Abstract
Duration of developmental stages in animals evolves under contrasting selection pressures of age-specific mortality and growth requirements. When relative importance of these effects varies across environments, evolution of developmental periods is expected to be slow. In birds, maternal effects on egg-laying order and offspring growth, two proximate determinants of nestling period, should enable rapid adjustment of developmental periods to even widely fluctuating mortality rates. We test this hypothesis in a population of house finches (Carpodacus mexicanus) breeding under two contrasting mortality risks: (i) a nest mite-free condition when selection on offspring survival favors a longer time in the nest; and (ii) a mite infestation when selection favors a shorter nest tenure. Mites affected survival of sons more than daughters, and females breeding under mite infestation laid male eggs last and female eggs first in the clutch, thereby reducing sons' exposure to mites and associated mortality. Strong sex bias in laying order and growth patterns enabled mite-infested offspring to achieve similar fledging size, despite a shorter nest tenure, compared with mite-free conditions. In mite-infested nests, male nestlings hatched at larger sizes, completed growth earlier, and had faster initial growth compared with mite-free nests, whereas mite-infested females grew more slowly but for a longer period of time. A combination of heavily sex-biased laying order and sex differences in growth patterns lowered mite-induced mortality by >10% in both sexes. Thus, strong maternal effects can account for frequently observed, but theoretically unexpected, concordance of mortality risks and growth patterns, especially under fluctuating ecological conditions.
Keywords: life history, logistic growth, parasites, sexual dimorphism
Developmental periods, such as incubation and nestling stages in birds, are complex life history traits shaped by the interaction of physiological tradeoffs of growth patterns and age-specific mortality (1–3). Although the time-dependent mortality associated with predation and parasitism favors shorter development and rapid growth (4–6), faster development increases energetic demands of growth and compromises offspring immunocompetence and functional performance (7, 8). These contrasting extrinsic and intrinsic pressures constrain the evolution of developmental periods and growth rates in response to ecological conditions (9, 10). A resolution of these competing demands is provided by epigenetic mechanisms, such as maternal effects on offspring growth, that can rapidly and reversibly modify duration of developmental stages in response to even widely fluctuating mortality risks (11, 12). For example, in response to increased predation risk, passerine birds increase egg allocation of maternal steroids, enabling faster offspring growth and shorter developmental periods (13, 14).
Time-dependent mortality resulting from nest ectoparasites is a powerful selection pressure on nestling growth and developmental periods, and maternal strategies that minimize this mortality range from transfer of growth-affecting and immunological substances into eggs (15, 16) and greater provisioning of infested offspring (17, 18) to selection of parasite-free nest sites or parasite-repellent nest material (19, 20). Moreover, egg-laying females are often exposed to the same nest ectoparasites that will attack their nestlings, enabling females to induce parasite responses and resistance in their offspring. For example, maternal exposure to ectoparasites before egg-laying reduces the effects of these parasites on nestlings (21) through greater allocation of hormones and immunoglobulins to eggs (22). Although maternal effects are uniquely placed to modify offspring developmental periods and growth patterns in response to variation in ectoparasite-induced mortality, their contribution to the evolution of age and size at fledging is poorly understood.
Maternal effects on offspring growth reduce parasite-induced mortality when they shorten the nestling period, increase growth rate, or modify allometry of growing offspring to enable greater allocation to traits that facilitate early fledging (23, 24). However, differences between the sexes in growth patterns, susceptibility to ectoparasites, and sensitivity to maternally transferred hormones limit the effectiveness of maternal adjustment of offspring growth (25), especially when parasite-induced maternal effects are a passive consequence of the mother's own defense (26). Modification of the sequence in which breeding females produce sons and daughters in a clutch, a widespread phenomenon in birds (27), enables both sex-specific allocation of maternal substances, even when such allocation is passive (28, 29), and sex-specific modification of growth rate and duration (30–32), which is important when sexes differ in sensitivity to ectoparasites.
