Abstract
There is a pressing need for adjuvants that will enhance the effectiveness of genetic vaccines. This is particularly important in cancer and infectious disease such as HIV and malaria for which successful vaccines are desperately needed. Here, we describe an approach to enhance immunogenicity that involves the activation of NF-κB by the transgenic expression of an intracellular signaling molecule, NF-κB-inducing kinase (NIK). In vitro, NIK increases dendritic cell antigen presentation in allogeneic and antigen-specific T cell proliferation assays by potently activating NF-κB and consequently up-regulating the expression of cytokines (TNF-α, IL-6, IL-12, IL-15, and IL-18), chemokines [IL-8, RANTES (regulated on activation, normal T cell expressed and secreted), macrophage inflammatory protein-1α, monocyte chemoattractant protein-1, and monocyte chemoattractant protein-3], MHC antigen-presenting molecules (class I and II), and costimulatory molecules (CD80 and CD86). In vivo, NIK enhances immune responses against a vector-encoded antigen and shifts them toward a T helper 1 immune response with increased IgG2a levels, T cell proliferation, IFN-γ production, and cytotoxic T lymphocyte responses more potently than complete Freund's adjuvant, a very efficacious T helper 1-inducing adjuvant. These findings define NIK, and possibly other inducers of NF-κB activation, as a potent adjuvant strategy that offers great potential for genetic vaccine development.
Keywords: dendritic cells, antigen presentation, immunogenicity, adenovirus
Despite the sequencing of the genome of many pathogens, and the development of sophisticated computer programs that can predict which types of proteins are encoded by the genes, where they are located, whether they cross-react with “self” proteins, whether they have minimal interstrain variation, and whether they are “good” vaccine candidates, there are still no effective vaccines for major existing and emerging global infectious diseases such as malaria, tuberculosis, influenza, and AIDS or for cancer (1). This problem is largely because most antigens against which immune responses can be induced through subunit or gene-based vaccines are insufficiently immunogenic. In contrast to live attenuated vaccines of the past, subunit or gene-based vaccines are poorly immunogenic and rarely induce the type of immune response required for protection, especially when cell-mediated immunity is needed. Thus, novel rational strategies to enhance the immunogenicity of pathogen-specific antigens and cancer-specific antigens are needed.
Several immunological adjuvants have been recently described and include bacterial molecules or derivatives such as unmethylated DNA (CpGs) and lipid particles. Cytokines [such as IL-2, IL-12, TNF-α, and granulocyte–macrophage colony-stimulating factor (GM-CSF)], chemokines [such as RANTES (regulated on activation, normal T cell expressed and secreted) and macrophage inflammatory protein (MIP)-1α], and costimulatory or adhesion molecules (such as CD80 and lymphocyte function-associated antigen-3) have also been used to enhance the immunogenicity of vaccine preparations either as recombinant proteins or cDNA sequences incorporated into gene-based vaccines (1–3). Although the incorporation of appropriate cytokines, chemokines, or costimulatory molecules enhances vaccine immunogenicity, improved efficacy is only achieved when combinations of them are used. Indeed, it was recently demonstrated that the combination of GM-CSF, TNF-α, and IL-12 was the most effective for protection against viral challenge in mice, presumably by increasing the number and activity of dendritic cells (DCs), resulting in increased IFN-γ production (4).
In this study, we describe an approach for enhancing vaccine immunogenicity by using our increasing understanding of the intracellular molecular mechanisms regulating DC antigen presentation, the limiting step in the generation of adaptive immune responses and adaptive immunity (5). Recent observations have demonstrated that the transcription factor NF-κB is essential for optimal DC function as inhibition of NF-κB or its upstream activator IκB kinase 2 (IKK2) blocks DC antigen presentation both in vitro and in vivo (6–9). Several immunological adjuvants have been shown to activate NF-κB among their multiple actions, but they are, however, limited in use by their lack of specific, localized, and coordinated effects and consequently by toxicity (10). We investigated whether the specific activation of NF-κB through the use of NF-κB-inducing kinase (NIK), a potent intracellular activator of both the canonical and noncanonical NF-κB pathway when overexpressed (11, 12), is sufficient to up-regulate DC antigen-presenting function and enhance immunity in vitro and in vivo.
Results
NIK Induces both the Canonical and Noncanonical Pathways of NF-κB Activation in DCs.
First, we examined the optimal way of activating NF-κB in DCs through the use of intracellular signaling molecules. We used replication-deficient adenoviruses to express WT forms of NIK (AdNIK), IκB kinase 2 (AdIKK2), MEKK-1 (AdMEKK-1), all known NF-κB activators (13–16), or the control proteins β-gal (Adβ-gal) and GFP (AdGFP) into DCs. We found that in DCs only NIK was a powerful activator of NF-κB (Fig. 1A).
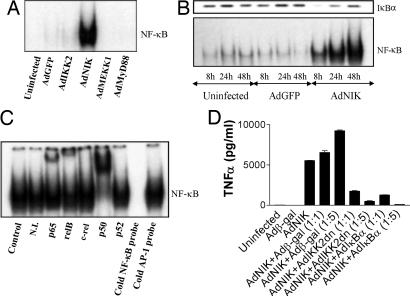
Fig. 1.
