Abstract
Endogenous retroviruses (ERVs) are fixed and abundant in the genomes of vertebrates. Circumstantial evidence suggests that ERVs play a role in mammalian reproduction, particularly placental morphogenesis, because intact ERV envelope genes were found to be expressed in the syncytiotrophoblasts of human and mouse placenta and to elicit fusion of cells in vitro. We report here in vivo and in vitro experiments finding that the envelope of a particular class of ERVs of sheep, endogenous Jaagsiekte sheep retroviruses (enJSRVs), regulates trophectoderm growth and differentiation in the periimplantation conceptus (embryo/fetus and associated extraembryonic membranes). The enJSRV envelope gene is expressed in the trophectoderm of the elongating ovine conceptus after day 12 of pregnancy. Loss-of-function experiments were conducted in utero by injecting morpholino antisense oligonucleotides on day 8 of pregnancy that blocked enJSRV envelope protein production in the conceptus trophectoderm. This approach retarded trophectoderm outgrowth during conceptus elongation and inhibited trophoblast giant binucleate cell differentiation as observed on day 16. Pregnancy loss was observed by day 20 in sheep receiving morpholino antisense oligonucleotides. In vitro inhibition of the enJSRV envelope reduced the proliferation of mononuclear trophectoderm cells isolated from day 15 conceptuses. Consequently, these results demonstrate that the enJSRV envelope regulates trophectoderm growth and differentiation in the periimplantation ovine conceptus. This work supports the hypothesis that ERVs play fundamental roles in placental morphogenesis and mammalian reproduction.
Keywords: development, placenta, sheep, trophectoderm
The sheep genome contains ≈20 copies of endogenous retroviruses (ERVs) highly related to the exogenous and pathogenic Jaagsiekte sheep retrovirus (JSRV) (1–3). Endogenous JSRVs (enJSRVs) are abundantly expressed in the epithelia of the female genital tract (4). In the placenta, enJSRVs are expressed in the mononuclear trophectoderm cells of the conceptus (embryo/fetus and associated extraembryonic membranes) and are most abundant in the trophoblast giant binucleate cells (BNCs) and multinucleated syncytial plaques of the placentomes (5–7). The temporal expression of the enJSRV envelope (env) gene in the trophectoderm is coincident with key events in the development of the sheep conceptus (8). enJSRV env mRNAs are first detected at day 12 (5), when the blastocyst begins the process of elongation, involving the intense proliferation and outgrowth of mononuclear trophectoderm cells producing IFN-τ, the antiluteolytic signal for pregnancy recognition in ruminants (9, 10).
In sheep, trophoblast giant BNCs differentiate from mononuclear trophectoderm cells beginning on day 14, migrate, and then fuse with the uterine luminal epithelium, as well as each other, to form multinucleated syncytial plaques that ultimately form the cotyledonary portions of the placenta (11). The BNCs derive from the mononuclear trophectoderm cells by a poorly characterized mechanism presumably involving mitotic polyploidy, whereas the syncytial plaques are thought to develop by cell–cell fusion (12). Hyaluronidase 2 (HYAL2) is a glycosylphosphatidylinositol-anchored cell-surface protein that can serve as a cellular receptor for exogenous JSRV Env as well as for retroviral vectors pseudotyped by enJSRV Env (13, 14). By RT-PCR analyses, HYAL2 mRNA is first detected in the conceptus on day 16, which is associated with the onset of BNC differentiation (5). Throughout pregnancy, HYAL2 mRNA can be detected in the BNCs and multinucleated syncytia of sheep placentomes but not in the mononuclear trophectoderm cells of the conceptus or any cells of the endometrium.
Of great interest for comparative physiology is that enJSRV env expression in the developing ovine placenta is strikingly similar to that observed for syncytin 1 and 2, products of human ERV env in humans and primates (15–19) and possibly of two related env genes (syncytin A and syncytin B) in mice (20). Syncytins encode highly fusogenic retroviral envelope proteins that are expressed in the syncytiotrophoblast layer generated by mononuclear cytotrophoblast cell fusion at the maternal–fetal interface. Syncytins are fusogenic when expressed in vitro, thereby advancing the hypothesis that they are involved in placental morphogenesis (15–19). Thus, circumstantial evidence gleaned from studies of primates, sheep, and rodents supports the concept that independently acquired ERVs have been positively selected for a convergent physiological role in placental morphogenesis (21, 22).
In these studies we tested the hypothesis that enJSRV Env has a biological role in periimplantation ovine conceptus development and placental morphogenesis by using an in vivo morpholino loss-of-function approach (23) to block enJSRV Env production in utero.
Results
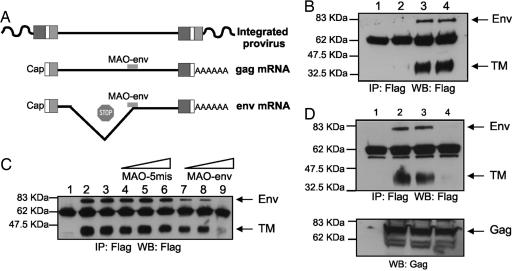
A morpholino antisense oligonucleotide (MAO) was designed to specifically inhibit expression of enJSRV env mRNAs (MAO-env) (Fig. 1A). MAOs inhibit RNA splicing and/or translation by a steric block mechanism that is RNase H-independent (23). Morpholinos are effective only when designed to complement the nucleotide region around the start codon and/or possible splicing sites of a given gene mRNA. The nucleotide sequence around the splice acceptor and start codon of the exogenous JSRV env and the known enJSRV loci are highly conserved, indicating that one common MAO should inhibit splicing and translation of most enJSRV proviral loci expressing an intact env gene (6). To examine morpholino effectiveness, we conducted a series of in vitro studies in transiently transfected 293T cells (Fig. 1 B–D). MAO-env effectively inhibited expression of enJS5F16 (one of the cloned enJSRV loci) (6) Env (Fig. 1B, lane 2) in a dose-dependent fashion (Fig. 1C). A five-mismatch (MAO-5mis) and a standard (MAO-std) control morpholino had no effect on the expression of enJS5F16 Env (Fig. 1 B and C). As expected, MAO-env and the control morpholinos did not affect expression of enJSRV Gag (the polyprotein forming the retroviral capsid) in transfected 293T cells (Fig. 1D), because the retroviral Gag is produced from a full-length genomic mRNA, whereas Env is produced only from correctly spliced mRNA (24) (Fig. 1A). Thus, MAO-env specifically and effectively inhibits enJSRV env mRNA translation.