Here we show that, in a native population of house finches (Carpodacus mexicanus), simultaneous adjustment of laying order of male and female eggs and sex-specific growth patterns enabled shorter exposure to nest ectoparasites and lesser associated mortality without compromising size at fledging. In the desert of southwestern Arizona, house finches breed under two contrasting conditions, in late winter when ectoparasites are rare, and in late spring when most nests are heavily infested with a hematophagous ectoparasitic nest mite, Pellonyssus reedi. First, we control for seasonal effects to show that selection for offspring survival favors longer nest tenure under mite-free conditions and shorter nest tenure under mite infestation. Second, we document strong sex bias in egg-laying order by females breeding under mite infestation but not under mite-free conditions, and show that the resulting modification of exposure to nest mites and changes in growth patterns significantly reduces mite-caused mortality. We discuss the evolutionary benefits of epigenetic adjustment of offspring growth and developmental periods when extrinsic mortality varies widely across environments experienced during the breeding cycle.
Results
Probability of Survival Depends on Nest Tenure.
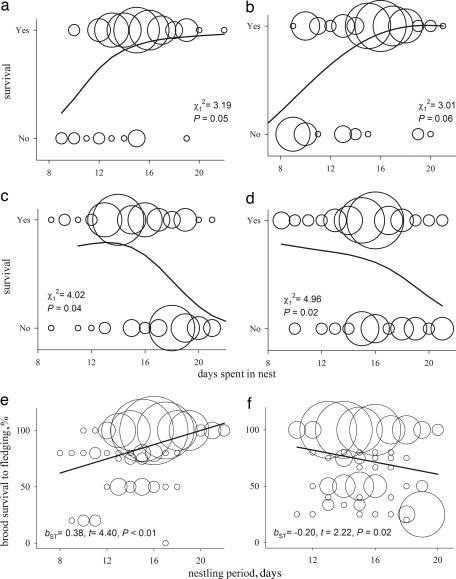
In nests without mites, longer time spent in the nest (nest tenure) was associated with greater survival in females, and marginally better survival in males (Fig. 1a and b). In nests infested with mites, longer nest tenure strongly reduced survival probability in both sexes (Fig. 1 c and d). Similarly, greater brood survival to fledging was associated with a longer nestling period under mite-free conditions and with a shorter nestling period under mite infestation (Fig. 1 e and f). Probability of mite infestation increased as breeding season progressed. However, when mite presence in the nest was statistically controlled, nest initiation date did not affect either individual nestlings' mortality or the percentage of brood that survived to fledging (nest initiation date, F1,197 = 0.14, P = 0.71; and F1,197 = 1.79, P = 0.19).
Fig. 1.
Nestling survival in relation to mite presence. (a–d) Probability of survival as a function of days spent in the nests without mites for male (a) and female (b) nestlings, and in nests with mites for male (c) and female (d) nestlings. (e and f) The relationship between nestling period and percentage of brood survived to fledging in nests without (e) and with (f) mites. Curves are splines. Straight lines are least-squared regressions. Bubble radius is proportional to the number of overlapping data points.
Sex Bias in Laying Order Modifies Nest Tenure for Males and Females.
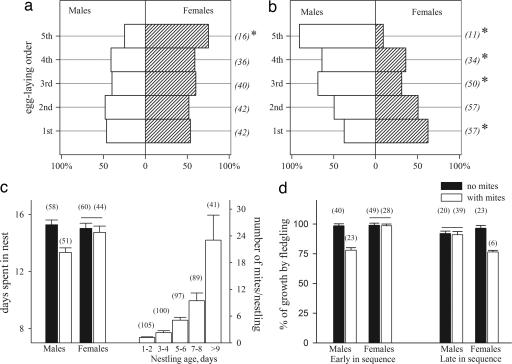
Clutches laid during mite-free and mite-infested periods had strongly different orders of male and female eggs (Fig. 2; mite season, Satterthwaite F = 22.14, P < 0.001; nest initiation date, F = 2.07, P = 0.16). Nests without mites had no sex bias in egg-laying order (Fig. 2a; first: χ12 = 0.74, P = 0.39; second: χ12 = 0.19, P = 0.67; third: χ12 = 3.08, P = 0.08; fourth: χ12 = 1.67, P = 0.20), except for female-biased last-laid egg (χ12 = 4.68, P = 0.03). In mite-infested nests, the first egg was strongly female-biased and the last three eggs were strongly male-biased (Fig. 2b; first: χ12 = 10.87, P = 0.002; second: χ12 = 0.01, P = 0.92; third: χ12 = 12.89, P < 0.001; fourth: χ12 = 8.17, P = 0.01; fifth: χ12 = 18.97, P < 0.001). In mite-free nests, male and female nestlings spent equal time in nests (Fig. 2c), but in mite-infested nests, strong sex bias in egg-laying order led to significantly longer nest tenure of females compared with males (Fig. 2c Left; males: Kruskal–Wallis χ12 = 15.09, P < 0.001; females: χ12 = 0.89, P = 0.82).