NIK activates both the canonical and noncanonical pathways of NF-κB in DCs. DCs were generated from peripheral blood after culture with GM-CSF and IL-4. (A) EMSA analysis indicating NF-κB DNA binding activity of DCs infected with recombinant adenoviruses for 24 h. (B) Western blot analysis of cytosolic IκBα expression (Upper) and EMSA analysis of NF-κB DNA binding activity (Lower) of DCs infected with AdGFP or AdNIK for 6, 24, or 48 h. (C) Antibody supershift/EMSA analysis indicating the presence of p65, relB, and p50 in the NF-κB DNA binding activity of AdNIK-infected DCs for 24 h. (D) NIK-mediated induction of TNF-α in DCs uses IKK2 and NF-κB. DCs were infected with AdNIK or Adβ-gal (multiplicity of infection 100) or combinations of AdNIK with Adβ-gal, AdIKK2dn, or AdIκBα at the indicated ratios, and mean cytokine production (±SD) of triplicate cultures was determined after 24 h. This is representative of four independent experiments from unrelated donors.
NIK activated both the canonical (classical) and noncanonical pathways of NF-κB activation in DCs. Classical IκBα degradation was seen 6 h after adenoviral gene transfer of NIK into DCs, which returned to its prestimulation levels after 48 h (Fig. 1B), presumably through an NF-κB-dependent mechanism (17). IκBα degradation was followed by increased NF-κB activation that was apparent at 6 h but maximal after 48 h when measured by EMSA (Fig. 1B). When AdNIK-induced NF-κB activity was examined by EMSA and supershift analysis, we found that the major NF-κB subunits present were p65, p50, and relB, with the strongest supershift being for p50 (Fig. 1C). However, this observation may be related to the relative effectiveness of the antibodies for each subunit–DNA complex rather than a true reflection of actual subunit abundance. As relB activation has been shown to be independent of the canonical pathway and the inhibitory proteins IκBα, IκBβ, or IκBε, and to depend solely on the noncanonical pathway (12, 18), these results indicated that NIK also activates the noncanonical pathway of NF-κB in DCs.
AdNIK-mediated DC activation such as NF-κB-dependent TNF-α production was blocked by coadministration of the inhibitors of the canonical pathway of NF-κB activation, IκBα and dominant negative IKK2 (IKK2dn) but not β-gal (Fig. 1D), further confirming the specific mechanism of action of NIK, through the activation of NF-κB rather than another pathway.
NIK Coordinates the Up-Regulation of Cytokines, Chemokines, MHC Antigen-Presenting Molecules, and Costimulatory Molecules and Induces Morphological Changes in DCs.
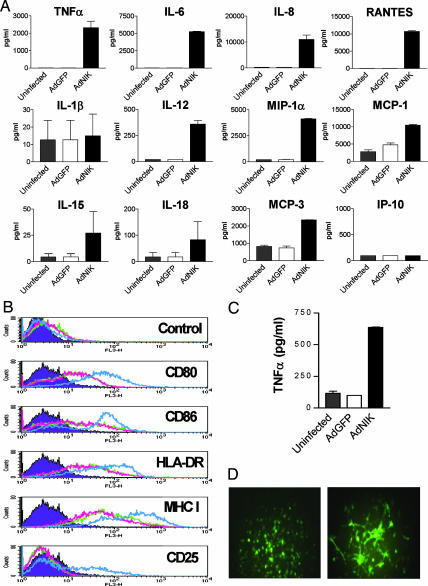
We then examined the effects of NIK-driven NF-κB activation in DCs. We found that AdNIK but not the control virus AdGFP resulted in the up-regulation of TNF-α, IL-6, IL-12, IL-15, and IL-18 with no effect on the production of IL-1β (Fig. 2A). The up-regulation of the T helper 1 (Th1)-promoting cytokines IL-12, IL-15, and IL-18 suggested that NIK would efficiently induce Th1 responses. NIK also up-regulated the production of the chemokines IL-8, MIP-1α/β, monocyte chemoattractant protein (MCP)-1, and MCP-3, but had no effect on IFN-inducible protein 10 (IP-10) (Fig. 2A). AdNIK also up-regulated the expression of the antigen-presenting molecules MHC class I and II, the costimulatory molecules CD80 and CD86, and the IL-2 receptor α chain (CD25) (Fig. 2B). Moreover, AdNIK had a profound effect on DC morphology when compared with GFP (AdGFP) by inducing dendrites and protrusions visible by fluorescence microscopy (data not shown).
Fig. 2.
NIK activates both human and mouse DCs. (A) Cytokine and chemokine production of human monocyte-derived DCs infected with AdGFP or AdNIK for 24 h. Mean cytokine production (±SD) of triplicate cultures is shown and is representative of four independent experiments. (B) FACS analysis of antigen-presenting and costimulatory molecule expression of human DCs infected with AdGFP (green line) or AdNIK (blue line) for 48 h. Uninfected cells (red line) and isotype control antibody (purple) are also shown. (C) TNF-α production of mouse bone marrow-derived DCs infected with AdGFP or AdNIK for 24 h. Mean production (±SD) of triplicate cultures is shown and is representative of four independent experiments. (D) Phenotypic appearance of AdNIK-infected mouse macrophages showing a DC-like phenotype with extended dendrites and protrusions. Mouse peritoneal macrophages were infected with AdGFP (Left) or AdNIK(GFP) (Right) for 48 h and examined by fluorescence microscopy. (Magnification: ×20.)
