Abstract
Genetic susceptibility to rheumatoid arthritis (RA) is associated with certain MHC class II molecules. To clarify the role of these determinants in RA, we generated the D1CC transgenic mouse that expressed genes involved in antigen processing and presentation by the MHC class II pathway in joints. The class II transactivator, which was transcribed from the rat collagen type II promoter and enhancer, directed the expression of these genes. In D1CC mice congenic for the H-2q (DBA/1) background, small amounts of bovine collagen type II in adjuvant induced reproducibly an inflammatory arthritis resembling RA. Importantly, these stimuli had no effect in DBA/1 mice. Eighty-nine percent of D1CC mice developed chronic disease with joint swelling, redness, and heat in association with synovial proliferation as well as pannus formation and mononuclear infiltration of synovial membranes. Granulomatous lesions resembling rheumatoid nodules and interstitial pneumonitis also were observed. As in patients with RA, anticyclic citrullinated peptide antibodies were detected during the inflammatory stage. Finally, joints in D1CC mice displayed juxtaarticular demineralization, severe joint space narrowing, and erosions, which led to ankylosis, but without the appearance of osteophytes. Thus, aberrant expression of MHC class II in joints facilitates the development of severe erosive inflammatory polyarthritis, which is very similar to RA.
Keywords: autoimmunity, class II transactivator, transgenic mouse, nodules, pneumonitis
Rheumatoid arthritis (RA) is a chronic inflammatory disease with symmetrical inflammatory and erosive polyarthritis of synovial joints and a variety of extraarticular manifestations. Particular MHC class II alleles such as DRB0401, DRB0404, and DQ8 are linked to RA in 30–50% of cases (1–3). To examine possible mechanisms of RA, many models of inflammatory arthritis have been developed in the mouse and rat (4, 5). Among these models, collagen-induced arthritis (CIA) leads to acute inflammation, osteophytosis, and destruction of bone in DBA/1 and B10.Q (H2q) strains of mice (6, 7). In these mice, passively transferred autoantibodies against CII, or the injection of a combination of certain monoclonal anti-CII antibodies, also induce rapid inflammation of joints (8, 9). A recently discovered model, the SKG mouse, contains mutations in the ζ chain-associated protein of 70 kDa (ZAP-70) that is involved in the signaling from the T cell antigen receptor (10). In these mice, infection with fungi or immunization with zymosan can induce a chronic inflammatory arthritis (11). In K/BxN mice, chronic arthritis develops spontaneously because anti-G6PI antibodies accumulate in serum and joints, leading to inflammatory arthritis and bone destruction (12, 13). In all these mice and several other models, the onset and progression of inflammatory arthritis have been well characterized and analyzed. However, how faithfully these small animal models resemble RA and what roles MHC class II play in their disease have remained elusive.
MHC class II, DM, and invariant chain (Ii) genes are all regulated at the transcriptional level by the class II transactivator (CIITA) (14). These genes share cis-acting sequences (S, X, and Y boxes) in their promoters and enhancers that bind regulatory factor X (RFX) and nuclear factor (NF-Y) that, in turn recruit CIITA (15, 16). CIITA then binds many coactivators that increase rates of initiation and elongation of MHC class II, DM, and Ii genes. RFX and NF-Y are expressed ubiquitously. In sharp contrast, the expression of CIITA is restricted to antigen-presenting cells and mature B cells (17). Thus, CIITA is the master transcriptional integrator that leads to the expression of genes required for antigen processing and presentation by the MHC class II pathway. Interestingly, the cytokine IFN-γ induces the synthesis of CIITA and, thereby, of MHC class II in many somatic cells, which transforms them to “professional” antigen-presenting cells at sites of inflammation (18).
To determine whether the aberrant expression of MHC class II in joints can lead to or potentiate inflammatory arthritis in mice, we linked the human CIITA gene to the rat CII promoter and enhancer (19). This plasmid construction then was used to create transgenic DBA/1, CII promoter/enhancer-driven CIITA (D1CC) mice, which were analyzed further.
Results
D1CC Mice Express MHC Class II in Joints and Are Highly Susceptible to Immunization with Low Doses of Bovine CII (bCII).