Fig. 2.
Sex ratio and growth changes in relation to mite presence. Sex ratio in relation to egg-laying order in mite-free (a) and mite-infested (b) nests. Asterisks indicate significant deviation from an equal sex ratio. (c Left) Sex difference in nest tenure during mite-free (black bars) and mite-infested (white bars) periods. (c Right) Number of mites per nestling in relation to nestling age. (d) Percentage of growth completed by fledging by male and female nestlings hatched early (first and second positions) and late in the laying sequence (third position and later) under mite-free (black bars) and mite-infested (white bars) conditions. Shown are means ± 1 SE. Numbers in parentheses are sample sizes. Horizontal lines in c and d indicate means that are not significantly different between mite-free and mite-infested periods.
Male and Female Growth Are Differentially Affected by Mites.
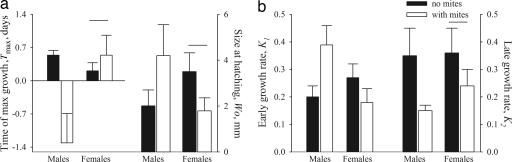
Within nests, the abundance of mites increased with nestling age and reached its maximum 9 days after hatching (Fig. 2c Right, “>9” vs. earlier ages: χ12 = 4.33, P = 0.03), and the effect of mites on the size at fledging depended on the nestling's hatching order and sex (Fig. 2d). In nests with mites, early-hatched (first or second) males and late-hatched (third and later) females completed the least percentage of growth by fledging, whereas late-hatching males and early-hatching females did not differ in percentage of completed growth from the mite-free condition (Fig. 2d; early in the egg-laying sequence, males: χ12 = 10.14, P < 0.001; females: χ12 = 0.94, P = 0.93; late in the egg-laying sequence, males: χ12 = 0.77, P = 0.63; females: χ12 = 17.83, P < 0.001). This difference between the sexes was because of different growth strategies of males and females in relation to mite presence (Fig. 3). In nests with mites, males hatched at larger sizes and had faster and earlier initial growth, but slower late growth compared with males growing under mite-free conditions (Fig. 3 a and b; Tmax: χ12 = 9.46, P < 0.01; W0: χ12 = 3.75, P = 0.05; K1: χ12 = 5.03, P < 0.05; K2: χ12 = 7.00, P < 0.01). Such patterns of growth, combined with overproduction of males in the last three laying positions, resulted in significantly shorter overlap of intensive growth and high mite abundance within a nest (Figs. 2d and 3a). In females, hatching size as well as initial and late growth tended to decrease in nests with mites compared with females growing under mite-free conditions (Fig. 3; Tmax: χ12 = 2.34, P = 0.07; W0: χ12 = 3.51, P = 0.06; K1: χ12 = 3.72, P = 0.05; K2: χ12 = 3.09, P = 0.06).
Fig. 3.
Nestling growth under mite-free (black bars) and mite-infested (white bars) conditions. Shown are time of maximum growth and size at hatching (a), and early and late growth of male and female nestlings (b). Shown are means ± 1 SE. Horizontal lines indicate means that are not significantly different between mite-free and mite-infested conditions. Sample sizes are shown in Fig. 2c.
Sex Bias in Laying Order and Nestling Growth Lowers Mite-Induced Mortality.