Similar effects were also seen in mouse antigen-presenting cells. We found that NIK activated mouse peritoneal exudate cells (data not shown) and bone marrow-derived DCs (Fig. 2C) to produce cytokines such as TNF-α and to undergo morphological changes indicative of an activated phenotype with extended dendrites and protrusions (Fig. 2D).
NIK Enhances DC Antigen Presentation in both Allogeneic and Antigen-Specific T Cell Proliferation Assays in Vitro.
In functional assays we ascertained that NIK-induced NF-κB activation also augments DC antigen-presenting function. We found that NIK potently enhanced the ability of immature DCs to induce allogeneic T cell proliferation (up to 20-fold at low DC numbers) (Fig. 3A). The magnitude of the response with NIK paralleled that seen when known optimal maturation stimuli such as LPS and monocyte-conditioned medium (MCM) (19, 20) were used to enhance DC antigen-presenting function. This enhancing effect of NIK could be blocked by IKK2dn or IκBα (data not shown), confirming that it was caused by NF-κB activation.
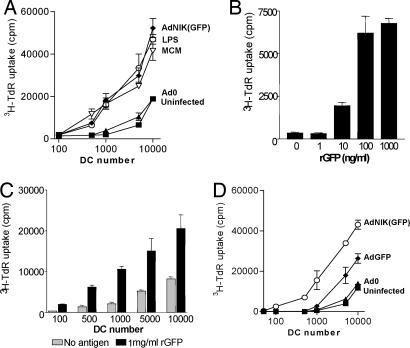
Fig. 3.
NIK enhances DC antigen presentation in allogeneic and antigen-specific T cell proliferation assays. (A) Proliferation of allogeneic T cells induced by untreated DCs, DCs treated with 100 ng/ml LPS or 50% (vol/vol) MCM, or DCs infected with Ad0 or AdNIK. (B) Proliferation of GFP-specific T cells induced by unpulsed or GFP-pulsed autologous irradiated peripheral blood mononuclear cells. (C) Proliferation of GFP-specific T cells by GFP-pulsed LPS-matured DC. (D) Proliferation of GFP-specific T cells induced by irradiated adenovirus-infected or control uninfected DC. Mean proliferation (±SEM) of triplicate cultures is shown and is representative of at least four independent experiments from unrelated donors.
We also examined whether NIK can enhance immune responses against specific antigens by taking advantage of the immunogenicity of GFP (21, 22), a jellyfish protein already incorporated into our adenoviral vectors. We generated human GFP-specific T cell lines by culturing peripheral blood mononuclear cells (PBMCs) with recombinant GFP protein and IL-2, as described for tetanus-specific T cell lines (23). GFP-specific T cell lines proliferated vigorously to restimulation with antigen and irradiated PBMCs (Fig. 3B) or LPS-matured DCs pulsed with GFP (Fig. 3C). GFP-specific T cells also proliferated in response to AdGFP-infected DCs, but not to Ad0-infected or uninfected control DCs (except at high DC–T cell ratios where antigen-independent proliferation can occur) (Fig. 3D). This response was greatly enhanced when DCs infected with AdNIK(GFP) were used (Fig. 3D), demonstrating that NIK is also capable of enhancing antigen-specific T cell proliferation.
NIK Enhances Antigen-Specific Antibody Responses Against a Vector-Encoded Antigen in Vivo and Skews Them Toward a Th1 Type.
To test whether NIK can also enhance antigen-presenting function in vivo and be a useful adjuvant for vaccines, we examined the effect of NIK on the immune response against GFP in vivo (Fig. 4A). As vaccine vectors we used recombinant adenoviruses as they are commonly used for this purpose (24, 25). We also used BALB/c mice as these animals induce strong immune responses against GFP (21) and s.c. injections at the base of the tail as this site is rich in skin DCs and inguinal lymph nodes that can be assayed (26).
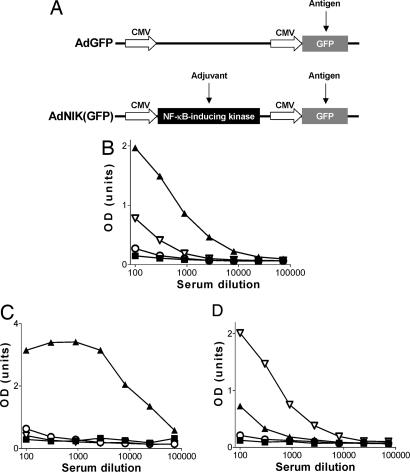
Fig. 4.
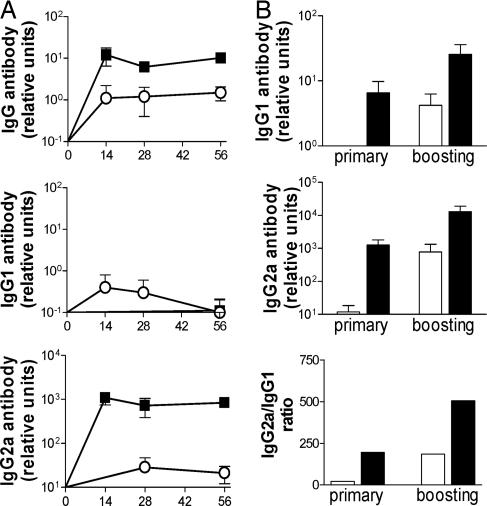
NIK increases total and IgG2a antibody responses against the vector-encoded antigen GFP in vivo. (A) Schematic presentation of AdGFP and AdNIK(GFP) viral vectors. (B–D) ELISA of GFP-specific IgG (B), IgG1 (C), and IgG2a (D) antibody levels in sera of mice 14 days after immunization with saline (■), GFP in CFA (▴), AdGFP (○), or AdNIK(GFP) (▵). Results are shown as mean OD units of five mice per group plotted against serum dilutions.