D1CC mice express CIITA and MHC class II in joints (Fig. 6, which is published as supporting information on the PNAS web site). Although spontaneous swelling and redness would occur in isolated joints of most mice, D1CC mice did not develop chronic symmetrical polyarthritis. Similar to SKG and DBA/1 mice (CIA model), the D1CC mice required a trigger to develop inflammatory arthritis. However, because of the aberrant expression of MHC class II in joints, we expected the D1CC mice to be more responsive to the immunization with bCII than parental DBA/1 mice. Thus, we tested progressively lower doses of bovine CII (loCII) in D1CC mice. We used the clinical score in Table 1, which is published as supporting information on the PNAS web site, to monitor the development of inflammatory arthritis (6). Indeed, the D1CC mice developed severe CIA after immunization with 5–10 μg of bCII (loCII-D1CC), a dose that never elicited detectable disease in DBA/1 mice (loCII-DBA/1; Fig. 1A). Thus, the conventional DBA/1 control mice required 20- to 40-fold higher amounts of bCII [200 μg of bCII, high-dose bCII (hiCII)] for the induction of CIA (hiCII-DBA/1, Fig. 1A).
Fig. 1.
Chronic arthritis in the loCII-D1CC mouse. To compare D1CC and DBA/1 mice receiving loCII or hiCII, clinical pictures of each mouse were monitored twice weekly and scored (Table 1). Eight-week-old mice were injected with bCII/complete Freund's adjuvant and boosted with bCII/incomplete Freund's adjuvant 3 weeks later (arrows below the graph). (A) loCII-D1CC but not loCII-DBA/1 mice develop polyarthritis, which persists for a longer period. Graphs are as follows: Line with filled circles, D1CC mouse injected with loCII (loCII-D1CC); bold line with filled squares, DBA/1 mice injected with loCII (loCII-DBA/1); dashed line with filled triangles: DBA/1 mice injected with hiCII (hiCII-DBA/1). Eighty-nine percent of loCII-D1CC but only 57% of hiCII-DBA.1 mice developed arthritis. Our clinical criteria focused on heat, swelling, redness, and functional impairment. (B–E) Joints of loCII-D1CC mouse are swollen. Although parental DBA/1 mice had no phenotype on loCII (D and E, fore limbs and hind limbs, respectively), loCII-D1CC mice developed swollen and red extremities on this regiment (B and C, fore limbs and hind limbs, respectively). (F and G) Joints of loCII-D1CC mouse are hot. Thermographs were performed with joints of loCII-D1CC and loCII-DBA/1 mice. Whereas the maximum temperature at the foot reached 25°C in average in the loCII-DBA/1 mouse (G), it reached 28°C on average in the loCII-D1CC mouse at 8 weeks (F). Arrows in F and G point in the direction of thermographs, from arrow point to arrowhead. Numbers below the graph indicate the distance from arrow point in millimeters. The thermal scale with corresponding temperatures (°C) is presented as colors changing from blue to red to the right of G.
The incidence of disease between sexes was not statistically different (data not presented). Moreover, in loCII-D1CC mice, the peak clinical score of inflammatory arthritis was the same as in hiCII-DBA/1 mice. However, the inflammatory period lasted 34.3 ± 3.7 days in loCII-D1CC mice versus 15.9 ± 2.7 days in hiCII-DBA/1 mice (Fig. 1A). Thus, the duration of inflammation was twice as long in loCII-D1CC mice. The Mann–Whitney U test was used to confirm the significance of this difference (P < 0.01). In addition, the disease incidence was increased from 57.1% in hiCII-DBA/1 mice to 89% in loCII-D1CC or hiCII-D1CC mice (Table 2, which is published as supporting information on the PNAS web site). Whereas redness and swelling were severe in loCII-D1CC mice, no obvious clinical symptoms were observed in loCII-DBA/1 mice (Fig. 1 B–E). We also examined temperatures at various limbs by thermography. Temperatures averaged ≈25°C in loCII-DBA/1 mice and increased up to 28°C in loCII-D1CC mice (Fig. 1 F and G and thermographs below). Taken together, D1CC mice developed chronic inflammatory arthritis with redness, swelling, and fever at the joint after immunization with minimal amounts of bCII. This finding indicates that the aberrant expression of MHC class II in synovial joints facilitates the development of chronic inflammatory arthritis in the D1CC mouse.
Histological Features of Articular Inflammation in loCII-D1CC Mice.
We also examined the progression of disease by histology. At 1 week after the second immunization, there was no inflammation in loCII-DBA/1 mice (Fig. 2 A and D and data not shown). At 8 weeks, infiltration of inflammatory cells, proliferation of synoviocytes with pannus formation, and erosion of bone were observed exclusively in loCII-D1CC, but not loCII-DBA/1 mice (Fig. 2 B and E). In the loCII-D1CC mice, the number of infiltrating cells declined dramatically at 15 weeks (Fig. 2C). However, an abundance of granulocytes (Fig. 2C) and mast cells (Fig. 7, which is published as supporting information on the PNAS web site) had migrated into the pannus at that point, and although the subchondral articular cartilage was severely disrupted at this stage, no subluxation and/or ankylosis was detected until much later. We also found granulomatous lesions resembling rheumatoid nodules in loCII-D1CC but not in loCII-DBA/1 mice (Fig. 2 G and H).