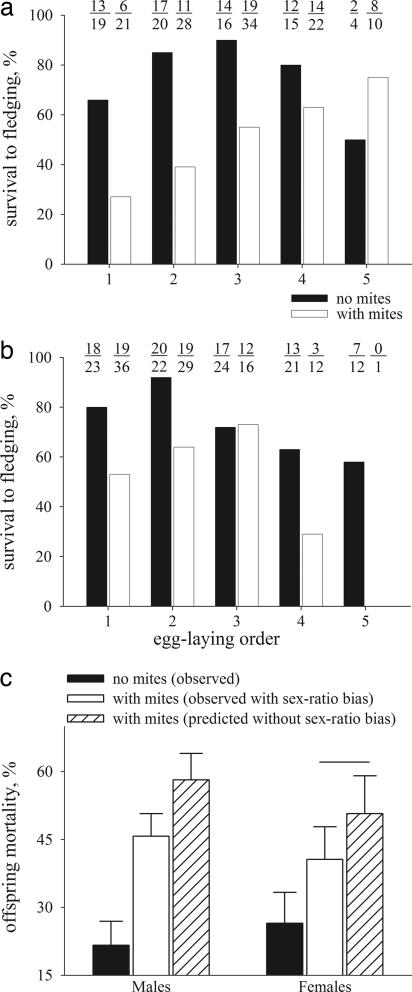
Mortality of both male and female nestlings increased with mite infestation, but the rate of this increase depended on nestling hatching order and sex (Fig. 4), in close concordance with the effects of nest tenure on nestling mortality (Figs. 1 c and d and 2d). In males, in mite-free nests, survival was the highest for the middle hatching positions, whereas in nests with mites, survival was the highest for last-hatched males and the lowest for first-hatched males (Fig. 4a). In females, in mite-free nests, the survival was the highest for the first two hatching positions, whereas in nests with mites, survival was the highest for the middle hatching positions (Fig. 4b; effects on mortality: sex: F1,197 = 3.41, P = 0.05; nest tenure: F1,197 = 4.23, P = 0.04; sex × egg-laying order: F4,197 = 5.08, P = 0.05; sex × mite presence: F1,197 = 4.08, P = 0.04; egg-laying order × mite presence: F1,197 = 12.11, P < 0.01; sex × mite presence × nest tenure: F1,197 = 4.63, P = 0.03). Thus, the most sex-biased laying positions (the last three for males and the first for females, Fig. 2a) were the most distinct between the sexes in mite-induced mortality (Fig. 4 a and b). Males hatched from the strongly male-biased last three eggs had the highest survival, whereas females in these positions had the lowest survival (Fig. 4 a and b). In nests with mites in the absence of sex bias in laying sequence, predicted mortality would have been 12.5% higher for male offspring and 10.1% higher for female offspring (in females, only “no mites” and “observed with mites” groups differed; K-ratio t = 5.06, P < 0.01; Fig. 4c).
Fig. 4.
Nestling survival in relation to mite presence. Survival of male (a) and female (b) offspring under mite-free (black bars) and mite-infested (white bars) conditions. Numbers above bars are sample sizes used to calculate survival (number survived/total number). (c) Observed offspring mortality under mite-free conditions (black bars), observed mite-induced mortality (white bars), and predicted mite-induced mortality assuming equal distribution of sexes among laying positions (hatched bars). Horizontal line indicates means that are not significantly different. SE of the predicted means are averages of values obtained for each laying position.
Discussion
When selection on morphology is strong but variable across environments and generations, it favors flexible allometric relationships among growing traits, thereby facilitating greater environmental and maternal effects on growth (33). Indeed, epigenetic modifications of growth rates and developmental periods contribute strongly to frequently observed, but theoretically unexpected, concordance of growth rates and mortality risks in birds (13, 14). However, despite frequent empirical demonstrations of strong maternal effects in birds, the evolutionary relationship between maternal strategies and resulting maternal effects is poorly understood. Especially puzzling is the evolution of precise sex-biased maternal effects, because sex-specific growth requirements of offspring should lower offspring sensitivity to maternal strategies and thus reduce the strength of maternal effects.
Coevolution of maternal adaptations and sex-specific maternal effects is frequently implicated in precise and adaptive maternal adjustment of offspring growth in response to changes in ecological or social aspects of breeding (31, 34–37). For example, maternal effects resulting from environmentally induced maternal adaptation played a key role in enabling initial colonization of highly divergent environments across North America by the house finch. Such maternal effects were caused by an interplay between maternal adaptations to novel environments (ambient temperature-induced incubation and associated hormonal changes) and sex-specific sensitivity of offspring to conditions during growth, resulting in opposite maternal effects between recently established populations at the north and south of the species' range (29, 30). Do these maternal effects represent an evolved strategy of dispersing populations or are they an induced response to novel breeding conditions? In this context, it is particularly instructive to examine the capacity of native and nondispersing populations to exhibit sex-biased maternal effects in response to seasonal changes in environment within a breeding cycle.