Immunization with 20 μg per mouse of GFP emulsified in complete Freund's adjuvant (CFA) induced strong GFP-specific IgG antibody responses with the major subclass being IgG1, but with IgG2a also present (Fig. 4 B–D). In contrast, immunization with AdGFP or AdNIK(GFP) at an adenoviral titer of 106 pfu per mouse did not induce measurable GFP-specific IgG antibody responses when examined at 14 days after immunization or at the later time points of up to 2 months (data not shown). However, when a 10-fold higher titer (107 pfu per mouse) of adenoviruses was used, AdGFP induced low, but detectable, IgG, IgG1, and IgG2a antibody responses that were greatly enhanced when AdNIK(GFP) was administered (Fig. 4 B–D).
Both AdGFP and AdNIK(GFP) immunization resulted in increased IgG2a/IgG1 ratios when compared with an arbitrarily defined ratio of 1 (100 relative units of IgG2a vs. 100 relative units of IgG1) of mice immunized with GFP and CFA, suggesting that adenovirally delivered GFP favored Th1-type immune responses. However, AdNIK(GFP) preferentially enhanced the IgG2a levels compared with AdGFP, thus shifting the IgG2a/IgG1 ratio from 22 seen with AdGFP to 195 (Table 1). Therefore, NIK incorporation into the adenoviral vector both enhanced the magnitude and skewed the immune response to the vector-encoded antigen GFP to a Th1-type cytokine profile.
Table 1.
Expression of IgG, IgG1, and IgG2a antibody levels of mice immunized with AdGFP or AdNIK (GFP) as relative units (±SEM) of five mice per group and corresponding IgG2a/IgG1 ratios
| Ig type | AdGFP | AdNIK(GFP) | GFP in CFA |
|---|---|---|---|
| IgG | 1.1 (±1.1) | 12.1 (±5.6) | 100 |
| IgG1 | 0.4 (±0.4) | 0 | 100 |
| IgG2a | 6.4 (±4.0) | 1,083.2 (±334.2) | 100 |
| IgG1/IgG2a | 22 | 195 | 1 |
NIK(GFP)-Induced GFP-Specific Antibody Responses Are Long-Lived and Can Be Boosted.
Subsequently, we examined the duration of these antibody responses and whether they can be boosted. We found that GFP-specific IgG, IgG1, and IgG2a antibody levels induced after immunization with 107 infectious units (i.u.) per mouse of AdGFP or AdNIK(GFP) remained high over a 2-month period (Fig. 5A). The mice were boosted with the lower titer of 106 i.u. per mouse (that does not induce a primary immune response) 56 days after primary immunization. Administration of AdNIK(GFP) to AdNIK(GFP)-primed mice further boosted IgG2a levels 10-fold, but had a variable effect on IgG1 levels (0- to 4-fold enhancement) (Fig. 5B). This process resulted in a further increase of the IgG2a/IgG1 ratio, suggesting that boosting AdNIK(GFP)-immunized mice with AdNIK(GFP) further skews the immune response to a Th1 type.
Fig. 5.
NIK-induced antibody responses are long-lived and can be boosted. (A) Time course of GFP-specific IgG, IgG1, and IgG2a antibody levels expressed as relative units (±SEM) of five mice per group immunized with AdGFP (○) or AdNIK(GFP) (■). (B) Effect of boosting immunizations with AdGFP (white) or AdNIK(GFP) (black) in GFP-specific IgG1 and IgG2a antibody responses and IgG2a/IgG1 ratios. Mice were immunized at day 0 and boosted at day 56 with AdGFP or AdNIK(GFP), and sera was collected at days 14 and 70. Mean relative antibody units (±SEM) of five mice per group are shown and are representative of three independent experiments.
NIK Enhances Antigen-Specific Cell-Mediated Immune Responses.
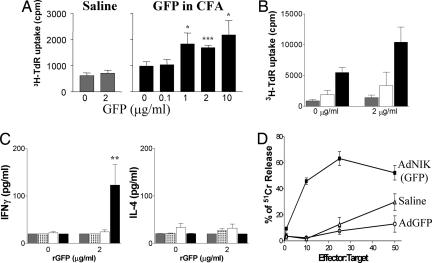
GFP-specific T cell responses of lymph node cells (LNC) from immunized animals were then assayed. Immunization of mice with GFP in CFA or 107 pfu AdGFP induced LNC proliferation, which was greatly increased in mice immunized with 107 pfu AdNIK(GFP) (Fig. 6A and B). As LNC proliferation depends on T cell proliferation and IL-2 production, this result indicated that NIK enhanced cell-mediated and Th1 responses in vivo. Indeed, IFN-γ production but not IL-4 was detected in supernatants from LNC cultures of mice immunized with AdNIK(GFP) but not AdGFP or GFP in CFA (Fig. 6C). This finding is consistent with the high levels of IgG2a antibody production.
Fig. 6.
