–rural DAS produced similar distributions; thus, only strategy 1 is plotted. (a) The percentage of HIV infections prevented by 2008; each urban–rural DAS prevents 21% (median) of infections, and the Durban-only strategy prevents 25% (median) of infections. (b) The level of transmitted resistance at 2008. (c) The percentage of AIDS-related deaths averted by 2008.
Fig. 3.
Map of the spatially explicit standardized risk of HIV infection per individual in rural communities in KwaZulu-Natal province. The pretreatment risk of infection for an individual in a rural community is standardized to the risk of HIV infection in Durban. This standardized risk is color-coded throughout the province where the highest risk (for communities located close to Durban) is red and the lowest risk is blue. The maximum standardized risk is not 100%, but ≈60%, because we plot only risk in rural communities. Risk sharply decreases with distance from Durban because the degree of sexual cross-mixing decreases.
Theoretical studies have shown that the highest level of transmitted resistance occurs where treatment rates are the highest (15). Therefore, it may be expected that the Durban-only DAS would generate the highest levels of transmitted resistance, because it would result in high levels of treatment in one location. Paradoxically, we found that the urban-only DAS would generate the lowest levels of transmitted resistance (Fig. 2b). By 2008, only 1% (median) of new infections would be because of transmitted resistance if the drugs are given only to Durban, whereas ≈2% (median) of new infections would be because of transmitted resistance if the drugs are shared between rural and urban areas (Fig. 2b). Although the differences in the percentages are small, the difference in absolute numbers is fairly high. If Durban received all of the drugs, then 5,400 cases of transmitted resistance would be avoided by 2008. The Durban-only DAS would generate the lowest levels of transmitted resistance, because this strategy would be the most effective in reducing transmission (Fig. 2). Our predicted levels of transmitted resistance are low, because our predictions are only short term (to 2008). Relatively high levels of acquired resistance would be likely to emerge after several years on treatment (3–6); as levels of acquired resistance rise, levels of transmitted resistance will increase (16, 17). For longer time periods, our model predicts higher levels of transmitted resistance.
The number of person-treatment-years is equivalent for all three DAS; each strategy would result in the treatment of ≈500,000 patients by 2008. However, the greatest number of AIDS-related deaths would be averted if the drugs were allocated only to Durban (Fig. 2c). This effect would occur for two reasons. Firstly, the urban-only strategy would be the most effective strategy for increasing average survival time by inducing the greatest heterogeneity in the death rate from the four infection states. Secondly, the urban-only strategy would maximize the reduction in transmission, which would translate into reduced AIDS-related deaths. The Durban-only DAS would avert 25% (median) of deaths by 2008 and would avert an additional 1,400 AIDS-related deaths in comparison with either of the two urban–rural strategies. Obviously, epidemiological differences induced by the three DAS would magnify over time.
Our predictions reveal that the urban-only DAS would have the most beneficial epidemiological impact by preventing infections, reducing transmitted resistance, and averting AIDS-related deaths. Decisions about allocating scarce drugs should consider the ethical consequences, as well as the epidemiological consequences. Obviously a Durban-only DAS would not lead to treatment equity and would exacerbate existing substantial inequalities in healthcare between urban and rural areas. Thus the Durban-only strategy would be the least ethical (in terms of equitable distribution of ART) (18) of the three DAS. DAS will directly and indirectly effect treatment equity. Direct effects on treatment equity are obvious. If drugs are allocated only to Durban, then ≈90% of the PLWHA who need treatment in Durban would receive ART, but none of the PLWHA in rural areas would receive ART. If the drugs are shared between urban and rural areas, then ≈50% of the PLWHA who need treatment (throughout the province) would receive ART. Indirect effects on treatment equity because of different DAS are less obvious. To evaluate these indirect effects on treatment equity, we compared our model's predictions, in terms of urban and rural disparities, for the Durban-only DAS with an urban–rural DAS (by using strategy 1; results are similar for strategies 1 and 2).