Fig. 1.
Design and effects of morpholinos on enJSRV Env expression in vitro. (A) MAO-env was designed to inhibit splicing and translation of enJSRV env mRNA but not expression of full-length genomic RNA (which expresses the viral Gag). (B) 293T cells were mock-transfected (lane 1) or transfected with pSV-En2EnvFlag, a simian virus 40-driven expression plasmid for enJS5F16 env cDNA tagged with a Flag epitope at the C terminus (lanes 2–4). Cells were then treated with MAO-env (lane 2) or MAO-5mis (lane 3) and MAO-std as controls (lane 4). All morpholinos were complexed with the Endo-Porter delivery reagent and used at a final concentration of 80 μM. After 48 h, enJS5F156 Env expression was determined by immunoprecipitation (IP) and Western blot analysis (WB). Note that the full-length retroviral Env is processed into a surface domain and a transmembrane domain (TM). (C) 293T cells were mock-transfected (lane 1) or transfected with pSV-En2EnvFlag as above. Cells were then treated with Endo-Porter alone (lane 2), MAO-std as a control (lane 3), MAO-5mis as a control (lanes 4–6; 20, 40, and 80 μM, respectively), or MAO-env (lanes 7–9; 20, 40, and 80 μM, respectively). All morpholinos were complexed with Endo-Porter delivery reagent. After 24 h, enJS5F16 Env expression was determined by immunoprecipitation and Western blot analysis as in B. (D) 293T cells were mock-transfected (lane 1) or cotransfected with pSV-En2EnvFlag and pCMV2 en56A1 expressing the full-length en56A1 clone (lanes 2–4). Cells were then treated Endo-Porter alone (lanes 1 and 2), MAO-5mis as a control (lane 3), or MAO-env (lane 4). All morpholinos were complexed with Endo-Porter delivery reagent and used at a final concentration of 80 μM. After 48 h, enJSRV Env expression (Upper) was determined by immunoprecipitation and Western blot analysis as in B and C and Gag expression by Western blot analysis (Lower).
To conduct in vivo loss-of-function studies, we injected the morpholinos into the lumen of the ovine uterus on day 8 after mating and determined their effect on conceptus development and pregnancy establishment on either day 16 (study one) or day 20 (study two). The blastocyst hatches from the zona pellucida on day 8 of pregnancy in sheep (10). The large size of the uterine horn (10–12 cm in length and 2–3 cm in width) makes intraluminal injection feasible in this large-animal model. The amount of morpholino used for these studies was extrapolated from work in the mouse (25). A dose–response study to determine the minimal amount of morpholino that can be used for in vivo studies was not conducted. Indeed, this amount may be gene-specific, given the relative differences in mRNA abundance for each transcribed gene. In study one, pregnancy rates were not different (P > 0.10) among the various treatments (Table 1). However, major differences were observed in conceptus development in ewes receiving MAO-env as compared with the MAO-5mis or MAO-std controls. The conceptuses from all ewes injected with the control morpholinos, MAO-5mis and MAO-std, were fully elongated and filamentous, which is typical of day 16 of pregnancy (Fig. 2A). Although the conceptuses recovered from ewes injected with MAO-env were elongated, they were fragile and substantially smaller than those from ewes injected with the control morpholinos (Fig. 2A). Histological examination found that conceptuses from ewes injected with either control morpholino were of normal appearance, containing many mononuclear trophectoderm cells (Fig. 2B). In contrast, conceptuses from MAO-env-treated ewes seemed to have fewer mononuclear trophectoderm cells compared with the conceptuses from control ewes. In addition, most mononuclear trophectoderm cells from the MAO-env-treated ewes displayed intracytoplasmic vacuoles (Fig. 2B).
Table 1.
Effect of morpholinos on pregnancy and conceptus development in sheep
| Morpholino | Study one: Day 16 after mating |
Study two: Day 20 after mating |
||||||
|---|---|---|---|---|---|---|---|---|
| Pregnancy rate,* % | Conceptus development | IFN-τ in uterine flush | BNCs, % | Pregnancy rate,* % | Conceptus development | IFN-τ in uterine flush | BNCs, % | |
| MAO-std | 100 (5/5) | Elongated, filamentous | 282 ± 50 | 12 ± 1 | 100 (5/5) | Elongated, filamentous | ND | ND |
| MAO-5mis | 100 (5/5) | Elongated, filamentous | 232 ± 50 | 10 ± 1 | 83 (5/6) | Elongated,filamentous | ND | ND |
| MAO-env | 100 (5/5) | Growth-retarded, filamentous† | 68 ± 10 | 1 ± 1 | 20 (1/5) | Not present‡ | ND | ND |
All ewes received intrauterine injections of morpholinos on day 8 after mating and were hysterectomized on either day 16 or 20. ND, not determined.
*Pregnancy rate data are expressed as percentage of ewes with a conceptus recovered from the uterine lumen by flush before hysterectomy (day 16) or present in the uterine lumen at hysterectomy (day 20) (pregnant/total).