NIK enhances Th1 and CTL immune responses against the vector-encoded antigen GFP in vivo. (A) Proliferation of inguinal LNC in response to GFP of mice immunized with saline or GFP in CFA. (B) Proliferation of inguinal LNC in response to GFP of mice immunized with saline (gray), AdGFP (white), or AdNIK(GFP) (black). (C) IFN-γ and IL-4 production of inguinal LNC in response to GFP of mice immunized with saline (gray), GFP in CFA (white with black dots), AdGFP (white), or AdNIK(GFP) (black). ∗, P < 0.05; ∗∗, P < 0.01 with the one-way ANOVA Dunnet's multiple comparisons test. (D) Spleen CTL responses against proleukemic GFP-expressing BM185 cells (targets) in a 51Cr release assay. Mice were immunized at day 0 and boosted at day 28 with AdGFP or AdNIK(GFP), and spleen cells were collected at day 35. Percentage ± SEM of specific 51Cr release is shown. Experiments are representative of two independent experiments of five mice per group.
Finally, GFP-specific cytotoxic T lymphocyte (CTL) responses were examined in mice immunized with 107 i.u. of AdGFP or AdNIK(GFP) and boosted after 28 days with 106 i.u. of the same viruses. It was found that AdNIK(GFP) induced CTL responses against the BM185 proleukemic B cells stably expressing GFP (21), whereas AdGFP had no greater effect than that seen with naïve mice (Fig. 6D). Collectively, these data suggest that NIK potently enhances cell-mediated immune responses in vivo, including the induction of cytotoxic T cells.
Discussion
There is an urgent need for new adjuvants that enhance vaccine immunogenicity and are compatible with new production technology such as gene-based vaccines. Here, we used NIK, an intracellular signaling molecule, to activate both the canonical and noncanonical pathways of NF-κB in a specific and contained manner and up-regulate in that way DC antigen-presenting function and antigen-specific immunity in vitro and in vivo.
There is ample evidence that NF-κB is essential for optimal DC function. Inhibition of NF-κB blocks DC antigen presentation both in vitro and in vivo (6–9, 27), and inhibition of relB results in the generation of IL-10-producing regulatory T cells and antigen-specific tolerance in vivo (28). Indeed, many immunological adjuvants share their ability to activate NF-κB among their multiple actions, but are often limited in use by their lack of specific, localized, and coordinated effects, and consequently by toxicity. In that respect, NIK differs as it is expressed together with antigen in cells and thus activates NF-κB only in these cells.
In DCs, NIK activates both the canonical and noncanonical pathways of NF-κB activation characterized by the nuclear translocation of the p65 and relB NF-κB subunits, respectively (11, 12). Nuclear localization of p65 and relB has been previously shown to correlate with DC maturation and efficient antigen presentation (29–31). The profound induction of both branches of NF-κB activation by NIK results in the up-regulation of several antigen-presenting and costimulatory molecules, cytokines, and chemokines that contain NF-κB sites in the promoters of their genes (32–37). Interestingly, IL-1β and IP-10 that also contain NF-κB sites in their promoters were not induced by NIK. As IL-1β is NF-κB-dependent and IP-10 is IRF3-dependent but NF-κB-independent in many but not all systems (38, 39), our data suggest that all of the signals required for the expression of these genes cannot be compensated by NIK alone.
The functional consequence of NIK-induced NF-κB activation is the significant enhancement of antigen presentation. NIK potently up-regulates DC antigen presentation in allogeneic and antigen-specific T cell proliferation assays to a similar extent seen with LPS or MCM, two of the most effective DC maturation stimuli described (19, 20). More importantly, NIK potently up-regulates antigen presentation in vivo in BALB/c mice, which is a “Th2 skewed” strain. When compared with vector alone or recombinant antigen emulsified in CFA (one of the most potent Th1-inducing adjuvants known), NIK enhances antigen-specific antibody responses several fold and shifts them to IgG2a, an isotype associated with Th1-type immunity. In boosting strategies, NIK further increases the IgG2a/IgG1 ratio and the Th1 shift. NIK also enhances antigen-specific LNC proliferation and IFN-γ production, probably reflecting in vivo the ability of NIK to induce high levels of the Th1-promoting cytokines IL-12 and IL-18 seen in vitro. Moreover, NIK induces antigen-specific CTL responses against antigen-expressing proleukemic B cells (21). This finding was not unexpected as Th1 cells (induced by NIK) are the most effective form of helper cells for CTL responses against experimental viral infections and tumors (5).
This study has major implications for the design and development of new-generation vaccines by using recombinant DNA technologies that are often limited by the poor immunogenicity of antigens and the limited efficacy of currently available adjuvants. We demonstrate that the specific activation of the NF-κB pathway (both the canonical and noncanonical branches) through the intracellular expression of NF-κB-activating molecules such as NIK is sufficient to activate the natural pathway of antigen presentation that coordinates the production of cytokines and chemokines and the expression of MHC antigen presenting and costimulatory molecules. We also put forward the idea of using intracellular signaling molecules in gene-based vaccines to enhance immunogenicity of vector-encoded antigens in a specific and contained manner, i.e., the same cell that also expresses the antigen or epitopes of interest. Finally, we provide a strategy of augmenting the efficacy of adenovirus-based vaccines for which there is increased interest because of the speed of the response induced and their ease of production. This may be useful for recently described adenovirus-vector vaccines against Ebola virus (24) and H5N1 avian flu (25) that use adenoviruses, but lack an additional adjuvant. Our strategy augments these advantages.
Materials and Methods
Reagents.