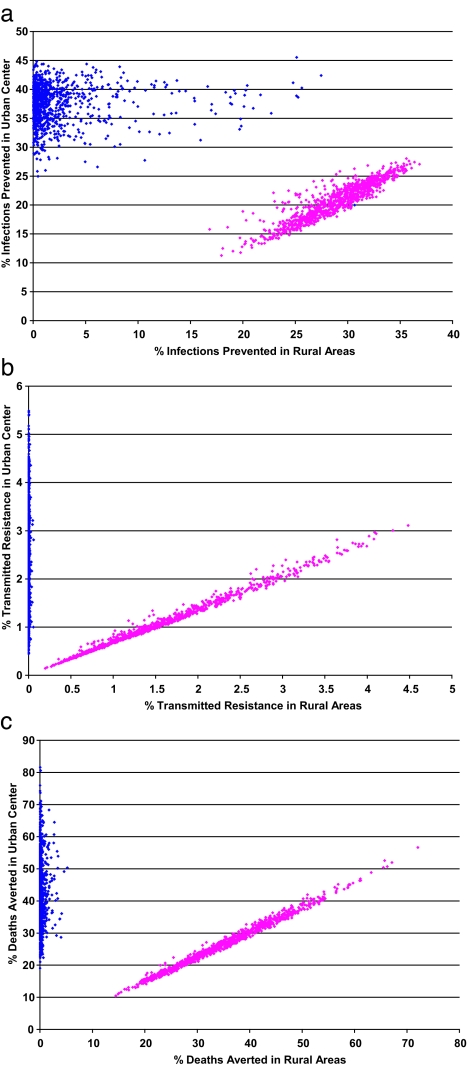
To evaluate the indirect effects on treatment equity, we analyzed the epidemiological impact of the DAS in urban and rural areas separately. Predictions were generated from our model by using an uncertainty analysis (described in Methods). Indirect effects on treatment equity (by 2008) because of the DAS are shown graphically as plots of the percentage of infections prevented (Fig. 4a), the percentage of transmitted resistance expected (Fig. 4b), and the percentage of deaths averted (Fig. 4c). If drugs are allocated only to Durban (blue data), the effects on transmission, transmitted resistance, and AIDS-related deaths would (not surprisingly) be dramatically different between urban and rural areas (Fig. 4). Transmission would decrease by 25–46% in Durban but by <5% in rural areas (Fig. 4a). Transmitted resistance would rise to 0.4–5.5% in Durban but would not emerge in rural areas (Fig. 4b). The AIDS-related death rate in Durban would decrease by 42% (median) but only by 0.1% (median) in rural areas (Fig. 4c). In contrast to the Durban-only DAS, if the drugs are shared between Durban and rural areas (pink data) the effects on transmission, transmitted resistance, and AIDS-related death rates in urban and rural areas would be similar (Fig. 4). Transmission would be reduced by 11–28% in Durban and by 17–37% in rural areas (Fig. 4a). Transmitted resistance would remain low (0.1–3%) in Durban and also in rural areas (0.2–4.5%) (Fig. 4b). The AIDS-related death rate would be reduced by 26% (median) in Durban and 34% (median) in rural communities (Fig. 4c). Thus, the mixed urban–rural DAS would not lead to either direct or indirect disparities in treatment equity between the urban and rural communities. However, the Durban-only DAS would lead to substantial direct and indirect disparities in treatment equity between the urban and rural communities. We also conducted a sensitivity analysis of our model to identify which parameters were of greatest importance in increasing (or decreasing) the magnitude (by 2008) of the cumulative number of infections prevented, the level of transmitted resistance, and the cumulative number of deaths (see Supporting Text and Fig. 5, which are published as supporting information on the PNAS web site).
Fig. 4.
Scatterplots for the predicted data from our spatially explicit model that show the indirect effect of treatment equity in urban and rural areas. These predicted data were calculated from an uncertainty analysis of our model and are based on 1,000 Monte Carlo simulations of parameter samples over the entire defined parameter ranges. Results shown are for an urban–rural DAS (pink data) and the Durban-only DAS (blue data). The two urban–rural DAS produced similar scatterplots; thus, only strategy 1 is plotted. Scatterplots show the epidemiological impact of each DAS in the urban and rural areas. (a) The percentage of infections prevented by 2008. (b) The percentage of transmitted resistance at 2008. (c) The percentage of deaths averted by 2008. In each scatterplot, the y axis shows the epidemiological impact of the different DAS on the urban center, and the x axis shows the epidemiological impact on the rural areas.