Here we documented that in a native population of house finches breeding under distinct mortality regimes, sex-specific adjustment of two proximate determinants of nestling period, egg-laying order and growth rate (Fig. 2), resulted in shorter exposure to nest ectoparasites and lesser associated mortality (Fig. 4) without compromising size at fledging (Fig. 2d). Although nestlings fledging from mite-infested nests had lower subsequent juvenile survival (T.L.H. and A.V.B., unpublished work), most of the mite-induced mortality, and most modifications of growth patterns, occurred in the nest. Similar size at fledging was enabled by a different combination of growth strategies. Males hatched at larger sizes but grew faster, whereas females grew more slowly but for a longer time (Figs. 2c and 3). The finding, that under certain breeding contexts a native population of an invasive species exhibits strongly sex-biased and reversible adjustment of laying order and offspring growth rates (Fig. 2), raises several questions. First, what are the mechanisms by which breeding females can sex-bias laying order and offspring growth under mite infestation? Second, why do sexes differ in growth requirements under mite infestation, and what enables rapid and sex-specific phenotypic adjustment of growth trajectories without apparent changes in fledging morphology? Third, what are the benefits of epigenetic effects on growth trajectories compared with genetic inheritance of growth trajectories, and how can such effects evolve?
Exposure to ectoparasites before egg-laying and associated endocrinological changes in breeding females can be one proximate mechanism enabling or triggering modifications in laying order and offspring growth (15, 21, 38). Although ectoparasite exposure of breeding females can induce greater egg allocation of maternal steroids that facilitate nestling growth or begging, such allocation can have detrimental effects on growth of the sexes if male and female offspring differ in growth requirements (39–41). Differential production of male and female eggs in relation to laying sequence, as observed in this study, not only enables mothers to allocate hormones differentially to each sex, but may also provide the mechanism by which hormone fluctuations in maternal plasma because of ectoparasite exposure produce sex-biased laying order. For example, similar acquisition of steroids by eggs of the same sex might be enabled by clustering of oocytes in the ovary or by temporal similarity among oocytes of the same sex in recruitment in relation to maternal hormonal profile (29, 42). Such a mechanism can both proximally link ectoparasite exposure by breeding females and sex bias in laying order and growth rates because of allocation of maternal steroids as well as enable frequently observed reversed sex-specific maternal effects in sequential breeding attempts.
Contrary to theoretical expectation of low phenotypic and genetic variation in the shape of avian growth trajectories (10, 43), empirical studies often document flexible growth strategies of parasitized nestlings when resources are preferentially allocated to traits facilitating fledging (19, 24, 44). Growth of male birds is more affected by nest parasites than growth of female birds (25), and consequently, the sexes in parasitized nests often differ in growth strategies (45) (Fig. 4). The effect of maternal steroids on differential and sex-specific distribution of growth hormone receptors across tissues can enable differential allocation across traits (25), whereas complexity and integration of growth trajectory components not only lend a multitude of targets to such epigenetic effects (3, 46) but also enable extensive postfledging compensatory growth (45, 47, 48).
The results of this study underscore the importance of environmentally induced maternal effects in the evolution of local adaptations (49). We showed that birds in a native population that exhibit no evidence of sex-biased maternal effects under parasite-free conditions are nevertheless able to rapidly and adaptively modify maternal effects on sex and growth of offspring under parasite infestation in a pattern similar to the ambient temperature-induced maternal effects in a dispersing population of this invasive species (30). Such rapid and sex-specific epigenetic adjustment of offspring growth to variable ecological conditions might be more common than previously recognized. Moreover, compensatory maternal effects on growth components, along with sex-biased mortality risk and frequently fluctuating ecological conditions of breeding, may ultimately hinder genetic coevolution of developmental periods and growth rates, explaining contrasting results of studies correlating growth rates, developmental periods, and extrinsic mortality (4, 8).
Materials and Methods
Study Organisms and Measurements.