Human recombinant GM-CSF was a kind gift of G. Larsen (Genetics Institute, Boston, MA). Human recombinant IL-2, IL-4, and mouse GM-CSF were purchased from PeproTech (London, U.K.). Escherichia coli LPS was obtained from Sigma (St. Louis, MO).
Adenoviral Vectors and Their Propagation.
Adenoviruses encoding GFP, β-gal, NIK, MyD88, IKK2, MEKK-1, and IκBα are E1/E3-deleted, belong to the Ad5 serotype, and have been described (13, 14, 39). The adenoviruses for IKK2dn and IκBα were kind gifts of R. De Martin (University of Vienna, Vienna, Austria), and the adenovirus for β-gal was from M. Wood (University of Oxford, Oxford, U.K.). All viruses were propagated, purified, and titered as before (40).
Generation of Human and Mouse DCs and Gene Transfer.
Human and mouse myeloid DCs were generated as described (8, 41). For gene transfer, human and mouse DCs were plated at 1 × 106 cells per ml in serum-free RPMI medium 1640 in 48- or 96-well plates (Falcon, Oxford, U.K.) and infected with adenoviruses at a multiplicity of infection of 100 for human (unless stated otherwise) and 500 for mouse DCs for 2 h as described (8). This method is highly efficient and nonperturbing to DCs (8, 42) and invariably results in >95% of the cells expressing the transgene of interest.
T Cell Proliferation Assays.
DCs matured with 100 ng/ml of LPS or 50% (vol/vol) MCM or infected with adenoviruses were cultured in graded doses with 1 × 105 purified allogeneic or GFP-specific T cells in 96-well plates. Proliferation was measured on day 5 for allogeneic T cells or day 3 for GFP-specific T cells after a 16-h pulse with [3H]thymidine (0.5 μCi per well; Amersham Life Sciences, Amersham, U.K.). GFP-specific T cell lines were generated as described (23). In all, T cell proliferation assays background T cell proliferation in the absence of antigen-presenting cells was <100 cpm.
Western Blotting and EMSA.
Cytosolic and nuclear extracts of DCs were prepared as described (43). Cytosolic proteins were analyzed by Western blotting for IκBα, and nuclear extracts (10 μg) for NF-κB DNA-binding activity were analyzed by EMSA. The antibodies for IκBα and p65 (H-286), relB (E-259), c-rel (H-085), p50 (H-119), and p52 (F-259) NF-κB subunits were purchased from Santa Cruz Biotechnology (Santa Cruz, CA).
Analysis of Cytokines.
Human monocyte-derived or mouse bone marrow-derived DCs were plated at 1 × 105 cells per well in 96-well tissue culture plates and either left uninfected or infected with recombinant adenoviruses. Supernatants were collected after 48 h and analyzed for TNF-α, IL-1β, IL-6, IL-8, IL-12, MIP-1α, RANTES, IP-10, MCP-1, and/or MCP-3 by ELISA (Pharmingen, Oxford, U.K.).
Analysis of Cell Surface Molecules by FACS.
Cell surface molecule expression of monocyte-derived DCs was analyzed by FACS. Directly conjugated antibodies for human CD80, CD86, HLA-A,B,C, and HLA-DR, and mouse CD80 and CD86, and appropriate isotype controls were purchased from Pharmingen.
Mice, Immunization, and Determination of Antibody Levels.
Eight- to 10-week-old BALB/c mice (Harlan, Oxon, U.K.) were immunized s.c. with 20 μg per mouse of recombinant GFP (Roche Molecular Biochemicals, Indianapolis, IN) emulsified in CFA, 106 or 107 i.u. of AdGFP, AdNIK(GFP), or Ad0. In some cases, adenovirus-immunized mice were boosted with 106 i.u. of adenovirus at day 56. Mice were tail-bled at days 14, 28, 56, and 70, and sera was collected and examined separately for the presence of GFP-specific antibody levels by using an ELISA-based assay. Relative antibody levels were determined by interpolation from the dilution curve of a reference serum sample (rGFP in CFA-immunized mice for 14 days, defined as 100 relative units) titrated in parallel to the test sera. Anti-mouse IgG, IgG1, and IgG2a antibodies were purchased from The Binding Site, Birmingham, U.K.
LNC Proliferation and CTL Assays.
Seven days after immunization inguinal lymph nodes were teased apart and cultured at 2.5 × 106 cells per ml with or without GFP. Proliferation was assessed at day 3 after overnight pulsing with 0.5 μCi per well of [3H]thymidine. Supernatants were also collected at day 3 and assayed for IFN-γ and IL-4 (R&D Systems, Abingdon, U.K.). For CTL assays, mice immunized with 107 i.u. and boosted at day 28 with 106 i.u. of adenovirus were killed at day 35, and spleen cells were cultured at 2 × 106 cells per ml with irradiated GFP-expressing B cells BM185-GFP kindly provided by D. Kohn (Children's Hospital, Los Angeles, CA) (21). After 2 days, 20 ng/ml IL-2 was added to expand GFP-specific T cells for 4 days. Serial dilutions of these expanded effector cells were cultured with 2 × 103 51Cr-labeled BM185-GFP cells (target cells) for 6 h, and supernatants were collected and assayed for gamma radiation counting. The percentage of specific release was calculated as [experimental release − spontaneous release/total release − spontaneous release)] × 100, with total counts corresponding to 1% Triton ×-100 lysed cells.