Discussion
City and villages models have been used to design vaccination strategies for other infectious diseases, notably measles (14, 19), but a city and villages spatially explicit model previously has not been developed and analyzed for HIV epidemics. Our methodology and analyses are innovative, because city and villages models have not previously been used to (i) evaluate the epidemiological impact of treatment strategies, (ii) evaluate the consequences of different drug allocation strategies, (iii) predict the evolution of drug resistance, and (iv) predict evolutionary trajectories for the HIV epidemic in KwaZulu-Natal. Furthermore, our model is the first model, to our knowledge, to combine a dynamic epidemiological model with an allocation model for scarce resources to evaluate ethical decision-making. We chose not to include vertical transmission (which is an important aspect of the African epidemic) in our current model, because we are making only short-term population-level predictions. Our results will be robust (for short-term predictions) to the inclusion of vertical transmission. We also have chosen to not include population demographics; including population demographics is unnecessary, because we only generate predictions to 2008. Future more elaborate models (that are based on our spatially explicit model) can be used to make longer-term predictions. These more detailed models can include different drug-regimens, increased health infrastructure (as it becomes available), changes in population demographics, and vertical transmission.
The effectiveness of targeting behavioral interventions to reduce epidemics is well known. Not everyone is equally at risk of infection; thus, it makes sense to target high-risk groups. Targeting maximizes the effectiveness of interventions without causing an ethical dilemma. The primary purpose of ART is therapeutic; thus, the preventative effect is only secondary. Therefore, targeting ART poses serious ethical dilemmas. Different ethical criteria can be used to decide how to allocate a scarce supply of ART, including justice as reciprocity or compensation, utilitarian principles of maximizing overall societal benefits, egalitarian principles of equity (distributing resources equally among groups), and the Maximin principle (which prioritizes individuals that are the least advantaged) (18). The results of our analyses highlight the contrast between DAS that are maximally effective and those that are equitable.
If government health policy officials in KwaZulu-Natal wish to apply the utilitarian principle (and minimize the epidemic), our results show that they should allocate all drugs to Durban. If they wish to apply the egalitarian principle, our previous results (18) show they should divide drugs between Durban and rural communities; however, as we have previously shown, it will be hard to achieve treatment equity in KwaZulu-Natal because very complicated DAS will need to be used. Finally, if the government health policy officials in KwaZulu-Natal wish to apply the Maximin principle, then our results show that they should choose to allocate all of the drugs to rural communities. Thus, a variety of different DAS can be justified depending on which ethical principle government health policy officials wish to use as a basis in their decision-making process. Most likely a balance will be desired between all of these ethical principles and primarily strategies that are maximally effective and those that are equitable will be used. Any balancing of drug allocation across multiple objectives will result in suboptimal tradeoffs in the effect of each of the respective objectives. Government health officials in KwaZulu-Natal and other resource-constrained regions must unfortunately face these difficult policy decisions, of which there will be no consensus. This scientific study assists these authorities so that they can make informed decisions, but in no way are our analyses to be construed as a policy statement for KwaZulu-Natal. We also note that if cost constraints were included in our current analyses, rather than only drug availability, then our results would be even more extreme (i.e., there would be a more severe tradeoff between effectiveness and equity) because it would cost more to distribute drugs in rural areas.
The rollout of antiretrovirals in Africa to deal with the HIV pandemic is the largest public health effort that has ever been attempted. The rollout has successfully begun in various resource-constrained settings, including small programs in South Africa (20–22). One of the main concerns of many public health officials is that increased use of antiretrovirals in Africa will lead to substantial increases in the levels of transmitted resistance. Our results show that different DAS will lead to differences in levels of transmitted resistance, with (unexpectedly) the lowest levels of transmitted resistance occurring if antiretrovirals are allocated only to Durban. Many financial, medical, logistical, scientific, and political obstacles have yet to be overcome for the successful rollout of antiretovirals in Africa (14). Disinhibition in sexual behavior may occur as the rollout proceeds and can be included in our model; so far disinhibition has almost exclusively occurred in gay communities (3), and these effects have been modeled previously (7). During the rollout, PLWHA may migrate from rural locations to urban centers to receive treatment (23–25); our current results are robust to migration (results not shown) unless migration rates are extremely high, because ≈55% of PLWHA already reside in Durban. Furthermore, migration rates are unlikely to be extremely high because HIV-infected individuals in rural areas would have to move permanently to Durban to receive the necessary treatment monitoring.