House finches were studied in a resident population in southwestern Arizona beginning in 2002. Birds were trapped year round and marked with a unique combination of one aluminum and three colored plastic rings. Eggs were numbered sequentially on the day of laying, and within 10 h of hatching nestlings' tarsi were colored with a marker in accordance with egg-laying order to enable individual identification until nestlings were old enough to band. Reliable estimation of growth parameters requires a trait that reaches asymptotic size before fledging under both mite-infested and mite-free conditions, and tarsus length satisfied this requirement (50). We measured tarsus length of each nestling to the base of the foot with an accuracy of 0.01 mm every second day from hatching, for at least four times until fledging. The average of left and right tarsus measures was used in the analyses. K.P.O. measured all nestlings used in this study. The sigmoid Gompertz curve describes growth of house finches (50):
where Wt is the measurement at age t; W0 is the estimated value at hatching: K1 is the initial specific growth rate constant (describes the first part of the growth curve, before the inflection); and K2 is the maturation rate of the exponential rate of decay of the specific growth-rate constant (i.e., 1/Wt × dWt/dt = K1 × e−K2t), describing the second part in which growth rate decreases until the observed asymptotic value (A) is reached. The age at the point where the growth rate is maximum (Tmax) was as follows:
We estimated tarsus growth parameters for each nestling by using the Marquardt algorithm of PROC NLIN of SAS 9.12 (SAS Institute, Cary, NC), which minimized the sum of squares between predicted and observed values of growth. The data set was composed of 49 mite-free nests and 57 mite-infested nests. All females were color-marked, and nests of females breeding under both mite-free and mite-infested periods were excluded in these analyses.
P. reedi is a hematophagous ectoparasitic nest mite that feeds primarily on nestling house finches. Mites were counted on each nestling every second day. To assess the final population of mites within a nest, nests were collected at fledging and placed individually on white paper in a Ziploc bag (S. C. Johnson, Racine, WI) with a chloroform-soaked cotton ball for 2 min. Mites were counted under an MZ 12.5 dissecting microscope (Leica, Deerfield, IL). Mite-free status of nests where no mites were recorded on nestlings was verified by placing nests in a Berlese funnel apparatus for 48 h with a 60-W light bulb. Visual assessment of mite presence on nestlings was an accurate representation of mite infestation status of the nest. We collected a 5 to 10-μl blood sample from nestlings by brachial venipuncture using Whatman 903 filter paper (Whatman, Florham Park, NJ), and genomic DNA was extracted with a Puregene kit (Gentra Systems, Minneapolis, MN). To assign sex to nestlings, we used PCR primers P8 and P2, which anneal to conserved exonic regions and amplify across an intron in both CHD1-Wand CHD1-Zgenes.
Statistical Analyses.
To analyze differences in sex ratio among clutches produced under mite-free and mite-infested conditions while accounting for interdependency of egg-laying positions within each clutch and for the binomial error variance of the sex data, we used the glimmix macro of SAS 9.12 (SAS Institute) with nest identity as a random effect, and mite season, nest initiation date, and egg-laying position as fixed effects (51). Within-clutch sex ratio bias for each egg-laying position was tested with the binomial test. The maximum likelihood estimates of probability of survival as a function of cumulative time spent in the nest was calculated with the PROBIT procedure of SAS 9.12 (SAS Institute). Probability of survival was fitted with a spline curve (52). For survival frequency across nestling period, we used the method of cross-validation to select the smoothing parameter with the maximum predictive power. This cross-validation was done by excluding, with replacement, all individuals in turn for different values of the nest tenure. We used Type III general linear models with nest identity, year, and nest initiation date as effects to estimate significance of egg-laying order, sex, and duration of nest tenure for offspring survival in nests with and without mites. Sex differences in nest tenure, percentage of growth completed, and growth parameters were tested with nonparametric two-tailed Kruskal–Wallis tests. Multiple comparisons were conducted with Waller–Duncan K-ratio t tests or t (least significant difference) multiple-range tests (α = 0.05). To assess the effect of sex bias in egg-laying order on offspring mortality in mite-infested nests, we first calculated the number of offspring produced in each laying order in nests with mites and then, assuming an equal sex ratio, calculated the expected number of male and female offspring. We then applied empirically observed levels of mite-induced mortality for each sex and laying order to the number of males and females expected in the absence of sex-biased egg-laying order and calculated expected total number of offspring. Predicted survival and associated standard errors were obtained by averaging data for all egg-laying positions.
Acknowledgments
We thank R. Duckworth, R. Young, L. Landeen, S. West, and an anonymous reviewer for helpful comments and discussion; many assistants for help in the field; and J. Hubbard, E. Lindstedt, and K. Soetart for help with molecular sexing analyses. This study was funded by National Science Foundation Grants IBN-0218313 and DEB-0077804 and the Packard Fellowship (to A.V.B.) and Silliman Memorial Research awards (to T.L.H. and K.P.O.).