Fig. 2.
Histology of the loCII-D1CC mouse. (A–C) Histology of joints from the loCII-D1CC mouse. Although no changes are observed 1 week after the boost (A), pannus formation over articular cartilage and proliferating synoviocytes (p and s, respectively; B), and thinning of articular cartilage (c; C) are observed at 8 and 15 weeks after the boost, respectively. Bone (b) also is labeled. (D–F) Histology of joints in the loCII-DBA/1 mouse shows no changes at 1 (D), 8 (E), and 15 (F) weeks after the boost. (G and H) Nodules are observed in the loCII-D1CC mouse. Three weeks after the boost, granulomas (g; G) and nodules (h; H) are observed only in the loCII-D1CC mouse.
Interstitial Pneumonitis in loCII-D1CC Mice.
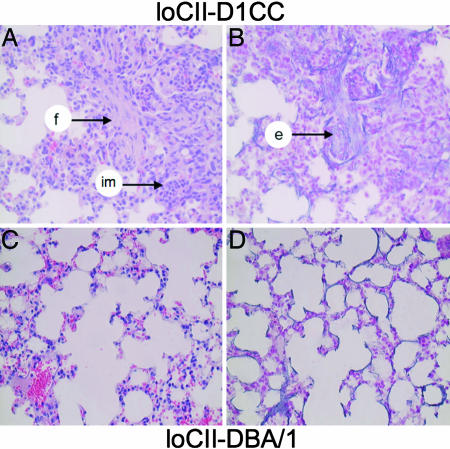
We found interstitial pneumonitis with fibrosis as an extraarticular manifestation of disease only in loCII-D1CC but not in hiCII-DBA/1 mice >6 months after the first immunization (Fig. 3 B and D). There was also a sizeable infiltration of mononuclear cells such as neutrophils and macrophages in these pulmonary lesions. However, because the mice were kept under specific pathogen-free (SPF) conditions and we did not find the deposition of fibrin in D1CC or DBA/1 mice, there was no indication that this inflammation could be due to infection (Fig. 3 A and C). However, newly synthesized elastic fibers were identified in loCII-D1CC mice by elastica and Kernechtrot staining (Fig. 3B). Because interstitial pneumonitis has not been described with CIA but can be found in severe RA, this feature again documents the similarities in inflammatory lesions between loCII-D1CC mice and RA in humans.
Fig. 3.
Interstitial pneumonitis is observed only in loCII-D1CC mice. Histology of lungs 6 months after the boost in loCII-D1CC and loCII-DBA/1 mice. Fibrosis, infiltrating mononuclear cells (f and im, arrow, respectively; A), and newly produced elastic fiber (e, arrow; B) are observed in lungs of the loCII-D1CC mouse but not in those of the loCII-DBA/1 mouse (C and D). A and C were stained with HE. B and D were stained with elastica and kernechtrot stains to visualize elastic fibers.
Bone Destruction and Decreased Mineral Density in loCII-D1CC Mice.
Next, we demonstrated the severity of articular pathology by radiography. In loCII-D1CC mice, decreased mineral density was observed at 18 and 39 weeks after the immunization but not at earlier stages of inflammation (Fig. 4 A–D). To substantiate further these radiographic findings, we also performed computed tomographic (CT) scans focused on the large knee joints. Bone destruction, joint space narrowing, and erosions were observed at the patella, distal femur, proximal tibia, and fibula (Fig. 4 E–J; see Movies 1–3, which are published as supporting information on the PNAS web site). In addition, relatively dark-gray areas that represent decreased mineral densities paralleled the progression of bone erosions. Osteophytes and “rebound” new bone formation such as arthrodysplasia were found only in hiCII-DBA/1 mice (Fig. 4 I and J and Movies 1–3) (7). To confirm the osteoporosis at the knee joint, the bone mineral density of cancellous bone in each mouse was calculated from CT scan data. The bone mineral density declined immediately after immunization in hiCII-DBA/1 mice (Fig. 4K). It decreased by ≈10% and then recovered at 3 months. In contrast, it decreased by 25% at 3 months and lasted at least 6 months in loCII-D1CC mice. At the terminal stage of chronic inflammatory arthritis, severe ankylosis also was observed at the proximal interphalangeal and metacarpophalangeal joints in loCII-D1CC mice (Fig. 5 A and B). Even though they were observed for >1 year, we did not detect any osteoporosis or bone erosions in loCII-DBA/1 mice. Thus, the articular deterioration of the loCII-D1CC mouse, in contrast to that of CIA, seems to be chronic and progressive, similar to the joint destruction seen in severe active RA.