†Conceptuses were growth-retarded, slightly elongated, and fragile with no detectable trophoblast giant BNCs.
‡The single recovered conceptus was severely growth-retarded as compared with conceptuses recovered from MAO-5mis and MAO-std controls and contained no detectable BNCs (data not shown).
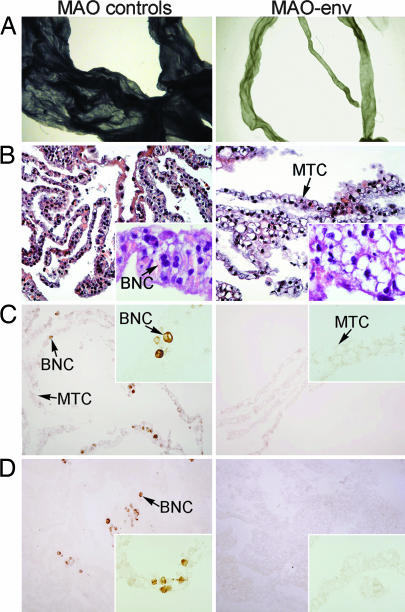
Fig. 2.
Effects of morpholinos on periimplantation conceptus trophoblast growth and differentiation. MAO-std, MAO-5mis, or MAO-env was injected into the uterine lumen on day 8 after mating, and conceptuses were recovered on day 16 (see Materials and Methods for experimental details). (A) Morphology of the conceptuses was examined by using an inverted microscope. Micrographs are shown at the same magnification. Note the retarded growth in the conceptus recovered from a MAO-env-treated ewe. (B) Portions of the conceptuses were fixed in paraformaldehyde, embedded in paraffin, sectioned, and stained with hematoxylin and eosin. (Width of each field of view is 420 μm with the Inset at 85 μm.) (C and D) Trophoblast giant binucleate cells (BNCs) in conceptuses were detected by pregnancy-associated glycoproteins (PAGs) (C) and CSH1 (alias placental lactogen) (D) in the conceptus. Immunoreactive PAG and CSH1 proteins were detected in paraformaldehyde-fixed, paraffin-embedded sections of conceptuses by using a rabbit anti-ovine PAG or anti-ovine CSH1 antibody. (Width of each field of view is 420 μm with the Inset at 210 μm.) Data are representative of conceptuses from all ewes. MTC, mononuclear trophectoderm cell.
To quantify the effect of MAO-env on mononuclear trophectoderm cell growth, we measured the levels of IFN-τ (which is produced solely by mononuclear trophectoderm cells) in uterine flushings (9). Consistent with retarded growth of the conceptus (Fig. 2A), the relative amounts of IFN-τ in the uterine flushings of MAO-env-injected ewes (68 ± 10 relative units) were considerably lower (P < 0.05) than MAO-5mis-injected (232 ± 50 relative units) and MAO-std-injected (282 ± 50 relative units) ewes (Table 1). Next, we evaluated conceptuses for the presence of trophoblast giant BNCs, which derive from mononuclear trophectoderm cells. Chorionic somatomammotropin hormone 1 (CSH1; alias placental lactogen) and pregnancy-associated glycoproteins (PAGs) are expressed only by BNCs and are useful markers for these cells (10). Immunostaining for CSH1 and PAG proteins found 10 ± 1% or 12 ± 1% BNCs in the conceptuses from control ewes (MAO-5mis and MAO-std, respectively), whereas BNCs were very scarce (1 ± 1%) or not present at all in conceptuses recovered from MAO-env-injected ewes (Fig. 2 C and D). Therefore, in vivo enJSRV Env knockdown in the conceptus retarded trophectoderm growth and prohibited differentiation of trophoblast giant BNCs.
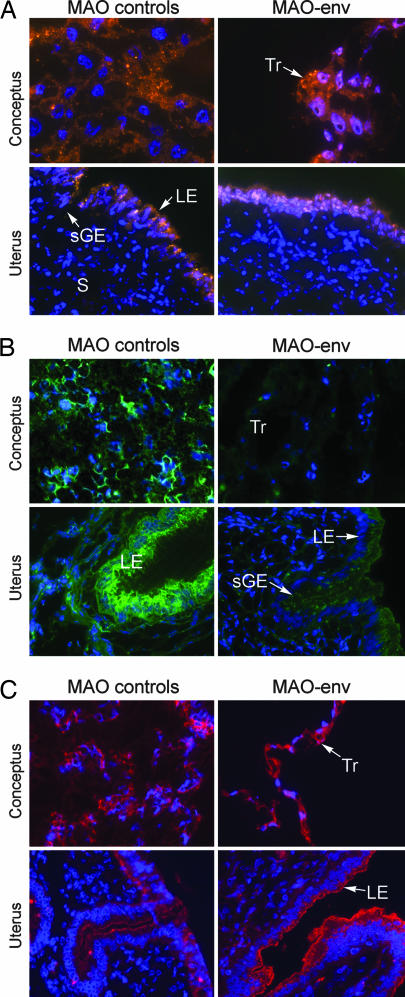
Next we confirmed that retarded development of conceptuses of MAO-env-treated ewes was associated with a reduction in enJSRV Env (Fig. 3). As shown in Fig. 3A, rhodamine-labeled morpholinos were observed in trophectoderm cells of the conceptus as well as in luminal epithelium and superficial glandular epithelium of the endometrium. In conceptuses recovered from ewes injected with MAO-5mis and MAO-std controls, abundant enJSRV Env protein was observed at the apical surfaces of the mononuclear trophectoderm cells, as well as the luminal epithelium and glandular epithelium of the endometrium. As assessed by immunofluorescence analysis, enJSRV Env expression was almost completely diminished in the trophectoderm of day 16 conceptuses recovered from ewes injected with MAO-env and was also substantially decreased in the uterine luminal epithelium (Fig. 3B). Very little or no background binding was observed in negative controls in which rabbit IgG was substituted for the primary antibody or the primary antibody was omitted in the procedure (data not shown). Immunofluorescence analyses are required for evaluation of enJSRV Env abundance with the rabbit anti-JSRV Env antibody because it does not recognize the Env protein under Western blot conditions (M.P., M.V., and T.E.S., unpublished results). No differences in enJSRV Env were observed in the middle to deep uterine glandular epithelia, which was expected because of the inability of the morpholinos to be delivered to those cells (data not shown). As expected from in vitro results (Fig. 1), expression of enJSRV Gag protein in the conceptus trophectoderm and endometrial epithelia was not affected by the morpholinos (Fig. 3C). Thus, the observed alterations in conceptus development, trophectoderm growth, and BNC differentiation in MAO-env-treated ewes could be directly correlated with the inhibition of enJSRV Env by the MAO-env approach.