Footnotes
The authors declare no conflict of interest.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Arendt JD. Q Rev Biol. 1997;72:149–177. [Google Scholar]
- 2.Case TJ. Q Rev Biol. 1978;53:243–282. doi: 10.1086/410622. [DOI] [PubMed] [Google Scholar]
- 3.Roff DA, Remeš V, Martin TE. J Evol Biol. 2005;18:1425–1433. doi: 10.1111/j.1420-9101.2005.00958.x. [DOI] [PubMed] [Google Scholar]
- 4.Remes V, Martin TE. Evolution (Lawrence, Kans) 2002;56:2505–2518. doi: 10.1111/j.0014-3820.2002.tb00175.x. [DOI] [PubMed] [Google Scholar]
- 5.Møller AP. Oikos. 2005;111:291–301. [Google Scholar]
- 6.Clayton DH, Moore J. Host–Parasite Evolution: General Principles and Avian Models. Oxford: Oxford Univ Press; 1997. [Google Scholar]
- 7.Ricklefs RE. Proc Natl Acad Sci USA. 1992;89:4722–4725. doi: 10.1073/pnas.89.10.4722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ricklefs RE, Starck JM, Konarzewski M. In: Avian Growth and Development. Starck JM, Ricklefs RE, editors. New York: Oxford Univ Press; 1998. pp. 266–287. [Google Scholar]
- 9.Starck JM. In: Avian Growth and Development. Starck JM, Ricklefs RE, editors. New York: Oxford Univ Press; 1998. pp. 59–88. [Google Scholar]
- 10.Ricklefs RE. Ibis. 1968;110:418–451. [Google Scholar]
- 11.Rossiter M. Annu Rev Ecol Syst. 1996;27:451–476. [Google Scholar]
- 12.Badyaev AV. Proc R Soc London Ser B; 2005. pp. 877–886. [Google Scholar]
- 13.Gorman KB, Williams TD. Biol Lett. 2005;1:461–464. doi: 10.1098/rsbl.2005.0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwabl H, Palacios MG, Martin TE. Evolution (Lawrence, Kans) 2006 in press. [Google Scholar]
- 15.Tschirren B, Richner H, Schwabl H. Proc R Soc London Ser B; 2004. pp. 1371–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saino N, Dall'ara P, Martinelli R, Møller AP. J Evol Biol. 2002;15:735–743. [Google Scholar]
- 17.Johnson LS, Albrecht DJ. Oikos. 1993;66:255–262. [Google Scholar]
- 18.Tripet F, Richner H. Oikos. 1997;78:557–561. [Google Scholar]
- 19.Møller AP. Ecology. 1990;71:2345–2357. [Google Scholar]
- 20.Proctor H, Owens I. Trends Ecol Evol. 2000;15:358–364. doi: 10.1016/s0169-5347(00)01924-8. [DOI] [PubMed] [Google Scholar]
- 21.Heeb P, Werner I, Kolliker M, Richner H. Proc R Soc London Ser B; 1998. pp. 51–56. [Google Scholar]
- 22.Buechler K, Fitze PS, Gottstein B, Jacot A, Richner H. J Anim Ecol. 2002;71:247–252. [Google Scholar]
- 23.Szep T, Møller AP. Oecologia. 1999;119:9–15. doi: 10.1007/s004420050755. [DOI] [PubMed] [Google Scholar]
- 24.Saino N, Calza S, Møller AP. Oikos. 1998;81:217–228. [Google Scholar]
- 25.Badyaev AV. Trends Ecol Evol. 2002;17:369–378. [Google Scholar]
- 26.Uller T. Biol Rev Camb Philos Soc. 2006;81:207–217. doi: 10.1017/S1464793105006962. [DOI] [PubMed] [Google Scholar]
- 27.Pike TW, Petrie M. Biol Rev Camb Philos Soc. 2003;78:553–574. doi: 10.1017/s1464793103006146. [DOI] [PubMed] [Google Scholar]