Statistical Methods.
For statistical analysis, a two-sided Student's t test of paired comparisons or a one-way ANOVA Dunnet's multiple comparisons test was used to compare the nonimmunized control group against the others (GraphPad, San Diego, CA).
Acknowledgments
We thank Prof. E. Simpson for critically reading the manuscript and Mr. P. Warden and Mr. S. Ahmed for assistance in the in vivo work. This study was funded by the Arthritis Research Campaign of the United Kingdom and the Kennedy Institute of Rheumatology Trustees.
Abbreviations
- NIK
NF-κB-inducing kinase
- MCP
monocyte chemoattractant protein
- MIP
macrophage inflammatory protein
- Th1
T helper 1
- CTL
cytotoxic T lymphocyte
- GM-CSF
granulocyte–macrophage colony-stimulating factor
- DC
dendritic cell
- IKK2
IκB kinase 2
- IKK2dn
dominant negative IKK2
- IP-10
IFN-inducible protein 10
- MCM
monocyte-conditioned medium
- CFA
complete Freund's adjuvant
- LNC
lymph node cells
- i.u.
infectious units.
Footnotes
Conflict of interest statement: The subject of this research has been filed in patents that have been licensed to Synovis Ltd. of which M.F. and B.M.F. are founders and shareholders.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Berzofsky JA, Ahlers JD, Belyakov IM. Nat Rev Immunol. 2001;1:209–219. doi: 10.1038/35105075. [DOI] [PubMed] [Google Scholar]
- 2.Ahlers JD, Belyakov IM, Berzofsky JA. Curr Mol Med. 2003;3:285–301. doi: 10.2174/1566524033479843. [DOI] [PubMed] [Google Scholar]
- 3.Moingeon P. J Biotechnol. 2002;98:189–198. doi: 10.1016/s0168-1656(02)00131-1. [DOI] [PubMed] [Google Scholar]
- 4.Ahlers JD, Belyakov IM, Matsui S, Berzofsky JA. Int Immunol. 2001;13:897–908. doi: 10.1093/intimm/13.7.897. [DOI] [PubMed] [Google Scholar]
- 5.Steinman RM, Pope M. J Clin Invest. 2002;109:1519–1526. doi: 10.1172/JCI15962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ardeshna KM, Pizzey AR, Devereux S, Khwaja A. Blood. 2000;96:1039–1046. [PubMed] [Google Scholar]
- 7.Rescigno M, Martino M, Sutherland CL, Gold MR, Ricciardi-Castagnoli P. J Exp Med. 1998;188:2175–2180. doi: 10.1084/jem.188.11.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andreakos E, Smith C, Monaco C, Brennan FM, Foxwell BM, Feldmann M. Blood. 2003;101:983–991. doi: 10.1182/blood-2002-06-1835. [DOI] [PubMed] [Google Scholar]
- 9.Yoshimura S, Bondeson J, Foxwell BM, Brennan FM, Feldmann M. Int Immunol. 2001;13:675–683. doi: 10.1093/intimm/13.5.675. [DOI] [PubMed] [Google Scholar]
- 10.Brennan FR, Dougan G. Vaccine. 2005;23:3210–3222. doi: 10.1016/j.vaccine.2004.11.072. [DOI] [PubMed] [Google Scholar]
- 11.Malinin NL, Boldin MP, Kovalenko AV, Wallach D. Nature. 1997;385:540–544. doi: 10.1038/385540a0. [DOI] [PubMed] [Google Scholar]
- 12.Solan NJ, Miyoshi H, Carmona EM, Bren GD, Paya CV. J Biol Chem. 2002;277:1405–1418. doi: 10.1074/jbc.M109619200. [DOI] [PubMed] [Google Scholar]
- 13.Smith C, Andreakos E, Crawley JB, Brennan FM, Feldmann M, Foxwell BM. J Immunol. 2001;167:5895–5903. doi: 10.4049/jimmunol.167.10.5895. [DOI] [PubMed] [Google Scholar]
- 14.Conron M, Andreakos E, Pantelidis P, Smith C, Beynon HL, Dubois RM, Foxwell BM. Am J Respir Crit Care Med. 2002;165:996–1004. doi: 10.1164/ajrccm.165.7.2107058. [DOI] [PubMed] [Google Scholar]
- 15.Andreakos E, Sacre SM, Smith C, Lundberg A, Kiriakidis S, Stonehouse T, Monaco C, Feldmann M, Foxwell BM. Blood. 2004;103:2229–2237. doi: 10.1182/blood-2003-04-1356. [DOI] [PubMed] [Google Scholar]
- 16.Andreakos E, Smith C, Kiriakidis S, Monaco C, de Martin R, Brennan FM, Paleolog E, Feldmann M, Foxwell BM. Arthritis Rheum. 2003;48:1901–1912. doi: 10.1002/art.11044. [DOI] [PubMed] [Google Scholar]
- 17.Sun SC, Ganchi PA, Ballard DW, Greene WC. Science. 1993;259:1912–1915. doi: 10.1126/science.8096091. [DOI] [PubMed] [Google Scholar]
- 18.Muller JR, Siebenlist U. J Biol Chem. 2003;278:12006–12012. doi: 10.1074/jbc.M210768200. [DOI] [PubMed] [Google Scholar]