Fig. 3.
Delivery and effectiveness of morpholinos in vivo. MAO-std, MAO-5mis, or MAO-env was injected into the uterine lumen of sheep on day 8 after mating, and the conceptuses were removed on day 16 (see Materials and Methods for experimental details). (A) Portions of the conceptuses were frozen in optimal cutting temperature (OCT) compound and sectioned. Sections were rinsed in PBS, and a coverslip was affixed by using DAPI-containing mounting medium. Fluorescence microscopy was used to detect the rhodamine-labeled morpholino (orange/red) and DAPI nuclei (blue). (B and C) Conceptuses and uteri were sectioned and analyzed for enJSRV Env protein by immunofluorescence analysis using a rabbit antiserum toward the JSRV Env (B) or Gag (C) with a FITC-labeled secondary antibody. (Width of each field of view is 140 μm.) Data are representative of conceptuses from all ewes. LE, luminal epithelium; sGE, superficial glandular epithelium; S, stroma; Tr, trophectoderm.
In study two, we determined whether conceptuses from MAO-env-treated ewes could establish pregnancy. Ewes were injected with morpholinos on day 8 after mating, and effects were assessed on day 20. Early pregnancy loss occurred in almost all MAO-env-injected ewes, but not in control ewes (Table 1). Pregnancy rates of 100% and 83% were not different (P > 0.10) in control ewes receiving MAO-std or MAO-5mis control morpholinos, respectively, but were substantially reduced (P < 0.025) to 20% in MAO-env-treated ewes (Table 1). The single recovered conceptus from a MAO-env-injected ewe was severely growth-retarded and contained no detectable BNCs (data not shown). Most of the ewes injected with MAO-env exhibited estrus at day 17–18 after mating, which is indicative of early pregnancy loss because of inadequate IFN-τ production by the conceptus. At hysterectomy, a normal elongated, filamentous, and implanting conceptus was observed in the ligated uterine horn of ewes injected with control morpholinos (data not shown).
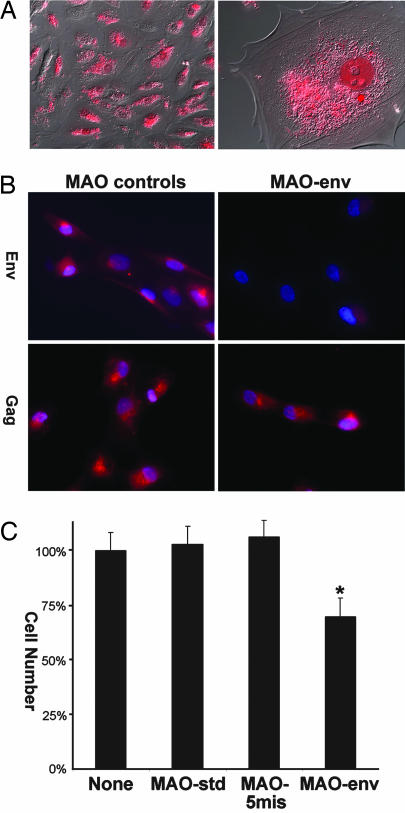
To complement the in vivo data from studies one and two, we isolated mononuclear trophectoderm cells from day 15 conceptuses and cultured them in vitro. As assessed by RT-PCR analysis, the cultured mononuclear trophectoderm cells expressed enJSRV env and gag mRNAs as well as IFN-τ mRNA, whereas CSH1 and PAG mRNAs were not detected (data not shown). Morpholinos were effectively delivered to >95% of the trophectoderm cells in vitro (Fig. 4A). Immunofluorescence analyses found that treatment of trophectoderm cells in vitro with MAO-env inhibited expression of enJSRV Env, whereas the MAO-std and MAO-5mis controls had no effect on enJSRV Env abundance (Fig. 4B). As expected, none of the morpholinos affected enJSRV Gag abundance (Fig. 4B). Trophectoderm cell number was reduced (P < 0.05) by 33% in cultures treated with the MAO-env, whereas MAO-std and MAO-5mis controls had no effect on cell proliferation (Fig. 4C).
Fig. 4.