We have shown (for the first time) that there will be different epidemiological outcomes depending on the DAS that is used; allocation decisions should only be made after the ethical consequences have been carefully considered by health policy officials. Difficult decisions will soon have to be made by these officials in KwaZulu-Natal as they decide how to allocate ART. Allocating all of the drugs to Durban will ensure that the drugs have the maximum impact on reducing the epidemic. However, this strategy is highly inequitable and would further exacerbate the existing substantial urban–rural healthcare disparities in KwaZulu-Natal. Our analyses highlight the difficult choices between epidemiological and ethical objectives that will have to be made by the governments and health policy officials of resource-constrained countries to decide how to divide the scarce drugs between the urban and rural areas in their countries. We suggest that, before these critical treatment decisions are made, spatially explicit mathematical models be used to predict the epidemiological (and ethical) consequences of drug allocation decisions.
Methods
Mathematical Structure of the Spatially Explicit HIV Urban–Rural Model.
Our spatially explicit urban–rural model for linked HIV epidemics in multiple communities is specified by Eq. 1 through 5.
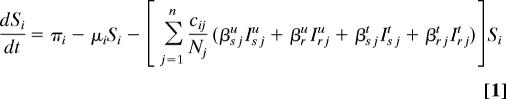 |
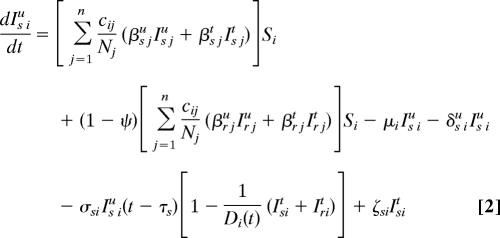 |
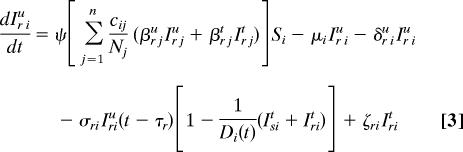 |
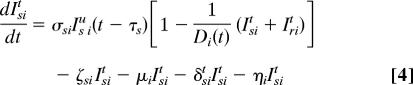 |
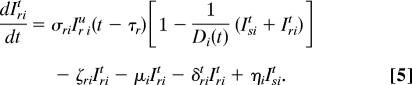 |
Ni = Si + ISiu + Iriu + ISit + Irit is the total population size of community i (i = 1, …, n), where n specifies the number of communities. Within each community in our model, we track susceptible individuals (Si) (Eq. 1), untreated individuals infected with wild-type strains (ISiu) (Eq. 2) or drug-resistant strains (Iriu) (Eq. 3), and individuals on ART infected with wild-type strains (ISit) (Eq. 4) or drug-resistant strains (Irit) (Eq. 5). We assume that individuals infected with drug-resistant strains can transmit both wild-type and drug-resistant strains; the probability that the resistant strains are transmitted (as opposed to wild-type strains) is denoted by ψ. Individuals join the sexually active population at rate π and leave at rate μ.
The summation terms in Eq. 1 specify the per capita force of infection in each community; β specifies the transmission probability and cij specifies the degree of sexual cross-mixing (i.e., the average rate of acquiring new sex partners per unit time that people in community i choose from community j). The degree of sexual cross-mixing was calculated for each of m rural communities using Eq. 6:
where n refers to the urban community, c represents the total number of new sex partners acquired per person per year, di specifies the distance of the ith rural community from Durban, and f is an “accessibility factor”; f incorporates factors other than distance that can decrease sexual cross-mixing, such as heterogeneities in space and movement of people in the province that are because of road networks and transport availability. Eq. 6 gives average sexual cross-mixing proportions consistent with studies of urban–rural mixing (26). Individuals in rural communities also choose sex partners from their own community (cii = c − cin, i = 1 … m). We define cni = cin(Ni/Nn) to balance sex partnerships between the urban and rural communities and cm = c − Σi=1mcni.
We let Di(t) be the maximum number of people that can be on ART in community i at time t because of drug availability due to the DAS that is being modeled, and the per capita rate of ART initiation for the treatment-eligible HIV-infected individuals is represented by σ. This rate is calculated from the number of HIV-infected individuals that are expected to be treated per year according to the South African government's plan. For the purpose of modeling this treatment plan (and to include treatment in the equations that specify our model), we needed to convert the expected numbers treated per year into a per capita treatment rate. Individuals that are treatment-eligible according to the World Health Organization's medical criteria (8) (which occurs τs years after seroconversion, on average) may go on treatment, but this treatment initiation is limited by the drug availability, Di(t), according to the DAS. The total number of people in community i on treatment according to the DAS is ISit + Irit, which very rapidly saturates (dynamically) to Di(t). Individuals in each infected state die because of AIDS at rates δ. Expected survival time for individuals infected with wild-type and drug-resistant HIV is calculated by 1/δst = [psα5 + (1 − ps)] (1/δsu − τs) and 1/δrt = [prα5 + (1 − pr)] (1/δru − τr), respectively. Here, ps and pr specify the fractions of individuals infected with wild-type and drug-resistant virus, respectively, that achieve viral suppression on ART, and τ is the expected time between seroconversion and treatment eligibility. Expected extension of life because of ART is modeled by α5. The rate of giving up ART is specified by ς. Treated individuals infected with wild-type virus develop drug resistance at rate η.