Fig. 4.
Joint space narrowing, erosions, and osteoporosis in the loCII-D1CC mouse. (A–D) Progressive joint damage in the loCII-D1CC mouse. Radiographic examination reveals the decline of bone mineral density and joint space narrowing and erosions in the fore limbs at 0 (A), 9 (B), 18 (C), and 39 (D) weeks after the first injection. (E–J) Joint space narrowing, erosions, and osteoporosis in the knee of the loCII-D1CC mouse 24 weeks after the boost. (E and F) CT scans are presented for the distal femur, the knee joint, the proximal tibia, and the fibula. Note the narrowing of the joint space (E, left arrow), erosions, and osteoporosis (E and F, right arrows). None of these changes are observed in the parental loCII-DBA/1 mouse treated likewise (G and H). In sharp contrast, hiCII-DBA/1 mice developed erosions and formed new bone resembling osteophytes 12 weeks after the boost (I and J, arrows). (K) Progressive osteoporosis in the loCII-D1CC mouse. Presented is the comparison of decreased periarticular bone mineral density over time between ldCIl-D1CC and hiCII-DBA/1 mice. Ten sections of cancellous bone measuring 0.1 mm each were analyzed by densitometry of CT scans from both knees in each mouse. Times after the boost are presented below the bar graphs. Data are presented as percentage of bone mineral density of loCII-D1CC (black bars) and hiCII-DBA/1 (gray bars) compared with the untreated parental DBA/1 mouse (WT was given the value of 100%; open bar) over time. Six mice were studied in each group. Error bars reflect SEM. All values were calculated by using data from three or four mice. Student's t test was performed for each value between loCII-D1CC and hiCII-DBA/1 mice (∗, P < 0.05).
Fig. 5.
Fore limbs of the loCII-D1CC mouse reveal extensive joint destruction. Fifteen months after the boost, fore limbs of loCII-D1CC (A) and loCII-DBA/1 (B) mice were stained with Alizarin and Alcian blue, which stain bone red and cartilage blue. Note the complete destruction of all joints (ankylosis, a, arrows) and shortening of phalanges in the loCII-D1CC mouse and normal joints in loCII-DBA/1 mice.
Discussion
In this report, we present the D1CC small animal model of inflammatory arthritis. In these mice, the entire machinery for antigen processing and presentation by the MHC class II pathway was activated aberrantly via transgenic expression of CIITA. First, the D1CC mice containing ≈10 copies of the CIITA transgene expressed easily detectable CIITA transcripts and MHC class II in joints (Fig. 6). Second, chronic inflammatory arthritis developed in 89% of male and female loCII-D1CC mice (as contrasted to 57% of CIA in hiCII-DBA/1 mice) after immunization with small doses of bCII that had no effect on parental DBA/1 mice. Third, not only did this arthritis start slower and last longer than CIA in hiCII-DBA/1 mice, but it resulted in characteristic articular and extraarticular manifestations very closely resembling those of severe RA in humans. In loCII-D1CC mice, chronic symmetrical polyarthritis eventually led to ankylosis and destruction of affected joints. Finally, as in RA, D1CC mice developed antibodies to CCP (Table 3, which is published as supporting information on the PNAS web site). Importantly, unlike other models of inflammatory arthritis (5), we did not introduce any mutations into the mouse. Thus, our work returns the emphasis of RA back to the normal and aberrant immune responses in the host. We propose that D1CC mice may represent an attractive additional animal model for the study of RA.
Histologically, inflammatory infiltrates and proliferating synoviocytes leading to granulomatous lesions resembling rheumatoid nodules were observed only in loCII-D1CC mice. Even though the presence of palisading histiocytes surrounding necrotic nodules could not be ascertained, similar pathology has been reported with pristane-induced arthritis, MRL-lpr/lpr, and SKG mice but not with CIA in DBA/1 mice (11, 20, 21). In contrast to the SKG mice, the D1CC mice had no skin inflammation. Slowly progressive inflammatory arthritis also led to easily identifiable migration of mast cells into the pannus. Because these mast cells expressed MHC class II and B7.1, they might potentially function as antigen-presenting cells (Fig. 7; refs. 22 and 23).