Effects of morpholinos on in vitro ovine trophectoderm growth. Mononuclear trophectoderm cells were isolated from day 15 conceptuses. (A) Morpholino delivery. Rhodamine-labeled MAO-std was complexed with Endo-Porter aqueous delivery reagent and added to cells in culture. Fluorescence microscopy was used to visualize the labeled MAO in cells. [Width of each field of view is 870 μm (Left) and 90 μm (Right).] (B) Effect of morpholinos on enJSRV Env and Gag protein in cultured trophectoderm cells. Cells were grown on glass slides and mock-treated or treated with MAO-std, MAO-5mis, or MAO-env for 48 h. Immunofluorescence analysis determined that synthesis of enJSRV Env, but not Gag, protein was inhibited in cells treated with MAO-env but not the other morpholinos. Results are representative of three experiments. (Width of each field of view is 140 μm.) (C) Effect of morpholinos on trophectoderm cell growth. Cells were grown in culture dishes until 30% confluency and mock-treated (no morpholino) or treated with MAO-std, MAO-5mis, or MAO-env for 48 h. Cell number was reduced (P < 0.05) by 33% in cultures treated with the MAO-env relative to control morpholinos. Results are from three independent experiments, and data are expressed as the percentage of cell number in mock-treated cultures.
Discussion
These results demonstrate an essential role for ERVs in placental morphogenesis by in vivo experimentation. When a morpholino loss-of-function approach in utero was used, enJSRV Env knockdown caused a reduction in trophectoderm outgrowth during blastocyst elongation and formation of the conceptus. In sheep, pregnancy recognition and establishment involve elongation of the spherical blastocyst to a filamentous conceptus between days 12 and 16 and production of IFN-τ by the conceptus (26, 27). IFN-τ is a developmentally regulated gene that is expressed only in the mononuclear trophectoderm cells of the sheep conceptus (28) from days 10 to 20 with a peak on day 16 (29–31). IFN-τ is antiluteolytic and acts on the endometrium to inhibit development of the luteolytic mechanism, thereby maintaining corpus luteum function and ensuring continued production of progesterone, which is necessary for pregnancy (32). Here, a reduced amount of IFN-τ was found in the uterine flushes of MAO-env-treated ewes containing a growth-retarded conceptus with fewer mononuclear trophectoderm cells. Thus, the pregnancy loss observed before day 20 in MAO-env-treated ewes is likely attributable to an inability of the growth-retarded conceptus to produce sufficient IFN-τ to abrogate development of the endometrial luteolytic mechanism, resulting in luteolysis and a return to estrus (33).
The in vivo and in vitro loss-of-function studies presented here strongly support the hypotheses that enJSRVs play a fundamental role in mononuclear trophectoderm cell outgrowth and differentiation of trophoblast giant BNCs during the periimplantation period of pregnancy (8). Unfortunately, little is known of the cellular and molecular mechanisms regulating trophectoderm proliferation and differentiation during early pregnancy in ruminants (34). Further, the cellular and molecular mechanism(s) whereby enJSRV Env has biological effects within cells is unknown. It is of interest that JSRV env (the exogenous counterpart of enJSRVs) encodes a functional structural protein that is a dominant oncoprotein, a unique feature among oncogenic retroviruses (35). Thus, it is possible that enJSRV and JSRV Env proteins share common mechanisms by which they influence the cell cycle and cell proliferation, but only the Env of the exogenous JSRV evolved to become truly oncogenic. It is also possible that enJSRV Env is essential for trophoblast giant BNCs and formation of multinucleated syncytia by eliciting cell–cell fusion similar to proposed actions of syncytins in humans and mice (15–20). However, results of study one clearly indicate that enJSRV Env influences the mononuclear trophectoderm cell growth and differentiation during conceptus elongation that precedes the formation of multinucleated syncytia. None of the enJSRV Env cloned to date elicits syncytia formation in vitro when ovine, human, or mouse cell lines expressing ovine HYAL2 are used (M.P., M.V., M. Golder, K.A.D., and T.E.S., unpublished results). However, cloning of all sheep enJSRV loci will be necessary to investigate enJSRV Env structure, function, and distribution among Artiodactyla, given that the sheep genome has not been sequenced. Available data based on zoo blots suggest that enJSRVs are present in most Caprinae (36); however, enJSRV locus-specific PCR analyses will be needed to assess the evolution and distribution of the biologically relevant proviruses.
From an evolutionary point of view, we speculate that the protection of the host against related exogenous retroviruses was a driving force influencing the fixation of ERVs in the genome of various mammals (21, 22). For example, enJSRVs interfere with exogenous JSRV at both early and late steps of the replication cycle (14, 37). After the fixation of ERVs in the germ line of the host, their expression in the placenta may have favored conceptus development and increased reproductive efficiency (22, 38, 39). Given that enJSRVs are important for conceptus growth and differentiation, it is likely that some of the host mechanisms governing these reproductive processes may have been lost later during evolution. In conclusion, available evidence obtained in vivo in sheep and in vitro in primates and rodents strongly supports the hypothesis that independently acquired ERVs were positively selected for a convergent physiological role in placental morphogenesis (22). The enormous structural variability of placentae among major taxa supports a model where retroviruses have conferred increased diversity and functionality during evolution (21).
Materials and Methods
Morpholino Design.
Morpholino oligonucleotides were designed and synthesized by Gene Tools (Philomath, OR). The MAO-env had the sequence GCTTC GGCAT CCTGT GGAAA AACAC and targeted to the enJSRVs env RNA overlapping the splice donor and acceptor region (see Fig. 1A). The MAO-5mis control morpholino had the sequence GGTTC GCCAT CCTCT GCAAA AAGAC (italic type indicates differences from MAO-env). The MAO-std had the sequence CCTCT TACCT CAGTT ACAAT TTATA and targeted to a splice site mutant of Homo sapiens hemoglobin β-chain (HBB) gene (GenBank accession no. AY605051). All morpholinos were synthesized with a 5′ rhodamine modification for convenient detection.