Time-Dependent Uncertainty and Sensitivity Analyses.
Our model was parameterized to reflect the demography of KwaZulu-Natal; hence, the source rate, πi, was set to reflect the population size in this province. We performed an uncertainty analysis by setting ranges (based on published studies and clinical expertise) on all model parameters (see Tables 1 and 2, which are published as supporting information on the PNAS web site) and carrying out 1,000 simulations by using Latin Hypercube sampling (27). Ranges in parameter estimates accounted for uncertainty in values and intrinsic biological heterogeneity. Because the population of Durban is five times greater than the population of any other city in KwaZulu-Natal, we assumed that, effectively, there is one major urban center in the province. The exact number of rural communities in KwaZulu-Natal is unknown; thus, we divided the population into n communities (one urban center and m = n − 1 rural communities) and varied m between 400 and 4,000; the average population per rural community varied from ≈1,300 to 13,000 (reasonable estimates for the communities of KwaZulu-Natal). We estimated the proximity of village i to the urban center by sampling di from an exponential distribution with mean d∈(10,100) km, consistent with the size of KwaZulu-Natal. We varied the accessibility factor f (that influenced sexual cross-mixing) as a random variable between 0 and 1. Parameter c (the average number of new sex partners per person per year) was sampled from a range of 0.5–1.5. Average length of time that individuals remain in the sexually active population (where they acquire new sex partners) was sampled from a range of 2–20 years. For additional parameter ranges used in our analyses, see Tables 1 and 2.
We made 1,000 predictions for each of the three DAS that we investigated. Specifically, we used our model to predict (to 2008) three variables: the percentage of HIV infections prevented, the level of transmitted drug resistance, and the percentage of AIDS-related deaths averted. We then used our spatially explicit model to subdivide these three variables into urban and rural locations. We also conducted a time-dependent sensitivity analysis by calculating partial rank correlation coefficients and plotting these results as tornado plots (27). Results were similar for the two urban–rural DAS; thus, only the results for one DAS are shown.
Supplementary Material
Acknowledgments
We thank members of S.M.B.'s research group and Timothy Pylko for useful discussions during the course of this work. This work was supported by National Institutes of Health National Institute of Allergy and Infectious Diseases Grant R01 AI041935 (to S.M.B.).
Abbreviations
- ART
antiretroviral therapy
- DAS
drug allocation strategy/strategies
- PLWHA
people living with HIV/AIDS.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Shisana O, Simbayi L. Nelson Mandela/HSRC Study of HIV/AIDS. Cape Town, South Africa: Human Sciences Research Council; 2002. pp. 45–67. [Google Scholar]
- 2.South African Department of Health. [Accessed August 28, 2006];Operational Plan for Comprehensive HIV and AIDS Care, Management, and Treatment for South Africa. 2003 :248. Available at www.info.gov.za/issues/hiv/careplan.htm. [Google Scholar]
- 3.Blower S, Bodine E, Kahn J, McFarland W. AIDS. 2005;19:1–14. doi: 10.1097/00002030-200501030-00001. [DOI] [PubMed] [Google Scholar]
- 4.Blower S, Farmer P. AIDScience. 2003;3 http://aidscience.org/Articles/AIDScience033.asp. [Google Scholar]