We also observed interstitial pneumonitis with fibrosis and deposition of newly synthesized elastic fibers in the lungs of loCII-D1CC mice. These lesions lacked fibrin, which can be related to infection. Serological analyses revealed the presence of anti-CII and CCP antibodies in sera of both loCII-D1CC and hiCII-DBA/1 mice (Table 3). Notably, the concentration of anti-CCP antibodies was significantly higher in loCII-D1CC than in hiCII-DBA/1 mice, suggesting a more sustained inflammatory process in joints of loCII-D1CC mice. However, attempts to measure the concentration of cytokines and anti-CII antibodies in the synovial fluid were not successful. Radiographic examinations and CT scans revealed further similarities between the arthritis in the loCII-D1CC mouse and RA. These data included periarticular osteoporosis, joint space narrowing, and severe destruction of bone without osteophytosis, all of which led eventually to ankylosis. Although the erosive destruction of bone started in the earlier stages of inflammation at 8 weeks, this bone erosion could only be detected by histology and not yet radiographically (Figs. 2B and 4 A–D and K). Recently, similar microscopic joint damage was detected by MRI in RA patients (24–26).
In a subgroup of RA patients, MHC class II represent a critical genetic risk factor for the susceptibility to the disease (27). Moreover, the expression of these HLAs (HLA class II) have been detected in chondrocytes of RA patients (28, 29). From our study, we hypothesize that the expression of MHC class II on target organs can increase their sensitivity to arthritogenic stimuli in general. This observation might explain the spontaneous inflammation of individual joints without immunization in D1CC mice, some of which persisted for prolonged periods of time and could have resulted from local trauma (data not presented). Moreover, increased sensitivity also might explain the rather severe bone destruction we found in loCII-D1CC mice. In contrast, acute inflammation induces osteophytosis in CIA in DBA/1 mice, where bone resorption is more transient and new bone formation is enhanced soon thereafter (7, 30). Finally, joints in loCII-D1CC but not loCII-DBA/1 progressed to ankylosis and severe functional impairment. We found severe bone destruction in almost all metacarpophalangeal joint and in >60–80% of the proximal interphalangeal joints. These data suggest that lower doses of bCII lead to a more chronic arthritis in the more susceptible D1CC than DBA/1 mice, which is reminiscent of a slow smoldering course of progressive joint destruction seen in severe RA (4, 31).
D1CC mice also provide an excellent breeding partner for other models of inflammatory arthritis in the mouse. Because the balance between Th1 and Th2 was not significantly different in D1CC mice, aberrant expression of MHC class II together with abnormal selection of T cells such as survival of autoreactive clones will be investigated further. For example, the hCIITA transgene also might influence other types of arthritis in mice and possibly render the arthritis in other animal models, such as SKG mouse, spontaneous (20, 32, 33). Moreover to create even better and more mouse models of RA, D1CC mice may be backcrossed with the DRB1*0401 or DRB1*0402, and human CD4 double-transgenic mice, which lack I-Aβ−/− on the DBA/1 background (34, 35). Both DR genes are expressed from the mouse I-Eα promoter and enhancer, thus aberrant CIITA expression will induce the expression of DR*0401 or DRB1*0402 in their chondrocytes (36). DRB1*0401 is not only one of the susceptible genes for RA but is also the molecule that presents citrullinated arthritogenic antigens. However, DRB1*0402 does not present the same peptides. Thus, these DR alleles will help us to dissect the contributions between specific arthritogenic stimuli and MHC class II from RA patients. Also, specific peptides that are presented by aberrantly expressed MHC class II in humanized D1CC mice will be used to tolerize these mice to these antigens, which then can be evaluated for effects of such therapeutic vaccination on their disease.
Materials and Methods
CIA.
For the induction of CIA, D1CC and DBA/1 mice at 7 to 9 weeks of age were housed in a pathogen-free animal care facility of Nagoya City University Graduate School of Medical Sciences in accordance with institutional guidelines. These mice were anesthetized with diethyl ether before induction of CIA. At that point, they were immunized with bCII (5–10 μg, called loCII for D1CC and DBA/1 mice and 200 μg, called hiCII for DBA/1 mice) (Collagen Research Center, Tokyo, Japan), which was emulsified with an equal volume of complete Freund's adjuvant (Difco Laboratories, Detroit, MI). Mice were injected intradermally at the base of the tail, near inguinal and axillary lymph nodes on day 0 as the first injection. On day 21, the mice were boosted with the bCII in the same manner except that incomplete Freund's adjuvant (Difco Laboratories) was used as the solvent.
Evaluation of Joint Arthritis.