In Vitro Transfection Studies.
pSV-En2EnvFlag expresses, under the control of the simian virus 40 promoter, the Env of enJS5F16 (6) tagged with a Flag epitope at the C terminus. pCMV2en56A1 expresses the full-length enJS56A1 locus (6). Human 293T cells were transfected with pSV-En2EnvFlag or cotransfected with pSV-En2EnvFlag and pCMV2 en56A1 by using Lipofectamine (Invitrogen, Carlsbad, CA). After 3 h, cells were washed with PBS and incubated with Endo-Porter aqueous delivery reagent (Gene Tools) (8 μl/ml medium) and 20–80 μM MAO-env, MAO-5mis, or MAO-std. After 48 h, transfected cells were lysed, and cell lysates were analyzed for the presence of enJSRV Env or Gag by immunoprecipitation and/or Western blotting employing an anti-Flag antibody (Sigma–Aldrich, St. Louis, MO) and an anti-JSRV p23 (Matrix Science, London, U.K.) as previously described (37).
In Vivo Studies.
All animal experiments were approved by the Institutional Animal Care and Use Committee of Texas A&M University. Suffolk cross-bred ewes were mated at estrus (day 0) and on day 1 to rams of proven fertility. On day 8 after mating, the ewes were subjected to a midventral laparotomy. The base of the uterine horn ipsilateral to the corpus luteum was double-ligated by using nonabsorbable umbilical tape to prevent migration and growth of the conceptus through the uterine body into the contralateral uterine horn. This surgical procedure does not affect conceptus implantation or fetal development in sheep (40). MAO-std, MAO-5mis, and MAO-env (100 nmol) were complexed with Gene Tools Endo-Porter delivery reagent (50 μl) and diluted to a 1-ml final volume with OPTI-MEM (Invitrogen). The complex was then introduced into the lumen of the uterus (n = 5–6 ewes per morpholino) via the uterotubal junction by using a 1-ml syringe fitted with a 20-gauge catheter. The volume of the uterine lumen of one horn is ≈250–500 μl. After the morpholinos had been discharged into the uterine lumen, the catheter was withdrawn, and the uterine horn was gently massaged to distribute the morpholinos throughout the uterine lumen. The outside of the uterus was rinsed with sterile 5% (vol/vol) glycerol in saline to prevent the formation of adhesions and placed back in the body cavity.
For study one, the morpholino-injected ewes were hysterectomized on day 16. The uterine horn injected with the morpholinos was flushed with 10 ml of sterile PBS. If the conceptus was present, its morphology was recorded (spherical, tubular, or elongated). The conceptus was immediately removed from the uterine flush with a pipette, and the volume of the flush was recorded. Photomicrographs of the conceptus were obtained by using an inverted microscope fitted with a digital camera. Portions of the conceptus were then placed in optimal cutting temperature (OCT) compound (Miles, Oneonta, NY), frozen in liquid nitrogen, and stored at −80°C. Another portion of the conceptus was fixed in freshly prepared 4% (wt/vol) paraformaldehyde in PBS and embedded in paraffin wax. The uterine flush was clarified by centrifugation (5,000 × g for 15 min at 4°C), aliquoted, and stored at −80°C. The amount of IFN-τ in the uterine flush was quantified by semiquantitative Western blot analysis as described in ref. 41.
For study two, the morpholino-injected ewes were hysterectomized on day 20. The uterine horn injected with the morpholinos was not flushed but rather opened along the mesometrial border to expose the conceptus. Portions of the conceptus (if present) and sections of the uterine horn containing the conceptus were frozen in optimal cutting temperature (OCT) compound or fixed in 4% (vol/vol) paraformaldehyde for histological analysis.
Histology, Immunohistochemistry, and Immunofluorescence.
Effective delivery of the rhodamine-labeled morpholinos was analyzed by fluorescence microscopy. Cryosections of the uteri and conceptuses were prepared, and a DAPI-containing mounting medium was used to visualize nuclei. The enJSRV Env and Gag proteins were evaluated in frozen conceptus tissue sections by immunofluorescence staining using a rabbit antiserum toward the JSRV Env or Gag as described previously (6, 37). Negative controls included substituting rabbit IgG in place of the primary antibody as well as removal of the primary antibody. Filter checks were used to ensure specific signals. Immunoreactive CSH1 and PAG proteins were assessed in the conceptus by using standard immunohistochemical procedures with antibodies to ovine CSH1 (rabbit polyclonal antibody was kindly provided by Russ V. Anthony, Colorado State University, Fort Collins, CO) and ovine PAG (rabbit anti-recombinant PAG3 polyclonal antibody was kindly provided by Jonathan A. Green, University of Missouri-Columbia, Columbia, MO). Rabbit IgG was used in place of the relevant primary antibody as a negative control. The number of BNCs was quantified by determining the number of CSH1- and PAG-immunostained BNCs relative to mononuclear trophoblast cells in at least five nonsequential sections of each conceptus from each ewe. The nuclei were visualized after applying hematoxylin counterstain. Sections from all sheep and treatment groups were analyzed in duplicate in the same run, and images were captured by using standardized procedures.
In Vitro Ovine Mononuclear Trophectoderm Cell Culture and Proliferation Assay.
Conceptuses from day 15 pregnant ewes were recovered by sterile flush. The inner cell mass was removed by dissection, and trophectoderm cells were isolated and cultured by using methods described previously (42). Rhodamine-labeled MAO-std (100 nmol) was complexed with Endo-Porter aqueous delivery reagent (6 μl/ml of medium) and added to cells in culture. Fluorescence microscopy was used to visualize the labeled MAO in cells 24 h after treatment by using a Zeiss Stallion Double Detector Imaging system (Carl Zeiss Microimaging, Thornwood, NY).