- 19.Sallusto F, Cella M, Danieli C, Lanzavecchia A. J Exp Med. 1995;182:389–400. doi: 10.1084/jem.182.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Romani N, Reider D, Heuer M, Ebner S, Kampgen E, Eibl B, Niederwieser D, Schuler G. J Immunol Methods. 1996;196:137–151. doi: 10.1016/0022-1759(96)00078-6. [DOI] [PubMed] [Google Scholar]
- 21.Stripecke R, Carmen Villacres M, Skelton D, Satake N, Halene S, Kohn D. Gene Ther. 1999;6:1305–1312. doi: 10.1038/sj.gt.3300951. [DOI] [PubMed] [Google Scholar]
- 22.Steinbauer M, Guba M, Cernaianu G, Kohl G, Cetto M, Kunz-Schughart LA, Geissler EK, Falk W, Jauch KW. Clin Exp Metastasis. 2003;20:135–141. doi: 10.1023/a:1022618909921. [DOI] [PubMed] [Google Scholar]
- 23.Lanzavecchia A. Nature. 1985;314:537–539. doi: 10.1038/314537a0. [DOI] [PubMed] [Google Scholar]
- 24.Sullivan NJ, Geisbert TW, Geisbert JB, Xu L, Yang ZY, Roederer M, Koup RA, Jahrling PB, Nabel GJ. Nature. 2003;424:681–684. doi: 10.1038/nature01876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoelscher MA, Garg S, Bangari DS, Belser JA, Lu X, Stephenson I, Bright RA, Katz JM, Mittal SK, Sambhara S. Lancet. 2006;367:475–481. doi: 10.1016/S0140-6736(06)68076-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hammond SA, Guebre-Xabier M, Yu J, Glenn GM. Crit Rev Ther Drug Carrier Syst. 2001;18:503–526. [PubMed] [Google Scholar]
- 27.Yoshimura S, Bondeson J, Brennan FM, Foxwell BM, Feldmann M. Eur J Immunol. 2001;31:1883–1893. doi: 10.1002/1521-4141(200106)31:6<1883::aid-immu1883>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 28.Martin E, O'Sullivan B, Low P, Thomas R. Immunity. 2003;18:155–167. doi: 10.1016/s1074-7613(02)00503-4. [DOI] [PubMed] [Google Scholar]
- 29.Neumann M, Fries H, Scheicher C, Keikavoussi P, Kolb-Maurer A, Brocker E, Serfling E, Kampgen E. Blood. 2000;95:277–285. [PubMed] [Google Scholar]
- 30.Pettit AR, Quinn C, MacDonald KP, Cavanagh LL, Thomas G, Townsend W, Handel M, Thomas R. J Immunol. 1997;159:3681–3691. [PubMed] [Google Scholar]
- 31.Mintern JD, Belz G, Gerondakis S, Carbone FR, Heath WR. J Immunol. 2002;168:3283–3287. doi: 10.4049/jimmunol.168.7.3283. [DOI] [PubMed] [Google Scholar]
- 32.Barnes PJ, Karin M. N Engl J Med. 1997;336:1066–1071. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- 33.Blanchet O, Bourge JF, Zinszner H, Israel A, Kourilsky P, Dausset J, Degos L, Paul P. Proc Natl Acad Sci USA. 1992;89:3488–3492. doi: 10.1073/pnas.89.8.3488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Israel A, Le Bail O, Hatat D, Piette J, Kieran M, Logeat F, Wallach D, Fellous M, Kourilsky P. EMBO J. 1989;8:3793–3800. doi: 10.1002/j.1460-2075.1989.tb08556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blanar MA, Burkly LC, Flavell RA. Mol Cell Biol. 1989;9:844–846. doi: 10.1128/mcb.9.2.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao J, Freeman GJ, Gray GS, Nadler LM, Glimcher LH. J Exp Med. 1996;183:777–789. doi: 10.1084/jem.183.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li J, Liu Z, Jiang S, Cortesini R, Lederman S, Suciu-Foca N. J Immunol. 1999;163:6386–6392. [PubMed] [Google Scholar]
- 38.Kawai T, Takeuchi O, Fujita T, Inoue J, Muhlradt PF, Sato S, Hoshino K, Akira S. J Immunol. 2001;167:5887–5894. doi: 10.4049/jimmunol.167.10.5887. [DOI] [PubMed] [Google Scholar]
- 39.Ciesielski CJ, Andreakos E, Foxwell BM, Feldmann M. Eur J Immunol. 2002;32:2037–2045. doi: 10.1002/1521-4141(200207)32:7<2037::AID-IMMU2037>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 40.Graham FL, Prevec L. Mol Biotechnol. 1995;3:207–220. doi: 10.1007/BF02789331. [DOI] [PubMed] [Google Scholar]
- 41.James E, Scott D, Chai JG, Millrain M, Chandler P, Simpson E. Int Immunol. 2002;14:1333–1342. doi: 10.1093/intimm/dxf093. [DOI] [PubMed] [Google Scholar]
- 42.Zhong L, Granelli-Piperno A, Choi Y, Steinman RM. Eur J Immunol. 1999;29:964–972. doi: 10.1002/(SICI)1521-4141(199903)29:03<964::AID-IMMU964>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 43.Clarke CJ, Taylor-Fishwick DA, Hales A, Chernajovsky Y, Sugamura K, Feldmann M, Foxwell BM. Eur J Immunol. 1995;25:2961–2966. doi: 10.1002/eji.1830251037. [DOI] [PubMed] [Google Scholar]