DBA/1 and D1CC mice were monitored from the onset of disease to the end of active inflammation twice weekly. The clinical severity of arthritis was quantified according to this simple scoring system: 0, no clinical symptom; 1, swelling and redness of one or two joints; 2, moderate swelling and redness of more than three joints; 3, severe swelling and redness of entire paw (6). All scores were added to the total clinical score, which reached the maximum at 12 (four severely affected joints, 4 × 3). The onset of arthritis was defined as the number of days between the first immunization with bCII and the occurrence of arthritis with a clinical score of >3. The end of acute arthritis was noted when the clinical score was reduced to 3. Body temperatures were measured at all extremities by using the thermograph (Neo Thermo; Nippon Anionics, Tokyo, Japan).
Immunohistochemistry.
For immunohistochemical staining, the avidin–biotin–peroxidase complex (ABC) method was used according to protocols from the manufacturer (Vector Laboratories, Burlingame, CA) (37). Paraffin sections were rehydrated and washed with tap water for 5 min. The endogenous peroxidase activity was inhibited by first incubating samples in 0.05% of H2O2 solution for 30 min and then washing with PBS for 10 min at room temperature. Thin sections were incubated with buffer C (1.5% normal serum in PBS) for 60 min, and primary antibodies (biotin-conjugated mouse anti-mouse I-Aq monoclonal antibody, BD Biosciences, San Jose, CA) were diluted to 1:6 with the blocking buffer (supplied by Vector Laboratories) for 30 min and washed with PBS for 10 min. All incubation steps with antibodies were performed in a humid chamber. Signals were detected by using a commercial ABC kit and DAB solution (Mouse on Mouse Immunodetection kits PK-2200 and SK-4100, respectively; Vector Laboratories). All specimens were counterstained with hematoxylin and mounted in VectaMount (H-5000; Vector Laboratories). As the negative control, isotype-matched antibodies (biotin-conjugated mouse IgG2b, monoclonal Ig isotype control; BD Biosciences Pharmingen) were used.
Radiographic Technique.
The computed radiographs (CR) were obtained by using the CR console (FCR5000plus; Fujifilm, Tokyo, Japan) and the high-resolution CR cassette (Fujifilm). The source of x-rays was a conventional radiographic unit and exposure factors were 50 kV, 50 mA, and 40 ms (Shimadzu, Kyoto, Japan).
Bone Mineral Density and CT.
We used peripheral quantitative computed tomography with a fixed x-ray fan beam of 10-mm spot size, at 1 mA and 50kVp (LaTheta LCT-100S; Aloka, Tokyo, Japan). Eighty slices (480 × 480-pixel matrix per slice, 0.1 mm thickness, a voxel size of 65 × 65 × 65 μm3) covered the entire knee joint and recreated the 3D CT picture of the joint by using the VGStudio MAX1.2 software (Nihon Visual Science, Tokyo, Japan). To measure the mineral density of bones at the joint from each mouse, data were recalculated from the distal femur and the proximal tibia, both adjoining the articular cartilage.
Histochemical Staining.
For H&E staining, paraffin sections were rehydrated and immersed first into the Mayer's hematoxylin solution (Muto Pure Chemicals Co., Ltd., Tokyo, Japan) for 5 min and then into the eosin solution (Muto Pure Chemicals Co., Ltd.) for 2 min. For elastical and kernechtrot staining, paraffin sections also were rehydrated. After washing with 1% of hydrochloric acid and 70% ethanol for 5 min, sections were incubated with resorcin fuchsin solution (Muto Pure Chemicals Co., Ltd.) for 60 min, washed with ethanol, and counterstained with Kernechtrot stain solution (Muto Pure Chemicals Co., Ltd.) for 2 min. For toluidine blue staining, sections were rehydrated and washed with tap water for 5 min and incubated with 0.05% of toluidine blue O solution (Waldeck, Muenster, Germany) for 10 min. All sections were dehydrated with ethanol and mounted in VectaMount. To count the number of infiltrated mast cells in the joint, the five most-visible areas of mast cells were selected in toluidine blue stained histological slides. The number of mast cells then were counted in all extremities in D1CC and DBA/1 mice and visualized in a bar graph. For cartilage and bone staining, specimens were immersed in 95% of ethanol for >5 days and in acetone for another 2 days. Skin, muscle, and fat were removed carefully and stained with Alcian blue (0.3% of Alcian blue 8GX; Sigma–Aldrich, St. Louis, MO) and Alizarin red (0.1% Alizarin sodium monosulfonate; Sigma–Aldrich) for 3 days. Finally, specimens were immersed in the 1% KOH solution and glycerol for 2 days.
Statistical Analyses.
All measurements were performed in duplicate, and all experiments were repeated at least three times. All error bars give SEM. Mann–Whitney U test was used for the statistical analysis of disease-related parameters between control and arthritic mice. The histomorphometric data and the serum titers of anti-CII antibodies between the control and the arthritic mice were compared by Student's t test. Values of P < 0.05 were considered to be statistically significant.