For immunofluorescence analyses, cells were grown on two-well Lab-Tek Coverglass Chambered slides (Nalge Nunc, Rochester, NY) and mock-treated (no morpholino) or treated with MAO-std, MAO-5mis, or MAO-env (100 nmol) complexed with Endo-Porter aqueous delivery reagent for 48 h. Immunoreactive enJSRV Env and Gag proteins were analyzed by immunofluorescence staining using a rabbit antiserum toward the JSRV Env or Gag as described previously (6, 37); rabbit IgG was used in place of the primary antibody as a negative control. Cells were sequentially imaged by using a Cy3 filter set followed by differential interference contrast optics using either a Plan-Apochromat (10×; n.a., 0.45) or a C-Apochromat (63× water correction; n.a., 1.2) objective lens. The experiment was independently repeated three times.
To determine the effects of morpholinos on trophectoderm cell proliferation, cells were grown in six-well culture dishes until 30% confluency and mock-treated (no morpholino) or treated with MAO-std, MAO-5mis, or MAO-env (100 nmol complexed to Endo-Porter aqueous delivery reagent) in triplicate for 48 h. A colorimetric assay using Janus green dye was used to assess cell numbers (43). The experiment was independently repeated three times.
Statistics.
Pregnancy rate data were analyzed by χ2 test. All quantitative data were subjected to least-squares ANOVA by using the General Linear Models procedures of the Statistical Analysis System (SAS Institute, Cary, NC). Statistical models accounted for sources of variation, including the main effects of morpholino type and, where appropriate, histological section (BNC quantification) or replicate (proliferation assays). The following data are presented as least-squares means with SEM: (i) data for IFN-τ in uterine flush and the number of BNCs in Table 1 and (ii) data for cell number in Fig. 4C.
Acknowledgments
We thank F. W. Bazer, J. C. Neil, and O. Jarrett for useful discussion and R. V. Anthony and J. L. Green for provision of reagents. This work was supported in part by the Wellcome Trust (to M.P.) and grants from the National Cancer Institute (to M.P.) and National Institute of Environmental Health Sciences (to T.E.S. and R.C.B.). M.P. is a Wolfson–Royal Society Research Merit Awardee.
Abbreviations
- ERV
endogenous retrovirus
- JSRV
Jaagsiekte sheep retrovirus
- enJSRV
endogenous retrovirus related to JSRV
- BNC
binucleate cell
- MAO
morpholino antisense oligonucleotide
- CSH
chorionic somatomammotropin hormone
- PAG
pregnancy-associated glycoprotein.
Footnotes
The authors declare no conflict of interest.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.DeMartini JC, Carlson JO, Leroux C, Spencer T, Palmarini M. Curr Top Microbiol Immunol. 2003;275:117–137. doi: 10.1007/978-3-642-55638-8_5. [DOI] [PubMed] [Google Scholar]
- 2.Palmarini M, Sharp JM, De las Heras M, Fan H. J Virol. 1999;73:6964–6972. doi: 10.1128/jvi.73.8.6964-6972.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palmarini M, Mura M, Spencer TE. J Gen Virol. 2004;85:1–13. doi: 10.1099/vir.0.19547-0. [DOI] [PubMed] [Google Scholar]
- 4.Palmarini M, Gray CA, Carpenter K, Fan H, Bazer FW, Spencer TE. J Virol. 2001;75:11319–11327. doi: 10.1128/JVI.75.23.11319-11327.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dunlap KA, Palmarini M, Adelson DL, Spencer TE. Biol Reprod. 2005;73:271–279. doi: 10.1095/biolreprod.105.039776. [DOI] [PubMed] [Google Scholar]
- 6.Palmarini M, Hallwirth C, York D, Murgia C, de Oliveira T, Spencer T, Fan H. J Virol. 2000;74:8065–8076. doi: 10.1128/jvi.74.17.8065-8076.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanna E, Sanna MP, Loddo C, Sanna L, Mura M, Cadelano T, Leoni A. Eur J Histochem. 2002;46:273–280. doi: 10.4081/1689. [DOI] [PubMed] [Google Scholar]
- 8.Dunlap KA, Palmarini M, Spencer TE. Placenta. 2006;27(Suppl A):5135–5140. doi: 10.1016/j.placenta.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 9.Roberts RM, Ealy AD, Alexenko AP, Han CS, Ezashi T. Placenta. 1999;20:259–264. doi: 10.1053/plac.1998.0381. [DOI] [PubMed] [Google Scholar]
- 10.Guillomot M. J Reprod Fertil Suppl. 1995;49:39–51. [PubMed] [Google Scholar]
- 11.Wooding FB. Am J Anat. 1984;170:233–250. doi: 10.1002/aja.1001700208. [DOI] [PubMed] [Google Scholar]
- 12.Wooding FB. Placenta. 1992;13:101–113. doi: 10.1016/0143-4004(92)90025-o. [DOI] [PubMed] [Google Scholar]
- 13.Miller AD. Curr Top Microbiol Immunol. 2003;275:179–199. doi: 10.1007/978-3-642-55638-8_7. [DOI] [PubMed] [Google Scholar]