- 5.Blower S, Ma L, Farmer P, Koenig S. Curr Drug Targets Infect Disord. 2003;3:345–353. doi: 10.2174/1568005033480999. [DOI] [PubMed] [Google Scholar]
- 6.Blower SM, Aschenbach AN, Gershengorn HB, Kahn JO. Nat Med. 2001;7:1016–1020. doi: 10.1038/nm0901-1016. [DOI] [PubMed] [Google Scholar]
- 7.Blower SM, Gershengorn HB, Grant RM. Science. 2000;287:650–654. doi: 10.1126/science.287.5453.650. [DOI] [PubMed] [Google Scholar]
- 8.WHO. Scaling Up Antiretroviral Therapy in Resource-Limited Settings: Treatment Guidelines for a Public Health Approach. Geneva: WHO; 2003. [Accessed August 28, 2006]. pp. 9–11. Available at www.who.int/3by5/publications/documents/arv_guidelines/en/index.html. [Google Scholar]
- 9.Wood E, Hogg RS, Yip B, Harrigan PR, O'Shaughnessy MV, Montaner JS. Ann Intern Med. 2003;139:810–816. doi: 10.7326/0003-4819-139-10-200311180-00008. [DOI] [PubMed] [Google Scholar]
- 10.King MS, Brun SC, Kempf DJ. J Infect Dis. 2005;191:2046–2052. doi: 10.1086/430387. [DOI] [PubMed] [Google Scholar]
- 11.Bangsberg DR, Charlebois ED, Grant RM, Holodniy M, Deeks SG, Perry S, Conroy KN, Clark R, Guzman D, Zolopa A, et al. AIDS. 2003;17:1925–1932. doi: 10.1097/00002030-200309050-00011. [DOI] [PubMed] [Google Scholar]
- 12.Harrigan PR, Hogg RS, Dong WW, Yip B, Wynhoven B, Woodward J, Brumme CJ, Brumme ZL, Mo T, et al. J Infect Dis. 2005;191:339–347. doi: 10.1086/427192. [DOI] [PubMed] [Google Scholar]
- 13.Sethi AK, Celentano DD, Gange SJ, Moore RD, Gallant JE. Clin Infect Dis. 2003;37:1112–1118. doi: 10.1086/378301. [DOI] [PubMed] [Google Scholar]
- 14.Anderson R, May R. Infectious Diseases of Humans: Dynamics and Control. Oxford: Oxford Univ Press; 1991. [Google Scholar]
- 15.Phillips AN, Dunn D, Sabin C, Pozniak A, Matthias R, Geretti AM, Clarke J, Churchill D, Williams I, Hill T, et al. AIDS. 2005;19:487–494. doi: 10.1097/01.aids.0000162337.58557.3d. [DOI] [PubMed] [Google Scholar]
- 16.Koenig SP, Leandre F, Farmer PE. AIDS. 2004;18(Suppl 3):S21–S25. doi: 10.1097/00002030-200406003-00005. [DOI] [PubMed] [Google Scholar]
- 17.Walton DA, Farmer PE, Lambert W, Leandre F, Koenig SP, Mukherjee JS. J Public Health Policy. 2004;25:137–158. doi: 10.1057/palgrave.jphp.3190013. [DOI] [PubMed] [Google Scholar]
- 18.Wilson DP, Blower SM. PLoS Med. 2005;2:132–141. doi: 10.1371/journal.pmed.0020050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bolker BM, Grenfell BT. Proc R Soc London Ser B; 1993. pp. 75–81. [Google Scholar]
- 20.Curran J, Debas H, Arya M, Kelley P, Knobler S, Pray L, editors. Institute of Medicine Committee on Examining the Probable Consequences of Alternative Patterns of Widespread Antiretroviral Drug Use in Resource-Constrained Settings. Scaling Up Treatment for the Global AIDS Pandemic: Challenges and Opportunities. Washington, DC: Natl Acad Press; 2004. [Google Scholar]
- 21.Anonymous. Lancet. 2005;365:1597. [Google Scholar]
- 22.Ooms G, Schrecker T. Lancet. 2005;365:1821–1823. doi: 10.1016/S0140-6736(05)66586-5. [DOI] [PubMed] [Google Scholar]
- 23.Voeten HA, Egesah OB, Habbema JD. Sex Transm Dis. 2004;31:481–487. doi: 10.1097/01.olq.0000135989.14131.9d. [DOI] [PubMed] [Google Scholar]
- 24.Bourne D, Sayed AR. Urban Health Newsl. 1996;30:29–32. [PubMed] [Google Scholar]
- 25.Zere E, McIntyre D. Int J Equity Health. 2003;2:7. doi: 10.1186/1475-9276-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blower SM, Boe C. J Acquired Immune Defic Syndr. 1993;6:1347–1352. [PubMed] [Google Scholar]
- 27.Blower SM, Dowlatabadi H. Int Stat Rev. 1994;62:229–243. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.