Supporting Information.
Additional data can be found in Supporting Materials and Methods, which is published as supporting information on the PNAS web site.
Supplementary Material
Acknowledgments
We thank M. Vadeboncoeur for the generation of D1CC mice; S. Fujii, K. Yuzawa, M. Sakamoto, Y. Miyahara, and S. Imai for outstanding technical support; H. Otsuka and Y. Tachikawa for quantitative CT analyses (Aloka, Tokyo, Japan); and William Seaman and Hugh O. McDevitt for critical discussions and comments on the manuscript. This work was supported by grants-in-aid from the Ministry of Health, Labor, and Welfare and Ministry of Education, Culture, Sports, Science, and Technology (Japan), and the Nora Eccles Treadwell Foundation and National Institutes of Health Grants R01 AI050770 and AR44647.
Abbreviations
- CII
collagen type II
- bCII
bovine CII
- hiCII
high-dose bCII
- loCII
lower doses of bCII
- CIITA
class II transactivator
- CCP
cyclic citrullinated peptide
- CIA
collagen-induced arthritis
- CT
computed tomographic
- D1CC
DBA/1, CII promoter/enhancer-driven CIITA
- RA
rheumatoid arthritis.
Footnotes
The authors declare no conflict of interest.
References
- 1.van Zeben D, Hazes JM, Zwinderman AH, Cats A, Schreuder GM, D'Amaro J, Breedveld FC. Arthritis Rheum. 1991;34:822–830. doi: 10.1002/art.1780340707. [DOI] [PubMed] [Google Scholar]
- 2.Feldmann M, Brennan FM, Maini RN. Cell. 1996;85:307–310. doi: 10.1016/s0092-8674(00)81109-5. [DOI] [PubMed] [Google Scholar]
- 3.Silman AJ, Pearson JE. Arthritis Res. 2002;4(Suppl 3):S265–S272. doi: 10.1186/ar578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Firestein GS. J Clin Invest. 2004;114:471–474. doi: 10.1172/JCI22651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Monach PA, Benoist C, Mathis D. Adv Immunol. 2004;82:217–248. doi: 10.1016/S0065-2776(04)82005-4. [DOI] [PubMed] [Google Scholar]
- 6.Wooley PH, Luthra HS, Stuart JM, David CS. J Exp Med. 1981;154:688–700. doi: 10.1084/jem.154.3.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanyu T, Chotanaphuti T, Arai K, Tanaka T, Takahashi HE. Bone. 1999;24:485–490. doi: 10.1016/s8756-3282(99)00006-x. [DOI] [PubMed] [Google Scholar]
- 8.Holmdahl R, Jansson L, Larsson A, Jonsson R. Scand J Immunol. 1990;31:147–157. doi: 10.1111/j.1365-3083.1990.tb02754.x. [DOI] [PubMed] [Google Scholar]
- 9.Terato K, Hasty KA, Reife RA, Cremer MA, Kang AH, Stuart JM. J Immunol. 1992;148:2103–2108. [PubMed] [Google Scholar]
- 10.Sakaguchi N, Takahashi T, Hata H, Nomura T, Tagami T, Yamazaki S, Sakihama T, Matsutani T, Negishi I, Nakatsuru S, Sakaguchi S. Nature. 2003;426:454–460. doi: 10.1038/nature02119. [DOI] [PubMed] [Google Scholar]
- 11.Yoshitomi H, Sakaguchi N, Kobayashi K, Brown GD, Tagami T, Sakihama T, Hirota K, Tanaka S, Nomura T, Miki I, et al. J Exp Med. 2005;201:949–960. doi: 10.1084/jem.20041758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Korganow AS, Ji H, Mangialaio S, Duchatelle V, Pelanda R, Martin T, Degott C, Kikutani H, Rajewsky K, Pasquali JL, et al. Immunity. 1999;10:451–461. doi: 10.1016/s1074-7613(00)80045-x. [DOI] [PubMed] [Google Scholar]
- 13.Matsumoto I, Staub A, Benoist C, Mathis D. Science. 1999;286:1732–1735. doi: 10.1126/science.286.5445.1732. [DOI] [PubMed] [Google Scholar]
- 14.Fontes JD, Kanazawa S, Nekrep N, Peterlin BM. Microbes Infect. 1999;1:863–869. doi: 10.1016/s1286-4579(99)00232-4. [DOI] [PubMed] [Google Scholar]