- 14.Spencer TE, Mura M, Gray CA, Griebel PJ, Palmarini M. J Virol. 2003;77:749–753. doi: 10.1128/JVI.77.1.749-753.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blaise S, de Parseval N, Benit L, Heidmann T. Proc Natl Acad Sci USA. 2003;100:13013–13018. doi: 10.1073/pnas.2132646100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blond JL, Lavillette D, Cheynet V, Bouton O, Oriol G, Chapel-Fernandes S, Mandrand B, Mallet F, Cosset FL. J Virol. 2000;74:3321–3329. doi: 10.1128/jvi.74.7.3321-3329.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frendo JL, Olivier D, Cheynet V, Blond J-L, Bouton O, Vidaud M, Rabreau M, Evain-Brion D, Mallet F. Mol Cell Biol. 2003;23:3566–3574. doi: 10.1128/MCB.23.10.3566-3574.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mallet F, Bouton O, Prudhomme S, Cheynet V, Oriol G, Bonnaud B, Lucotte G, Duret L, Mandrand B. Proc Natl Acad Sci USA. 2004;101:1731–1736. doi: 10.1073/pnas.0305763101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mi S, Lee X, Li X, Veldman GM, Finnerty H, Racie L, LaVallie E, Tang XY, Edouard P, Howes S, et al. Nature. 2000;403:785–789. doi: 10.1038/35001608. [DOI] [PubMed] [Google Scholar]
- 20.Dupressoir A, Marceau G, Vernochet C, Benit L, Kanellopoulos C, Sapin V, Heidmann T. Proc Natl Acad Sci USA. 2005;102:725–730. doi: 10.1073/pnas.0406509102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harris JR. BioEssays. 1998;20:307–316. doi: 10.1002/(SICI)1521-1878(199804)20:4<307::AID-BIES7>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 22.Prudhomme S, Bonnaud B, Mallet F. Cytogenet Genome Res. 2005;110:353–364. doi: 10.1159/000084967. [DOI] [PubMed] [Google Scholar]
- 23.Summerton J. Biochim Biophys Acta. 1999;1489:141–158. doi: 10.1016/s0167-4781(99)00150-5. [DOI] [PubMed] [Google Scholar]
- 24.Vogt VM. In: Retroviruses. Coffin JM, Hughes SH, Varmus HE, editors. Cold Spring Harbor, NY: Cold Spring Harbor Lab Press; 1997. pp. 27–69. [Google Scholar]
- 25.Luu KC, Nie GY, Salamonsen LA. Proc Natl Acad Sci USA. 2004;101:8028–8033. doi: 10.1073/pnas.0401069101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spencer TE, Bazer FW. Reprod Biol Endocrinol. 2004;2:49. doi: 10.1186/1477-7827-2-49. Available at www.rbej.com/content/2/1/49. [DOI] [PMC free article] [PubMed]
- 27.Roberts RM, Ealy AD, Alexenko AP, Han CS, Ezashi T. Placenta. 1999;20:259–264. doi: 10.1053/plac.1998.0381. [DOI] [PubMed] [Google Scholar]
- 28.Farin CE, Imakawa K, Hansen TR, McDonnell JJ, Murphy CN, Farin PW, Roberts RM. Biol Reprod. 1990;43:210–218. doi: 10.1095/biolreprod43.2.210. [DOI] [PubMed] [Google Scholar]
- 29.Ashworth CJ, Bazer FW. Biol Reprod. 1989;40:425–433. doi: 10.1095/biolreprod40.2.425. [DOI] [PubMed] [Google Scholar]
- 30.Farin CE, Imakawa K, Roberts RM. Mol Endocrinol. 1989;3:1099–1107. doi: 10.1210/mend-3-7-1099. [DOI] [PubMed] [Google Scholar]
- 31.Guillomot M, Michel C, Gaye P, Charlier N, Trojan J, Martal J. Biol Cell. 1990;68:205–211. doi: 10.1016/0248-4900(90)90309-q. [DOI] [PubMed] [Google Scholar]
- 32.Spencer TE, Ott TL, Bazer FW. Proc Soc Exp Biol Med; 1996. pp. 215–229. [DOI] [PubMed] [Google Scholar]
- 33.Mann GE, Lamming GE. Reprod Domest Anim. 1999;34:269–274. doi: 10.1111/j.1439-0531.2005.00565.x. [DOI] [PubMed] [Google Scholar]
- 34.Cammas L, Reinaud P, Dubois O, Bordas N, Germain G, Charpigny G. Biol Reprod. 2005;72:960–967. doi: 10.1095/biolreprod.104.034801. [DOI] [PubMed] [Google Scholar]
- 35.Fan H. Jaagsiekte Sheep Retrovirus and Lung Cancer. Berlin: Springer; 2003. [Google Scholar]
- 36.Hecht SJ, Stedman KE, Carlson JO, DeMartini JC. Proc Natl Acad Sci USA. 1996;93:3297–3302. doi: 10.1073/pnas.93.8.3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mura M, Murcia P, Caporale M, Spencer TE, Nagashima K, Rein A, Palmarini M. Proc Natl Acad Sci USA. 2004;101:11117–11122. doi: 10.1073/pnas.0402877101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Villarreal LP. J Virol. 1997;71:859–865. doi: 10.1128/jvi.71.2.859-865.1997. and erratum (1998) 72:6277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stoye JP, Coffin JM. Nature. 2000;403:715–717. doi: 10.1038/35001700. [DOI] [PubMed] [Google Scholar]
- 40.Bazer FW, Roberts RM, Basha SM, Zavy MT, Caton D, Barron DH. J Anim Sci. 1979;49:1522–1527. doi: 10.2527/jas1979.4961522x. [DOI] [PubMed] [Google Scholar]
- 41.Satterfield MC, Bazer FW, Spencer TE. Biol Reprod. 2006;75:289–296. doi: 10.1095/biolreprod.106.052944. [DOI] [PubMed] [Google Scholar]
- 42.Johnson GA, Bazer FW, Jaeger LA, Ka H, Garlow JE, Pfarrer C, Spencer TE, Burghardt RC. Biol Reprod. 2001;65:820–828. doi: 10.1095/biolreprod65.3.820. [DOI] [PubMed] [Google Scholar]
- 43.Raspotnig G, Fauler G, Jantscher A, Windischhofer W, Schachl K, Leis HJ. Anal Biochem. 1999;275:74–83. doi: 10.1006/abio.1999.4309. [DOI] [PubMed] [Google Scholar]