- 28.Badyaev AV, Acevedo Seaman D, Navara KJ, Hill GE, Mendonça MT. J Evol Biol. 2006;19:1044–1057. doi: 10.1111/j.1420-9101.2006.01106.x. [DOI] [PubMed] [Google Scholar]
- 29.Badyaev AV, Schwabl H, Young RL, Duckworth RA, Navara K, Parlow AF. Proc R Soc London Ser B; 2005. pp. 2165–2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Badyaev AV, Hill GE, Beck ML, Dervan AA, Duckworth RA, McGraw KJ, Nolan PM, Whittingham LA. Science. 2002;295:316–318. doi: 10.1126/science.1066651. [DOI] [PubMed] [Google Scholar]
- 31.Andersson M, Wallander J, Oring L, Akst E, Reed JM, Fleischer RC. J Evol Biol. 2003;16:510–515. doi: 10.1046/j.1420-9101.2003.00533.x. [DOI] [PubMed] [Google Scholar]
- 32.Daan S, Dijkstra C, Weissing FJ. Behav Ecol. 1996;7:426–430. [Google Scholar]
- 33.Cheverud JM. Am J Phys Anthropol. 1982;59:139–149. doi: 10.1002/ajpa.1330590204. [DOI] [PubMed] [Google Scholar]
- 34.Dijkstra C, Daan S, Buker JB. Funct Ecol. 1990;4:143–147. [Google Scholar]
- 35.Rosivall B, Torok J, Hasselquist D, Bensch S. Behav Ecol Sociobiol. 2004;56:346–351. [Google Scholar]
- 36.Uller T. Evolution (Lawrence, Kans) 2003;57:927–931. doi: 10.1111/j.0014-3820.2003.tb00303.x. [DOI] [PubMed] [Google Scholar]
- 37.West SA, Sheldon BC. Science. 2002;295:1685–1688. doi: 10.1126/science.1069043. [DOI] [PubMed] [Google Scholar]
- 38.Love OP, Chin EH, Wynne-Edwards KE, Williams TD. Am Nat. 2005;166:751–766. doi: 10.1086/497440. [DOI] [PubMed] [Google Scholar]
- 39.Tschirren B, Saladin V, Fitze PS, Schwabl H, Richner H. J Anim Ecol. 2005;74:675–682. [Google Scholar]
- 40.Burke WH. Poult Sci. 1992;71:1933–1938. doi: 10.3382/ps.0711933. [DOI] [PubMed] [Google Scholar]
- 41.Saino N, Ferrari RP, Romano M, Martinelli R, Lacroix A, Gil D, Møller AP. Behav Ecol. 2006;17:172–181. [Google Scholar]
- 42.Badyaev AV, Oh KP, Mui R. J Evol Biol. 2006;19:909–921. doi: 10.1111/j.1420-9101.2005.01041.x. [DOI] [PubMed] [Google Scholar]
- 43.Kirkpatrick M, Lofsvold D. Evolution (Lawrence, Kans) 1992;46:954–971. doi: 10.1111/j.1558-5646.1992.tb00612.x. [DOI] [PubMed] [Google Scholar]
- 44.Berggren A. New Zealand J Ecol. 2005;29:243–250. [Google Scholar]
- 45.Potti J, Merino S. Proc R Soc London Ser B; 1996. pp. 9–12. [Google Scholar]
- 46.West GB, Brown JH, Enquist BJ. Nature. 2001;413:628–631. doi: 10.1038/35098076. [DOI] [PubMed] [Google Scholar]
- 47.Cooch EG, Lank DB, Cooke F. J Anim Ecol. 1996;65:439–450. [Google Scholar]
- 48.Badyaev AV, Martin TE. J Evol Biol. 2000;13:290–301. [Google Scholar]
- 49.Mousseau TA, Fox CW. Trends Ecol Evol. 1998;13:403–407. doi: 10.1016/s0169-5347(98)01472-4. [DOI] [PubMed] [Google Scholar]
- 50.Badyaev AV, Whittingham LA, Hill GE. Evolution (Lawrence, Kans) 2001;55:176–189. doi: 10.1111/j.0014-3820.2001.tb01282.x. [DOI] [PubMed] [Google Scholar]
- 51.Krackow S, Tkadlec E. Behav Ecol Sociobiol. 2001;50:293–301. [Google Scholar]
- 52.Schluter D. Evolution (Lawrence, Kans) 1988;42:849–861. doi: 10.1111/j.1558-5646.1988.tb02507.x. [DOI] [PubMed] [Google Scholar]