- 15.Jabrane-Ferrat N, Fontes JD, Boss JM, Peterlin BM. Mol Cell Biol. 1996;16:4683–4690. doi: 10.1128/mcb.16.9.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu XS, Linhoff MW, Li G, Chin KC, Maity SN, Ting JP. Mol Cell Biol. 2000;20:6051–6061. doi: 10.1128/mcb.20.16.6051-6061.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanazawa S, Peterlin BM. Microbes Infect. 2001;3:467–473. doi: 10.1016/s1286-4579(01)01402-2. [DOI] [PubMed] [Google Scholar]
- 18.Steimle V, Siegrist CA, Mottet A, Lisowska-Grospierre B, Mach B. Science. 1994;265:106–109. doi: 10.1126/science.8016643. [DOI] [PubMed] [Google Scholar]
- 19.Horton W, Miyashita T, Kohno K, Hassell JR, Yamada Y. Proc Natl Acad Sci USA. 1987;84:8864–8868. doi: 10.1073/pnas.84.24.8864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wooley PH, Seibold JR, Whalen JD, Chapdelaine JM. Arthritis Rheum. 1989;32:1022–1030. doi: 10.1002/anr.1780320812. [DOI] [PubMed] [Google Scholar]
- 21.Hang L, Theofilopoulos AN, Dixon FJ. J Exp Med. 1982;155:1690–1701. doi: 10.1084/jem.155.6.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee DM, Friend DS, Gurish MF, Benoist C, Mathis D, Brenner MB. Science. 2002;297:1689–1692. doi: 10.1126/science.1073176. [DOI] [PubMed] [Google Scholar]
- 23.Tetlow LC, Woolley DE. Ann Rheum Dis. 1995;54:896–903. doi: 10.1136/ard.54.11.896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Forslind K, Larsson EM, Eberhardt K, Johansson A, Svensson B. Scand J Rheumatol. 2004;33:154–161. doi: 10.1080/03009740410006862. [DOI] [PubMed] [Google Scholar]
- 25.Ostergaard M, Ejbjerg B, Szkudlarek M. Best Pract Res Clin Rheumatol. 2005;19:91–116. doi: 10.1016/j.berh.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 26.Guermazi A, Taouli B, Lynch JA, Peterfy CG. Semin Musculoskelet Radiol. 2004;8:269–285. doi: 10.1055/s-2004-861575. [DOI] [PubMed] [Google Scholar]
- 27.Ridgway WM, Fasso M, Fathman CG. Science. 1999;284:749–751. doi: 10.1126/science.284.5415.749. [DOI] [PubMed] [Google Scholar]
- 28.Allard SA, Muirden KD, Camplejohn KL, Maini RN. Rheumatol Int. 1987;7:153–159. doi: 10.1007/BF00270363. [DOI] [PubMed] [Google Scholar]
- 29.Lance EM, Kimura LH, Manibog CN. Clin Orthop Relat Res. 1993;291:266–282. [PubMed] [Google Scholar]
- 30.Enokida M, Yamasaki D, Okano T, Hagino H, Morio Y, Teshima R. Bone. 2001;28:87–93. doi: 10.1016/s8756-3282(00)00406-3. [DOI] [PubMed] [Google Scholar]
- 31.Weyand CM, Hicok KC, Conn DL, Goronzy JJ. Ann Intern Med. 1992;117:801–806. doi: 10.7326/0003-4819-117-10-801. [DOI] [PubMed] [Google Scholar]
- 32.Keystone EC, Schorlemmer HU, Pope C, Allison AC. Arthritis Rheum. 1977;20:1396–1401. doi: 10.1002/art.1780200714. [DOI] [PubMed] [Google Scholar]
- 33.Eming R, Visconti K, Hall F, Sekine C, Kobayashi K, Chen Q, Cope A, Kanazawa S, Peterlin M, Rijnders A, et al. Arthritis Res. 2002;4(Suppl 3):S133–S140. doi: 10.1186/ar580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sonderstrup G. Springer Semin Immunopathol. 2003;25:35–45. doi: 10.1007/s00281-003-0129-z. [DOI] [PubMed] [Google Scholar]
- 35.Fugger L, Michie SA, Rulifson I, Lock CB, McDevitt GS. Proc Natl Acad Sci USA. 1994;91:6151–6155. doi: 10.1073/pnas.91.13.6151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kouskoff V, Fehling HJ, Lemeur M, Benoist C, Mathis D. J Immunol Methods. 1993;166:287–291. doi: 10.1016/0022-1759(93)90370-m. [DOI] [PubMed] [Google Scholar]
- 37.Ando K, Kanazawa S, Tetsuka T, Ohta S, Jiang X, Tada T, Kobayashi M, Matsui N, Okamoto T. Oncogene. 2003;22:7796–7803. doi: 10.1038/sj.onc.1206965. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.